Metal Complexes Bearing Sulfur-Containing Ligands as Catalysts in the Reaction of CO2 with Epoxides
Abstract
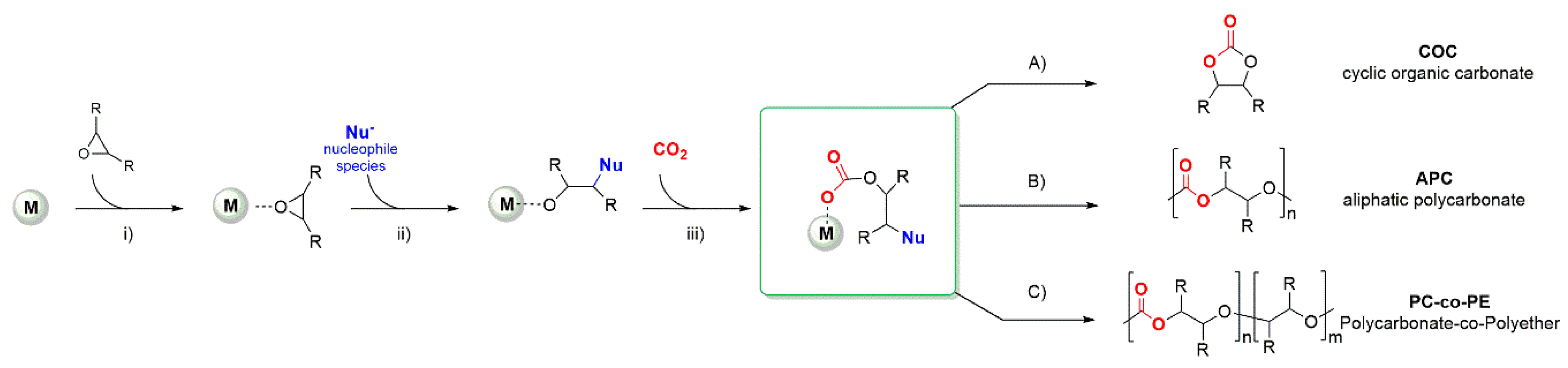
1. Introduction
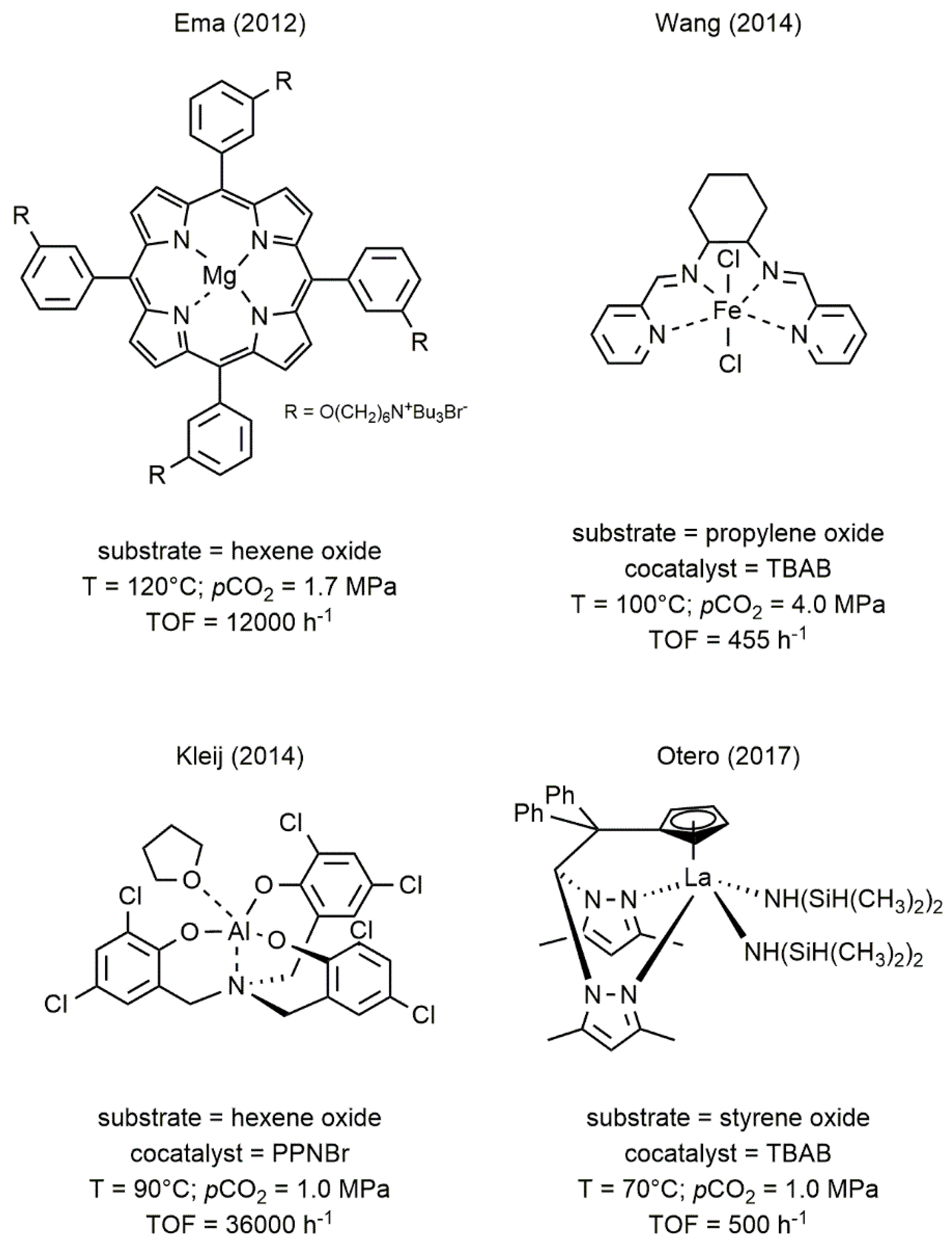
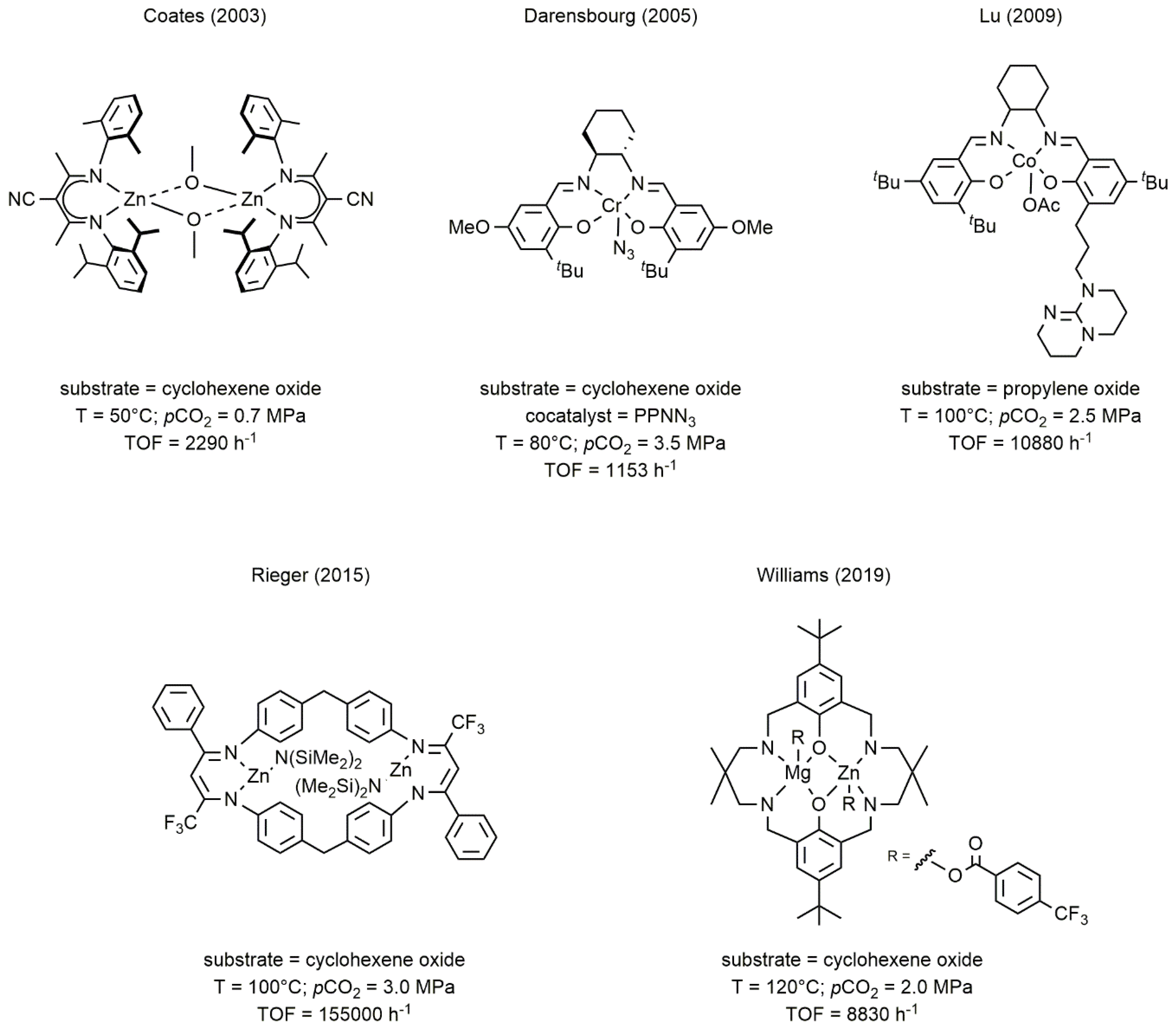
2. Metal Complexes Bearing Sulfur-Containing Ligands Active in the Coupling of CO2 and Epoxides
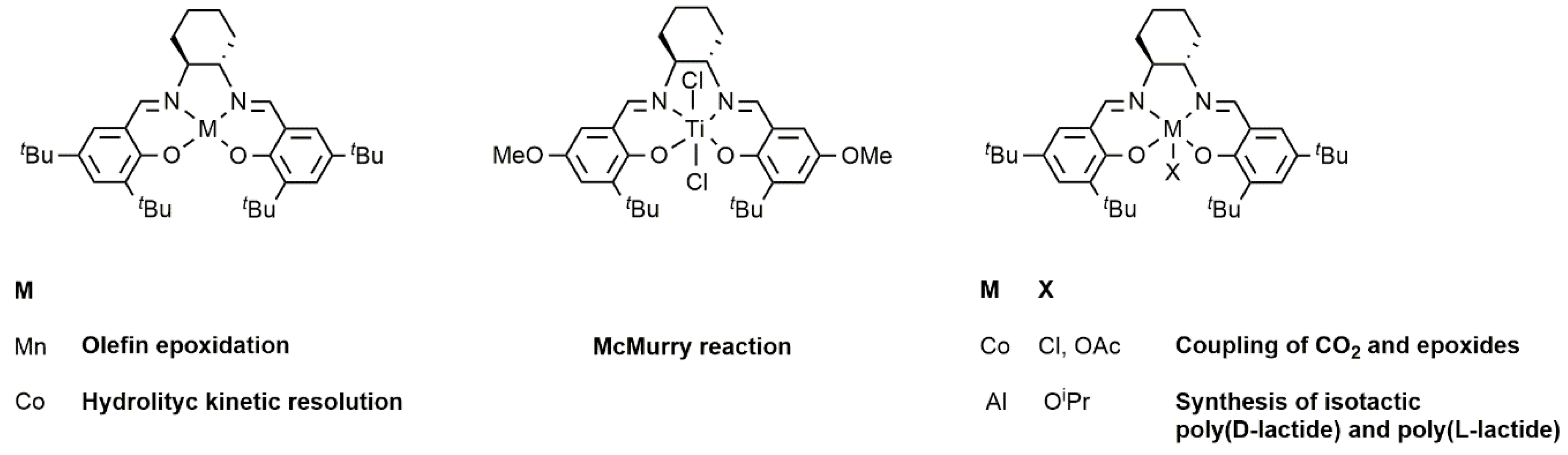
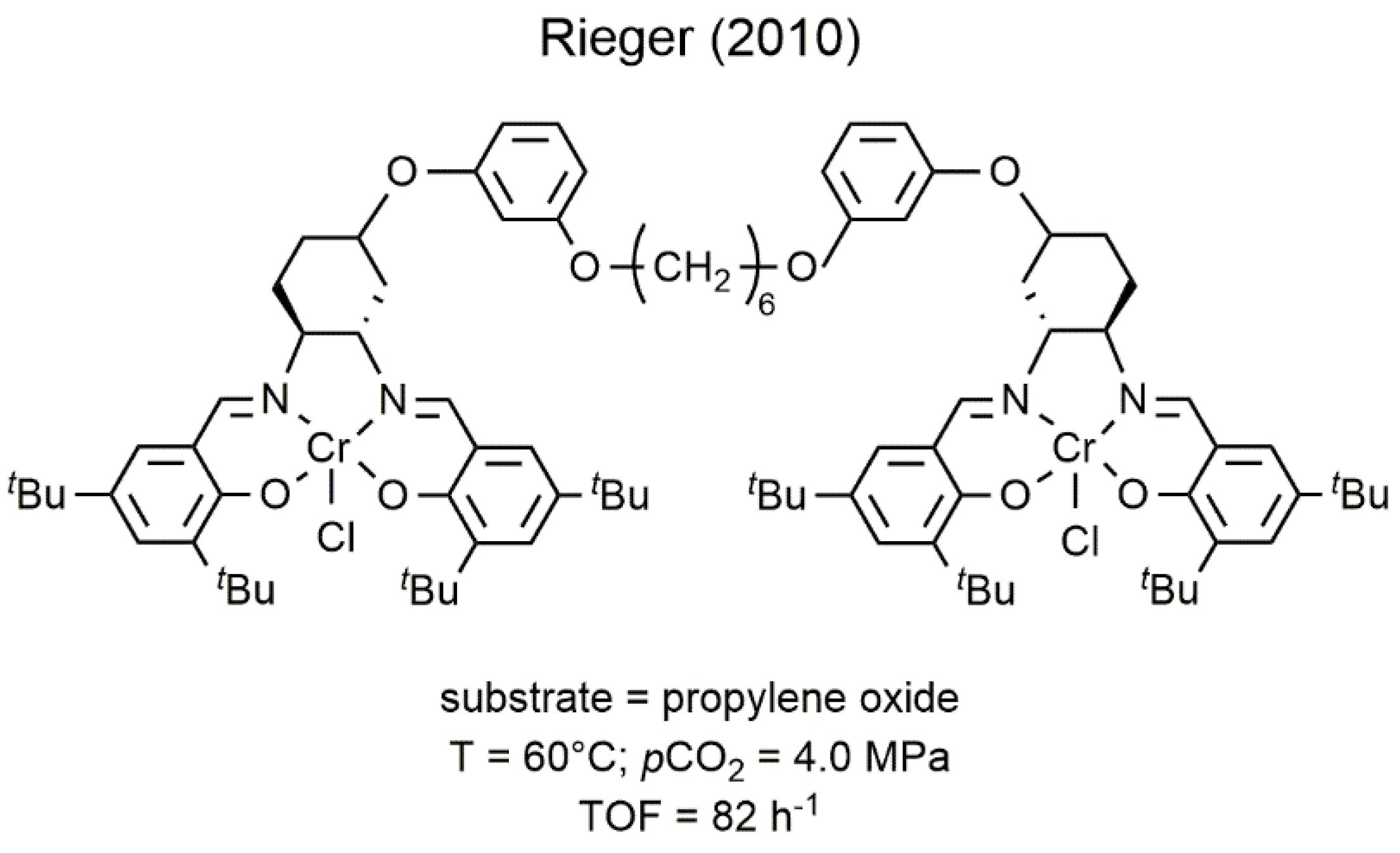
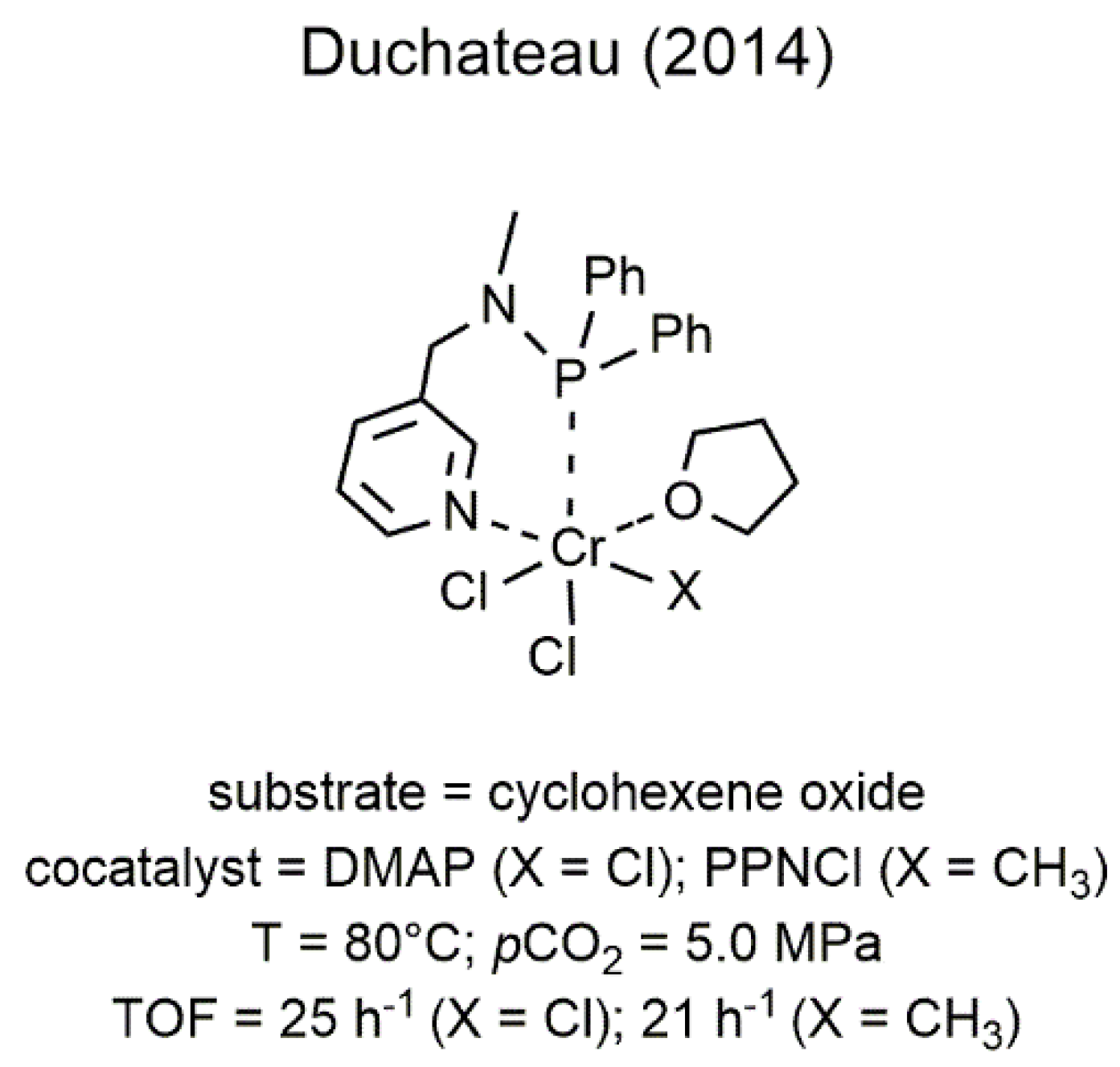
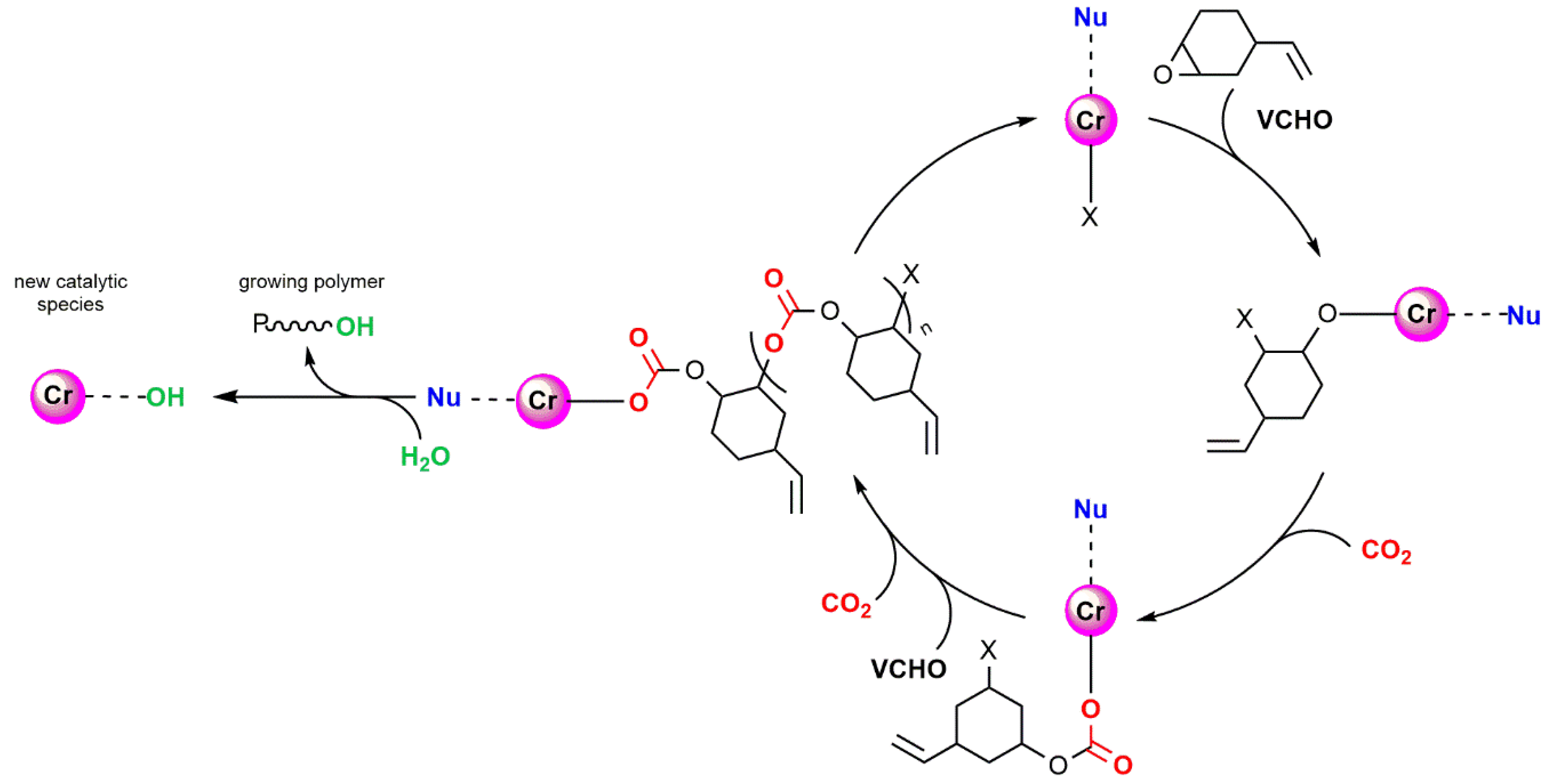
2.1. Chromium-Based Systems
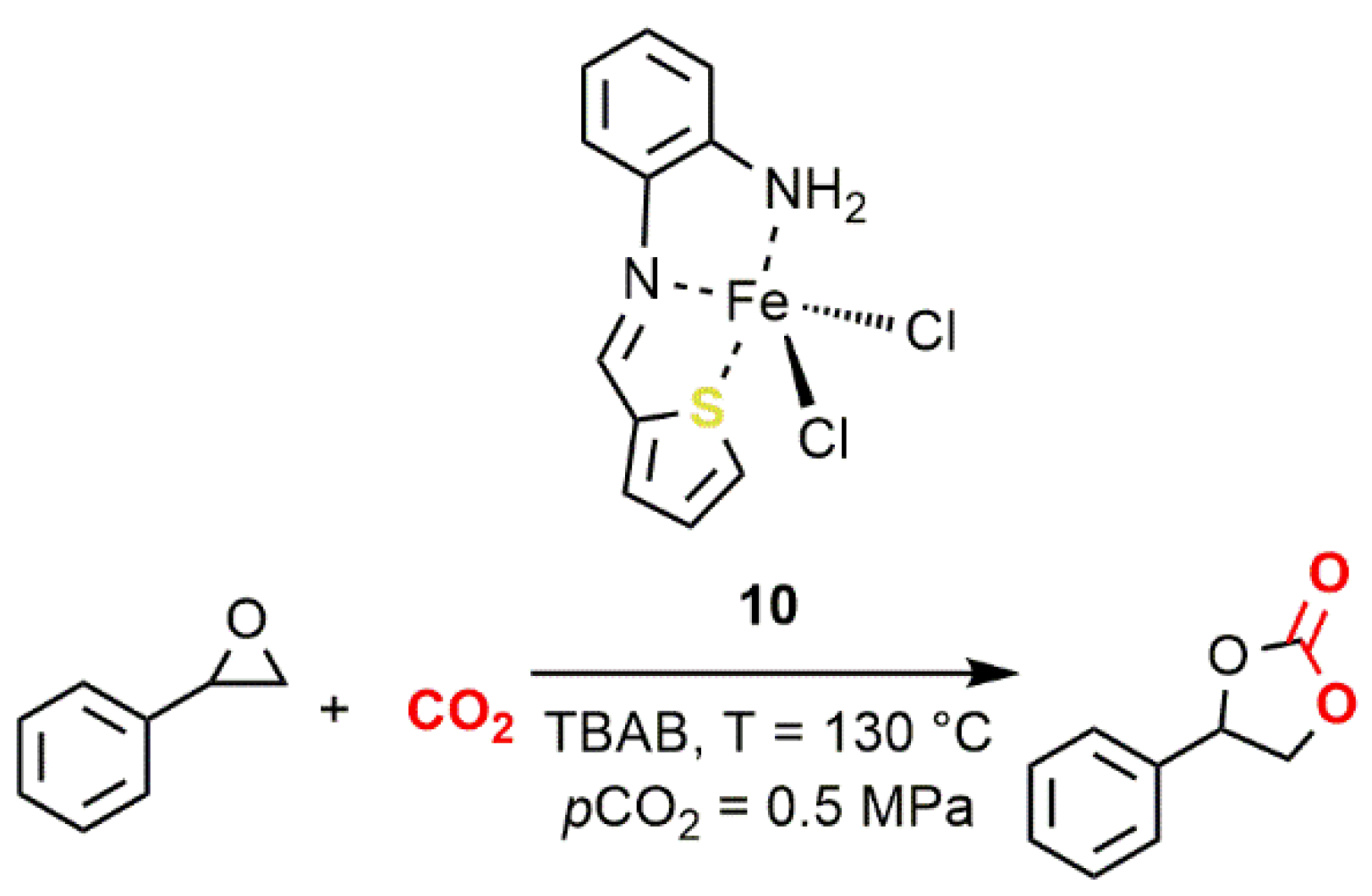
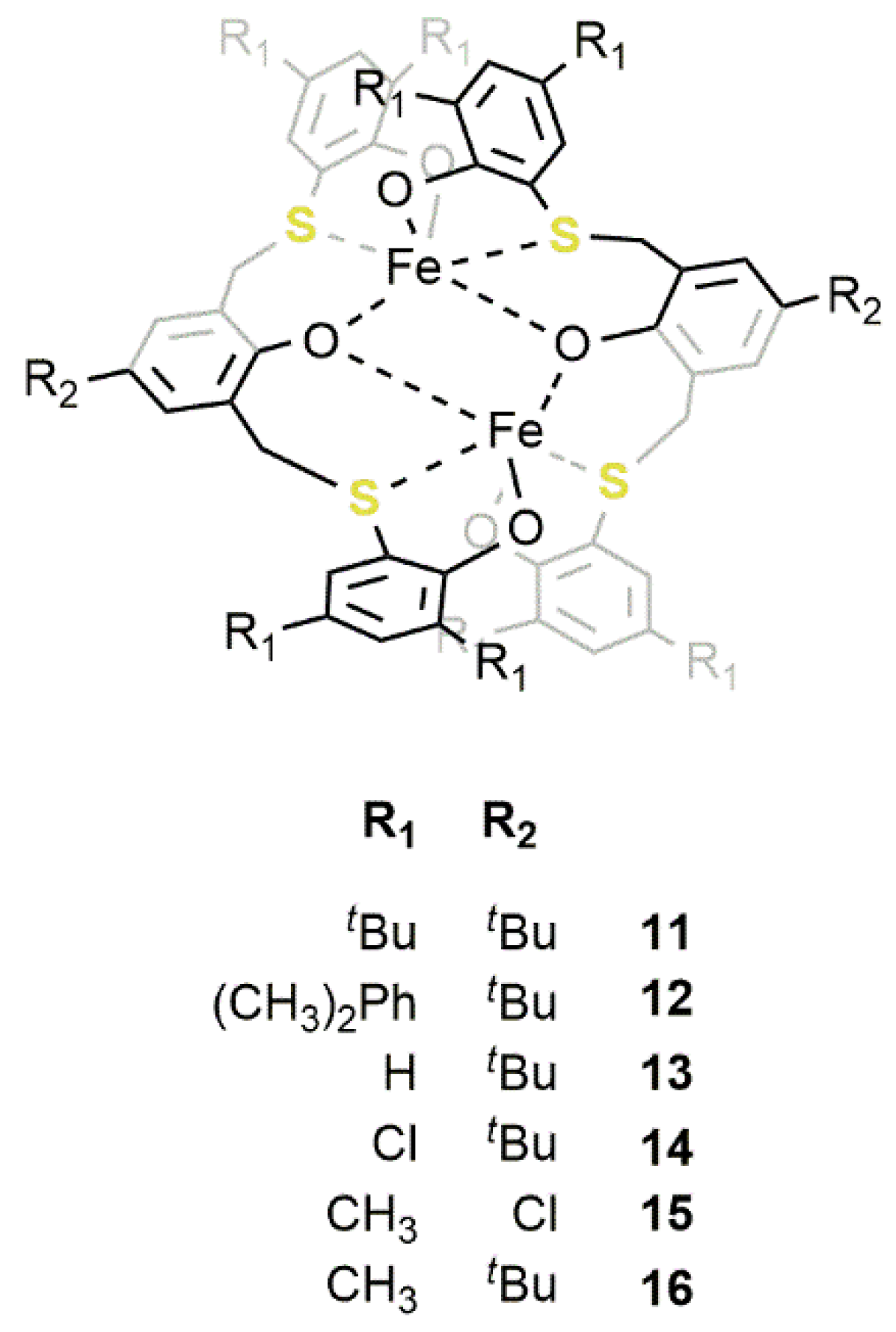
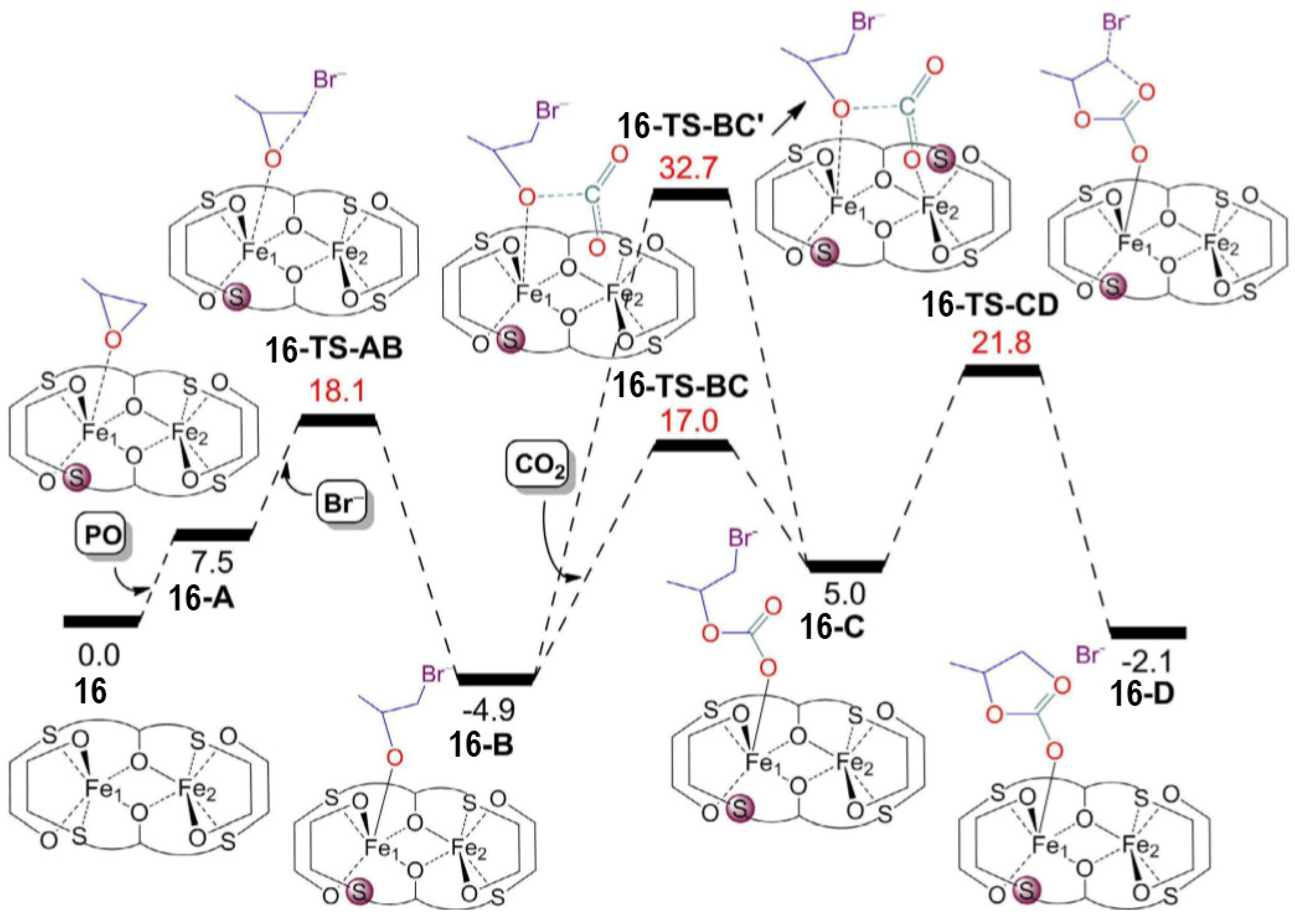
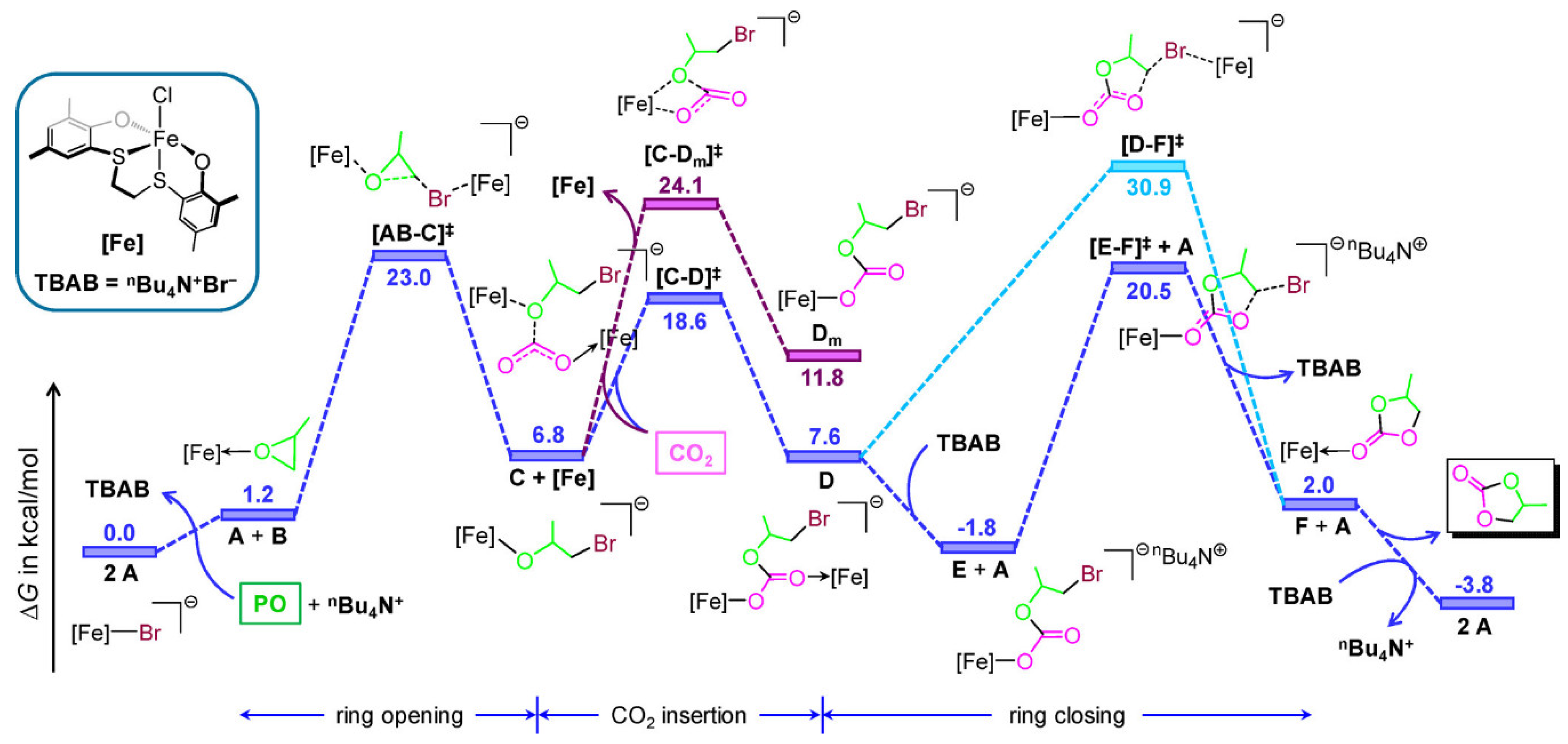
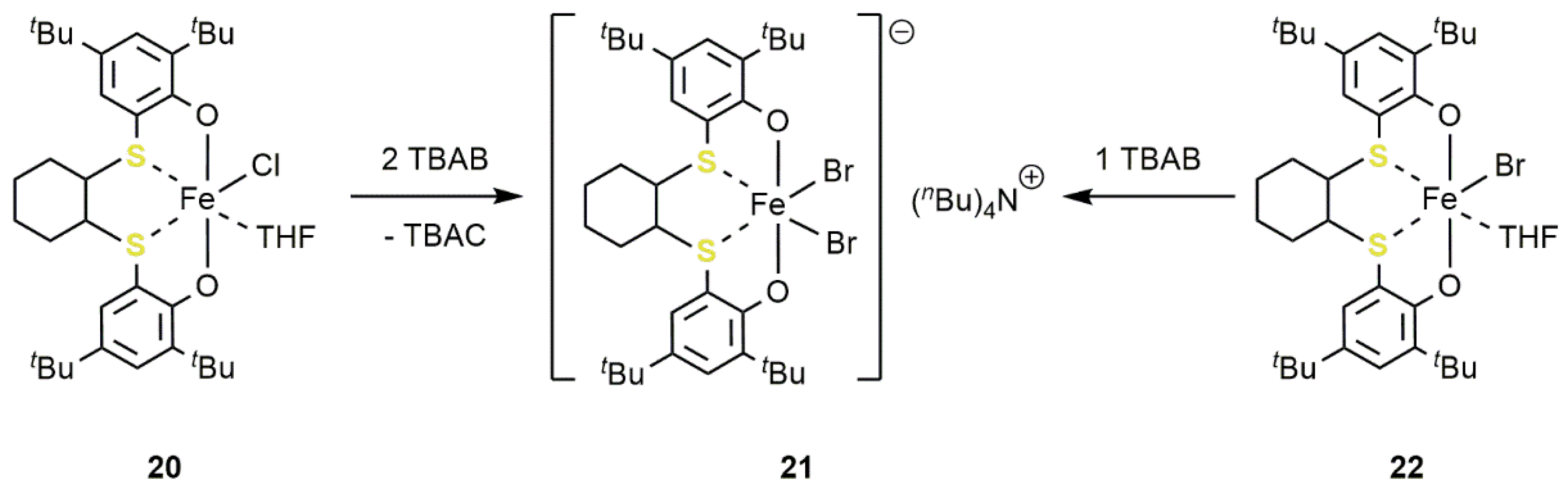
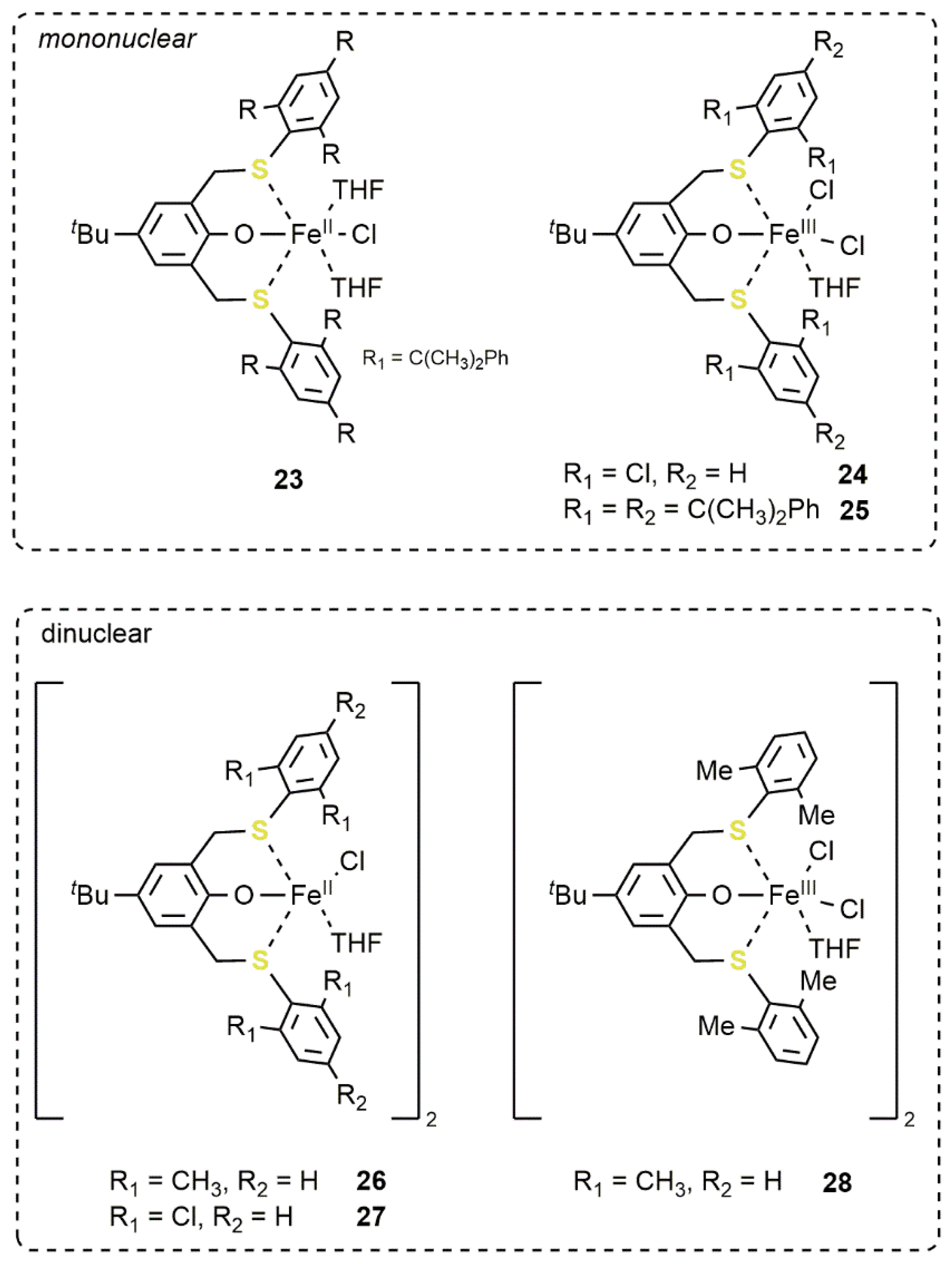
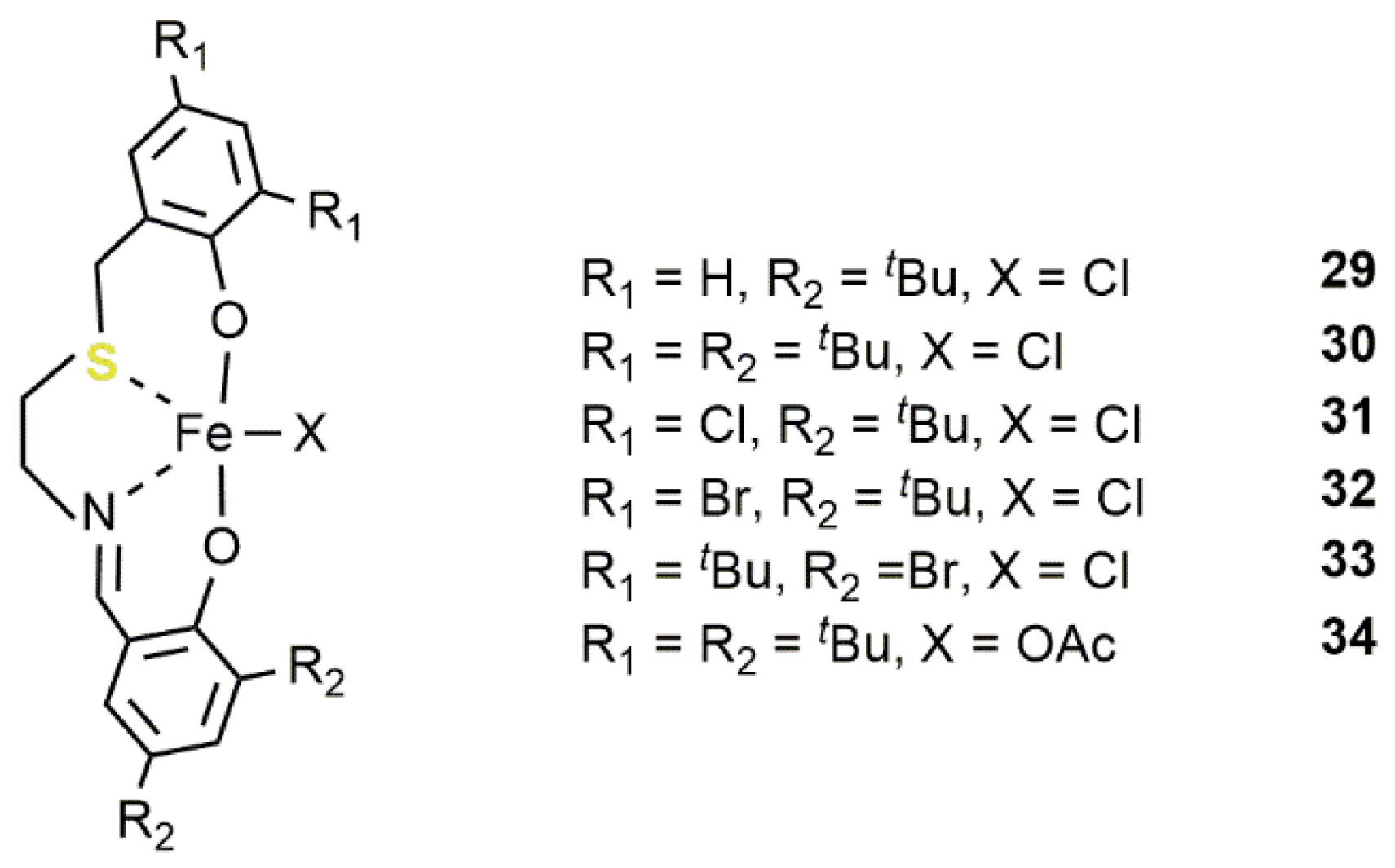
2.2. Iron-Based Systems
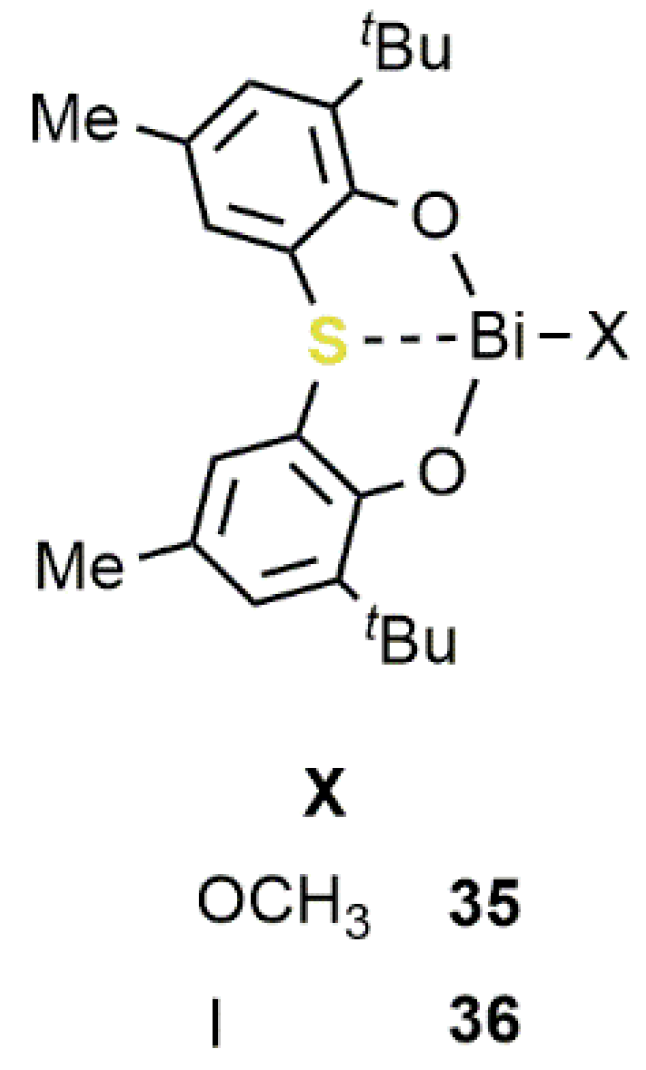
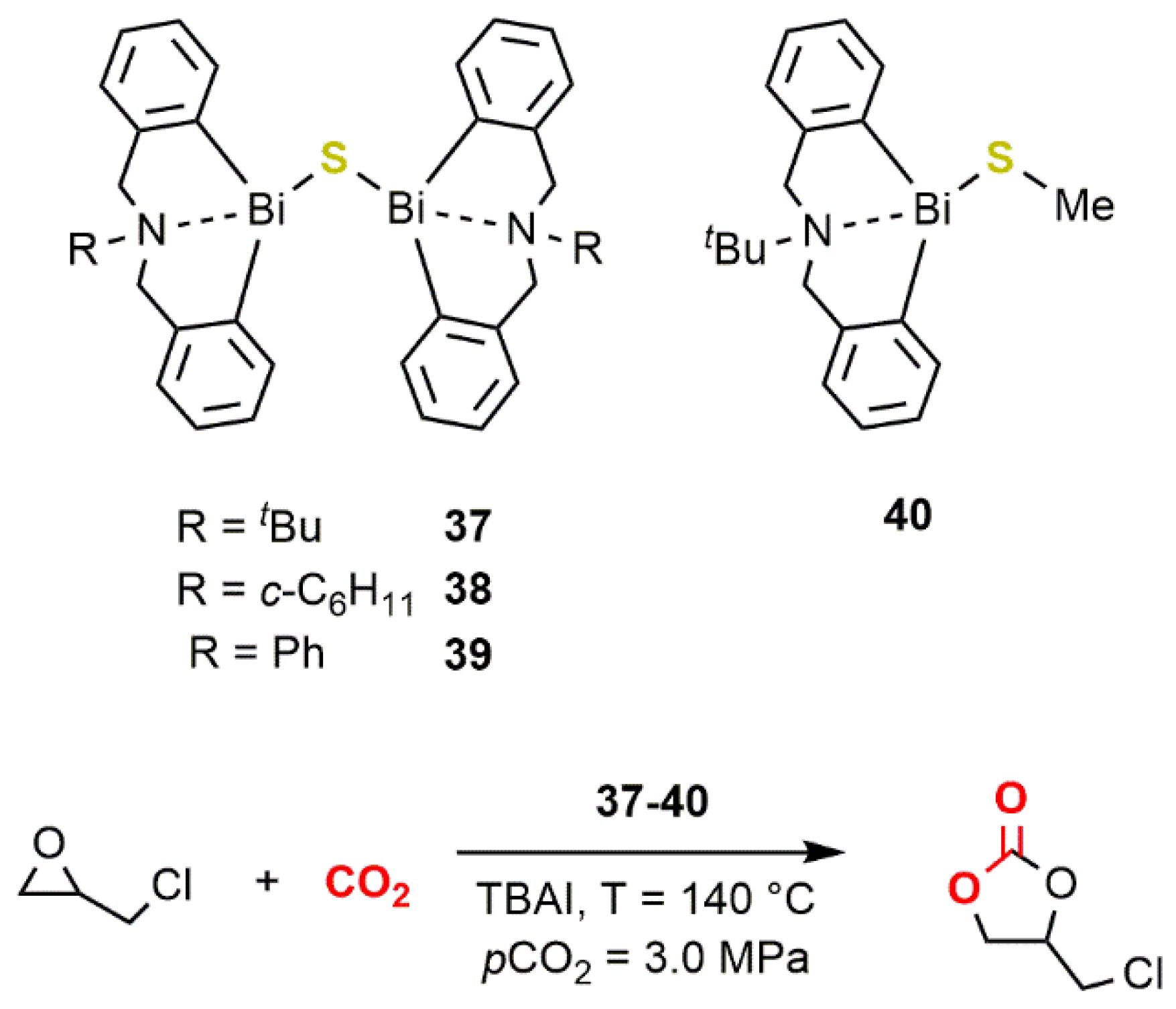
2.3. Bismuth-Based Systems
3. Conclusions
Funding
Conflicts of Interest
References
- Cornils, B.; Herrmann, W.A.; Beller, M.; Paciello, R. (Eds.) Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Four Volumes, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; ISBN 978-3-527-32897-0. [Google Scholar]
- Bhaduri, S.; Mukesh, D. Homogeneous Catalysis: Mechanisms and Industrial Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-87251-2. [Google Scholar]
- van Leeuwen, P.W.N.M. Homogeneous Catalysis: Understanding the Art; Springer Netherlands: Dordrecht, The Netherlands, 2004; ISBN 978-1-4020-3176-2. [Google Scholar]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- Dai, W.-L.; Luo, S.-L.; Yin, S.-F.; Au, C.-T. The direct transformation of carbon dioxide to organic carbonates over heterogeneous catalysts. Appl. Catal. A Gen. 2009, 366, 2–12. [Google Scholar] [CrossRef]
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew. Chem. Int. Ed. 2010, 49, 9822–9837. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wilson, S.J. What’s new with CO2? Recent advances in its copolymerization with oxiranes. Green Chem. 2012, 14, 2665. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Technol. 2012, 2, 2169. [Google Scholar] [CrossRef]
- Sakakura, T.; Kohno, K. The synthesis of organic carbonates from carbon dioxide. Chem. Commun. 2009, 1312. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete Metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Buonerba, A.; Capacchione, C. Homogeneous iron catalysts in the reaction of epoxides with carbon dioxide. Adv. Synth. Catal. 2019, 361, 265–282. [Google Scholar] [CrossRef]
- Poland, S.J.; Darensbourg, D.J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem. 2017, 19, 4990–5011. [Google Scholar] [CrossRef]
- Della Monica, F.; Kleij, A.W. Mechanistic guidelines in nonreductive conversion of CO2: The case of cyclic carbonates. Catal. Sci. Technol. 2020, 10, 3483–3501. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Castro-Gómez, F.; Martin, E.; Escudero-Adán, E.C.; Bo, C.; Kleij, A.W. Highly active aluminium catalysts for the formation of organic carbonates from CO2 and oxiranes. Chem. Eur. J. 2014, 20, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 1482. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Song, Q.-W.; Liu, X.-F.; Ma, R.; He, L.-N. Cooperative calcium-based catalysis with 1,8-diazabicyclo[5.4.0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure. Green Chem. 2016, 18, 2871–2876. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Castro-Osma, J.A.; Lara-Sánchez, A.; Otero, A. An efficient and versatile lanthanum heteroscorpionate catalyst for carbon dioxide fixation into cyclic carbonates. ChemSusChem 2017, 10, 2886–2890. [Google Scholar] [CrossRef]
- Chen, A.; Chen, C.; Xiu, Y.; Liu, X.; Chen, J.; Guo, L.; Zhang, R.; Hou, Z. Niobate salts of organic base catalyzed chemical fixation of carbon dioxide with epoxides to form cyclic carbonates. Green Chem. 2015, 17, 1842–1852. [Google Scholar] [CrossRef]
- Chen, F.; Liu, N.; Dai, B. Iron(II) bis-cnn pincer complex-catalyzed cyclic carbonate synthesis at room temperature. ACS Sustain. Chem. Eng. 2017, 5, 9065–9075. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Coupling reaction between CO2 and cyclohexene oxide: Selective control from cyclic carbonate to polycarbonate by ligand design of salen/salalen titanium complexes. Catal. Sci. Technol. 2014, 4, 3964–3972. [Google Scholar] [CrossRef]
- Sheng, X.; Qiao, L.; Qin, Y.; Wang, X.; Wang, F. Highly efficient and quantitative synthesis of a cyclic carbonate by iron complex catalysts. Polyhedron 2014, 74, 129–133. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Escudero-Adán, E.C.; Martin, E.; Kleij, A.W. A powerful aluminum catalyst for the synthesis of highly functional organic carbonates. J. Am. Chem. Soc. 2013, 135, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Cheng, M.; Lobkovsky, E.B.; Coates, G.W. Electronic and steric effects on catalysts for CO2/epoxide polymerization: Subtle modifications resulting in superior activities. Angew. Chem. Int. Ed. 2002, 41, 2599–2602. [Google Scholar] [CrossRef]
- Cheng, M.; Lobkovsky, E.B.; Coates, G.W. Catalytic reactions involving C1 feedstocks: New high-activity zn(ii)-based catalysts for the alternating copolymerization of carbon dioxide and epoxides. J. Am. Chem. Soc. 1998, 120, 11018–11019. [Google Scholar] [CrossRef]
- Moore, D.R.; Cheng, M.; Lobkovsky, E.B.; Coates, G.W. Mechanism of the alternating copolymerization of epoxides and CO2 using β-Diiminate zinc catalysts: Evidence for a bimetallic epoxide enchainment. J. Am. Chem. Soc. 2003, 125, 11911–11924. [Google Scholar] [CrossRef]
- Kissling, S.; Lehenmeier, M.W.; Altenbuchner, P.T.; Kronast, A.; Reiter, M.; Deglmann, P.; Seemann, U.B.; Rieger, B. Dinuclear zinc catalysts with unprecedented activities for the copolymerization of cyclohexene oxide and CO2. Chem. Commun. 2015, 51, 4579–4582. [Google Scholar] [CrossRef] [PubMed]
- Trott, G.; Garden, J.A.; Williams, C.K. Heterodinuclear zinc and magnesium catalysts for epoxide/CO2 ring opening copolymerizations. Chem. Sci. 2019, 10, 4618–4627. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Mackiewicz, R.M.; Billodeaux, D.R. Pressure dependence of the carbon dioxide/cyclohexene oxide coupling reaction catalyzed by chromium salen complexes. optimization of the comonomer-alternating enchainment pathway. Organometallics 2005, 24, 144–148. [Google Scholar] [CrossRef]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistic aspects of the copolymerization of CO2 with epoxides using a thermally stable single-site cobalt(III) catalyst. J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Dagorne, S. Coordination chemistry and applications of salen, salan and salalen metal complexes. In PATAI’S Chemistry of Functional Groups; Rappoport, Z., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; p. pat0611. ISBN 978-0-470-68253-1. [Google Scholar]
- Kakugo, M.; Miyatake, T.; Mizunuma, K. Polymerization of styrene and copolymerization of styrene with olefin in the presence of soluble ziegler-natta catalysts. In Studies in Surface Science and Catalysis; Elsevier: Tokyo, Japan, 1990; Volume 56, pp. 517–529. ISBN 978-0-444-98747-1. [Google Scholar]
- Song, T.; He, J.; Liang, L.; Liu, N.; Li, F.; Tong, X.; Mu, X.; Mu, Y. Titanium and zirconium complexes bearing new tridentate [OSO] bisphenolato-based ligands: Synthesis, characterization and catalytic properties for alkene polymerization. Dalton Trans. 2019, 48, 13719–13731. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Ishii, A. Recent advances in the chemistry of Group 4 metal complexes incorporating [OSSO]-type bis(phenolato) ligands as post-metallocene catalysts. Polym. Chem. 2011, 2, 1597. [Google Scholar] [CrossRef]
- Miyatake, T.; Mizunuma, K.; Seki, Y.; Kakugo, M. 2,2′-thiobis(6-tert-butyl-4-methylphenoxy)titanium or zirconium complex-methylalumoxane catalysts for polymerization of olefins. Makromol. Chem. Rapid Commun. 1989, 10, 349–352. [Google Scholar] [CrossRef]
- Capacchione, C.; Proto, A.; Ebeling, H.; Mülhaupt, R.; Möller, K.; Spaniol, T.P.; Okuda, J. Ancillary ligand effect on single-site styrene polymerization: Isospecificity of group 4 metal bis(phenolate) catalysts. J. Am. Chem. Soc. 2003, 125, 4964–4965. [Google Scholar] [CrossRef]
- Meppelder, G.-J.M.; Fan, H.-T.; Spaniol, T.P.; Okuda, J. Synthesis, structure, and olefin polymerization activity of titanium complexes bearing asymmetric tetradentate [OSNO]-type bis(phenolato) ligands. Inorg. Chem. 2009, 48, 7378–7388. [Google Scholar] [CrossRef]
- Mella, M.; Izzo, L.; Capacchione, C. Role of the metal center in the ethylene polymerization promoted by group 4 complexes supported by a tetradentate [OSSO]-type bis(phenolato) ligand. ACS Catal. 2011, 1, 1460–1468. [Google Scholar] [CrossRef]
- Buonerba, A.; Fienga, M.; Milione, S.; Cuomo, C.; Grassi, A.; Proto, A.; Capacchione, C. Binary copolymerization of p-Methylstyrene with butadiene and isoprene catalyzed by titanium compounds showing different stereoselectivity. Macromolecules 2013, 46, 8449–8457. [Google Scholar] [CrossRef]
- Ma, H.; Spaniol, T.P.; Okuda, J. Rare earth metal complexes supported by 1,ω-dithiaalkanediyl-bridged bis(phenolato) ligands: Synthesis, characterization and ring-opening polymerization catalysis of l-lactide. Dalton Trans. 2003, 4770–4780. [Google Scholar] [CrossRef]
- Meppelder, G.-J.M.; Halbach, T.S.; Spaniol, T.P.; Mülhaupt, R.; Okuda, J. A vanadium(V) complex with a tetradentate [OSSO]-type bis(phenolato) ligand: Synthesis, structure, and ethylene polymerization activity. J. Organomet. Chem. 2009, 694, 1235–1237. [Google Scholar] [CrossRef]
- Della Monica, F.; Luciano, E.; Buonerba, A.; Grassi, A.; Milione, S.; Capacchione, C. Poly(lactide-co-ε-caprolactone) copolymers prepared using bis-thioetherphenolate group 4 metal complexes: Synthesis, characterization and morphology. RSC Adv. 2014, 4, 51262–51267. [Google Scholar] [CrossRef]
- Lian, B.; Spaniol, T.P.; Horrillo-Martínez, P.; Hultzsch, K.C.; Okuda, J. Imido and amido titanium complexes that contain a [OSSO]-type bis(phenolato) ligand: Synthesis, structures, and hydroamination catalysis. Eur. J. Inorg. Chem. 2009, 2009, 429–434. [Google Scholar] [CrossRef]
- Konkol, M.; Kondracka, M.; Voth, P.; Spaniol, T.P.; Okuda, J. Rare-Earth metal alkyl and hydrido complexes containing a thioether-functionalized bis(phenolato) ligand: Efficient Catalysts for olefin hydrosilylation. Organometallics 2008, 27, 3774–3784. [Google Scholar] [CrossRef]
- Della Monica, F.; Maity, B.; Pehl, T.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Rieger, B.; Cavallo, L.; Capacchione, C. [OSSO]-type iron(III) complexes for the low-pressure reaction of carbon dioxide with epoxides: Catalytic activity, reaction kinetics, and computational study. ACS Catal. 2018, 8, 6882–6893. [Google Scholar] [CrossRef]
- Della Monica, F.; Luciano, E.; Roviello, G.; Grassi, A.; Milione, S.; Capacchione, C. Group 4 metal complexes bearing thioetherphenolate ligands. coordination chemistry and ring-opening polymerization catalysis. Macromolecules 2014, 47, 2830–2841. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Mackiewicz, R.M.; Rodgers, J.L.; Fang, C.C.; Billodeaux, D.R.; Reibenspies, J.H. Cyclohexene Oxide/CO2 copolymerization catalyzed by chromium(iii) salen complexes and n-methylimidazole: Effects of varying salen ligand substituents and relative cocatalyst loading. Inorg. Chem. 2004, 43, 6024–6034. [Google Scholar] [CrossRef]
- Paddock, R.L.; Nguyen, S.T. Chemical CO2 fixation: Cr(III) Salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J. Am. Chem. Soc. 2001, 123, 11498–11499. [Google Scholar] [CrossRef]
- Vagin, S.I.; Reichardt, R.; Klaus, S.; Rieger, B. Conformationally flexible dimeric salphen complexes for bifunctional catalysis. J. Am. Chem. Soc. 2010, 132, 14367–14369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coord. Chem. Rev. 2018, 372, 85–100. [Google Scholar] [CrossRef]
- Gurnham, J.; Gambarotta, S.; Korobkov, I.; Jasinska-Walc, L.; Duchateau, R. Chromium-catalyzed CO2–epoxide copolymerization. Organometallics 2014, 33, 4401–4409. [Google Scholar] [CrossRef]
- Si, G.; Zhang, L.; Han, B.; Zhang, H.; Li, X.; Liu, B. Chromium complexes containing a tetradentate [OSSO]-type bisphenolate ligand as a novel family of catalysts for the copolymerization of carbon dioxide and 4-vinylcyclohexene oxide. RSC Adv. 2016, 6, 22821–22826. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Kyran, S.J.; Liu, B.; Duan, Z.; Darensbourg, D.J. Copolymerization of carbon dioxide and cyclohexene oxide catalyzed by chromium complexes bearing semirigid [ONSO]-type ligands. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1938–1944. [Google Scholar] [CrossRef]
- Della Monica, F.; Paradiso, V.; Grassi, A.; Milione, S.; Cavallo, L.; Capacchione, C. A novel [OSSO]-type chromium(III) complex as a versatile catalyst for copolymerization of carbon dioxide with epoxides. Chem. Eur. J. 2020, 26, 5347–5353. [Google Scholar] [CrossRef]
- Nakano, K.; Kobayashi, K.; Ohkawara, T.; Imoto, H.; Nozaki, K. Copolymerization of epoxides with carbon dioxide catalyzed by iron–corrole complexes: Synthesis of a crystalline copolymer. J. Am. Chem. Soc. 2013, 135, 8456–8459. [Google Scholar] [CrossRef]
- Buchard, A.; Kember, M.R.; Sandeman, K.G.; Williams, C.K. A bimetallic iron(III) catalyst for CO2/epoxide coupling. Chem. Commun. 2011, 47, 212–214. [Google Scholar] [CrossRef]
- Laserna, V.; Fiorani, G.; Whiteoak, C.J.; Martin, E.; Escudero-Adán, E.; Kleij, A.W. Carbon dioxide as a protecting group: Highly efficient and selective catalytic access to cyclic cis-Diol scaffolds. Angew. Chem. Int. Ed. 2014, 53, 10416–10419. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Martin, E.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. An Efficient iron catalyst for the synthesis of five- and six-membered organic carbonates under mild conditions. Adv. Synth. Catal. 2012, 354, 469–476. [Google Scholar] [CrossRef]
- Sunjuk, M.; Abu-Surrah, A.S.; Al-Ramahi, E.; Qaroush, A.K.; Saleh, A. Selective coupling of carbon dioxide and epoxystyrene via salicylaldimine-, thiophenaldimine-, and quinolinaldimine-iron(II), iron(III), chromium(III), and cobalt(III)/Lewis base catalysts. Transit. Met. Chem. 2013, 38, 253–257. [Google Scholar] [CrossRef]
- Buonerba, A.; De Nisi, A.; Grassi, A.; Milione, S.; Capacchione, C.; Vagin, S.; Rieger, B. Novel iron(III) catalyst for the efficient and selective coupling of carbon dioxide and epoxides to form cyclic carbonates. Catal. Sci. Technol. 2015, 5, 118–123. [Google Scholar] [CrossRef]
- Della Monica, F.; Vummaleti, S.V.C.; Buonerba, A.; Nisi, A.D.; Monari, M.; Milione, S.; Grassi, A.; Cavallo, L.; Capacchione, C. Coupling of carbon dioxide with epoxides efficiently catalyzed by thioether-triphenolate bimetallic iron(III) complexes: Catalyst structure-reactivity relationship and mechanistic DFT study. Adv. Synth. Catal. 2016, 358, 3231–3243. [Google Scholar] [CrossRef]
- Buonerba, A.; Della Monica, F.; De Nisi, A.; Luciano, E.; Milione, S.; Grassi, A.; Capacchione, C.; Rieger, B. Thioether-triphenolate bimetallic Iron(III) complexes as robust and highly efficient catalysts for cycloaddition of carbon dioxide to epoxides. Faraday Discuss. 2015, 183, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Buonerba, A.; Paradiso, V.; Milione, S.; Grassi, A.; Capacchione, C. [OSSO]-type Fe(III) metallate as single-component catalyst for the CO2 cycloaddition to epoxides Adv. Synth. Catal. 2019, 361, 283–288. [Google Scholar] [CrossRef]
- Della Monica, F.; Leone, M.; Buonerba, A.; Grassi, A.; Milione, S.; Capacchione, C. CO2 cycloaddition to epoxides promoted by bis-thioether-phenolate Fe(II) and Fe(III) complexes. Mol. Catal. 2018, 460, 46–52. [Google Scholar] [CrossRef]
- Driscoll, O.J.; Stewart, J.A.; McKeown, P.; Jones, M.D. Salalen vs. thiolen: In the ring(-opening of epoxide and cyclic carbonate formation). New J. Chem. 2020, 44, 6063–6067. [Google Scholar] [CrossRef]
- Ollevier, T. New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 2013, 11, 2740. [Google Scholar] [CrossRef]
- Ollevier, T. (Ed.) Bismuth-Mediated Organic Reactions; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 311, ISBN 978-3-642-27238-7. [Google Scholar]
- Yamamoto, H.; Ishihara, K. (Eds.) Acid Catalysis in Modern Organic Synthesis; WILEY-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31724-0. [Google Scholar]
- Salvador, J.A.R.; Silvestre, S.M.; Pinto, R.M.A. Bismuth(III) reagents in steroid and terpene chemistry. Molecules 2011, 16, 2884–2913. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, R.; Xu, X.; Au, C.-T.; Yin, S.-F. Organoantimony and organobismuth complexes for CO2 fixation. RSC Adv. 2014, 4, 11907–11918. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, W.; Yin, S.; Luo, S.; Au, C.-T. Cationic organobismuth complex as an effective catalyst for conversion of CO2 into cyclic carbonates. Front. Environ. Sci. Eng. China 2009, 3, 32–37. [Google Scholar] [CrossRef]
- Yin, S.-F.; Shimada, S. Synthesis and structure of bismuth compounds bearing a sulfur-bridged bis(phenolato) ligand and their catalytic application to the solvent-free synthesis of propylene carbonate from CO2 and propylene oxide. Chem. Commun. 2009, 1136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Meng, Z.; Yin, S.; Song, X.; Tan, N.; Zhou, Y.; Yu, K.; Xu, X.; Luo, S.; Au, C.-T.; et al. Synthesis and structure of binuclear o/s-bridged organobismuth complexes and their cooperative catalytic effect on CO2 fixation. ChemPlusChem 2012, 77, 404–410. [Google Scholar] [CrossRef]


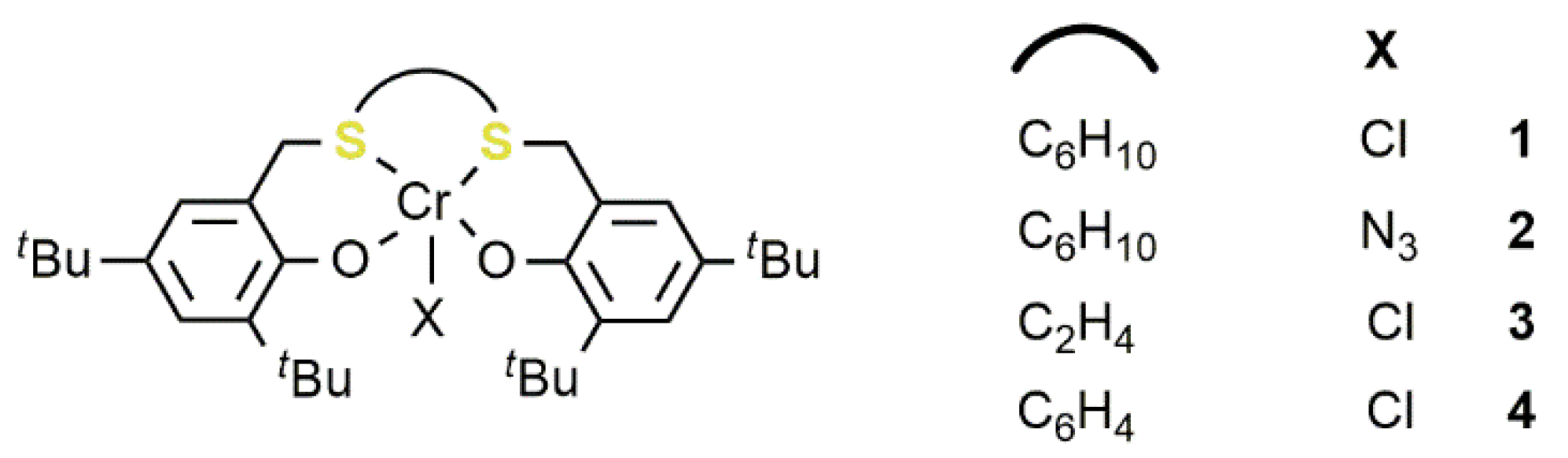
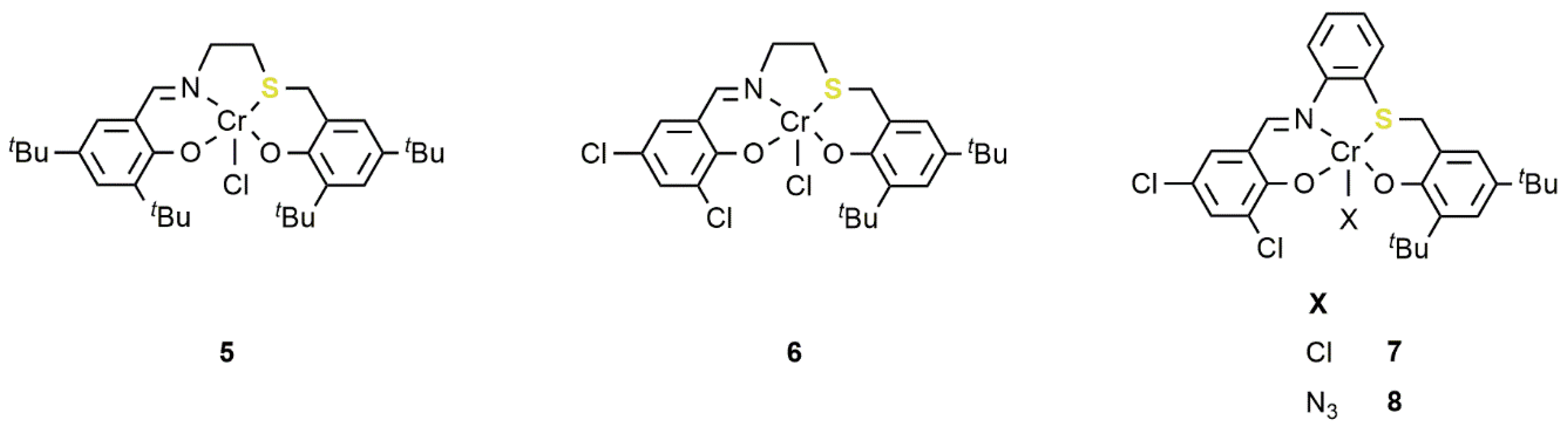
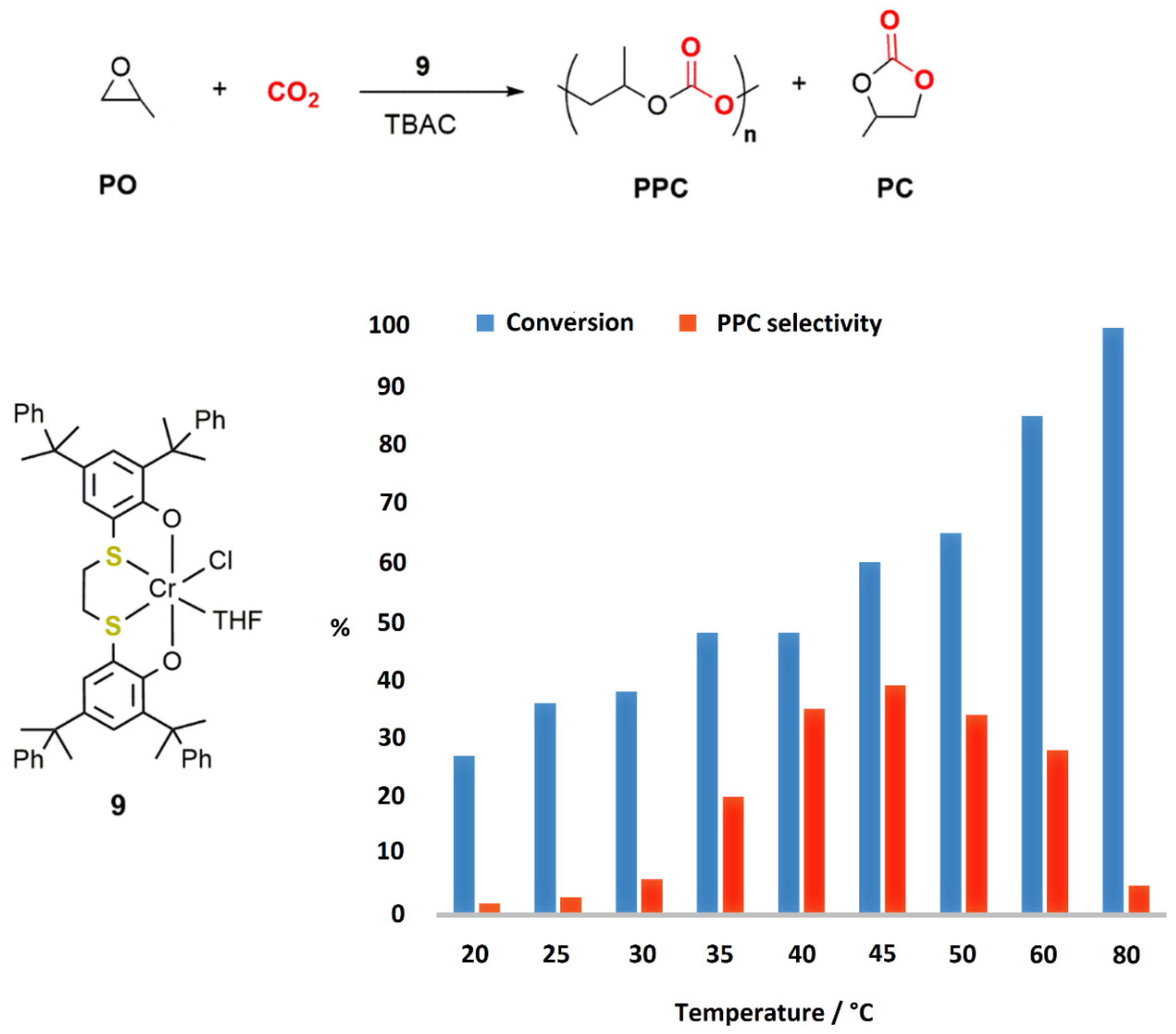
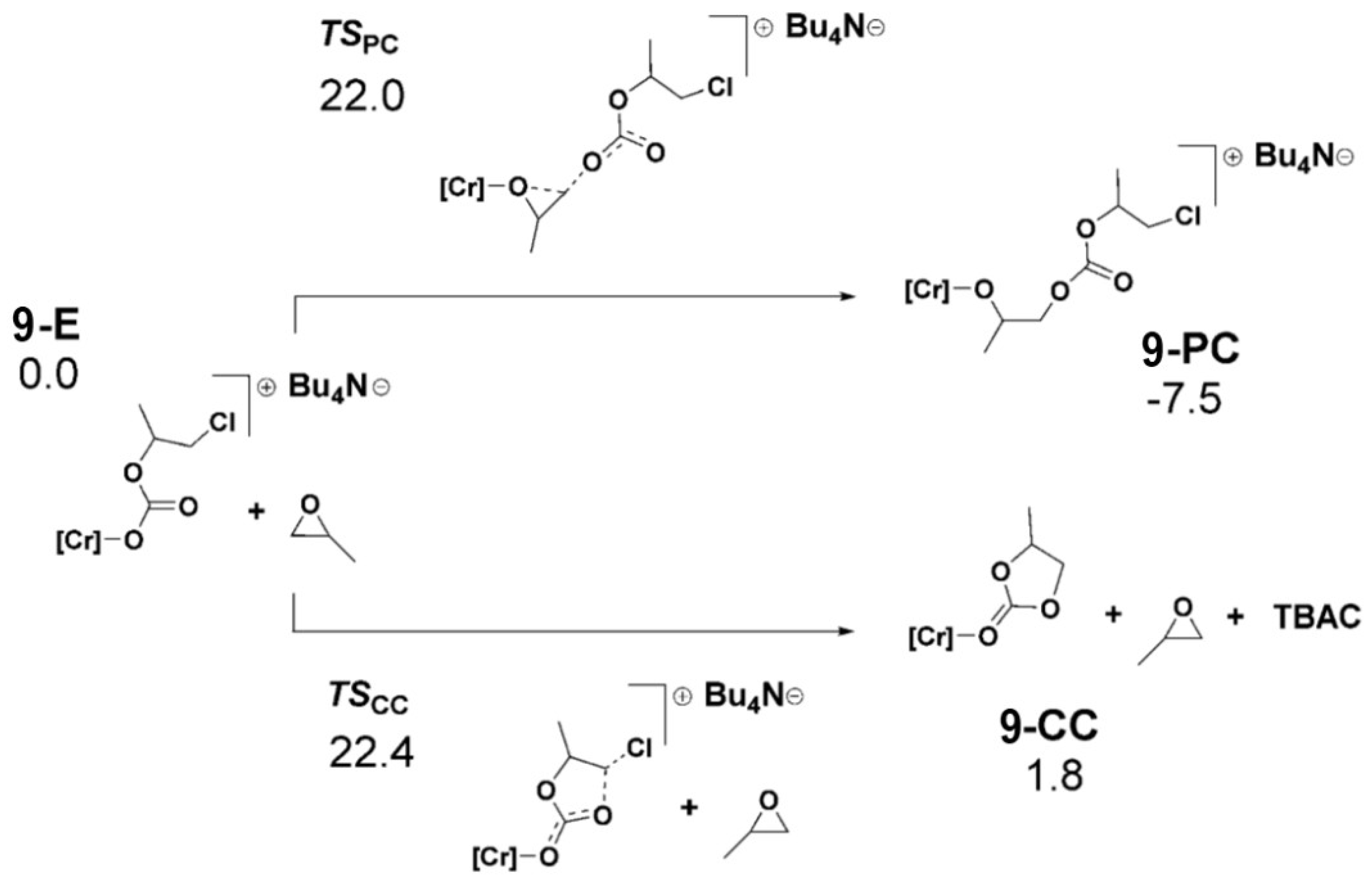
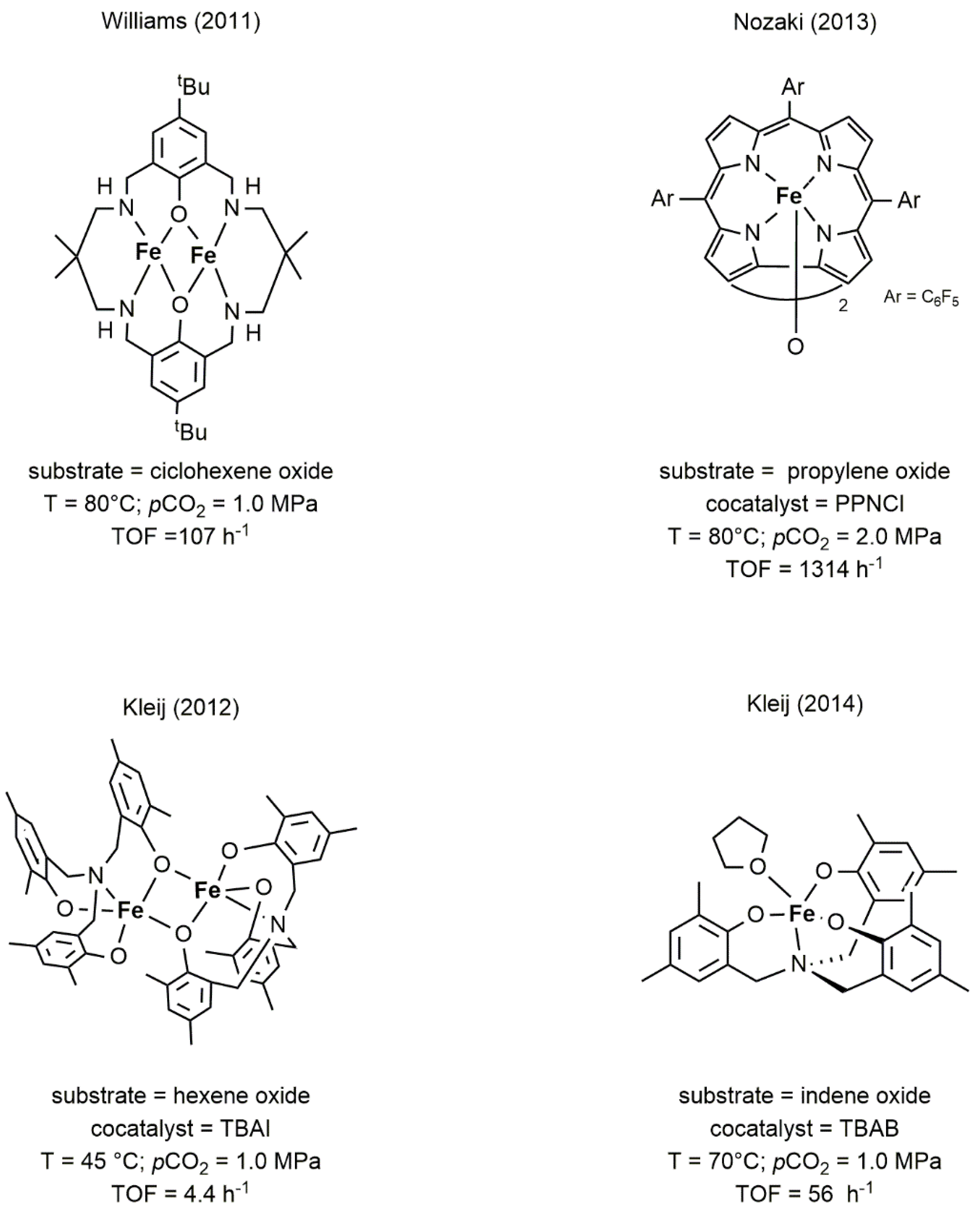

| Complex | TOF a (h−1) | Selectivity b (%) (Polymer-Carbonate Linkages) | Mnc (KDa) | Ðc |
|---|---|---|---|---|
| 1 | 74 | 73–97.5 | 15 | 1.30 |
| 2 | 82 | 68–98.0 | 20 | 1.35 |
| 3 | 78 | 71–99.0 | 19 | 1.27 |
| 4 | 13 | 44–95.5 | 6 | 1.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradiso, V.; Capaccio, V.; Lamparelli, D.H.; Capacchione, C. Metal Complexes Bearing Sulfur-Containing Ligands as Catalysts in the Reaction of CO2 with Epoxides. Catalysts 2020, 10, 825. https://doi.org/10.3390/catal10080825
Paradiso V, Capaccio V, Lamparelli DH, Capacchione C. Metal Complexes Bearing Sulfur-Containing Ligands as Catalysts in the Reaction of CO2 with Epoxides. Catalysts. 2020; 10(8):825. https://doi.org/10.3390/catal10080825
Chicago/Turabian StyleParadiso, Veronica, Vito Capaccio, David Hermann Lamparelli, and Carmine Capacchione. 2020. "Metal Complexes Bearing Sulfur-Containing Ligands as Catalysts in the Reaction of CO2 with Epoxides" Catalysts 10, no. 8: 825. https://doi.org/10.3390/catal10080825
APA StyleParadiso, V., Capaccio, V., Lamparelli, D. H., & Capacchione, C. (2020). Metal Complexes Bearing Sulfur-Containing Ligands as Catalysts in the Reaction of CO2 with Epoxides. Catalysts, 10(8), 825. https://doi.org/10.3390/catal10080825






