Formic Acid as a Hydrogen Source for the Additive-Free Reduction of Aromatic Carbonyl and Nitrile Compounds at Reusable Supported Pd Catalysts
Abstract
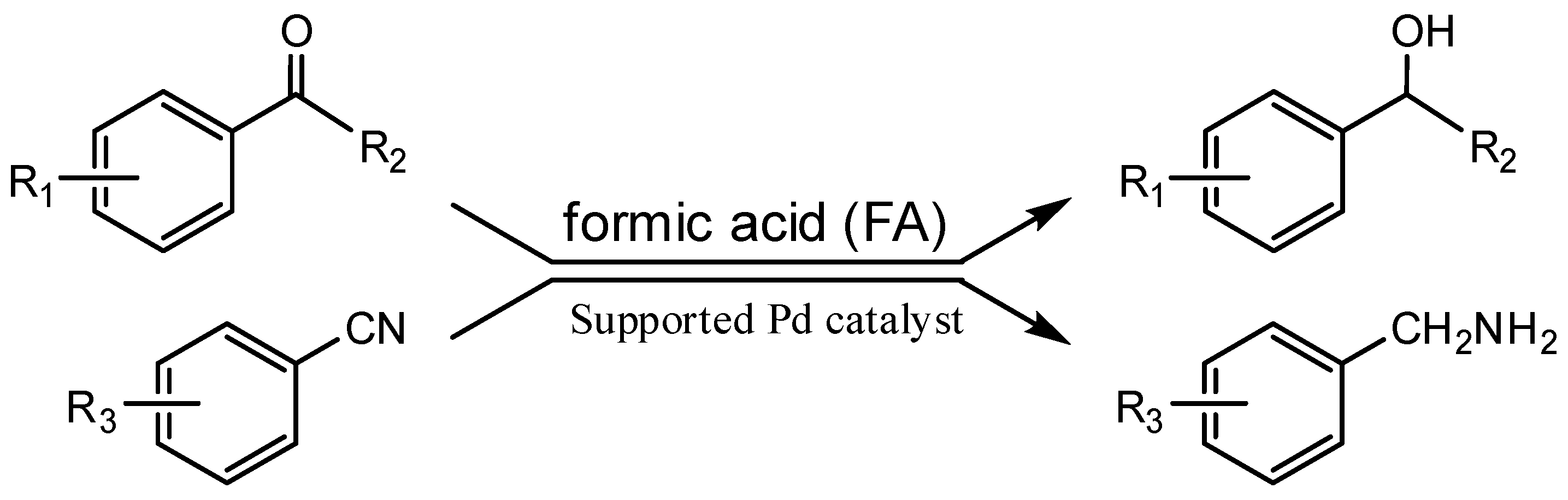
:1. Introduction
2. Results and Discussion
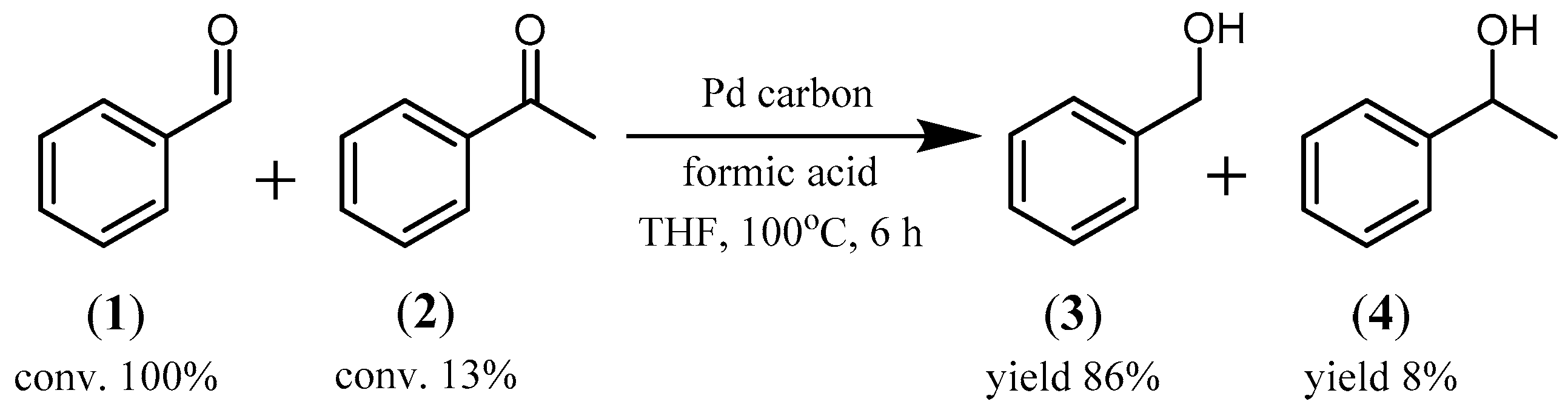
2.1. Reduction of Aromatic Carbonyl Compounds
2.2. Reduction of Aromatic Nitriles
3. Materials and Methods
3.1. Materials
3.2. Reaction Procedure
3.3. Product and FA Analysis
3.4. Catalyst Recycling Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Schmidt, I.; Müller, K.; Arlt, W. Evaluation of formic-acid-based hydrogen storage technologies. Energy Fuels 2014, 28, 6540–6544. [Google Scholar] [CrossRef]
- Ojeda, M.; Iglesia, E. Formic acid dehydrogenation on Au-based catalysts at near-ambient temperatures. Angew. Chem. Int. Ed. 2009, 48, 4800–4803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddien, A.; Loges, B.; Junge, H.; Gartner, F.; Noyes, J.R.; Beller, M. Continuous hydrogen generation from formic acid: Highly active and stable ruthenium catalysts. Adv. Synth. Catal. 2009, 351, 2517–2520. [Google Scholar] [CrossRef]
- Boddien, A.; Loges, B.; Gärtner, F.; Torborg, C.; Fumino, K.; Junge, H.; Ludwig, R.; Beller, M. Iron-catalyzed hydrogen production from formic acid. J. Am. Chem. Soc. 2010, 132, 8924–8934. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen from formic acid decomposition over Pd and Au catalysts. Catal. Today 2010, 154, 7–12. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Du, X.L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Efficient subnanometric gold-catalyzed hydrogen generation via formic acid decomposition under ambient conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef]
- Zhang, S.; Metin, Ö.; Su, D.; Sun, S. Monodisperse AgPd alloy nanoparticles and their superior catalysis for the dehydrogenation of formic acid. Angew. Chem. Int. Ed. 2013, 52, 3681–3684. [Google Scholar] [CrossRef]
- Yu, W.Y.; Mullen, G.M.; Flaherty, D.W.; Mullins, C.B. Selective hydrogen production from formic acid decomposition on Pd-Au bimetallic surfaces. J. Am. Chem. Soc. 2014, 136, 11070–11078. [Google Scholar] [CrossRef]
- Navlani-García, M.; Martis, M.; Lozano-Castelló, D.; Mori, K.; Yamashita, H. Investigation of Pd nanoparticles supported on zeolites for hydrogen production from formic acid dehydrogenation. Catal. Sci. Technol. 2015, 5, 364–371. [Google Scholar]
- Navlani-García, M.; Mori, K.; Nozaki, A.; Kuwahara, Y.; Yamashita, H. Screening of carbon-supported PdAg nanoparticles in the hydrogen production from formic acid. Ind. Eng. Chem. Res. 2016, 55, 7612–7620. [Google Scholar] [CrossRef]
- Wang, W.; He, T.; Liu, X.; He, W.; Cong, H.; Shen, Y.; Yan, L.; Zhang, X.; Zhang, J.; Zhou, X. Highly active carbon supported Pd–Ag nanofacets catalysts for hydrogen production from HCOOH. ACS Appl. Mater. Interfaces 2016, 8, 20839–20848. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, M.; Navlani-García, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Palladium nanoparticles supported on titanium-doped graphitic carbon nitride for formic acid dehydrogenation. Chem. Asian J. 2017, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Masuda, S.; Tanaka, H.; Yoshizawa, K.; Che, M.; Yamashita, H. Phenylamine-functionalized mesoporous silica supported PdAg nanoparticles: A dual heterogeneous catalyst for formic acid/CO2-mediated chemical hydrogen delivery/storage. Chem. Commun. 2017, 53, 4677–4680. [Google Scholar] [CrossRef] [PubMed]
- Reutemann, W.; Kieczka, H. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Isbell, H.S.; Frush, H.L. Reaction of carbohydrates with hydroperoxides: Part II. Oxidation of ketoses with the hydroperoxide anion. Carbohydr. Res. 1973, 28, 295–301. [Google Scholar] [CrossRef]
- Isbell, H.S.; Naves, R.G. Degradation of reducing disaccharides by alkaline hydrogen peroxide. Carbohydr. Res. 1974, 36, C1–C4. [Google Scholar] [CrossRef]
- Isbell, H.S. A diradical mechanism for the degradation of reducing sugars by oxygen. Carbohydr. Res. 1976, 49, C1–C4. [Google Scholar] [CrossRef]
- Kruse, A.; Gawlik, A. Biomass conversion in water at 330−410 °C and 30−50 MPa. identification of key compounds for indicating different chemical reaction pathways. Ind. Eng. Chem. Res. 2003, 42, 267–279. [Google Scholar] [CrossRef]
- Jin, F.; Yun, J.; Li, G.; Kishita, A.; Tohji, K.; Enomoto, H. Hydrothermal conversion of carbohydrate biomass into formic acid at mild temperatures. Green Chem. 2008, 10, 612–615. [Google Scholar] [CrossRef]
- Jin, F.; Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: Chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
- Wolfel, R.; Taccardi, N.; Bosmann, A.; Wasserscheid, P. Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen. Green Chem. 2011, 13, 2759–2763. [Google Scholar] [CrossRef]
- Ahlkvist, J.; Ajaikumar, S.; Larsson, W.; Mikkola, J.P. One-pot catalytic conversion of Nordic pulp media into green platform chemicals. Appl. Catal. A Gen. 2013, 454, 21–29. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Synthesis of high-value organic acids from sugars promoted by hydrothermally loaded Cu oxide species on magnesia. Appl. Catal. B Environ. 2015, 162, 1–10. [Google Scholar] [CrossRef]
- Sato, R.; Choudhary, H.; Nishimura, S.; Ebitani, K. Synthesis of formic acid from monosaccharides using calcined Mg-Al hydrotalcite as reusable catalyst in the presence of aqueous hydrogen peroxide. Org. Process. Res. Dev. 2015, 19, 449–453. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ohta, T.; Tsuji, Y.; Hiyoshi, T.; Tsuji, Y. Ruthenium catalyzed reduction of nitroarenes and azaaromatic compounds using formic acid. Bull. Chem. Soc. Jpn. 1984, 57, 2440–2444. [Google Scholar] [CrossRef]
- Wienhöfer, G.; Sorribes, I.; Boddien, A.; Westerhaus, F.; Junge, K.; Junge, H.; Llusar, R.; Beller, M. General and selective iron-catalyzed transfer hydrogenation of nitroarenes without base. J. Am. Chem. Soc. 2011, 133, 12875–12879. [Google Scholar] [CrossRef] [PubMed]
- Heeres, H.; Handana, R.; Chunai, D.; Rasrendra, C.B.; Girisuta, B.; Heeres, H.J. Combined dehydration/(transfer)-hydrogenation of C6-sugars (D-glucose and D-fructose) to γ-valerolactone using ruthenium catalysts. Green Chem. 2009, 11, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar] [CrossRef]
- Soltani, O.; Ariger, M.A.; Vázquez-Villa, H.; Carreira, E.M. Transfer hydrogenation in water: Enantioselective, catalytic reduction of α-cyano and α-nitro substituted acetophenones. Org. Lett. 2010, 12, 2893–2895. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Li, S.S.; Huang, J.; Liu, Y.M.; He, H.Y.; Cao, Y. Gold-catalyzed reductive transformation of nitro compounds using formic acid: Mild, efficient, and versatile. ChemSusChem 2015, 8, 3029–3035. [Google Scholar] [CrossRef]
- Son, P.A.; Nishimura, S.; Ebitani, K. Production of γ-valerolactone from biomass-derived compounds using formic acid as a hydrogen source over supported metal catalysts in water solvent. RSC Adv. 2014, 4, 10525–10530. [Google Scholar] [CrossRef]
- Tuteja, J.; Nishimura, S.; Ebitani, K. Base-free chemoselective transfer hydrogenation of nitroarenes to anilines with formic acid as hydrogen source by a reusable heterogeneous Pd/ZrP catalyst. RSC Adv. 2014, 4, 38241–38249. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Hydrothermal Preparation of a robust boehmite-supported N,N-dimethyldodecylamine N-oxide-capped cobalt and palladium catalyst for the facile utilization of formic acid as a hydrogen source. ChemCatChem 2015, 7, 2361–2369. [Google Scholar] [CrossRef]
- Casey, C.P.; Guan, H. An efficient and chemoselective iron catalyst for the hydrogenation of ketones. J. Am. Chem. Soc. 2007, 129, 5816–5817. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Srimani, D.; Chakraborty, S.; Ben-David, Y.; Milstein, D. Selective hydrogenation of nitriles to primary amines catalyzed by a cobalt pincer complex. J. Am. Chem. Soc. 2015, 137, 8888–8891. [Google Scholar] [CrossRef]
- Elangovan, S.; Topf, C.; Fischer, S.; Jiao, H.; Spannenberg, A.; Baumann, W.; Ludwig, R.; Junge, K.; Beller, M. Selective catalytic hydrogenations of nitriles, ketones, and aldehydes by well-defined manganese pincer complexes. J. Am. Chem. Soc. 2016, 138, 8809–8814. [Google Scholar] [CrossRef]
- The yields of benzyl alcohol were 93% (THF), 83% (toluene), 82% (1,4-dioxane) and 22% (ethanol), respectively.
- Ebitani, K.; Motokura, K.; Mizugaki, T.; Kaneda, K. Heterotrimetallic RuMnMn species on a hydrotalcite surface as highly efficient heterogeneous catalysts for liquid-phase oxidation of alcohols with molecular oxygen. Angew. Chem. Int. Ed. 2005, 44, 3423–3426. [Google Scholar] [CrossRef]
- Kaneda, K.; Kawanishi, Y.; Teranishi, S. Zr-catalyzed oxidation of alcohols to aldehydes in the presence of tBuOOH. high reactivity for primary and allylic hydroxyl functions. Chem. Lett. 1984, 13, 1481–1482. [Google Scholar] [CrossRef] [Green Version]
- The low catalysis of the Pd/carbon in the reduction of benzonitrile could be ascribed to the high adsorption ability of benzonitrile of the Pd/carbon. The Pd/carbon adsorbed 0.8 mmol g−1 benzonitrile in 2-PrOH at room temperature whereas the Pd/Al2O3 scarcely adsorbed benzonitrile under the same adsorption conditions.
- Effect of FA amount on the reduction of benzonitrile was investigated using EtOH solvent. See, Table S1. It is notable that use of excess amount of FA decreased the product yield.
- Therefore, 2-PrOH could not act as a hydrogen source for the reduction of benzonitrile under the present conditions.
- Additionally, ortho-phthalonitrile and hexane nitrile were not reduced into the corresponding amines using the present catalytic system.





| Entry | Catalyst | Conversion/% | Yield/% |
|---|---|---|---|
| 1 | Pd/carbon | 100 | 93 |
| 2 | Rh/carbon | 7 | 3 |
| 3 | Pt/carbon | 5 | 2 |
| 4 | Ru/carbon | 4 | 2 |
| Entry | Catalyst | Conversion/% | Yield/% |
|---|---|---|---|
| 1 | Pd/carbon | 100 | 93 |
| 2 | Pd/Al2O3 | 62 | 30 |
| 3 | Pd/SiO2 | 39 | 15 |
| 4 | Pd/MgO | 16 | 2 |
| 5 | Pd/CeO2 | 7 | 6 |
| 6 | Pd/TiO2 | 6 | 1 |
| 7 | Pd/ZrO2 | 2 | 1 |
| Entry | Substrate | Product | Conversion/% | Yield/% |
|---|---|---|---|---|
| 1 |  benzaldehyde |  benzylalcohol | >99 | 93 |
| 2 |  4-hydroxybenzaldehyde |  4-hydroxybenzylalcohol | 90 | 88 |
| 3 |  4-methoxybenzaldehyde |  4-methoxybenzylalcohol | 79 | 74 |
| 4 |  4-methylbenzaldehyde |  4-methylbenzylalcohol | 88 | 79 |
| 5 |  acetophenone |  1-phenyl ethanol | 95 | 86 |
| 6 |  benzophenone |  benzhydrol | 93 | 78 |
| 7 a |  3-pyridine carbaldehyde |  3-pyridine methanol | >99 | 93 |
| 8 b |  furfural |  furfurylalcohol | 56 | 30 |
| 9 a |  HMF |  2,5-dimethanol furan | 54 | 30 |

| R | Conversion/% | Yield/% | Selectivity/% |
|---|---|---|---|
| CH3 | >99 | >99 | >99 |
| OCH3 | >99 | 96 | 97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomar, P.; Nozoe, Y.; Ozawa, N.; Nishimura, S.; Ebitani, K. Formic Acid as a Hydrogen Source for the Additive-Free Reduction of Aromatic Carbonyl and Nitrile Compounds at Reusable Supported Pd Catalysts. Catalysts 2020, 10, 875. https://doi.org/10.3390/catal10080875
Tomar P, Nozoe Y, Ozawa N, Nishimura S, Ebitani K. Formic Acid as a Hydrogen Source for the Additive-Free Reduction of Aromatic Carbonyl and Nitrile Compounds at Reusable Supported Pd Catalysts. Catalysts. 2020; 10(8):875. https://doi.org/10.3390/catal10080875
Chicago/Turabian StyleTomar, Pooja, Yuou Nozoe, Naoto Ozawa, Shun Nishimura, and Kohki Ebitani. 2020. "Formic Acid as a Hydrogen Source for the Additive-Free Reduction of Aromatic Carbonyl and Nitrile Compounds at Reusable Supported Pd Catalysts" Catalysts 10, no. 8: 875. https://doi.org/10.3390/catal10080875
APA StyleTomar, P., Nozoe, Y., Ozawa, N., Nishimura, S., & Ebitani, K. (2020). Formic Acid as a Hydrogen Source for the Additive-Free Reduction of Aromatic Carbonyl and Nitrile Compounds at Reusable Supported Pd Catalysts. Catalysts, 10(8), 875. https://doi.org/10.3390/catal10080875






