Visible-Light Driven Photocatalytic Degradation of Pirimicarb by Pt-Doped AgInS2 Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
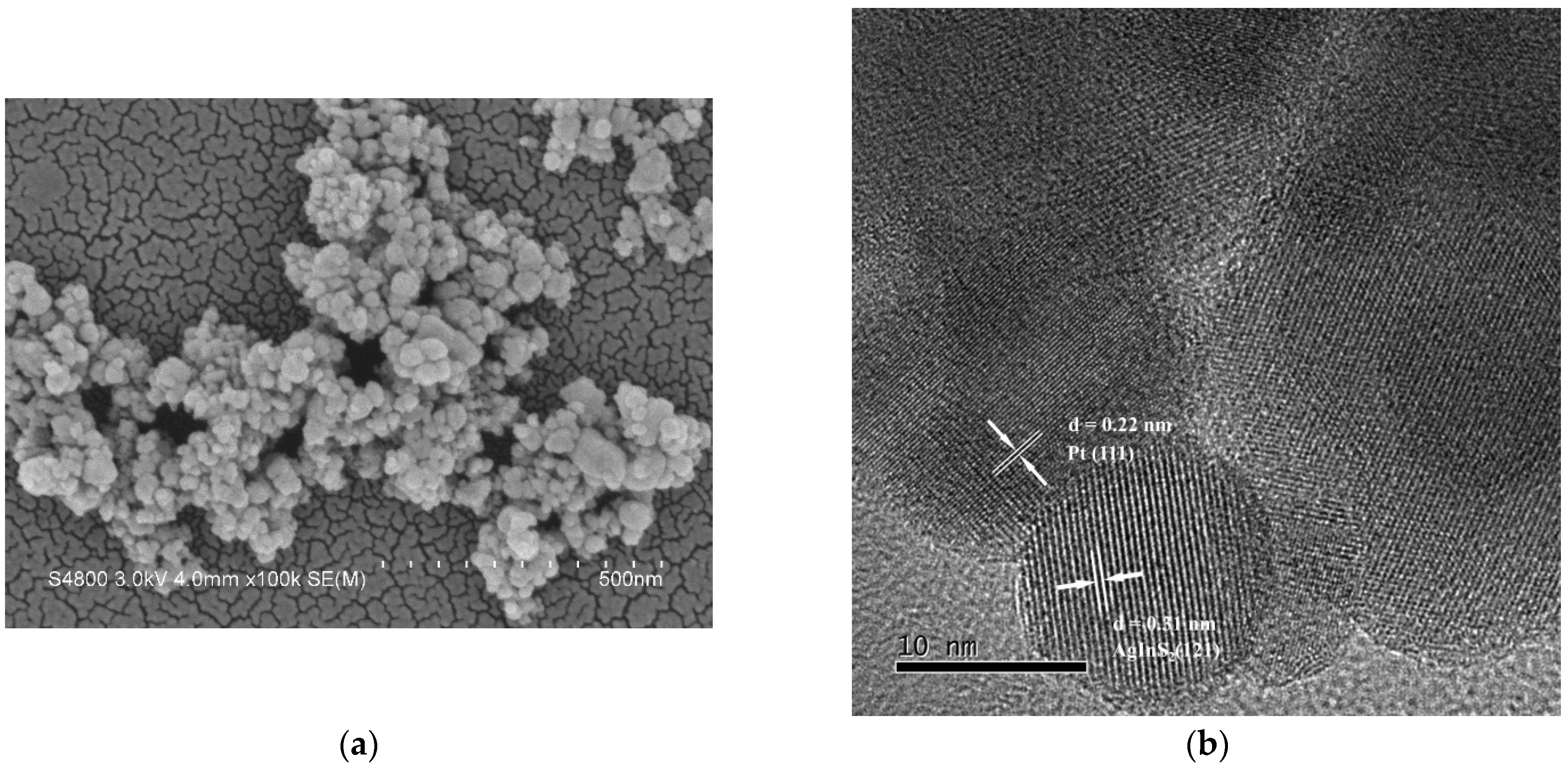
2.1. Characterization of the as-Prepared Undoped and Pt-doped AgInS2 samples
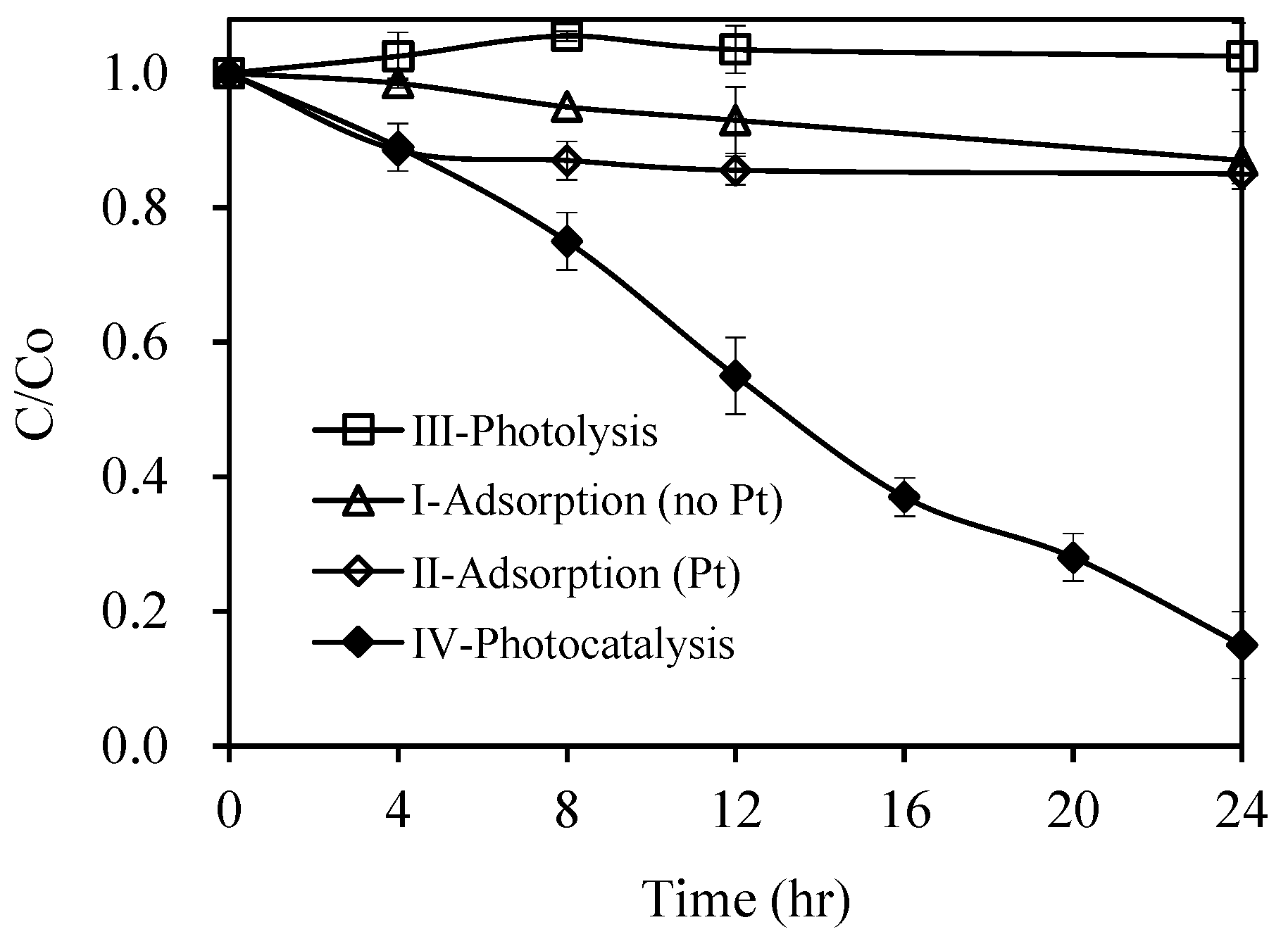
2.2. Photocatalytic Reaction
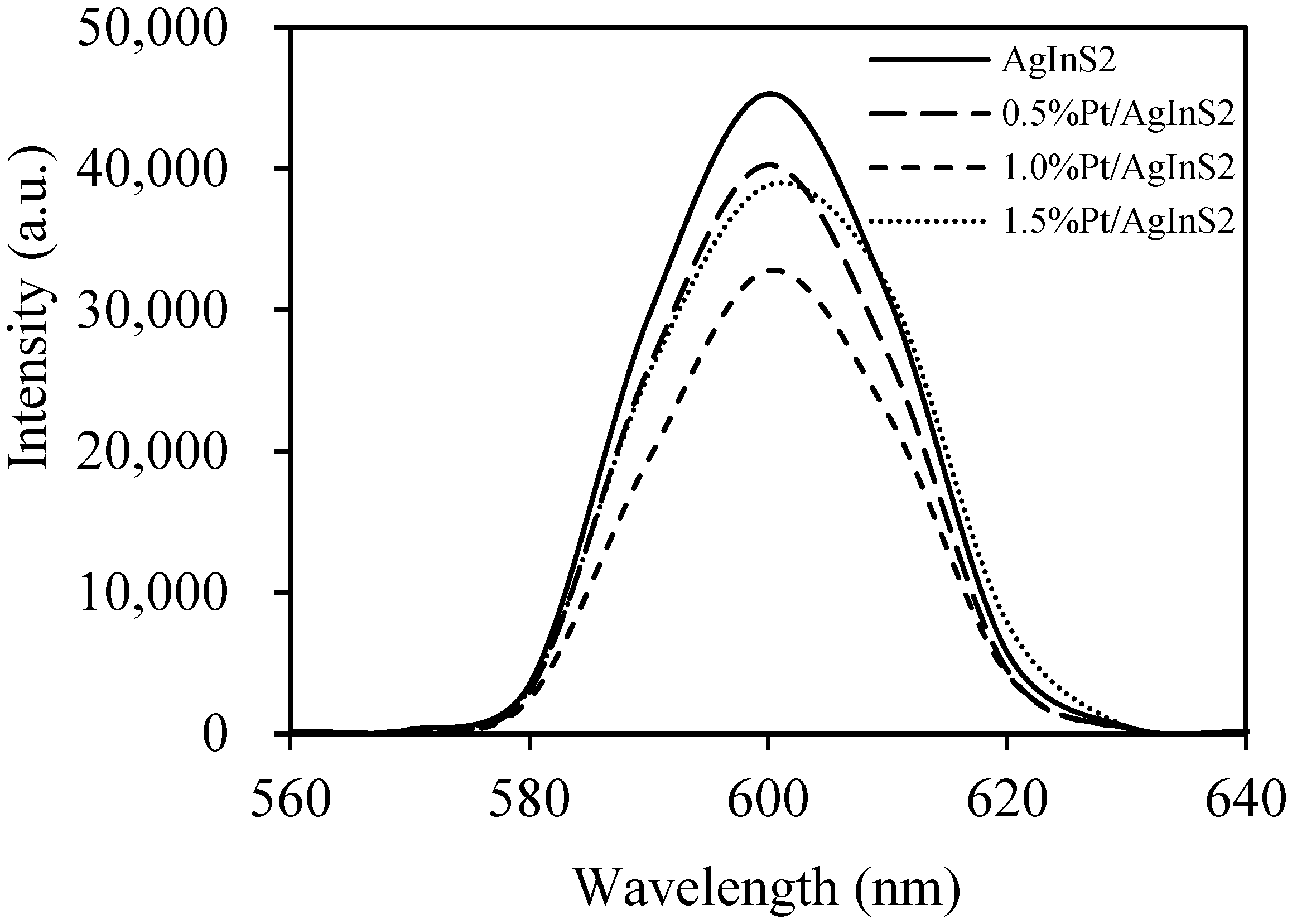
2.2.1. Effect of Pt Content
2.2.2. Effect of Catalyst Dosage
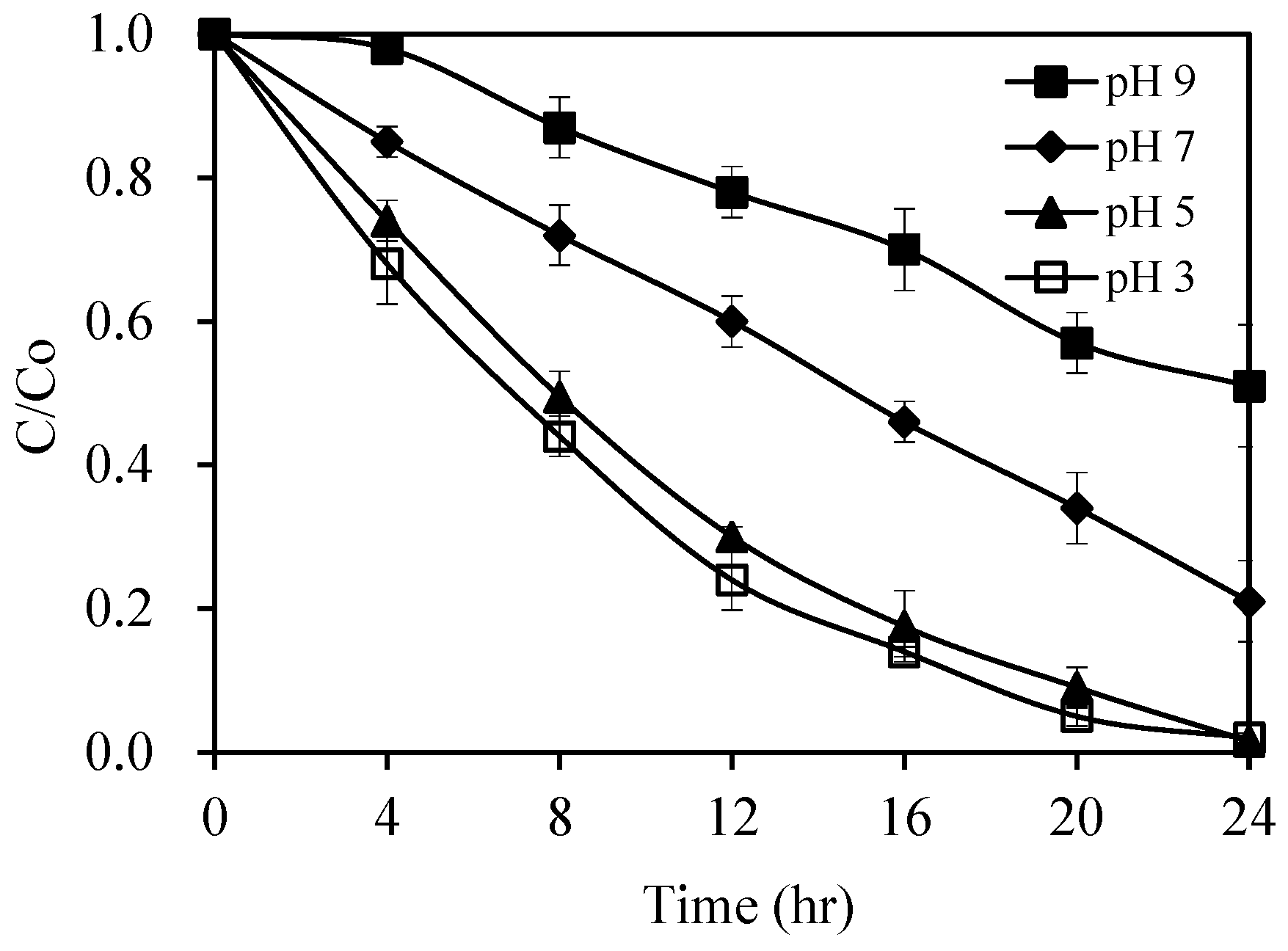
2.2.3. Effect of Initial Ph Value
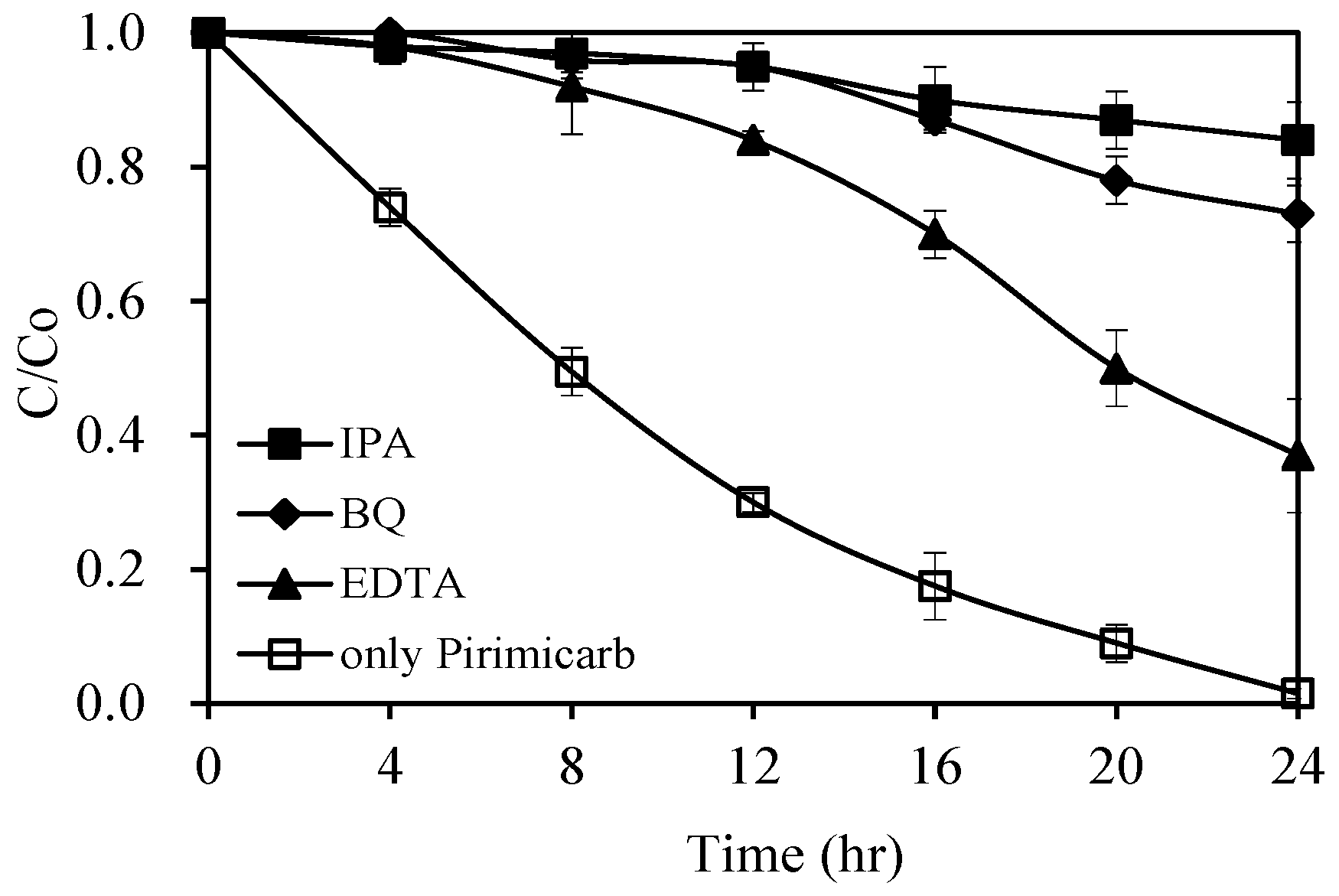
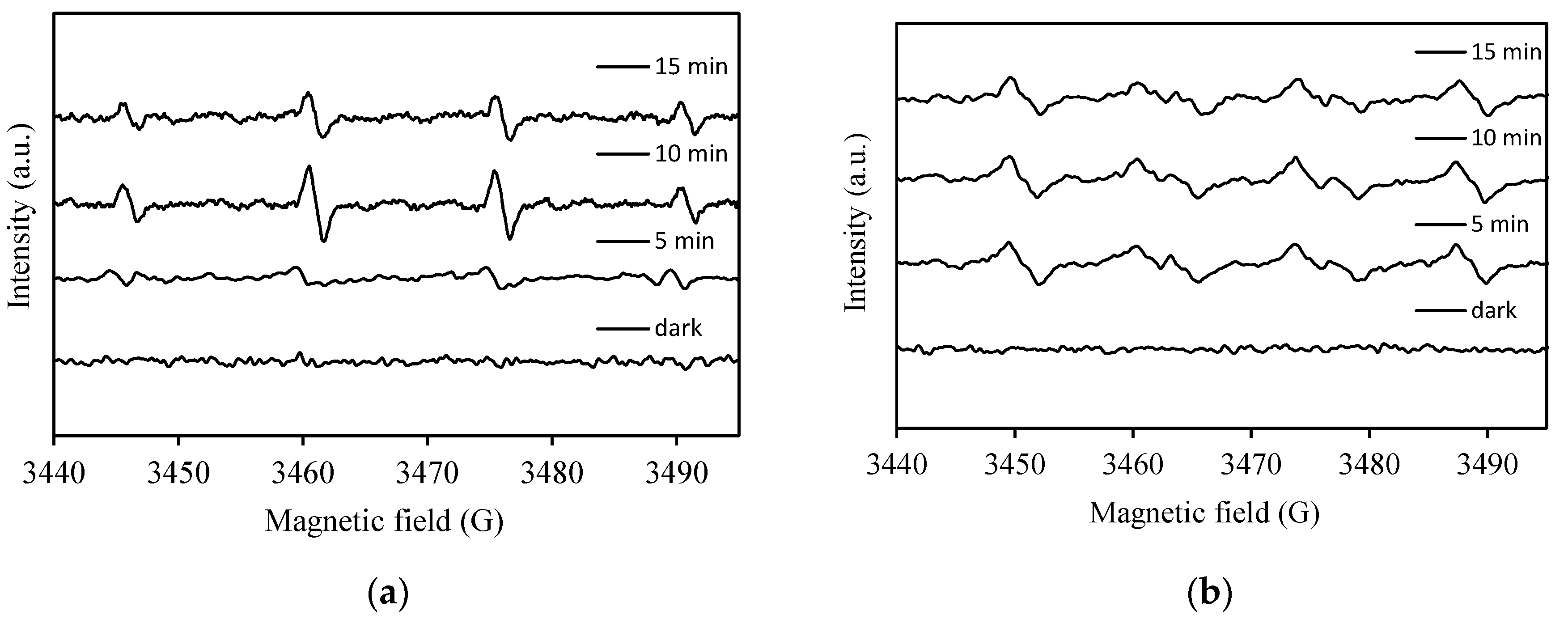
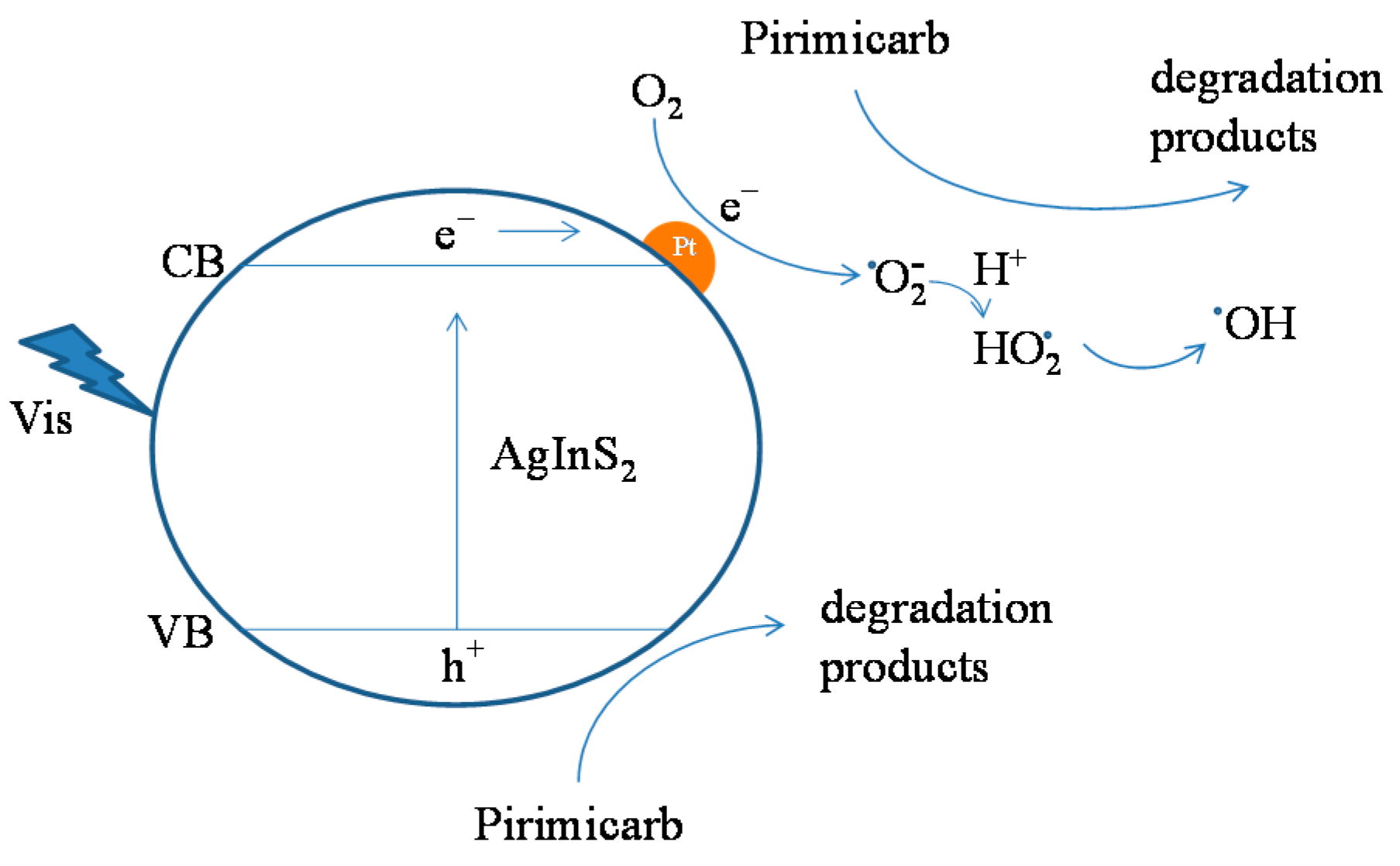
2.2.4. Identification of Active Species (Quenching and ESR Studies)
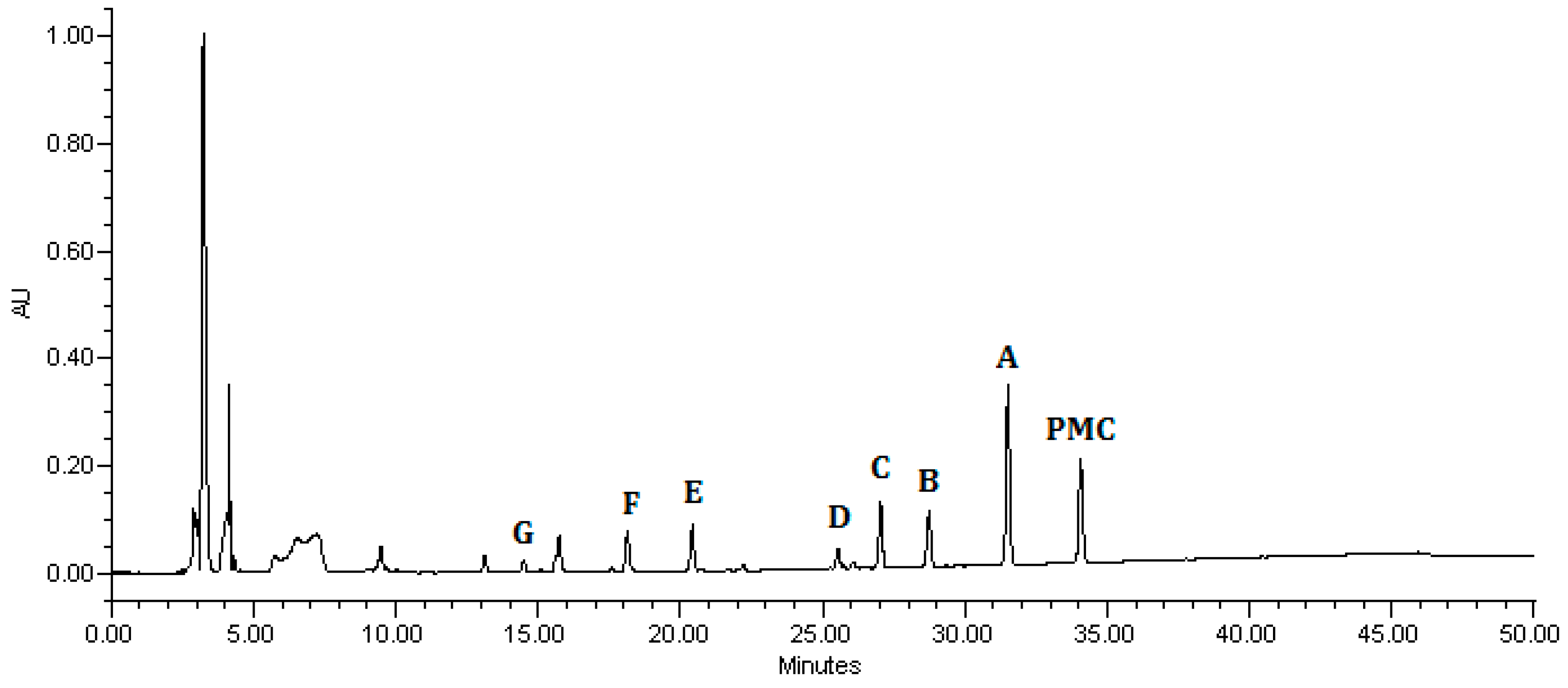
2.2.5. Reaction Pathway of Pirimicarb Degradation
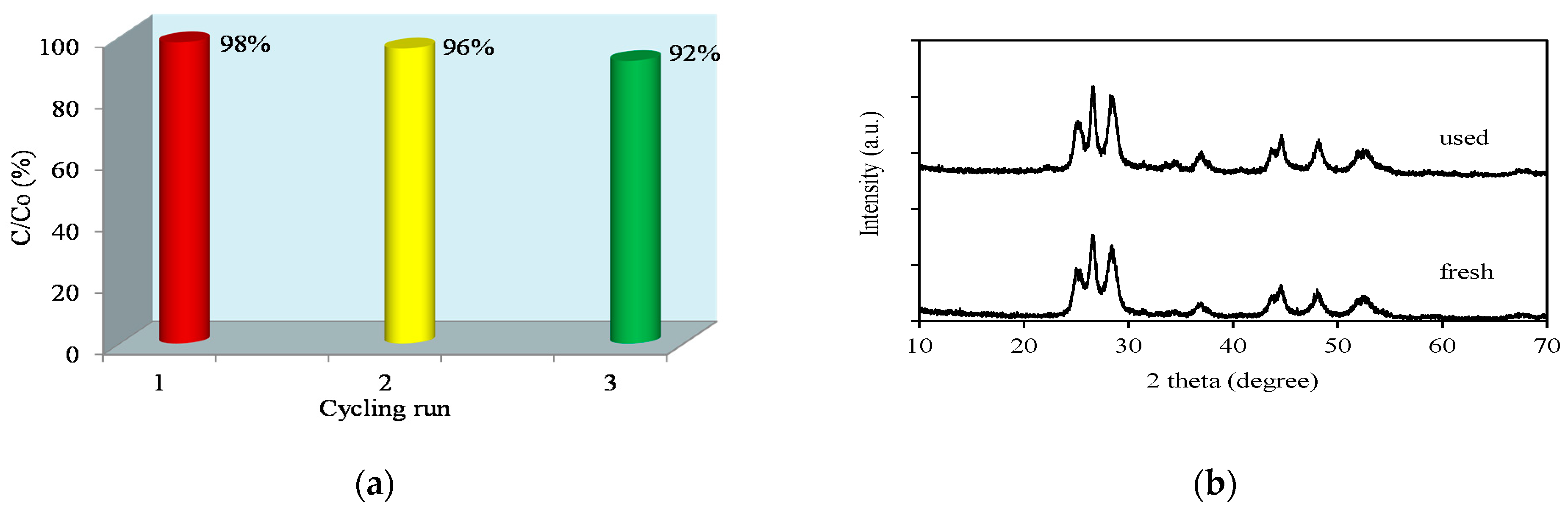
2.2.6. Efficiency of Recycled Catalyst
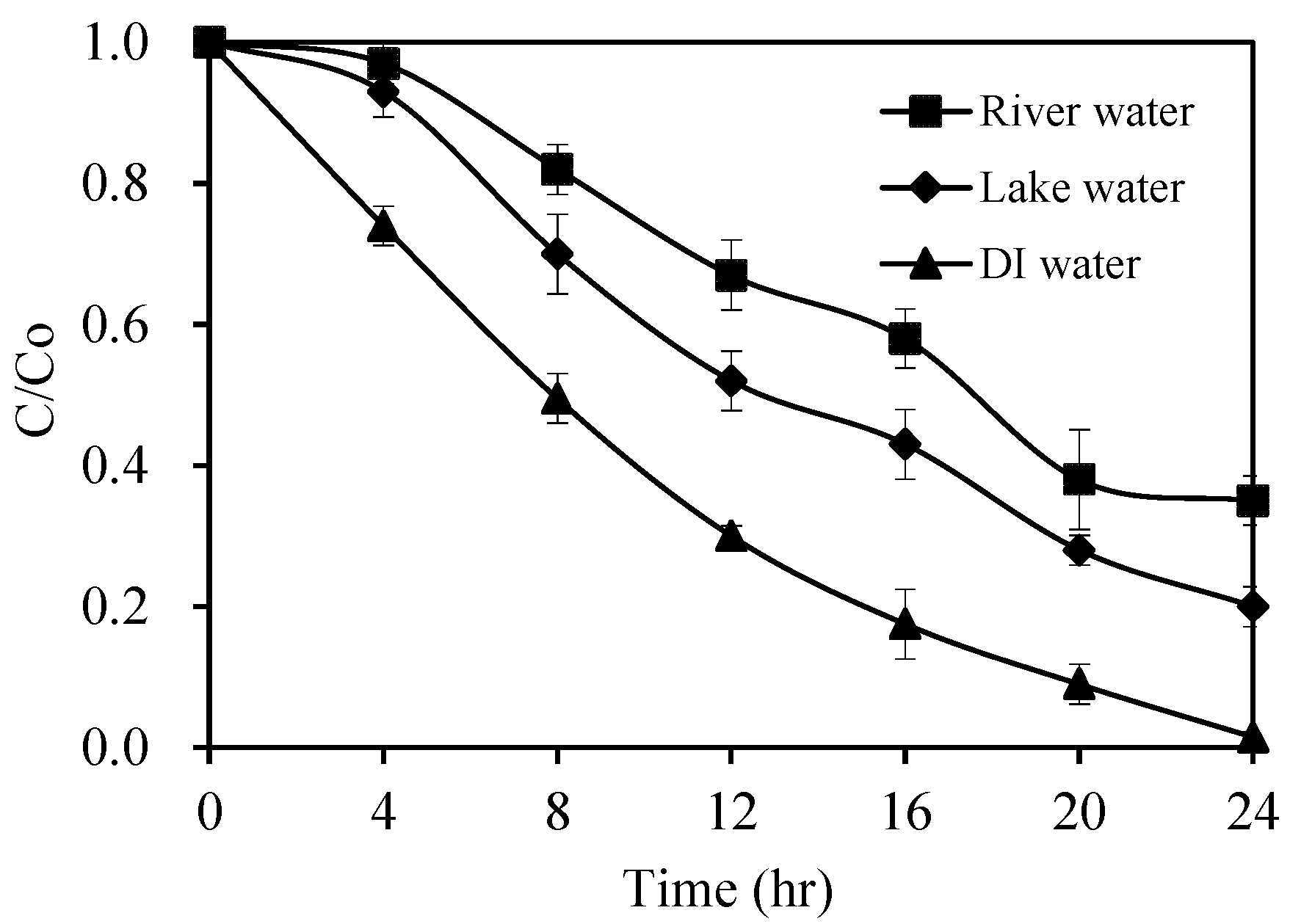
2.2.7. Photocatalytic Treatment of Natural Water Samples
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization Of Pt/Agins2
3.3. Apparatus and Instruments
3.4. Procedures and Analyses
3.5. Procedure for Degradation of Pirimicarb in Natural Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Diuzheva, A.; Dejmkova, H.; Fischer, J.; Andruch, V. Simultaneous determination of three carbamate pesticides using vortex-assisted liquid–liquid microextraction combined with HPLC-amperometric detection. Microchem. J. 2019, 150, 104071. [Google Scholar] [CrossRef]
- Wu, Q.; Chang, Q.; Wu, C.; Rao, H.; Zeng, X.; Wang, C.; Wang, Z. Ultrasound-assisted surfactant-enhanced emulsification microextraction for the determination of carbamate pesticides in water samples by high performance liquid chromatography. J. Chromatogr. A 2010, 1217, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Ruengprapavut, S.; Sophonnithiprasert, T.; Pongpoungphet, N. The effectiveness of chemical solutions on the removal of carbaryl residues from cucumber and chili presoaked in carbaryl using the HPLC technique. Food Chem. 2020, 309, 125659. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fu, F.; Chen, Z.; Li, D.; Zhang, L.; Chen, G. Study on the photodegradation and microbiological degradation of pirimicarb insecticide by using liquid chromatography coupled with ion-trap mass spectrometry. J. Chromatogr. A 2009, 1216, 3217–3222. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Rafique, U.; Akhtar, M.J. Removal of pirimicarb from agricultural waste water using cellulose acetate–modified ionic liquid membrane. Environ. Sci. Pollut. Res. 2019, 26, 15795–15802. [Google Scholar] [CrossRef]
- Champagne, P.-L.; Kumar, R.; Ling, C.-C. Multi-responsive self-assembled pyrene-appended β-cyclodextrin nanoaggregates: Discriminative and selective ratiometric detection of pirimicarb pesticide and trinitroaromatic explosives. Sens. Actuators B: Chem. 2019, 281, 229–238. [Google Scholar] [CrossRef]
- Soloneski, S.; Larramendy, M.L. Sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary (CHO-K1) cells treated with the insecticide pirimicarb. J. Hazard. Mater. 2010, 174, 410–415. [Google Scholar] [CrossRef]
- Fernández-Ramos, C.; Šatínský, D.; Solich, P. New method for the determination of carbamate and pyrethroid insecticides in water samples using on-line SPE fused core column chromatography. Talanta 2014, 129, 579–585. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, J.; Li, Q.; Zhang, S.; Liang, Y.; Zhang, H. Graphene based solid phase extraction combined with ultra high performance liquid chromatography–tandem mass spectrometry for carbamate pesticides analysis in environmental water samples. J. Chromatogr. A 2014, 1355, 219–227. [Google Scholar] [CrossRef]
- Omrani, N.; Nezamzadeh-Ejhieh, A. A comprehensive study on the mechanism pathways and scavenging agents in the photocatalytic activity of BiVO4/WO3 nano-composite. J. Water Process. Eng. 2020, 33, 101094. [Google Scholar] [CrossRef]
- Yang, B.; Park, H.-D.; Hong, S.W.; Lee, S.-H.; Park, J.-A.; Choi, J.-W. Photocatalytic degradation of microcystin-LR and anatoxin-a with presence of natural organic matter using UV-light emitting diodes/TiO2 process. J. Water Process. Eng. 2020, 34, 101163. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.; Saint, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Hsieh, Y.-H.; Chou, M.-Y.; Chang, C.-Y. Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution. Appl. Catal. B Environ. 1999, 21, 1–8. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Maurino, V.; Minero, C.; Carlin, V.; Tosato, M.L.; Pramauro, E.; Zerbinati, O. Photocatalytic degradation of atrazine and other s-triazine herbicides. Environ. Sci. Technol. 1990, 24, 1559–1565. [Google Scholar] [CrossRef]
- Chen, C.; Fan, H.; Shaya, J.; Chang, Y.; Golovko, V.B.; Toulemonde, O.; Huang, C.; Song, Y.; Lu, C.-S. Accelerated ZnMoO 4 photocatalytic degradation of pirimicarb under UV light mediated by peroxymonosulfate. Appl. Organomet. Chem. 2019, 33, 5113. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, C.; Huang, Y.; Lin, W.; Yen, Y.; Lu, C. Pirimicarb Degradation by BiVO 4 Photocatalysis: Parameter and Reaction Pathway Investigations. Sep. Sci. Technol. 2016, 51, 2284–2296. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chanéac, C.; Durupthy, O.; Mariey, L.; Maugé, F.; Brezová, V.; Jolivet, J.-P. New Insights Into BiVO4 Properties as Visible Light Photocatalyst. J. Phys. Chem. C 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Carra, I.; Ortega-Gómez, E.; Santos-Juanes, L.; López, J.L.C.; Sánchez-Pérez, J. Cost analysis of different hydrogen peroxide supply strategies in the solar photo-Fenton process. Chem. Eng. J. 2013, 224, 75–81. [Google Scholar] [CrossRef]
- Seitz, F.; Bundschuh, M.; Dabrunz, A.; Bandow, N.; Schaumann, G.E.; Schulz, R. Titanium dioxide nanoparticles detoxify pirimicarb under UV irradiation at ambient intensities. Environ. Toxicol. Chem. 2012, 31, 518–523. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, X.; Fan, K.; Peng, T.; Wei, B. Hydrothermal synthesis of single-walled carbon nanotube–TiO2 hybrid and its photocatalytic activity. Appl. Surf. Sci. 2013, 270, 238–244. [Google Scholar] [CrossRef]
- Li, S.; Tang, X.; Zang, Z.; Yao, Y.; Yao, Z.; Zhong, H.; Chen, B. I-III-VI chalcogenide semiconductor nanocrystals: Synthesis, properties, and applications. Chin. J. Catal. 2018, 39, 590–605. [Google Scholar] [CrossRef]
- Olejniczak, A.; Cichy, B.; Stręk, W. DFT calculations of metal-organic I-III-VI semiconductor clusters: Benchmark of exchange-correlation functionals and localized basis sets. Comput. Mater. Sci. 2019, 163, 186–195. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Salavati-Niasari, M. A simple microwave approach for synthesis and characterization of Ag2S–AgInS2 nanocomposites. Compos. Part B Eng. 2014, 56, 490–496. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Jafarisani, M. Photocatalytic degradation of 4-nitrophenol in aqueous solution by the AgInS2 nanoparticles synthesized via microwave heating technique. Int. J. Health Stud. 2017, 3, 18–24. [Google Scholar]
- Tang, X.; Choo, E.S.G.; Goh, G.K.L.; Yu, K.; Xu, Q.-H.; Xue, J. Synthesis and characterization of AgInS2–ZnS heterodimers with tunable photoluminescence. J. Mater. Chem. 2011, 21, 11239. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Liu, Q.; Zhu, Y.; Lu, Y. Density functional theory study on electronic and photocatalytic properties of orthorhombic AgInS2. Comput. Mater. Sci. 2014, 91, 159–164. [Google Scholar] [CrossRef]
- Zhang, W.; Li, D.; Chen, Z.; Sun, M.; Li, W.; Lin, Q.; Fu, X. Microwave hydrothermal synthesis of AgInS2 with visible light photocatalytic activity. Mater. Res. Bull. 2011, 46, 975–982. [Google Scholar] [CrossRef]
- Deng, F.; Zhong, F.; Lin, D.; Zhao, L.; Liu, Y.; Huang, J.; Luo, X.; Luo, S.; Dionysiou, D.D. One-step hydrothermal fabrication of visible-light-responsive AgInS2/SnIn4S8 heterojunction for highly-efficient photocatalytic treatment of organic pollutants and real pharmaceutical industry wastewater. Appl. Catal. B-Environ. 2017, 219, 163–172. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Sun, S.; Zhang, L.; Chang, J. Enhanced photocatalytic activity of Bi2WO6 loaded with Ag nanoparticles under visible light irradiation. Appl. Catal. B Environ. 2009, 92, 50–55. [Google Scholar] [CrossRef]
- Aazam, E. Photocatalytic oxidation of cyanide under visible light by Pt doped AgInS2 nanoparticles. J. Ind. Eng. Chem. 2014, 20, 4008–4013. [Google Scholar] [CrossRef]
- Deng, F.; Zhong, F.; Hu, P.; Pei, X.; Luo, X.; Luo, S. Fabrication of In-rich AgInS2 nanoplates and nanotubes by a facile low-temperature co-precipitation strategy and their excellent visible-light photocatalytic mineralization performance. J. Nanoparticle Res. 2017, 19, 14. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Liu, N.; Lun, N.; Qi, Y. Platinum-Nanoparticle-Modified TiO2 Nanowires with Enhanced Photocatalytic Property. ACS Appl. Mater. Interfaces 2010, 2, 3373–3377. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Teng, W.; Zhao, Q.; Shi, Y.; Yue, R.; Chen, Y. Efficient photocatalytic reduction of aqueous Cr(VI) over flower-like SnIn4S8 microspheres under visible light illumination. J. Hazard. Mater. 2013, 244, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, X.; Zhao, Q.; Ke, J.; Tadé, M.; Liu, S. Preparation of AgInS2/TiO2 composites for enhanced photocatalytic degradation of gaseous o-dichlorobenzene under visible light. Appl. Catal. B-Environ. 2016, 185, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Yue, E.; Lian, L.; Ma, W. Visible light induced dye-sensitized photocatalytic hydrogen production over platinized TiO2 derived from decomposition of platinum complex precursor. Int. J. Hydrogen Energy 2013, 38, 10746–10753. [Google Scholar] [CrossRef]
- Parayil, S.K.; Kibombo, H.S.; Wu, C.-M.; Peng, R.; Kindle, T.; Mishra, S.; Ahrenkiel, S.P.; Baltrusaitis, J.; Dimitrijevic, N.M.; Rajh, T.; et al. Synthesis-Dependent Oxidation State of Platinum on TiO2 and Their Influences on the Solar Simulated Photocatalytic Hydrogen Production from Water. J. Phys. Chem. C 2013, 117, 16850–16862. [Google Scholar] [CrossRef]
- Li, F.; Li, X. The enhancement of photodegradation efficiency using Pt–TiO2 catalyst. Chemosphere 2002, 48, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Qamar, M. Photodegradation of acridine orange catalyzed by nanostructured titanium dioxide modified with platinum and silver metals. Desalination 2010, 254, 108–113. [Google Scholar] [CrossRef]
- El-Maiss, J.; El Dine, T.M.; Lu, C.-S.; Karamé, I.; Kanj, A.; Polychronopoulou, K.; Shaya, J. Recent Advances in Metal-Catalyzed Alkyl–Boron (C(sp3)–C(sp2)) Suzuki-Miyaura Cross-Couplings. Catalysts 2020, 10, 296. [Google Scholar] [CrossRef] [Green Version]
- Shaya, J.; Deschamps, M.-A.; Michel, B.Y.; Burger, A. Air-Stable Pd Catalytic Systems for Sequential One-Pot Synthesis of Challenging Unsymmetrical Aminoaromatics. J. Org. Chem. 2016, 81, 7566–7573. [Google Scholar] [CrossRef]
- Huang, S.; Chen, C.; Tsai, H.; Shaya, J.; Lu, C. Photocatalytic degradation of thiobencarb by a visible light-driven MoS2 photocatalyst. Sep. Purif. Technol. 2018, 197, 147–155. [Google Scholar] [CrossRef]
- Lu, C.-S.; Shaya, J.; Fan, H.-J.; Chang, Y.-K.; Chi, H.-T.; Lu, C.-S. Silver vanadium oxide materials: Controlled synthesis by hydrothermal method and efficient photocatalytic degradation of atrazine and CV dye. Sep. Purif. Technol. 2018, 206, 226–238. [Google Scholar] [CrossRef]
- Annapoorani, R.; Dhananjeyan, M.; Renganathan, R. An investigation on ZnO photocatalysed oxidation of uracil. J. Photochem. Photobiol. A Chem. 1997, 111, 215–221. [Google Scholar] [CrossRef]
- Cunningham, J.; Corkery, S. Reactions involving electron transfer at semiconductor surfaces. VI. Electron spin resonance studies on dark and illuminated aqueous suspensions of zinc oxides. J. Phys. Chem. 1975, 79, 933–941. [Google Scholar] [CrossRef]
- Na, Y.; Kim, Y.-I.; Cho, D.W.; Pradhan, D.; Sohn, Y. Adsorption/photocatalytic performances of hierarchical flowerlike BiOBrxCl1−x nanostructures for methyl orange, Rhodamine B and methylene blue. Mater. Sci. Semicond. Process. 2014, 27, 181–190. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Photocatalytic Reactivity of Surface Platinized TiO2: Substrate Specificity and the Effect of Pt Oxidation State. J. Phys. Chem. B 2005, 109, 7399–7406. [Google Scholar] [CrossRef]
- Wang, X.; Waterhouse, G.I.N.; Mitchell, D.R.; Prince, K.; Caruso, R.A. Noble Metal-Modified Porous Titania Networks and their Application as Photocatalysts. ChemCatChem 2011, 3, 1763–1771. [Google Scholar] [CrossRef]
- Satra, J.; Mondal, P.; Ghorui, U.K.; Adhikary, B. In situ systematic metallic gold incorporation to optimize the photon conversion efficiency of AgInS2 thin film semiconductor. Sol. Energy Mater. Sol. Cells 2019, 195, 24–33. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D. One-step synthesis of mesoporous platinum/titania nanocomposites as photocatalyst with enhanced photocatalytic activity for methanol oxidation. Green Chem. 2011, 13, 428. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Choi, W. Effect of Platinum Deposits on TiO2on the Anoxic Photocatalytic Degradation Pathways of Alkylamines in Water: Dealkylation and N-Alkylation. Environ. Sci. Technol. 2004, 38, 4026–4033. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Wang, S.; Ge, J.; Qu, R.; Feng, M.; Sharma, V.K.; Wang, Z. Degradation kinetics and transformation products of chlorophene by aqueous permanganate. Water Res. 2018, 138, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lu, C.S.; Mai, F.D.; Weng, C.S. Photooxidative N-de-ethylation of anionic triarylmethane dye (sulfan blue) in titanium dioxide dispersions under UV irradiation. J. Hazard. Mater. 2006, 137, 1600–1607. [Google Scholar] [CrossRef] [PubMed]






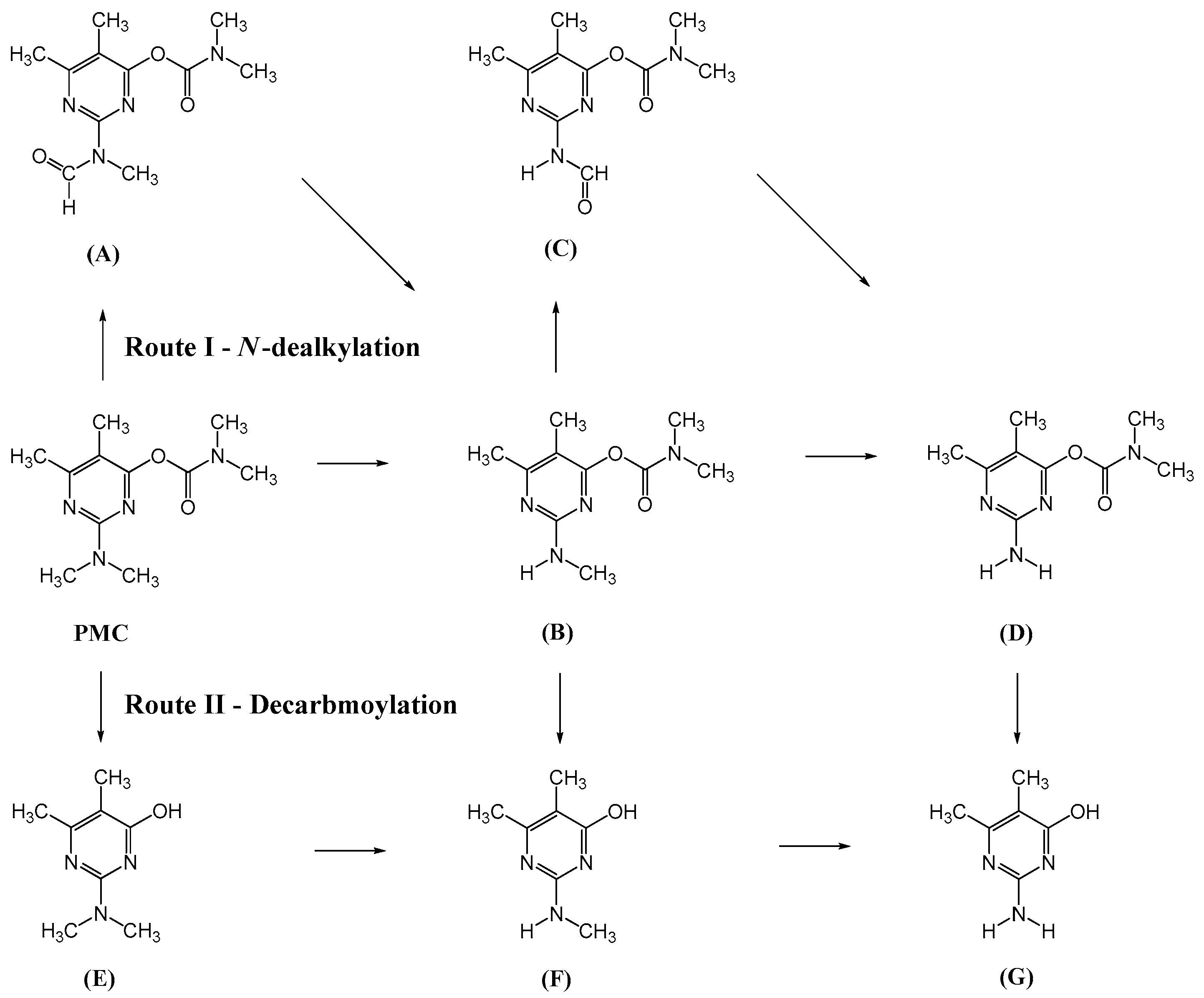
| Photocatalysts | Oxidants | Degradation Efficiency (%) | Degradation Time | Light Source | References |
|---|---|---|---|---|---|
| TiO2 | No oxidant | 50% | 72 h | UV light | [19] |
| Single-walled carbon nanotubes-TiO2 hybrid | No oxidant | 32–33% | 30 min | UV light (full light) | [20] |
| ZnMoO4 | No oxidant | 93% | 12 h | UV light | [15] |
| PMS | 98% | 3 h | UV light | [15] | |
| BiVO4 | No oxidant | <5% | 16 h | Visible light | [16] |
| H2O2 | 97.6% | 4 h | Visible light | [16] | |
| Pt/AgInS2 | No oxidant | 98% | 24 h | Visible light | Present work |
| Experiment | Conditions | Results (After 24 h) |
|---|---|---|
| I-Adsorption (no Pt) | ♦ Pirimicarb (10 mg L−1) ♦ AgInS2 (0.5 g L−1) (No Pt doping, no visible light) | Less than 15% of pirimicarb adsorption |
| II-Adsorption (Pt) | ♦ Pirimicarb (10 mg L−1) ♦ Pt/AgInS2 (0.5 g L−1) (No visible light) | Less than 15% of pirimicarb adsorption |
| III-Photolysis | ♦ Pirimicarb (10 mg L−1) ♦ Visible light (No Pt/AgInS2) | negligible pirimicarb degradation |
| IV-Photocatalysis | ♦ Pirimicarb (10 mg L−1) ♦ Pt/AgInS2 (0.5 g L−1) (Visible light) | 85% pirimicarb degradation |
| Pt content (wt%) | kapp (hr−1) | R2 |
|---|---|---|
| 0 | 0.015 | 0.97 |
| 0.25 | 0.043 | 0.96 |
| 0.5 | 0.045 | 0.98 |
| 1.0 | 0.092 | 0.93 |
| 1.5 | 0.055 | 0.94 |
| Structure | tR (min) | Characteristic Ions (m/z) |
|---|---|---|
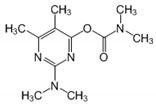 | 34.11 | [M]+ = 239, 195, 182 (PMC) 2-dimethylamino-5,6-dimethyl pyrimidin-4-yl-dimethylcarbamate |
 | 31.61 | [M]+ = 253, 225, 196 (A) 2-[(methylformyl)amino]-5,6-dimethyl pyrimidin-4-yl-dimethylcarbamate |
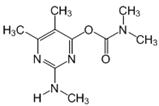 | 28.83 | [M]+ = 225, 180, 168 (B) 2-methylamino-5,6-dimethylpyrimidin- 4-yl-dimethylcarbamate |
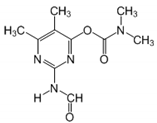 | 27.13 | [M]+ = 239, 211, 182 (C) 2-(formylamino)-5,6-dimethylpyrimidin- 4-yl-dimethylcarbamate |
 | 25.63 | [M]+ = 211, 166, 154 (D) 2-amino-5,6-dimethylpyrimidin- 4-yl-dimethylcarbamate |
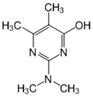 | 20.49 | [M]+ = 168, 123, 98 (E) 2-dimethylamino-5,6-dimethyl-4- hydroxypyrimidine |
 | 18.31 | [M]+ = 154, 137, 98 (F) 2-methylamino-5,6-dimethyl-4- hydroxypyrimidine |
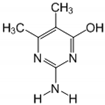 | 14.70 | [M]+ = 140, 123, 98 (G) 2-amino-5,6-dimethyl-4-hydroxy pyrimidine |
| Parameter | River Water | Lake Water |
|---|---|---|
| pH | 6.30 | 6.24 |
| Conductivity (μmho cm−1) | 420 | 378 |
| Turbidity (NTU) | 2.9 | 4.9 |
| TOC (mg L−1) | 13.43 | 3.44 |
| Sulfate (mg L−1) | 48.1 | 50.9 |
| Chloride (mg L−1) | 22.6 | 16.7 |
| Nitrate (mg L−1) | 84.8 | 2.9 |
| Nitrite (mg L−1) | 1.12 | 0.02 |
| Catalysts | Pt loading (wt%) | |
|---|---|---|
| Intended | Actual | |
| 0.25 wt% Pt/AgInS2 | 0.25 | 0.267 |
| 0.5 wt% Pt/AgInS2 | 0.5 | 0.586 |
| 1.0 wt% Pt/AgInS2 | 1.0 | 1.060 |
| 1.5 wt% Pt/AgInS2 | 1.5 | 1.530 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.; Shaya, J.; Tesana, S.; Golovko, V.B.; Wang, S.-Y.; Liao, Y.-Y.; Lu, C.-S.; Chen, C.-C. Visible-Light Driven Photocatalytic Degradation of Pirimicarb by Pt-Doped AgInS2 Nanoparticles. Catalysts 2020, 10, 857. https://doi.org/10.3390/catal10080857
Tsai H, Shaya J, Tesana S, Golovko VB, Wang S-Y, Liao Y-Y, Lu C-S, Chen C-C. Visible-Light Driven Photocatalytic Degradation of Pirimicarb by Pt-Doped AgInS2 Nanoparticles. Catalysts. 2020; 10(8):857. https://doi.org/10.3390/catal10080857
Chicago/Turabian StyleTsai, Hweiyan, Janah Shaya, Siriluck Tesana, Vladimir B. Golovko, Syuan-Yun Wang, Yi-Yen Liao, Chung-Shin Lu, and Chiing-Chang Chen. 2020. "Visible-Light Driven Photocatalytic Degradation of Pirimicarb by Pt-Doped AgInS2 Nanoparticles" Catalysts 10, no. 8: 857. https://doi.org/10.3390/catal10080857
APA StyleTsai, H., Shaya, J., Tesana, S., Golovko, V. B., Wang, S.-Y., Liao, Y.-Y., Lu, C.-S., & Chen, C.-C. (2020). Visible-Light Driven Photocatalytic Degradation of Pirimicarb by Pt-Doped AgInS2 Nanoparticles. Catalysts, 10(8), 857. https://doi.org/10.3390/catal10080857






