Highly Efficient Photocatalytic and Antimicrobial AgGaCl Tri-Doped ZnO Nanorods for Water Treatment under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. NRs Characterization
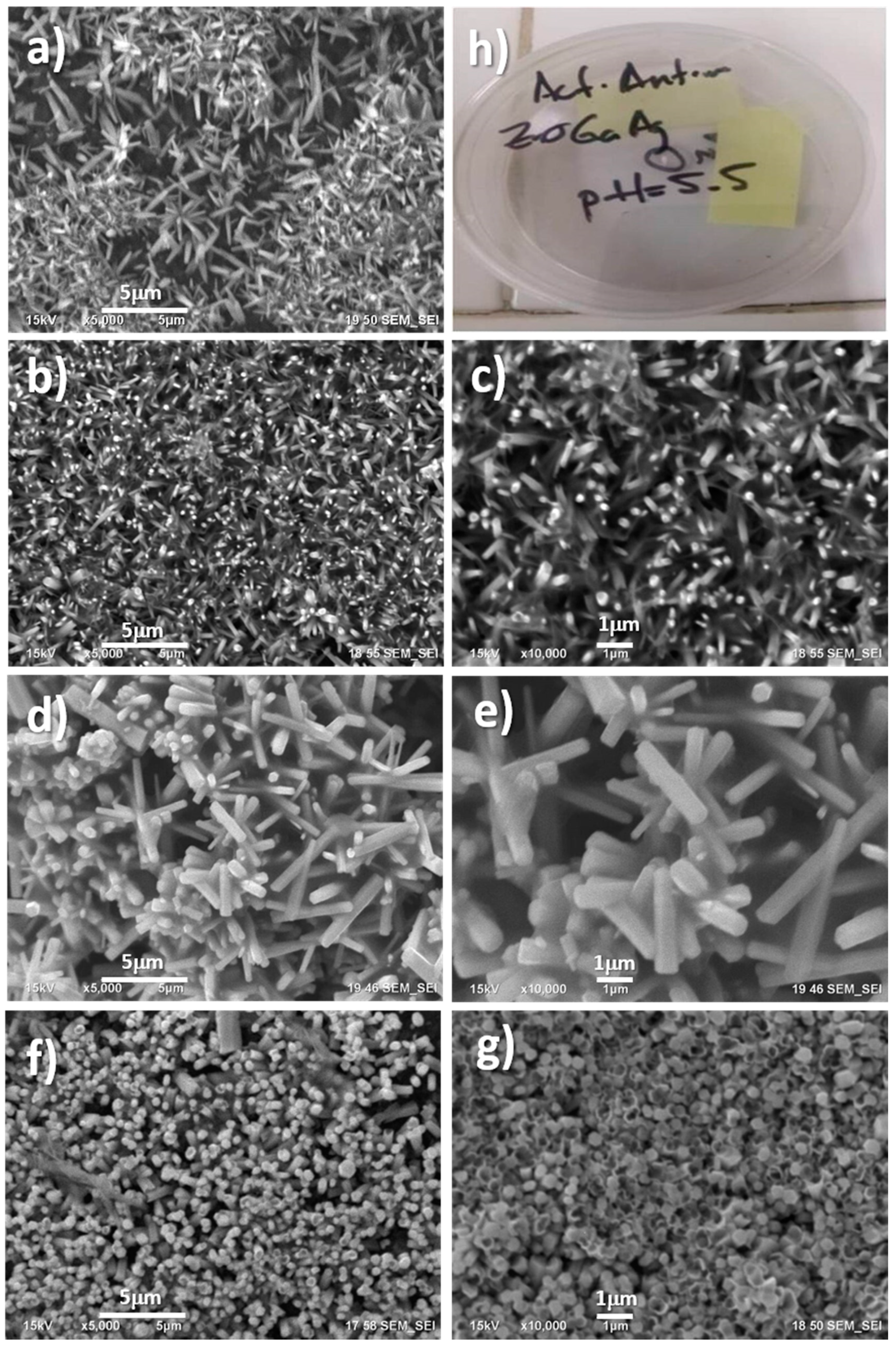
2.1.1. Morphology
2.1.2. Chemical Composition
2.1.3. Structure
2.1.4. Optical Properties
2.2. NRs Photocatalytic Activity
2.3. NRs Antimicrobial Activity
2.4. Comparative of Photocatalytic and Antimicrobial Properties
3. Materials and Methods
3.1. Microwave Assisted Doped ZnO NRs Synthesis on Polyethylene Substrate
3.2. NRs Characterization
3.3. NRs Photocatalytic Performance
3.4. Antimicrobial Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarzenbach, R.; Egli, T.; Hofstetter, T.B.; Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. J. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean Water 2019, 2, 8. [Google Scholar] [CrossRef]
- Dimapilis, E.A.; Hsu, C.; Mendoza, R.M.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review, Environ. Nanotech. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Gómez-Avilés, A.; Peñas-Garzón, M.; Rodriguez, J.J. Chapter 22—Semiconductor Photocatalysis for Water Purification, In Micro and Nano Technologies. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–651. [Google Scholar]
- Lin, J.; Luo, Z.; Liu, J.; Li, P. Photocatalytic degradation of methylene blue in aqueous solution by using ZnO-SnO2 nanocomposites. Mater. Sci. Semicond. Process. 2018, 87, 24–31. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B 2016, 194, 134–140. [Google Scholar] [CrossRef]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Ratova, M.; Redfern, J.; Verran, J.; Kelly, P.J. Highly efficient photocatalytic bismuth oxide coatings and their antimicrobial properties under visible light irradiation. Appl. Catal. B 2018, 239, 223–232. [Google Scholar] [CrossRef]
- Türkyılmaz, S.S.; Güy, O.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Ashebir, M.E.; Tesfamariam, G.M.; Nigussie, G.Y.; Tesfakiros, W.G. Structural, Optical, and Photocatalytic Activities of Ag-Doped and Mn-Doped ZnO Nanoparticles. J. Nanomater. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Cardoza-Contreras, M.; Vásquez-Gallegos, A.; Vidal-Limon, A.; Romo-Herrera, J.M.; Águila, S.; Contreras, O.E. Photocatalytic and antimicrobial properties of Ga doped and Ag doped ZnO nanorods for water treatment. Catalysts 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Durkin, D.P.; Elenewski, J.E.; Sun, Y.; Banek, N.A.; Hua, L.; Chen, H.; Wagner, M.J.; Zhang, W.; Shuai, D. Visible-Light-Responsive Graphitic Carbon Nitride: Rational Design and Photocatalytic Applications for Water Treatment. Environ. Sci. Technol. 2016, 50, 12938–12948. [Google Scholar] [CrossRef]
- Xua, J.; Shan, Y.; Sun, X.; Xu, J.; Yin, H.; Wang, L.; Wang, W. Highly photocatalytic activity of porous skeleton structure AlON powder synthesized by CRN under ultraviolet-light irradiation. Mol. Catal. 2018, 457, 17–23. [Google Scholar] [CrossRef]
- Islama, M.R.; Rahman, M.; Farhad, S.F.U.; Podder, J. Structural, optical and photocatalysis properties of sol–gel deposited Al doped ZnO thin films. Surf. Interfaces 2019, 16, 120–126. [Google Scholar] [CrossRef]
- Chitambar, C. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver Nanoparticles (AgNP) in the Environment: A Review of Potential Risks on Human and Environmental Health. Water Air Soil. Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Zhou, S.; Shao, Y.; Gao, N.; Li, L.; Deng, J.; Zhu, M.; Zhu, S. Effect of chlorine dioxide on cyanobacterial cell integrity, toxin degradation and disinfection by-product formation. Sci. Total Environ. 2014, 482, 208–213. [Google Scholar] [CrossRef]
- Slavin, Y.S.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odlaug, T.E. Antimicrobial activity of halogens. J. Food Prot. 1981, 44, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Maris, P. Modes of action of desinfectants. Rev. Sci. Tech. Off. Int. Epiz. 1995, 14, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a chemical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407–409. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurišić, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef] [PubMed]
- Tchelidze, T.; Chikoidze, E.; Gorochov, O.; Galtier, P. Perspectives of chlorine doping of ZnO. Thin Solid Film 2007, 515, 8744–8747. [Google Scholar] [CrossRef]
- Jiamprasertboon, A.; Powell, M.J.; Dixon, S.C.; Quesada-Cabrera, R.; Alotaibi, A.M.; Lu, Y.; Zhuang, A.; Sathasivam, S.; Siritanon, T.; Parkin, I.P.; et al. Photocatalytic and electrically conductive transparent Cl-doped ZnO thin films via aerosol assisted chemical vapour deposition. J. Mater. Chem. A 2018, 6, 12682. [Google Scholar] [CrossRef] [Green Version]
- Gray, N.F. Chapter Thirty-Two—Chlorine Dioxide. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Eds.; Academic Press: London, UK, 2014; pp. 591–598. ISBN 9780124158467. [Google Scholar]
- Backer, H.D. Chapter 7—Water Disinfection. In The Travel and Tropical Medicine Manual, 5th ed.; Sanford, C.A., Pottinger, P.S., Jong, E.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–111. ISBN 9780323375061. [Google Scholar]
- Ogata, N. Denaturation of Protein by Chlorine Dioxide: Oxidative Modification of Tryptophan and Tyrosine Residues. Biochemistry 2007, 46, 4898–4911. [Google Scholar] [CrossRef]
- Davies, J.M. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Therap. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Cho, M.; Lee, S.S.; Zhang, F.; Biswas, P.; Fortner, J.D. Crumpled Graphene Oxide Composite Membranes for Filtration and Disinfection Applications. Environ. Sci. Technol. 2016, 50, 2514–2521. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wen, J.Z.; Zhao, P.; Anderson, W.A. Synthesis of Vertically-Aligned Zinc Oxide Nanowires and Their Application as a Photocatalyst. Nanomaterials 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakhki, R.M.; Tayebee, R.; Ahsani, F. New and highly efficient Ag doped ZnO visible nano photocatalyst for removing of methylene blue. J. Mater. Sci. 2017, 28, 5941. [Google Scholar]






| Photocatalysts and/or Antimicrobial Materials | Suspended or Immobilized | Irradiation | Pollutant | Bacteria | % of Degraded Contaminant and Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|---|
| Transition metal doped ZnO NPs | Suspended | Visible | MB | E. coli | Cu-doped ZnO NPs 93% of MB degradation after 120 min (did not mention the initial MB concentration) and E. coli survival cell density reduction in 4 h. | [34] |
| Ag NPs by crumpled GO−TiO2 | Immobilized | Visible | None | E. coli and B. subtilis | Bacteria inactivation. Ag NPs regeneration and antifouling properties. | [35] |
| Bismuth tungstate and titanium dioxide coatings on glass | Immobilized | Visible | Rhodamine B | E. coli | Reduced the number of viable E. coli cells in suspension to below the limit of detection in the first 48 h of irradiation | [10] |
| ZnO nanowires on glass | Immobilized | UV | Methyl Orange (5 mg/L) | - | 96% of dye was degraded after 4 h of irradiation | [36] |
| ZnO and Ag doped ZnO nanoparticles | Suspended | Visible | MB (10−5 M) | - | 98% of dye was degraded by 0.5 mol% Ag-doped ZnO NPs in 180 min | [37] |
| Ag doped ZnO NRs and Ga-doped ZnO NRs on PE | Immobilized | Visible | MB (6 mg/L) | E. coli Vibrio spp. | 2 at% Ag doped ZnO NRs degraded 40% dye in 5 h. 2 at% Ag doped ZnO NRs and 2 at% Ga-doped ZnO NRs reduced 50% of bacteria growth on both strain cultures. | [13] |
| AgGaCl-doped ZnO NRs on PE | Immobilized | Visible | MB (100 mg/L) | Vibrio parahaemolyticus | AgGaCl-doped ZnO NRs (pH 5.5) degraded approximately 75% of MB solution in 5 h. AgGaCl-doped ZnO NRs completely inhibit the bacteria growth for 12 h. | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoza-Contreras, M.N.; Sánchez-Serrano, S.; Contreras, O.E. Highly Efficient Photocatalytic and Antimicrobial AgGaCl Tri-Doped ZnO Nanorods for Water Treatment under Visible Light Irradiation. Catalysts 2020, 10, 752. https://doi.org/10.3390/catal10070752
Cardoza-Contreras MN, Sánchez-Serrano S, Contreras OE. Highly Efficient Photocatalytic and Antimicrobial AgGaCl Tri-Doped ZnO Nanorods for Water Treatment under Visible Light Irradiation. Catalysts. 2020; 10(7):752. https://doi.org/10.3390/catal10070752
Chicago/Turabian StyleCardoza-Contreras, Marlene N., Samuel Sánchez-Serrano, and Oscar E. Contreras. 2020. "Highly Efficient Photocatalytic and Antimicrobial AgGaCl Tri-Doped ZnO Nanorods for Water Treatment under Visible Light Irradiation" Catalysts 10, no. 7: 752. https://doi.org/10.3390/catal10070752




