Biocatalytic Epoxidation of Cyclooctene to 1,2-Epoxycyclooctane by a Newly Immobilized Aspergillus niger Lipase
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Catalytic Performance of ANL@ZnGlu-MNPs on the Epoxidation of Different Substrates
2.2. Effect of Substrate Concentrations on the Epoxidation of Cyclooctene by ANL@ZnGlu-MNPs
2.3. Effect of Reaction Temperatures on the Epoxidation of Cyclooctene by ANL@ZnGlu-MNPs
2.4. Effect of ANL@ZnGlu-MNPs Doses on the Epoxidation of Cyclooctene
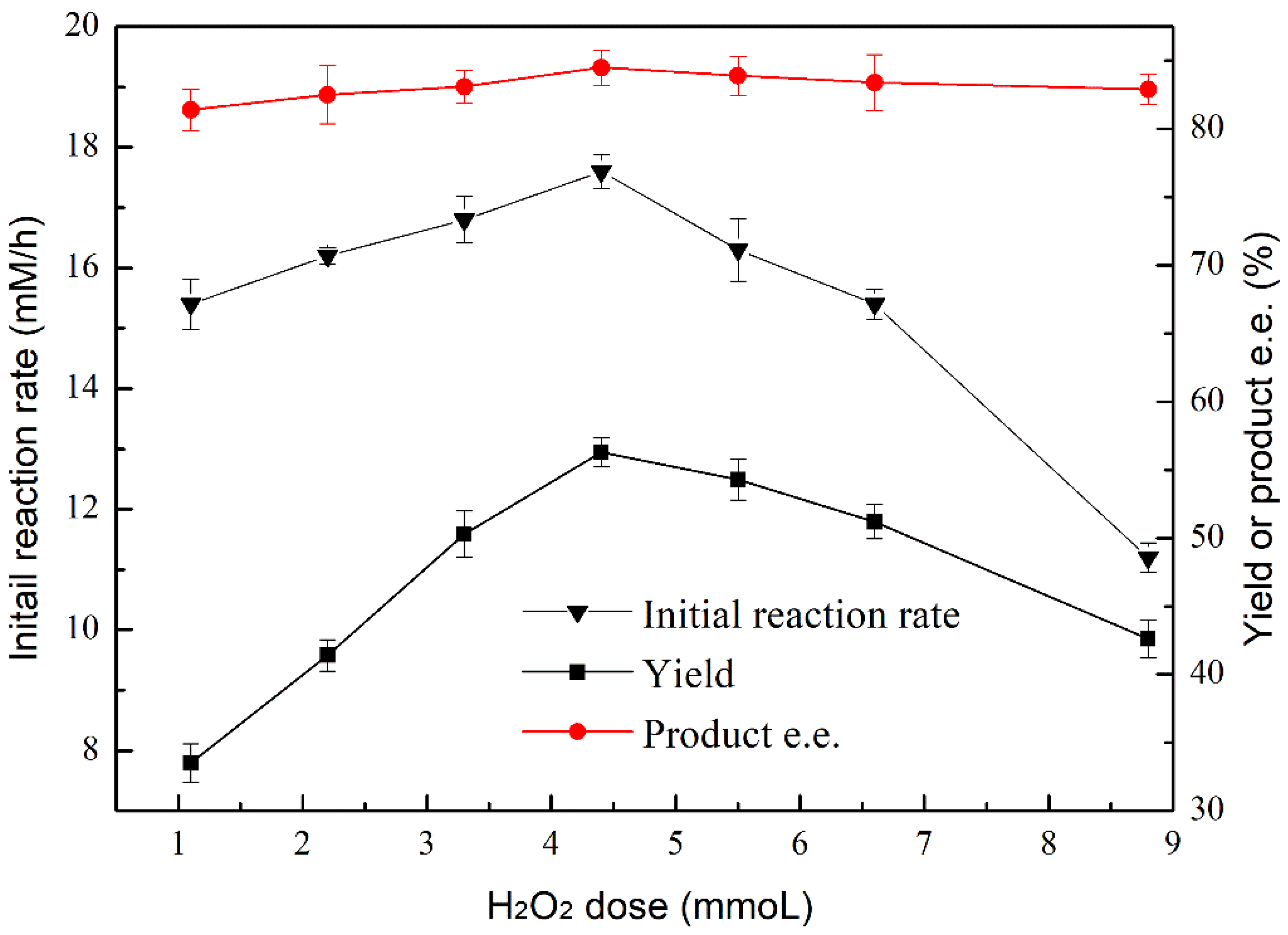
2.5. Effect of H2O2 Doses on the Epoxidation of Cyclooctene by ANL@ZnGlu-MNPs
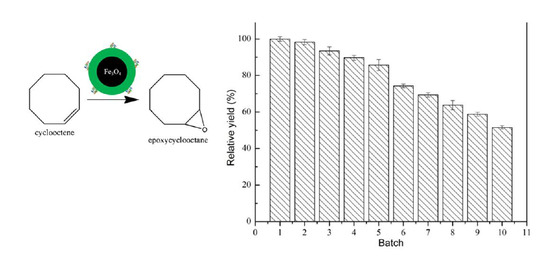
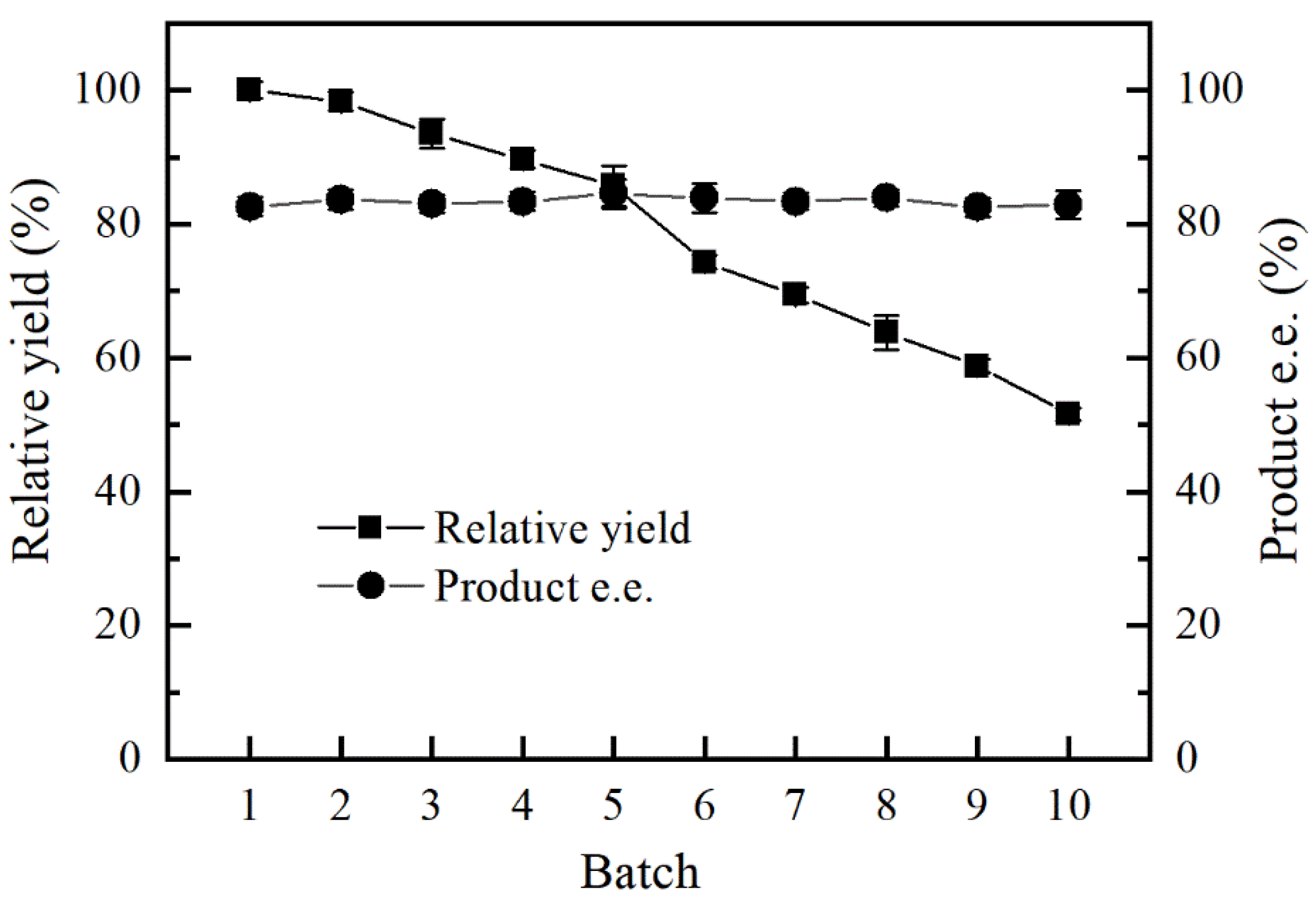
2.6. Operational Stability of ANL@ZnGlu-MNPs
3. Material and Methods
3.1. Material
3.2. Screening of Substrates
3.3. Reaction Parameter of ANL@ZnGlu-MNPs
3.4. Operational Stability of ANL@ZnGlu-MNPs
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| e.e. | enantiomeric excess |
| ANL | Aspergillus niger lipase |
| ZnGlu | Zn-glutamate |
| MNPs | magnetic iron oxide nanoparticles |
| ZnGlu-MNPs | ZnGlu-coated MNPs |
| ANL@ZnGlu-MNPs | ANL immobilized on ZnGlu-MNPs |
References
- Chen, W.J.; Lou, W.Y.; Zong, M.H. Efficient asymmetric hydrolysis of styrene oxide catalyzed by Mung bean epoxide hydrolases in ionic liquid-based biphasic systems. Bioresour. Technol. 2012, 115, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, Q.; Popowicz, G.M.; Yang, B.; Wang, Y. A mechanistic study into the epoxidation of carboxylic acid and alkene in a mono, di-acylglycerol lipase. Biochem. Biophys. Res. Commun. 2015, 460, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Xu, Y.; Mu, X.Q. Highly enantioselective conversion of racemic 1-phenyl-1,2-ethanediol by stereoinversion involving a novel cofactor-dependent oxidoreduction system of Candida parapsilosis CCTCC M203011. Org. Proc. Res. Dev. 2004, 8, 246–251. [Google Scholar] [CrossRef]
- Cao, S.L.; Yue, D.M.; Li, X.H.; Smith, T.J.; Li, N.; Zong, M.H.; Wu, H.; Lou, W.Y. Novel nano-/micro-biocatalyst: Soybean epoxide hydrolase immobilized on UiO-66-NH2 MOF for efficient biosynthesis of enantiopure (R)-1,2-octanediol in deep eutectic solvents. ACS Sustain. Chem. Eng. 2016, 4, 3586–3595. [Google Scholar] [CrossRef]
- Ren, W.M.; Liang, M.W.; Xu, Y.C.; Lu, X.B. Trivalent cobalt complex mediated formation of stereoregular CO2 copolymers from phenyl glycidyl ether. Polym. Chem. 2013, 4, 4425–4433. [Google Scholar] [CrossRef]
- Louis, E.C.; Washington, N.J. Manufacture of 1,2-Epoxycyclooctane. U.S. Patent 2571208, 16 October 1951. [Google Scholar]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.W.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Aouf, C.; Durand, E.; Lecomte, J.; Figueroa-Espinoza, M.C.; Dubreucq, E.; Fulcrand, H.; Villenuve, P. The use of lipases as biocatalysts for the epoxidation of fatty acids and phenolic compounds. Green Chem. 2014, 16, 1740–1754. [Google Scholar] [CrossRef] [Green Version]
- Zanette, A.F.; Zampakidi, I.; Sotiroudis, G.T.; Zoumpanioti, M.; Leal, I.C.R.; de Souza, R.O.M.A.; Cardozo-Filho, L.; Xenakis, A. Chemo-enzymatic epoxidation catalyzed by C. antarctica lipase immobilized in microemulsion-based organogels. J. Mol. Catal. B Enzym. 2014, 107, 89–94. [Google Scholar] [CrossRef]
- Abdullah, B.M.; Salih, N.; Salimon, J. Optimization of the chemoenzymatic mono-epoxidation of linoleic acid using D-optimal design. J. Saudi Chem. Soc. 2014, 18, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Ankudey, E.G.; Olivo, H.F.; Peeples, T.L. Lipase-mediate epoxidation utilizing urea-hydrogen peroxide in ethyl acetate. Green Chem. 2006, 8, 923–926. [Google Scholar] [CrossRef]
- Hua, X.; Ying, Y.; Zhang, X. Enhanced promiscuity of lipase-inorganic nanocrystal composites in the epoxidation of fatty acids in organic media. ACS Appl. Mater. Interfaces 2016, 8, 16257–16261. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Cui, L.; Jia, S.; Su, Z.; Zhang, S. Hybrid cross-linked lipase aggregates with magnetic nanoparticles: A robust and recyclable biocatalysis for epoxidation of oleic acid. J. Agric. Food Chem. 2016, 64, 7179–7187. [Google Scholar] [CrossRef]
- Cao, S.L.; Huang, Y.M.; Li, X.H.; Xu, P.; Wu, H.; Li, N.; Lou, W.Y.; Zong, M. Preparation and Characterization of Immobilized Lipase from Pseudomonas Cepacia onto Magnetic Cellulose Nanocrystals. Sci. Rep. 2016, 6, 20420. [Google Scholar] [CrossRef] [Green Version]
- Macrae, A.R.; Hammond, R.C. Present and future applications of lipases. Biotechnol. Genet. Eng. 1985, 3, 193–218. [Google Scholar] [CrossRef]
- Tudorache, M.; Gheorghe, A.; Viana, A.S.; Parvulescu, V.I. Biocatalytic epoxidation of α-pinene to oxy-derivatives over cross-linked lipase aggregates. J. Mol. Catal. B Enzym. 2016, 134, 9–15. [Google Scholar] [CrossRef]
- Silva, W.S.D.; Lapis, A.A.M.; Suarez, P.A.Z.; Neto, B.A.D. Enzyme-mediated epoxidation of methyl oleate supported by imidazolium-based ionic liquids. J. Mol. Catal. B Enzym. 2011, 68, 98–103. [Google Scholar] [CrossRef]
- Törnvall, U.; Orellana-Coca, C.; Hatti-Kaul, R.; Adlercreutz, D. Stability of immobilized Candida antarctica lipase B during chemo-enzymatic epoxidation of fatty acids. Enzym. Microb. Technol. 2007, 40, 447–451. [Google Scholar] [CrossRef]
- Wang, X.T.; Chen, X.H.; Xu, Y.; Lou, W.Y.; Wu, H.; Zong, M.H. Biocatalytic anti-prelog stereoselective reduction of ethyl acetoacetate catalyzed by whole cells of Acetobacter sp. CCTCC M209061. J. Biotechnol. 2013, 163, 292–300. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhang, X.Y.; Li, N.; Xu, P.; Lou, W.Y.; Zong, M.H. Biocatalytic reduction of hmf to 2,5-bis(hydroxymethyl)furan by HMF-tolerant whole cells. ChemSusChem 2017, 10, 372. [Google Scholar] [CrossRef]
- Vlček, T.; Petrović, Z.S. Optimization of the chemoenzymatic epoxidation of soybean oil. J. Am. Oil Chem. Soc. 2006, 83, 247–252. [Google Scholar] [CrossRef]
- Orellana-Coca, C.; Camocho, S.; Adlercreutz, D.; Mattiasson, B.; Hatti-Kaul, R. Chemo-enzymatic epoxidation of linoleic acid: Parameters influencing the reaction. Eur. J. Lipid Sci. Technol. 2005, 107, 864. [Google Scholar] [CrossRef]
- Xia, G.H.; Cao, S.L.; Xu, P.; Li, X.H.; Zhou, J.; Zong, M.H.; Lou, W.Y. Preparation of a nanobiocatalyst by efficiently immobilizing Aspergillus niger lipase onto magnetic metal-biomolecule frameworks (BioMOF). ChemCatChem 2017, 9, 1797–1800. [Google Scholar] [CrossRef]



| Olefins | Structure | Yield (%) | e.e. (%) |
|---|---|---|---|
| 1-octene |  | 33.8 | 56.3 (R) |
| 2-phenyl-1-propene | 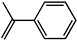 | 27.6 | 70.1 (S) |
| cyclooctene |  | 47.2 | 80.4 (1R,2R) |
| styrene |  | 23.4 | 85.8 (S) |
| 1-methyl-1-cyclohexene | 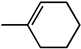 | 17.9 | 86.1 (1R,2R) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Peng, F.; Li, F.; Xia, G.; Zong, M.; Lou, W. Biocatalytic Epoxidation of Cyclooctene to 1,2-Epoxycyclooctane by a Newly Immobilized Aspergillus niger Lipase. Catalysts 2020, 10, 781. https://doi.org/10.3390/catal10070781
Chen Q, Peng F, Li F, Xia G, Zong M, Lou W. Biocatalytic Epoxidation of Cyclooctene to 1,2-Epoxycyclooctane by a Newly Immobilized Aspergillus niger Lipase. Catalysts. 2020; 10(7):781. https://doi.org/10.3390/catal10070781
Chicago/Turabian StyleChen, Qingsheng, Fei Peng, Fangzhou Li, Gaohui Xia, Minhua Zong, and Wenyong Lou. 2020. "Biocatalytic Epoxidation of Cyclooctene to 1,2-Epoxycyclooctane by a Newly Immobilized Aspergillus niger Lipase" Catalysts 10, no. 7: 781. https://doi.org/10.3390/catal10070781