Identifying Iron-Bearing Nanoparticle Precursor for Thermal Transformation into the Highly Active Hematite Photo-Fenton Catalyst
Abstract
:1. Introduction
2. Results and Discussions
2.1. The Crystalline Structure and Morphology of Iron-Bearing Nanoparticle Precursors and Photo-Fenton Catalysts
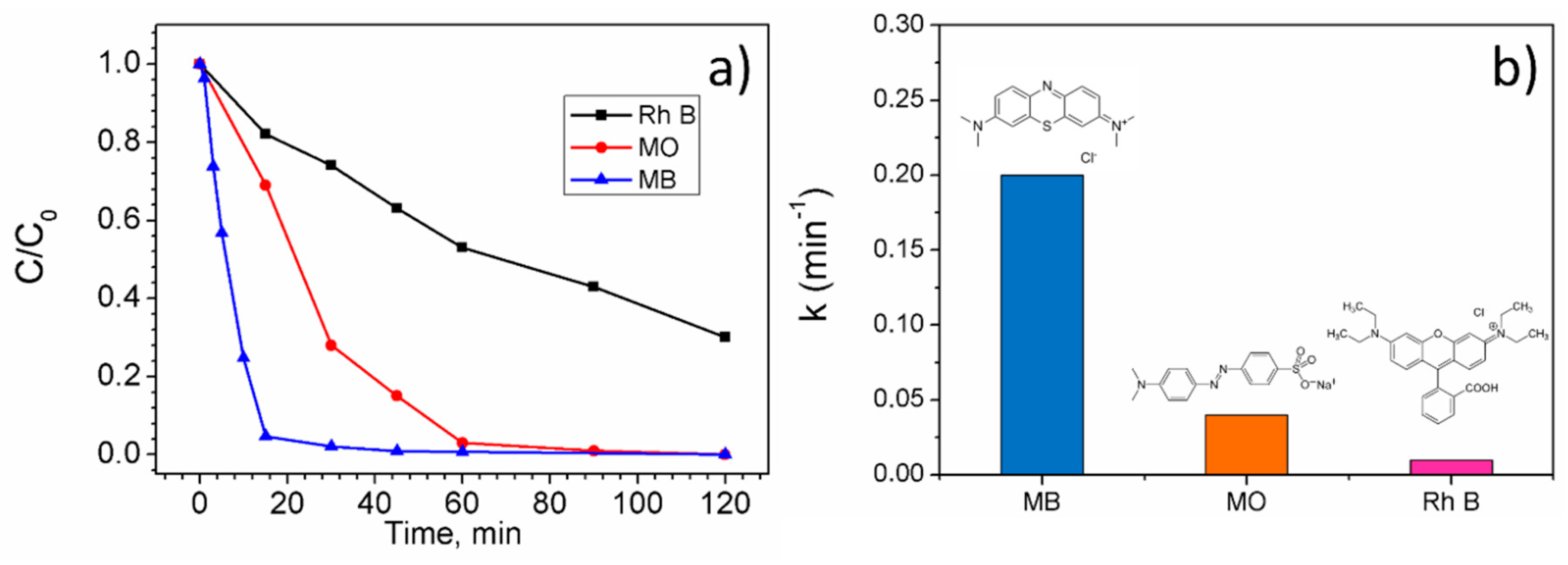
2.2. Photocatalytic and Photo-Fenton Activity
3. Materials and Methods
3.1. Sample Synthesis
3.2. Sample Characterisation
3.3. Photo-Fenton and Photocatalysis Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nidheesh, P. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, Y.; Huan, W.; Li, J.; Zhang, J.; Xing, M. Hollow-structured Fe2O3/Au/SiO2 nanorods with enhanced and recyclable photo-Fenton oxidation for the remediation of organic pollutants. Mater. Today Chem. 2019, 11, 86–93. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Chen, J.; Kong, L.; Lu, J.; Ding, F.; Xiong, Y. Fe2(MoO4)3 as an Effective Photo-Fenton-like Catalyst for the Degradation of Anionic and Cationic Dyes in a Wide pH Range. Ind. Eng. Chem. Res. 2013, 52, 13333–13341. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Cui, Z.; Wang, S.; Cao, M. Facet effect of a-Fe2O3 crystals on photocatalytic performance in the photo-Fenton reaction. RSC Adv. 2014, 4, 34387–34394. [Google Scholar] [CrossRef]
- Zhou, X.; Lan, J.; Liu, G.; Deng, K.; Yang, Y.; Nie, G.; Yu, J.; Zhi, L. Facet-mediated photodegradation of organic dye over hematite architectures by visible light. Angew. Chem. Int. Ed. 2012, 51, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Changotra, R.; Varshney, L.; Guin, J.P.; Dhir, A. Performance of hematite particles as an Iron source for the degradation of ornidazole in photo-fenton process. J. Sol-Gel Sci. Technol. 2018, 85, 203–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Wang, T.; Huang, H.; Chen, Y.; Li, Z.; Zou, Z. Heterogeneous degradation of organic contaminants in the photo-Fenton reaction employing pure cubic β-Fe2O3. Appl. Catal. B Environ. 2019, 245, 410–419. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Ge, M.; Wu, S.; Guan, J.; Tang, J.; Wu, X.; Ritchie, R.O. Facile self-assembly synthesis of γ Fe2O3/graphene oxide for enhanced photo-Fenton reaction. Environ. Pollut. 2019, 248, 229–237. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.; Sienkiewicz, A.; Bensimon, M.; Colmenares, J.J.V.; Benítez, N.; Pulgarin, C. Iron oxides semiconductors are efficients for solar water disinfection: A comparison with photo-Fenton processes at neutral Ph. Appl. Catal. B Environ. 2015, 166–167, 497–508. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Yuan, B.; Shen, C.; Fu, M.; Cui, H.; Sun, W. Large scale preparation of Cu-doped α-FeOOH nanoflowers and their photo-Fenton-like catalytic degradation of diclofenac sodium. Chem. Eng. J. 2016, 291, 174–183. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Fang, D.; Xu, J.; Liang, J.; Zhou, L. Microwave-ultrasound assisted synthesis of β-FeOOH and its catalytic property in a photo-Fenton-like process. Ultrason. Sonochem. 2015, 27, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Dükkancı, M. Sono-photo-Fenton oxidation of bisphenol-A over a LaFeO3 perovskite catalyst. Ultrason. Sonochem. 2018, 40, 110–116. [Google Scholar]
- Soltani, T.; Lee, B.-K. Enhanced formation of sulfate radicals by metal-doped BiFeO3 under visible light for improving photo-Fenton catalytic degradation of 2-chlorophenol. Chem. Eng. J. 2017, 313, 1258–1268. [Google Scholar] [CrossRef]
- Sharma, R.; Bansal, S.; Singhal, S. Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M = Cu, Zn, Ni and Co) in the structure. RSC Adv. 2015, 5, 6006–6018. [Google Scholar] [CrossRef]
- Anchieta, C.G.; Severo, E.C.; Rigo, C.; Mazutti, M.A.; Kuhn, R.C.; Müller, E.I.; Flores, E.M.; Moreira, R.D.F.P.M.; Foletto, E.L. Rapid and facile preparation of zinc ferrite (ZnFe2O4) oxide by microwave-solvothermal technique and its catalytic activity in heterogeneous photo-Fenton reaction. Mater. Chem. Phys. 2015, 160, 141–147. [Google Scholar] [CrossRef]
- Wen, Y.; Zhao, Y.; Guo, M.; Xu, Y. Synergetic effect of Fe2O3 and BiVO4 as photocatalyst nanocomposites for improved photo-Fenton catalytic activity. J. Mater. Sci. 2019, 54, 8236–8246. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Lu, F.; Qin, J.; Wei, F.; Xu, C.; Wang, S. Magnetic ZnFe2O4−C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light. Ind. Eng. Chem. Res. 2014, 53, 17294–17302. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, W.; Zhang, M.; Lin, Y.; Xu, N.; Chen, F.; Wang, D.; Chen, Z. Adsorption and synergetic Fenton-like degradation of methylene blue by a novel mesoporous α-Fe2O3/SiO2 at neutral pH. Ind. Eng. Chem. Res. 2018, 57, 5539–5549. [Google Scholar] [CrossRef]
- Zandi, O.; Hamann, T.W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 2016, 8, 778–783. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiangyong, H.; Chen, H. Elimination of estrogen and its estrogenicity by heterogeneous photo-Fenton catalyst β-FeOOH/resin. J. Photoch. Photobio. A Chem. 2012, 212, 94–100. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chang, Z.; Sun, X.; Li, Y. Crystal plane effect of Fe2O3 with various morphologies on CO catalytic oxidation. Catal. Commun. 2011, 12, 530–534. [Google Scholar] [CrossRef]
- Mao, Y.; Wong, S.S. Size-and shape-dependent transformation of nanosized titanate into analogous anatase titania nanostructures. J. Am. Chem. Soc. 2006, 128, 8217–8226. [Google Scholar] [CrossRef] [PubMed]
- Zboril, R.; Mashlan, M.; Petridis, D. Iron(III) oxides from thermal processes synthesis, structural and magnetic properties, Mössbaueer Spectroscopy Characterization, and Applications. Chem. Mater. 2002, 14, 969–982. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, R.; Li, J.; Su, Y.; Liu, X. Microwave-assisted synthesis and characterization of hexagonal Fe3O4 nanoplates. Solid State Sci. 2010, 12, 99–104. [Google Scholar] [CrossRef]
- Šutka, A.; Järvekülg, M.; Gross, K.A.; Kook, M.; Käämbre, T.; Visnapuu, M.; Trefalt, G.; Šutka, A. Visible light to switch-on desorption from goethite. Nanoscale 2019, 11, 3794–3798. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Bin Wu, H.; Yu, L.; Xu, R.; Lim, T.-T.; Lou, X.W. Template-free formation of uniform urchin-like α-FeOOH hollow spheres with superior capability for water treatment. Adv. Mater. 2012, 24, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.J.; Sun, L.D.; Yan, Z.G.; You, L.P.; Luo, F.; Han, X.D.; Yan, C.H.; Han, X.-D.; Pang, Y.-C.; Zhang, Z. Single-crystalline iron oxide nanotubes. Angew. Chem. Int. Ed. 2005, 44, 4328–4333. [Google Scholar] [CrossRef]
- Šutka, A.; Vanags, M.; Joost, U.; Smits, K.; Ruža, J.; Locs, J.; Kleperis, J.; Juhna, T. Aqueous synthesis of Z-scheme photocatalyst powders and thin-film photoanodes from earth abundant elements. J. Environ. Chem. Eng. 2018, 6, 2606–2615. [Google Scholar] [CrossRef]
- Cornell, R.M.; Giovanoli, R.; Schneider, W. Review of the hydrolysis of iron(III) and the crystallization of Amorphous Iron(III) Hydroxide Hydrate. J. Chem. Tech. Biotechnol. 1989, 46, 115–134. [Google Scholar] [CrossRef]
- Johnston, J.H.; Lewis, D.G. A detailed study of the transformation of ferrihydrite to haernatite in an aqueous medium at 92 °C. Geochim. Cosmochim. Acta 1983, 47, 1823–1831. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mat. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. Chem. Sus. Chem. 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Puma, G.L.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Leelavathi, A.; Madras, G.; Ravishankar, N. Origin of enhanced photocatalytic activity and photoconduction in high aspect ratio ZnO nanorods. Phys. Chem. Chem. Phys. 2013, 15, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH Verlag: Weinhein, Germany, 2003. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šutka, A.; Šutka, A.; Vanags, M.; Spule, A.; Eglītis, R.; Vihodceva, S.; Šmits, K.; Tamm, A.; Mežule, L. Identifying Iron-Bearing Nanoparticle Precursor for Thermal Transformation into the Highly Active Hematite Photo-Fenton Catalyst. Catalysts 2020, 10, 778. https://doi.org/10.3390/catal10070778
Šutka A, Šutka A, Vanags M, Spule A, Eglītis R, Vihodceva S, Šmits K, Tamm A, Mežule L. Identifying Iron-Bearing Nanoparticle Precursor for Thermal Transformation into the Highly Active Hematite Photo-Fenton Catalyst. Catalysts. 2020; 10(7):778. https://doi.org/10.3390/catal10070778
Chicago/Turabian StyleŠutka, Anna, Andris Šutka, Mārtiņš Vanags, Arnita Spule, Raivis Eglītis, Svetlana Vihodceva, Krišjānis Šmits, Aile Tamm, and Linda Mežule. 2020. "Identifying Iron-Bearing Nanoparticle Precursor for Thermal Transformation into the Highly Active Hematite Photo-Fenton Catalyst" Catalysts 10, no. 7: 778. https://doi.org/10.3390/catal10070778





