CoMn Catalysts Derived from Hydrotalcite-Like Precursors for Direct Conversion of Syngas to Fuel Range Hydrocarbons
Abstract
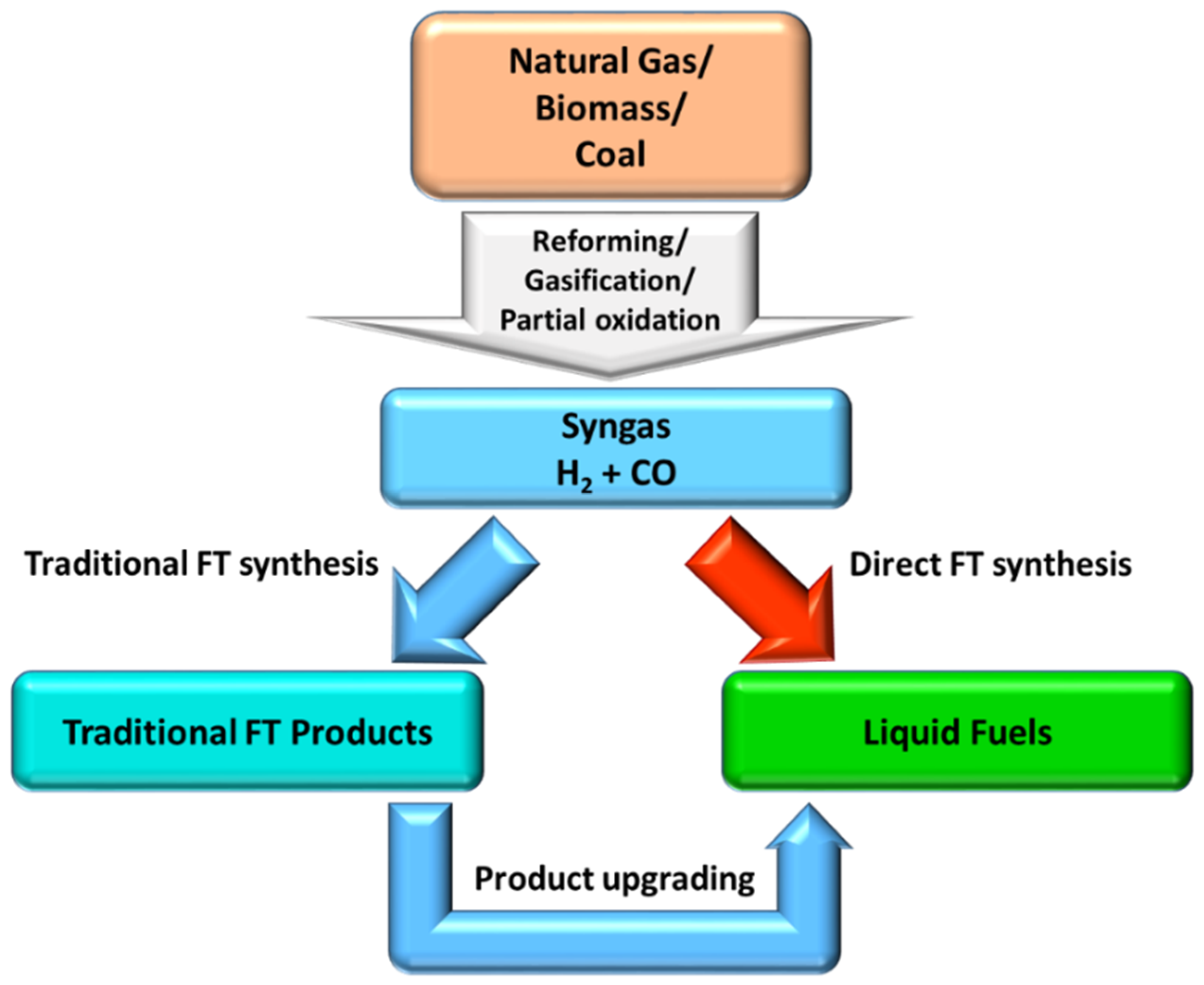
1. Introduction
2. Results and Discussion
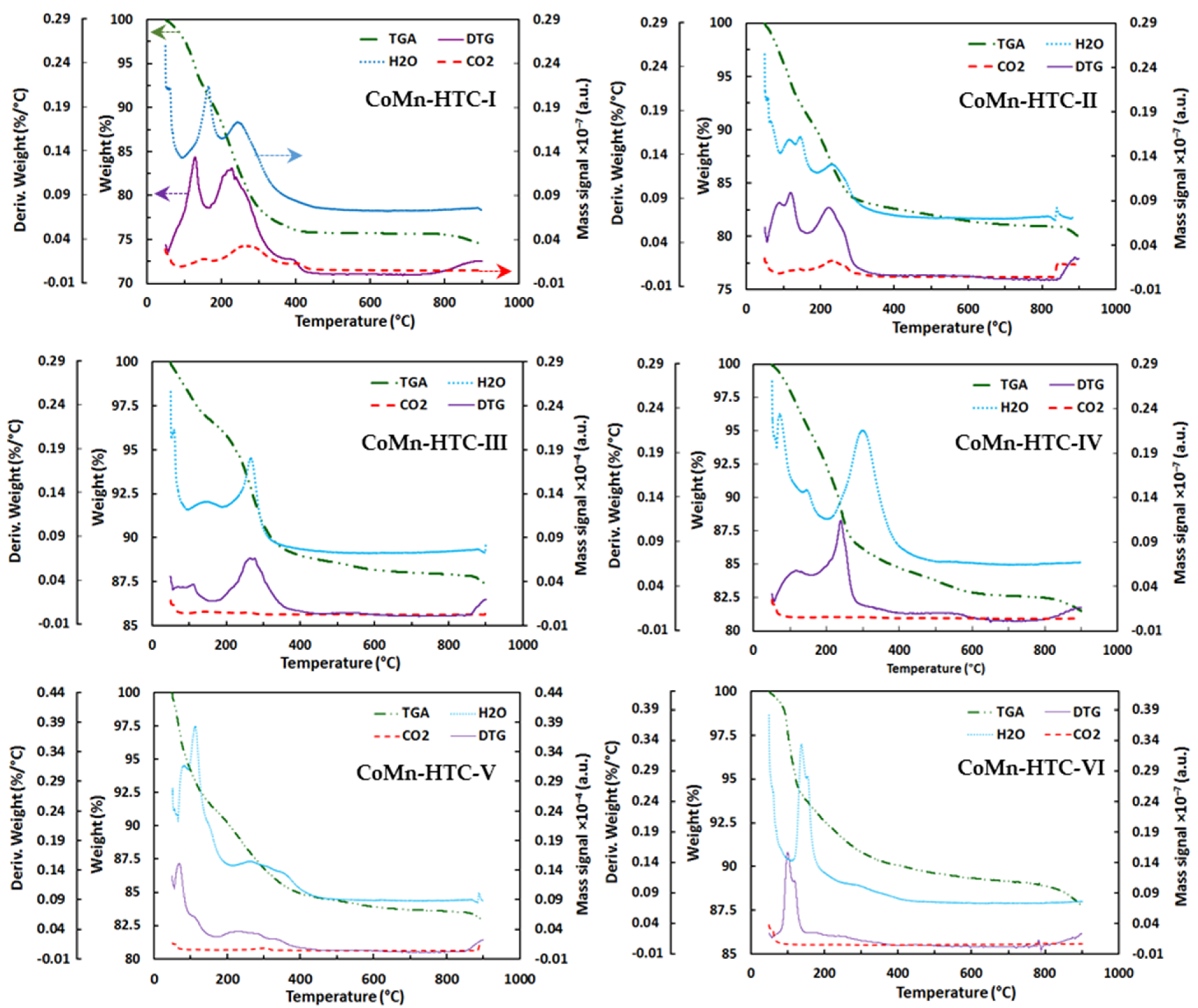
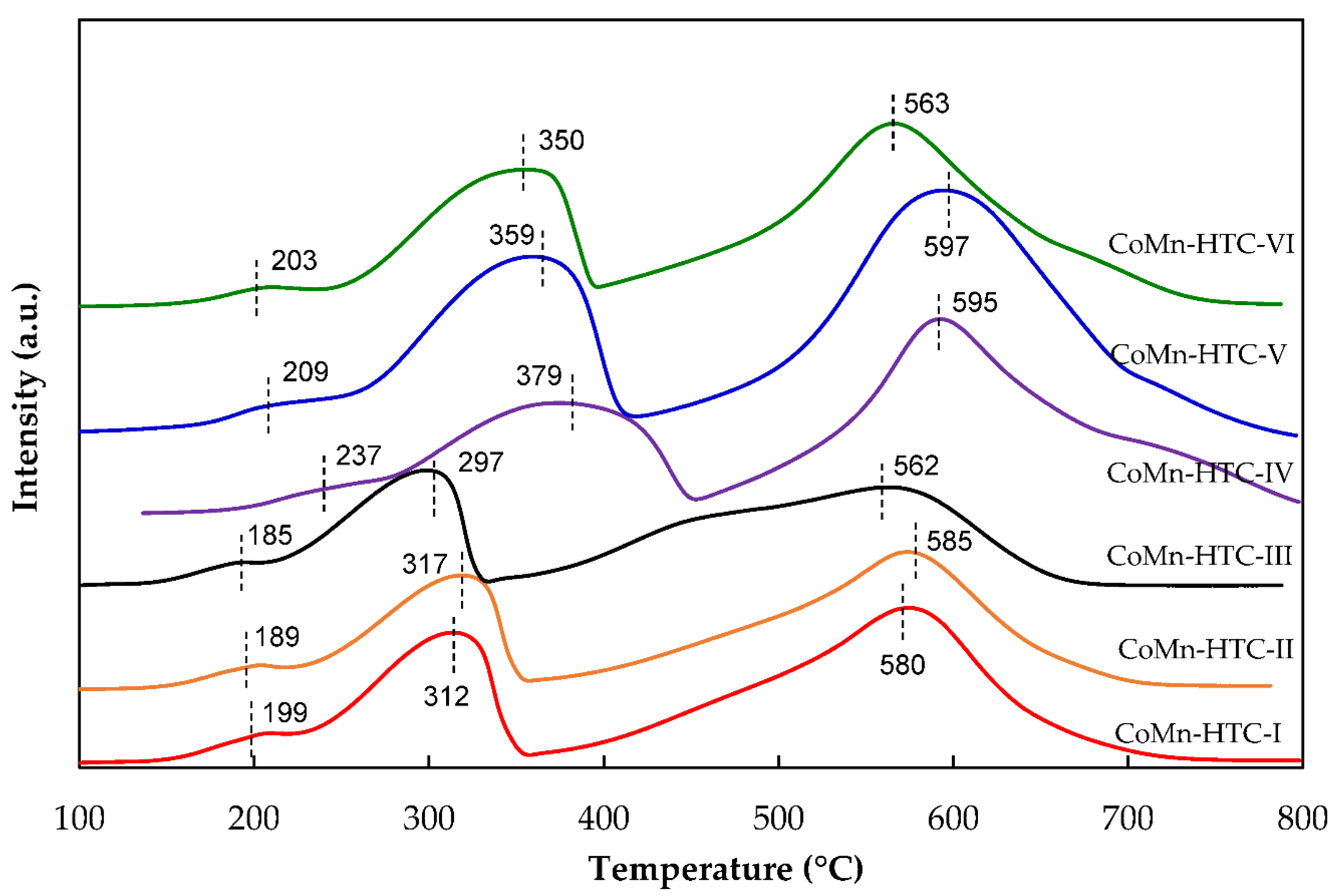
2.1. Characterization of Catalysts
2.2. Catalytic Evaluation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterizations
3.3. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vosoughi, V.; Badoga, S.; Dalai, A.K.; Abatzoglou, N. Modification of mesoporous alumina as a support for cobalt-based catalyst in Fischer-Tropsch synthesis. Fuel Process. Technol. 2017, 162, 55–65. [Google Scholar] [CrossRef]
- Pratt, J.W. A Fischer-Tropsch Synthesis Reactor Model Framework for Liquid Biofuels Production. SANDIA Report: SAND2012-7848, 2012. Available online: https://pdfs.semanticscholar.org/6093/1b3ffd88e3183156e132b694c50dc179833a.pdf (accessed on 15 May 2020).
- Okoye-Chine, C.G.; Moyo, M.; Liu, X.; Hildebrandt, D. A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol. 2019, 192, 105–129. [Google Scholar] [CrossRef]
- Vosoughi, V.; Badoga, S.; Dalai, A.K.; Abatzoglou, N. Effect of pretreatment on physicochemical properties and performance of multiwalled carbon nanotube supported cobalt catalyst for Fischer–Tropsch synthesis. Ind. Eng. Chem. Res. 2016, 55, 6049–6059. [Google Scholar] [CrossRef]
- Odunsi, A.O.; O’Donovan, T.S.; Reay, D.A. Dynamic modeling of fixed-bed Fischer-Tropsch reactors with phase change material diluents. Chem. Eng. Technol. 2016, 39, 2066–2076. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2020, 1–84. [Google Scholar] [CrossRef]
- Gholami, Z.; Zabidi, N.A.M.; Gholami, F.; Ayodele, O.B.; Vakili, M. The influence of catalyst factors for sustainable production of hydrocarbons via Fischer-Tropsch synthesis. Rev. Chem. Eng. 2017, 33, 337–358. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Jet fuel synthesis via Fischer–Tropsch synthesis with varied 1-olefins as additives using Co/ZrO2–SiO2 bimodal catalyst. Fuel 2016, 171, 159–166. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Sartipi, S.; van Dijk, J.E.; Gascon, J.; Kapteijn, F. Toward bifunctional catalysts for the direct conversion of syngas to gasoline range hydrocarbons: H-ZSM-5 coated Co versus H-ZSM-5 supported Co. Appl. Catal. A Gen. 2013, 456, 11–22. [Google Scholar] [CrossRef]
- Shi, D.; Faria, J.; Pham, T.N.; Resasco, D.E. Enhanced activity and selectivity of Fischer–Tropsch synthesis catalysts in water/oil emulsions. ACS Catal. 2014, 4, 1944–1952. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Sartipi, S.; Parashar, K.; Makkee, M.; Gascon, J.; Kapteijn, F. Breaking the Fischer–Tropsch synthesis selectivity: Direct conversion of syngas to gasoline over hierarchical Co/H-ZSM-5 catalysts. Catal. Sci. Technol. 2013, 3, 572–575. [Google Scholar] [CrossRef]
- Choudhury, H.A.; Moholkar, V.S. Synthesis of liquid hydrocarbons by Fischer–Tropsch process using industrial iron catalyst. Int. J. Innovat. Res. Sci. Eng. Technol. 2013, 2, 3493–3499. [Google Scholar]
- De La Ree, A.; Best, L.; Bradford, R.; Gonzalez-Arroyo, R.; Hepp, A. Fischer-Tropsch catalysts for aviation fuel production. In Proceedings of the 9th Annual International Energy Conversion Engineering Conference, San Diego, CA, USA, 31 July–3 August 2011; p. 5740. [Google Scholar]
- Cano, F.M.; Gijzeman, O.; De Groot, F.; Weckhuysen, B. Manganese promotion in cobalt-based Fischer-Tropsch catalysis. Stud. Surf. Sci. Catal. 2004, 147, 271–276. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Liao, P.; Zhang, C.; Zhang, L.; Yang, Y.; Zhong, L.; Wang, H.; Sun, Y. Higher alcohol synthesis via syngas over CoMn catalysts derived from hydrotalcite-like precursors. Catal. Today 2018, 311, 56–64. [Google Scholar] [CrossRef]
- He, L.; Berntsen, H.; Ochoa-Fernández, E.; Walmsley, J.C.; Blekkan, E.A.; Chen, D. Co–Ni catalysts derived from hydrotalcite-like materials for hydrogen production by ethanol steam reforming. Top. Catal. 2009, 52, 206–217. [Google Scholar] [CrossRef]
- Zhao, Q.; Ge, Y.; Fu, K.; Ji, N.; Song, C.; Liu, Q. Oxidation of acetone over Co-based catalysts derived from hierarchical layer hydrotalcite: Influence of Co/Al molar ratios and calcination temperatures. Chemosphere 2018, 204, 257–266. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Lauwaert, J.; Van Der Voort, P.; Verberckmoes, A. Recent advances on the utilization of layered double hydroxides (LDHs) and related heterogeneous catalysts in a lignocellulosic-feedstock biorefinery scheme. Green Chem. 2017, 19, 5269–5302. [Google Scholar] [CrossRef]
- Kuśtrowski, P.; Chmielarz, L.; Bożek, E.; Sawalha, M.; Roessner, F. Acidity and basicity of hydrotalcite derived mixed Mg–Al oxides studied by test reaction of MBOH conversion and temperature programmed desorption of NH3 and CO2. Mater. Res. Bull. 2004, 39, 263–281. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Tan, L.; Yan, H.; Zhao, Y.; Duan, H.; Song, Y.-F. Interface engineering of high-energy faceted Co3O4/ZnO heterostructured catalysts derived from layered double hydroxide nanosheets. Ind. Eng. Chem. Res. 2018, 57, 5259–5267. [Google Scholar] [CrossRef]
- Aider, N.; Touahra, F.; Bali, F.; Djebarri, B.; Lerari, D.; Bachari, K.; Halliche, D. Improvement of catalytic stability and carbon resistance in the process of CO2 reforming of methane by CoAl and CoFe hydrotalcite-derived catalysts. Int. J. Hydrog. Energy 2018, 43, 8256–8266. [Google Scholar] [CrossRef]
- Maggi, R.; Martens, J.A.; Poncelet, G.; Grange, P.; Jacobs, P.A.; Delmon, B. Preparation of Catalysts VII; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Abelló, S.; Bolshak, E.; Montané, D. Ni–Fe catalysts derived from hydrotalcite-like precursors for hydrogen production by ethanol steam reforming. Appl. Catal. A Gen. 2013, 450, 261–274. [Google Scholar] [CrossRef]
- Du, Y.-L.; Wu, X.; Cheng, Q.; Huang, Y.-L.; Huang, W. Development of Ni-based catalysts derived from hydrotalcite-like compounds precursors for synthesis gas production via methane or ethanol reforming. Catalysts 2017, 7, 70. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Gao, P.; Zhong, L.; Li, X.; Zhang, Z.; Wang, H.; Wei, W.; Sun, Y. Highly efficient Cu-based catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Today 2017, 281, 327–336. [Google Scholar] [CrossRef]
- Gao, P.; Xie, R.; Wang, H.; Zhong, L.; Xia, L.; Zhang, Z.; Wei, W.; Sun, Y. Cu/Zn/Al/Zr catalysts via phase-pure hydrotalcite-like compounds for methanol synthesis from carbon dioxide. J. CO2 Util. 2015, 11, 41–48. [Google Scholar] [CrossRef]
- Tanios, C.; Bsaibes, S.; Gennequin, C.; Labaki, M.; Cazier, F.; Billet, S.; Tidahy, H.L.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Syngas production by the CO2 reforming of CH4 over Ni–Co–Mg–Al catalysts obtained from hydrotalcite precursors. Int. J. Hydrog. Energy 2017, 42, 12818–12828. [Google Scholar] [CrossRef]
- Izquierdo-Colorado, A.; Dębek, R.; Da Costa, P.; Gálvez, M.E. Excess-methane dry and oxidative reforming on Ni-containing hydrotalcite-derived catalysts for biogas upgrading into synthesis gas. Int. J. Hydrog. Energy 2018, 43, 11981–11989. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The influence of nickel content on the performance of hydrotalcite-derived catalysts in CO2 methanation reaction. Int. J. Hydrog. Energy 2017, 42, 23548–23555. [Google Scholar] [CrossRef]
- Wang, L.; Cao, A.; Liu, G.; Zhang, L.; Liu, Y. Bimetallic CuCo nanoparticles derived from hydrotalcite supported on carbon fibers for higher alcohols synthesis from syngas. Appl. Surf. Sci. 2016, 360, 77–85. [Google Scholar] [CrossRef]
- Liao, P.-Y.; Zhang, C.; Zhang, L.-J.; Yang, Y.-Z.; Zhong, L.-S.; Guo, X.-Y.; Wang, H.; Sun, Y.-H. Influences of Cu Content on the Cu/Co/Mn/Al Catalysts Derived from Hydrotalcite-Like Precursors for Higher Alcohols Synthesis via Syngas. Acta Phys.-Chim. Sin. 2017, 33, 1672–1680. [Google Scholar] [CrossRef]
- Forgionny, A.; Fierro, J.; Mondragón, F.; Moreno, A. Effect of Mg/Al Ratio on catalytic behavior of Fischer–Tropsch cobalt-based catalysts obtained from hydrotalcites precursors. Top. Catal. 2016, 59, 230–240. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Yoon, W.-L.; Seo, Y.-S.; Rhee, Y.-W. The effect of precipitants on Ni-Al2O3 catalysts prepared by a co-precipitation method for internal reforming in molten carbonate fuel cells. Catal. Commun. 2012, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Rynkowski, J.; Gurgul, J.; Dzwigaj, S. Cobalt based catalysts supported on two kinds of beta zeolite for application in Fischer-Tropsch synthesis. Catalysts 2019, 9, 497. [Google Scholar] [CrossRef]
- Kang, S.-H.; Ryu, J.-H.; Kim, J.-H.; Jang, I.H.; Kim, A.R.; Han, G.Y.; Bae, J.W.; Ha, K.-S. Role of ZSM5 distribution on Co/SiO2 Fischer–Tropsch catalyst for the production of C5–C22 hydrocarbons. Energy Fuels 2012, 26, 6061–6069. [Google Scholar] [CrossRef]
- Kurian, M.; Thankachan, S.; Nair, D.S.; Aswathy, E.K.; Babu, A.; Thomas, A.; Krishna, K.T.B. Structural magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods. J. Adv. Ceram. 2015, 4, 199–205. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, N.; Wang, Z.; Sun, W.; Sun, K. Porous bimetallic Mn2Co1Ox catalysts prepared by a one-step combustion method for the low temperature selective catalytic reduction of NOx with NH3. Catal. Commun. 2015, 72, 111–115. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.B.; Zhou, F.; Lu, W.; Jin, J.; Ding, S.F. Promotional effect of H2O2 modification on the cerium-tungsten-titanium mixed oxide catalyst for selective catalytic reduction of NO with NH3. J. Phys. Chem. Solids 2018, 121, 360–366. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, S.G.; Lee, K.B. High-temperature CO2 sorption on hydrotalcite having a high Mg/Al molar ratio. ACS Appl. Mater. Interfaces 2016, 8, 5763–5767. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Ni, H.; Liu, D.; Sun, Z.; Li, P.; Yu, J. Enhancement of CO2 adsorption performance on hydrotalcites impregnated with alkali metal nitrate salts and carbonate salts. Ind. Eng. Chem. Res. 2020, 59, 6043–6052. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Wang, Y.; Tao, N.; Cai, H.; Liu, J.; Lv, J. Mg–Al Mixed oxide derived from hydrotalcites prepared using the solvent-free method: A stable acid–base bifunctional catalyst for continuous-flow transesterification of dimethyl carbonate and ethanol. Ind. Eng. Chem. Res. 2020, 59, 5591–5600. [Google Scholar] [CrossRef]
- Wiyantoko, B.; Kurniawati, P.; Purbaningtias, T.E.; Fatimah, I. Synthesis and characterization of hydrotalcite at different Mg/Al molar ratios. Procedia Chem. 2015, 17, 21–26. [Google Scholar] [CrossRef]
- Castaño-Robayo, M.-H.; Molina-Gallego, R.; Moreno-Guáqueta, S. Ethyl acetate oxidation over MnOx-CoOx. relationship between oxygen and catalytic activity. CT & F-Cienc. Tecnol. Futuro 2015, 6, 45–56. [Google Scholar]
- Gong, K.; Lin, T.; An, Y.; Wang, X.; Yu, F.; Wu, B.; Li, X.; Li, S.; Lu, Y.; Zhong, L.; et al. Fischer-Tropsch to olefins over CoMn-based catalysts: Effect of preparation methods. Appl. Catal. A Gen. 2020, 592, 117414. [Google Scholar] [CrossRef]
- Cui, M.; Hou, Y.; Zhai, Z.; Zhong, Q.; Zhang, Y.; Huang, X. Effects of hydrogen peroxide co-precipitation and inert N2 atmosphere calcination on CeZrLaNd mixed oxides and the catalytic performance used on Pd supported three-way catalysts. RSC Adv. 2019, 9, 8081–8090. [Google Scholar] [CrossRef]
- Cai, L.-N.; Guo, Y.; Lu, A.-H.; Branton, P.; Li, W.-C. The choice of precipitant and precursor in the co-precipitation synthesis of copper manganese oxide for maximizing carbon monoxide oxidation. J. Mol. Catal. A Chem. 2012, 360, 35–41. [Google Scholar] [CrossRef]





| Samples | Concentration (wt.%) | Concentration (mol%) | Molar Ratio | ||
|---|---|---|---|---|---|
| Co | Mn | Co | Mn | Co/Mn | |
| CoMn-HTC-I. | 41.3 | 14.7 | 0.70 | 0.27 | 2.62 |
| CoMn-HTC-II. | 41.0 | 15.2 | 0.70 | 0.28 | 2.51 |
| CoMn-HTC-III. | 42.3 | 19.5 | 0.72 | 0.35 | 2.02 |
| CoMn-HTC-IV. | 44.1 | 18.6 | 0.75 | 0.34 | 2.21 |
| CoMn-HTC-V. | 41.2 | 16.6 | 0.70 | 0.30 | 2.31 |
| CoMn-HTC-VI. | 43.3 | 18.6 | 0.73 | 0.34 | 2.17 |
| Catalyst | Acidic Site (mmol NH3/g) | ||
|---|---|---|---|
| First Peak | Second Peak | Total | |
| CoMn-HTC-I | 0.172 | 0.276 | 0.448 |
| CoMn-HTC-II | 0.147 | 0.201 | 0.348 |
| CoMn-HTC-III | 0.184 | 0.353 | 0.537 |
| Co-Mn-HTC-IV | 0.212 | 0.104 | 0.316 |
| CoMn-HTC-V | 0.208 | 0.196 | 0.404 |
| CoMn-HTC-VI | 0.073 | 0.151 | 0.224 |
| Catalyst | Precipitating Agent |
|---|---|
| CoMn-HTC-I (Group 1) | KOH + K2CO3 |
| CoMn-HTC-II (Group 1) | KOH + K2CO3 + air bubbling |
| CoMn-HTC-III (Group 1) | KOH + K2CO3 + H2O2 |
| CoMn-HTC-IV (Group 2) | KOH |
| CoMn-HTC-V (Group 2) | KOH + air bubbling |
| CoMn-HTC-VI (Group 2) | KOH + H2O2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, Z.; Tišler, Z.; Velvarská, R.; Kocík, J. CoMn Catalysts Derived from Hydrotalcite-Like Precursors for Direct Conversion of Syngas to Fuel Range Hydrocarbons. Catalysts 2020, 10, 813. https://doi.org/10.3390/catal10080813
Gholami Z, Tišler Z, Velvarská R, Kocík J. CoMn Catalysts Derived from Hydrotalcite-Like Precursors for Direct Conversion of Syngas to Fuel Range Hydrocarbons. Catalysts. 2020; 10(8):813. https://doi.org/10.3390/catal10080813
Chicago/Turabian StyleGholami, Zahra, Zdeněk Tišler, Romana Velvarská, and Jaroslav Kocík. 2020. "CoMn Catalysts Derived from Hydrotalcite-Like Precursors for Direct Conversion of Syngas to Fuel Range Hydrocarbons" Catalysts 10, no. 8: 813. https://doi.org/10.3390/catal10080813
APA StyleGholami, Z., Tišler, Z., Velvarská, R., & Kocík, J. (2020). CoMn Catalysts Derived from Hydrotalcite-Like Precursors for Direct Conversion of Syngas to Fuel Range Hydrocarbons. Catalysts, 10(8), 813. https://doi.org/10.3390/catal10080813






