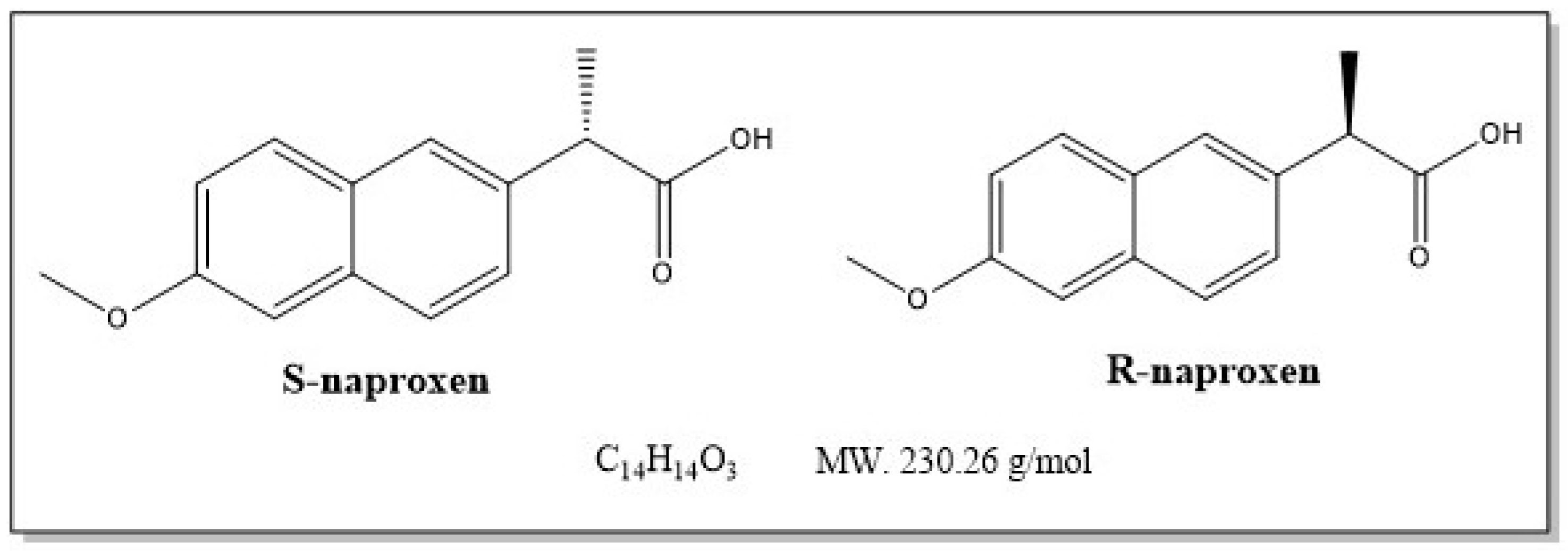
Enhanced Naproxen Elimination in Water by Catalytic Ozonation Based on NiO Films
Abstract
:1. Introduction
2. Results and Discussion
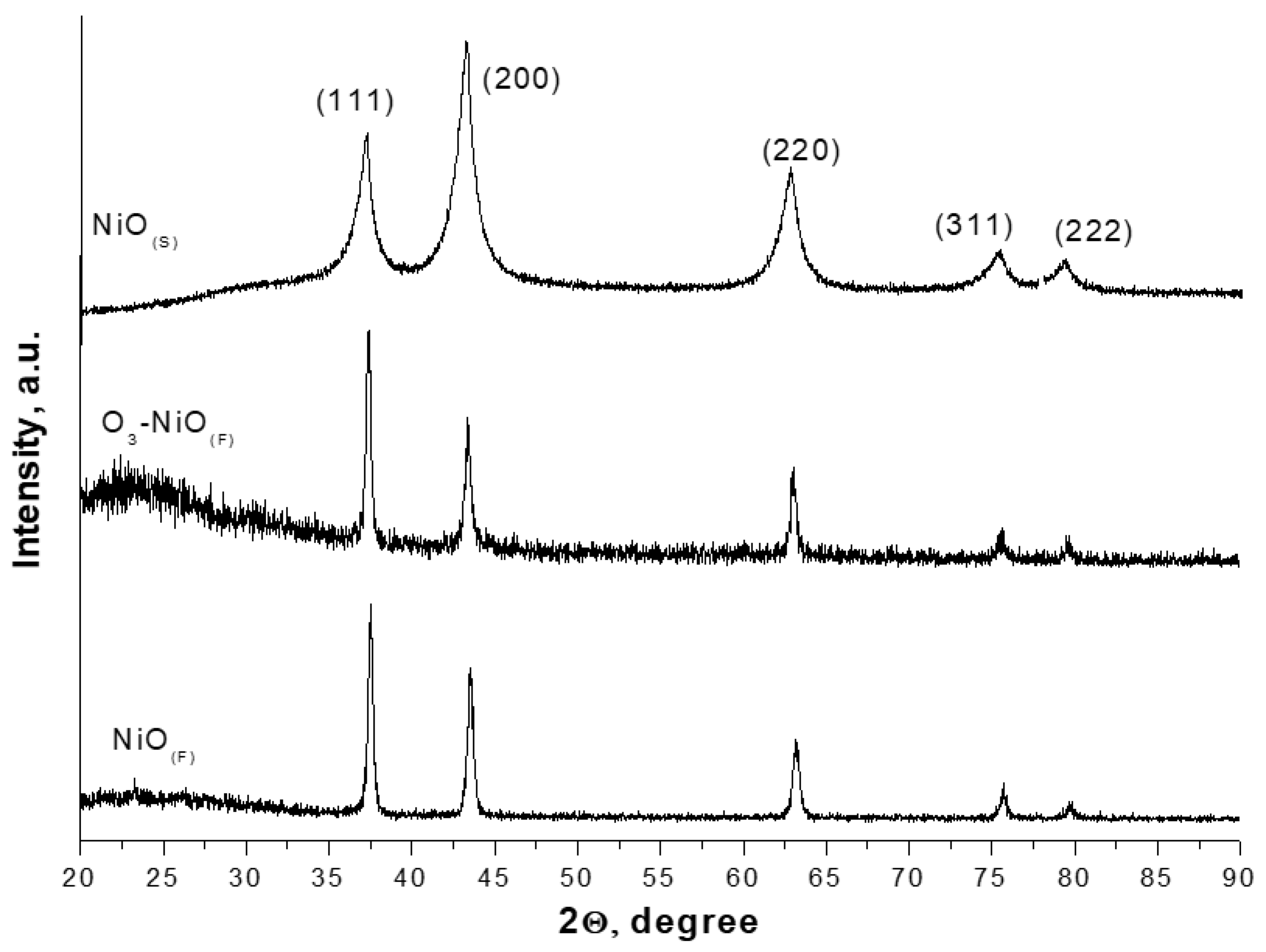
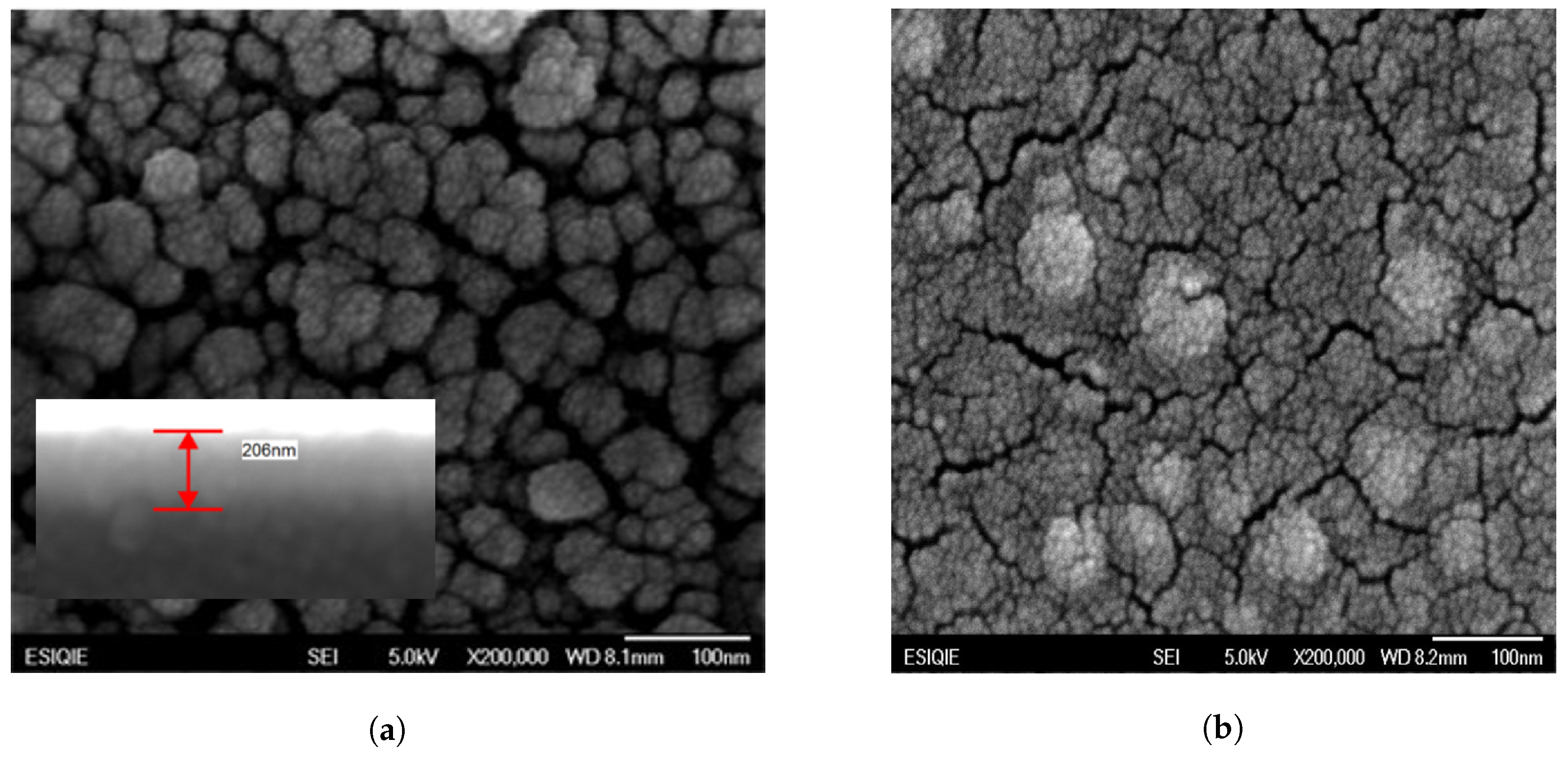
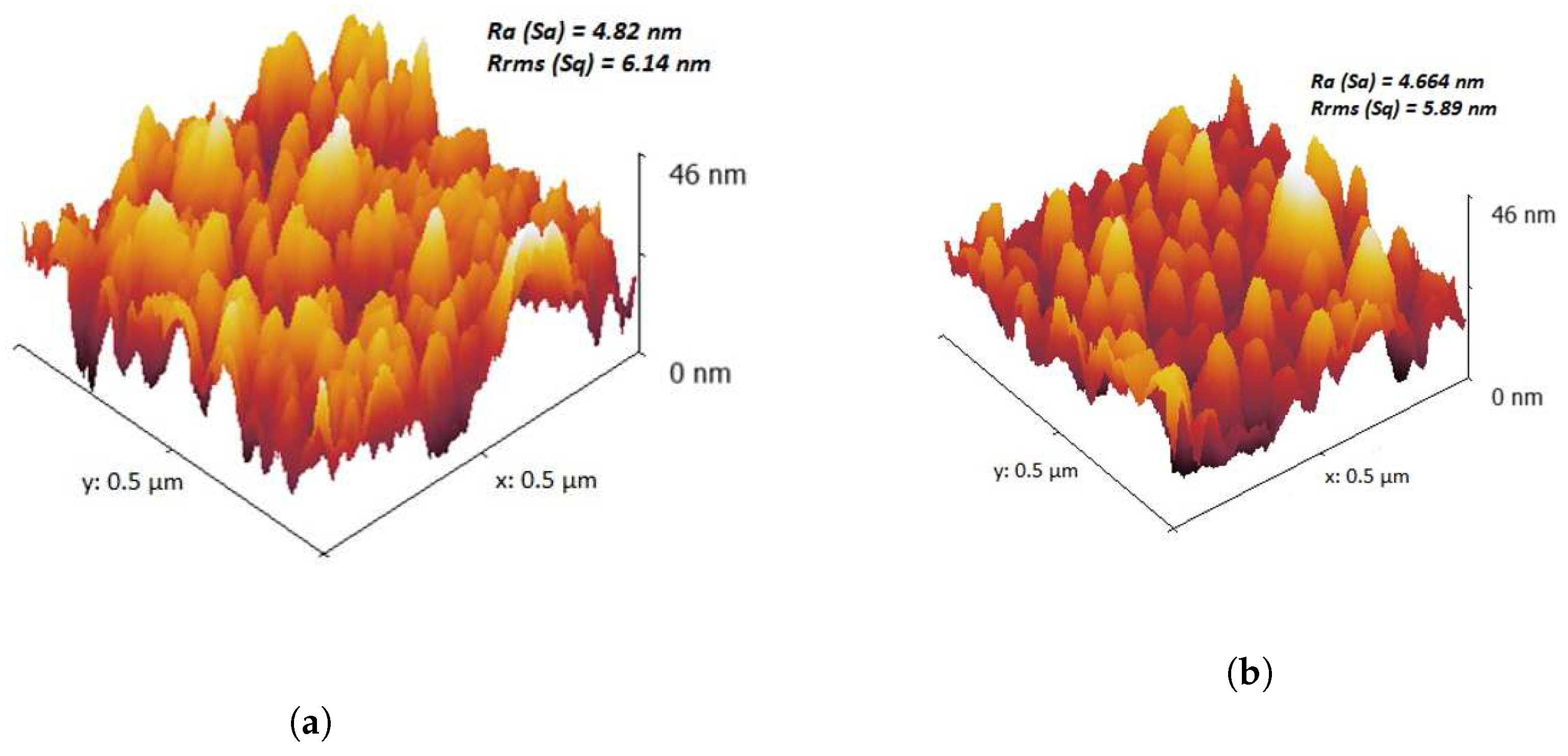
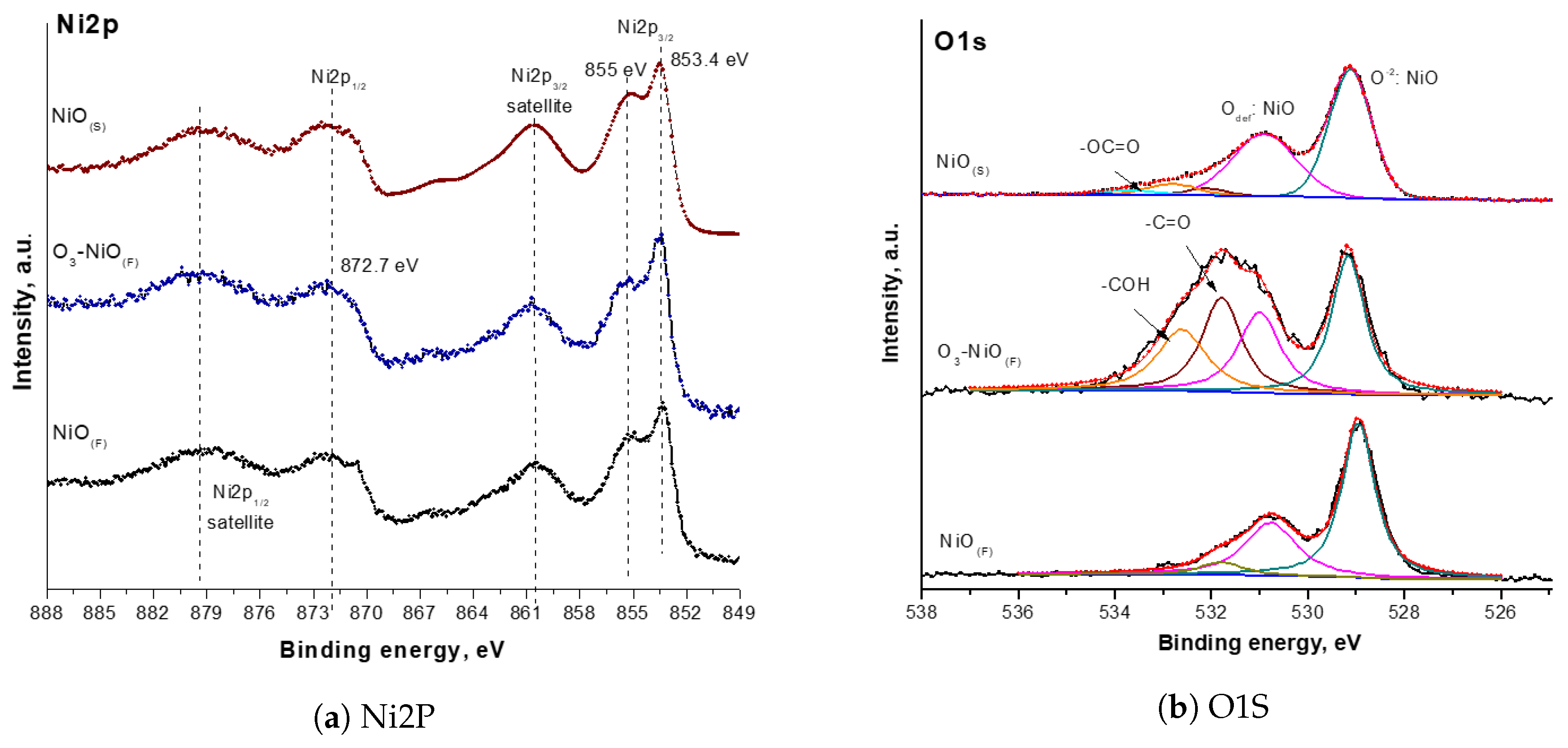
2.1. Characterization
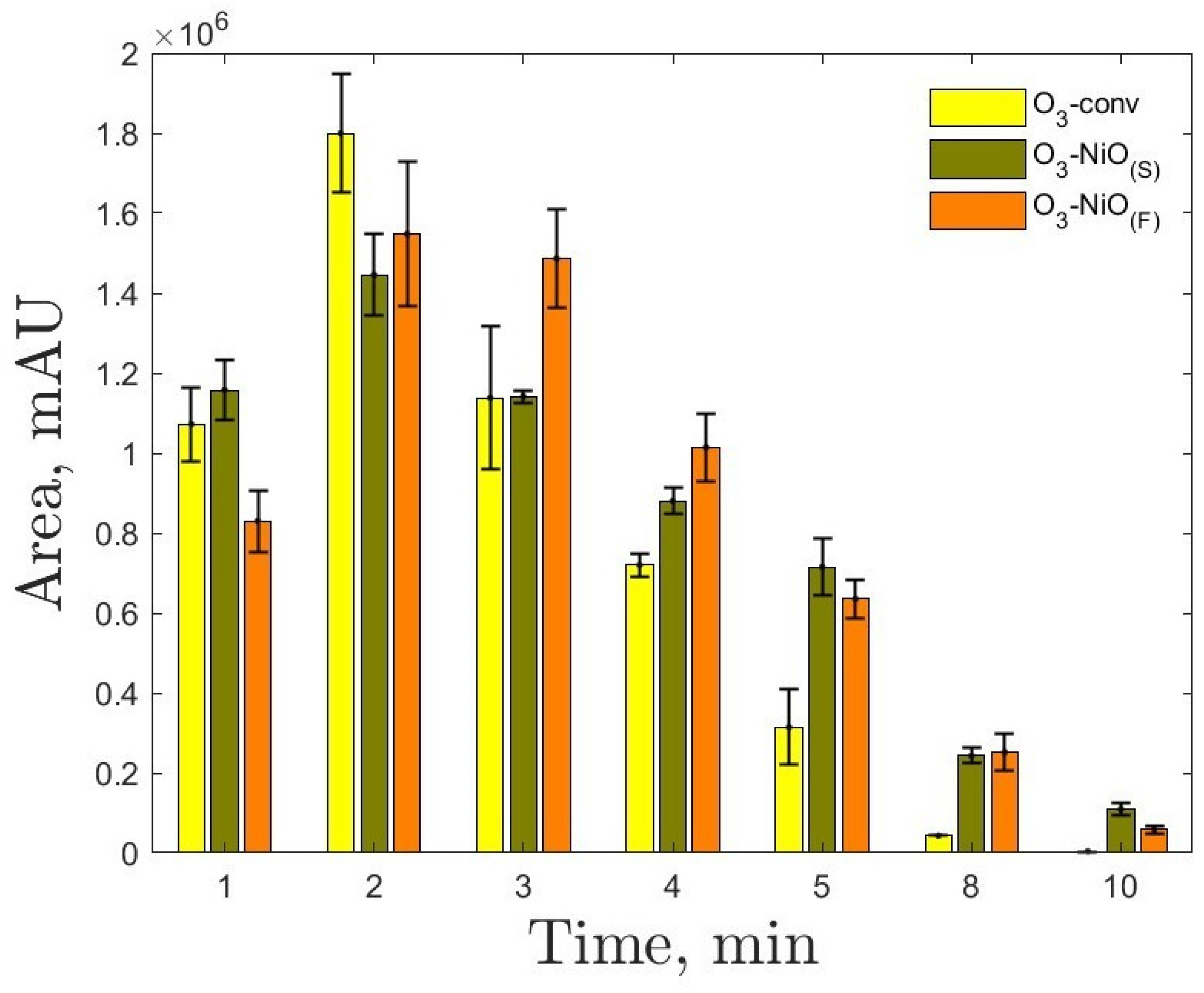
2.2. Evaluation of in the NP Removal Using Ozone
- (a)
- 1-(6-methoxynaphthalene-2-yl)ethylhydroperoxide,
- (b)
- 2-(3-(hydroxymethyl)-4-(2-methoxyethyl)phenyl)propanoic acid
- (c)
- 2-(4-(2-hydroxyvinyl)phenyl)acetic acid and
- (d)
- 4-Methylphenylacetic acid
2.3. Mathematical Modeling of the Reaction Dynamics
3. Materials and Methods
3.1. Synthesis of Nickel Oxide Films
3.2. Characterization
3.3. Catalytic Ozonation
3.4. Analytical Methods
3.5. Mathematical Modeling and Parameter Estimation
| Algorithm 1: Parameter estimation in the NP degradation by ozonation techniques |
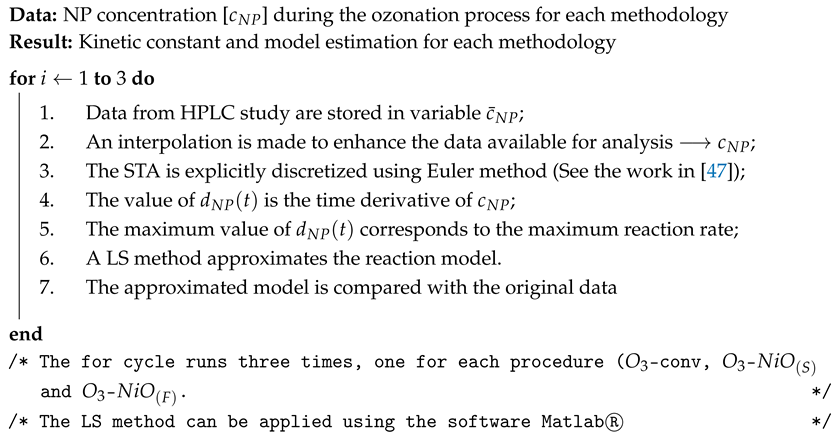 |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | Naproxen |
| CVD | Chemical vapor deposition |
| -conv | Conventional ozonation |
| NiO film | |
| - | Catalytic ozonation with suspension NiO |
| - | Catalytic ozonation with film NiO |
References
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.H.N.; Hanaor, D.A. The effects of copper doping on photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Quan, X.; Wang, D. Catalytic Ozonation in Arrayed Zinc Oxide Nanotubes as Highly Efficient Mini-Column Catalyst Reactors (MCRs): Augmentation of Hydroxyl Radical Exposure. Environ. Sci. Technol. 2018, 52, 8701–8711. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Gonzalo, S.; Santiago, J.; Rodríguez, A.; Perdigón-Melón, J.A.; García-Calvo, E. Kinetics and Mechanism of Catalytic Ozonation of Aqueous Pollutants on Metal Oxide Catalysts. Ozone Sci. Eng. 2011, 33, 434–440. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Lin, F.; Zhang, Z.; Yu, H.; Yan, B.; Wang, Z. Comparative investigation on catalytic ozonation of VOCs in different types over supported MnOx catalysts. J. Hazard. Mater. 2020, 391, 122218. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Álvarez, P.M.; Gimeno, O. Graphene-Based Catalysts for Ozone Processes to Decontaminate Water. Molecules 2019, 24, 3438. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Li, Y.; Nan, S.; Yoza, B.A.; Li, Y.; Zhan, Y.; Wang, Q.; Li, Q.X.; Guo, S.; Chen, C. Catalytic Ozonation of Nitrobenzene by Manganese-Based Y Zeolites. Front. Chem. 2020, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.L.; Valenzuela, M.A.; Poznyak, T.; Lartundo, L.; Chairez, I. Reactivity of NiO for 2,4-D degradation with ozone: XPS studies. J. Hazard. Mater. 2013, 262, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.M.; Vazquez-Arenas, J.; Castillo-Araiza, O.O.; Rodríguez, J.L.; Chairez, I.; Salinas, E.; Poznyak, T. Improving ozonation to remove carbamazepine through ozone-assisted catalysis using different NiO concentrations. Environ. Sci. Pollut. Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.M.; Rodríguez, J.L.; Chairez, I.; Tiznado, H.; Poznyak, T. Naphthalene degradation by catalytic ozonation based on nickel oxide: Study of the ethanol as cosolvent. Environ. Sci. Pollut. Res. Int. 2017, 24, 25550–25560. [Google Scholar] [CrossRef] [PubMed]
- Biard, P.F.; Werghi, B.; Soutrel, I.; Orhand, R.; Couvert, A.; Denicourt-Nowicki, A.; Roucoux, A. Efficient catalytic ozonation by ruthenium nanoparticles supported on SiO2 or TiO2: Towards the use of a non-woven fiber paper as original support. Chem. Eng. J. 2016, 289, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Shahamat, Y.D.; Farzadkia, M.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Esrafili, A. Magnetic heterogeneous catalytic ozonation: A new removal method for phenol in industrial wastewater. J. Environ. Health Sci. Eng. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battiato, S.; Giangregorio, M.M.; Catalano, M.R.; Nigro, R.L.; Losurdo, M.; Malandrino, G. Morphology-controlled synthesis of NiO films: The role of the precursor and the effect of the substrate nature on the films’ structural/optical properties. RSC Adv. 2016, 6, 30813–30823. [Google Scholar] [CrossRef]
- Xi, Y.Y.; Li, D.; Djurišić, A.B.; Xie, M.H.; Man, K.Y.K.; Chan, W.K. Hydrothermal Synthesis vs. Electrodeposition for High Specific Capacitance Nanostructured NiO Films. Electrochem. Solid State Lett. 2008, 11, D56. [Google Scholar] [CrossRef]
- Vargas Garcia, J.R.; Lazcano Ugalde, E.M.; Hernandez Santiago, F.; Hallen Lopez, J.M. Nanostructured nickel oxide films prepared by chemical vapor deposition and their electrochromic properties. J. Nanosci. Nanotechnol. 2008, 8, 2703–2706. [Google Scholar] [CrossRef]
- Pellegrino, F.; De Bellis, N.; Ferraris, F.; Prozzi, M.; Zangirolami, M.; Petriglieri, J.R.; Schiavi, I.; Bianco-Prevot, A.; Maurino, V. Evaluation of the Photocatalytic Activity of a Cordierite-Honeycomb-Supported TiO2 Film with a Liquid-Solid Photoreactor. Molecules 2019, 24, 4499. [Google Scholar] [CrossRef] [Green Version]
- Tekin, D.; Tekin, T.; Kiziltas, H. Photocatalytic degradation kinetics of Orange G dye over ZnO and Ag/ZnO thin film catalysts. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Guzmán, I.C.; Rodríguez, J.L.; Poznyak, T.; Chairez, I.; Hernández, I.; Hernández, R.T. Catalytic ozonation of 4-chlorophenol and 4-phenolsulfonic acid by CeO2 films. Catal. Commun. 2020, 133, 105827. [Google Scholar] [CrossRef]
- Muir, D.; Simmons, D.; Wang, X.; Peart, T.; Villella, M.; Miller, J.; Sherry, J. Bioaccumulation of pharmaceuticals and personal care product chemicals in fish exposed to wastewater effluent in an urban wetland. Sci. Rep. 2017, 7, 16999. [Google Scholar] [CrossRef]
- Arnold, K.E.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the environment: Assessing risks of pharmaceuticals to wildlife and ecosystems. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130569. [Google Scholar] [CrossRef] [Green Version]
- Emmanuel, E.; Perrodin, Y.; Keck, G.; Blanchard, J.M.; Vermande, P. Ecotoxicological risk assessment of hospital wastewater: A proposed framework for raw effluents discharging into urban sewer network. J. Hazard. Mater. 2005, 117, 1–11. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Biodegradation and biotransformation of polycyclic non-steroidal anti-inflammatory drugs. Rev. Environ. Sci. Biotechnol. 2015, 14, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, G.; Su, Q.; Lv, C.; Jin, X.; Wen, X. UV-Induced Photodegradation of Naproxen Using a Nano γ-FeOOH Composite: Degradation Kinetics and Photocatalytic Mechanism. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.K.; Dhakal, D.; Lee, S.W. Rapid degradation of naproxen by AgBr-α-NiMoO4 composite photocatalyst in visible light: Mechanism and pathways. Chem. Eng. J. 2018, 347, 836–848. [Google Scholar] [CrossRef]
- Arany, E.; Szabó, R.K.; Apáti, L.; Alapi, T.; Ilisz, I.; Mazellier, P.; Dombi, A.; Gajda-Schrantz, K. Degradation of naproxen by UV, VUV photolysis and their combination. J. Hazard. Mater. 2013, 262, 151–157. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Wu, Y.; Feng, L.; Zhang, L. Degradation of naproxen in chlorination and UV/chlorine processes: Kinetics and degradation products. Environ. Sci. Pollut. Res. Int. 2019, 26, 34301–34310. [Google Scholar] [CrossRef]
- Karaca, M.; Kıranşan, M.; Karaca, S.; Khataee, A.; Karimi, A. Sonocatalytic removal of naproxen by synthesized zinc oxide nanoparticles on montmorillonite. Ultrason. Sonochemistry 2016, 31, 250–256. [Google Scholar] [CrossRef]
- Tizhoosh, N.Y.; Khataee, A.; Hassandoost, R.; Soltani, R.D.C.; Doustkhah, E. Ultrasound-engineered synthesis of WS2@ CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochemistry 2020, 67, 105114. [Google Scholar] [CrossRef]
- Dulova, N.; Kattel, E.; Trapido, M. Degradation of naproxen by ferrous ion-activated hydrogen peroxide, persulfate and combined hydrogen peroxide/persulfate processes: The effect of citric acid addition. Chem. Eng. J. 2017, 318, 254–263. [Google Scholar] [CrossRef]
- van Kollenburg, G.H.; van Es, J.; Gerreten, J.; Lanters, H.; Bouman, R.; Koelewijn, W.; Davies, A.N.; Buydens, L.; van Manen, H.J.; Jansen, J.J. Understanding Chemical Production Processes by using PLS Path Model Parameters as Soft Sensors. Comput. Chem. Eng. 2020, 139, 106841. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Gonzalo, M.; García-Calvo, E. Catalytic ozonation of naproxen and carbamazepine on titanium dioxide. Appl. Catal. Environ. 2008, 84, 48–57. [Google Scholar] [CrossRef]
- Patil, V.P.; Pawar, S.; Chougule, M.; Godse, P.; Sakhare, R.; Sen, S.; Joshi, P. Effect of Annealing on Structural, Morphological, Electrical and Optical Studies of Nickel Oxide Thin Films. J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Chen, F.R.; Kai, J.J. Electrochromic properties of nano-composite nickel oxide film. Appl. Surf. Sci. 2008, 254, 3357–3363. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. TiO2 photocatalysis of naproxen: Effect of the water matrix, anions and diclofenac on degradation rates. Chemosphere 2015, 139, 579–588. [Google Scholar] [CrossRef]
- Mohamed, A.; Salama, A.; Nasser, W.S.; Uheida, A. Photodegradation of Ibuprofen, Cetirizine, and Naproxen by PAN-MWCNT/TiO2-NH2 nanofiber membrane under UV light irradiation. Environ. Sci. Eur. 2018, 30, 47. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Chairez, I.; Rodríguez, J.L.; Tiznado, H.; Santillán, R.; Arrieta, D.; Poznyak, T. Inhibition effect of ethanol in naproxen degradation by catalytic ozonation with NiO. RSC Adv. 2019, 9, 14822–14833. [Google Scholar] [CrossRef] [Green Version]
- Magallanes, D.; Rodríguez, J.L.; Poznyak, T.; Valenzuela, M.A.; Lartundo, L.; Chairez, I. Efficient mineralization of benzoic and phthalic acids in water by catalytic ozonation using a nickel oxide catalyst. New J. Chem. 2015, 39, 7839–7848. [Google Scholar] [CrossRef]
- Fuentes, I.; Rodriguez, J.L.; Tiznado, H.; Romo-Herrera, J.M.; Chairez, I.; Poznyak, T. Terephthalic acid decomposition by photocatalytic ozonation with VxOy/ZnO under different UV-A LEDs distributions. Chem. Eng. Commun. 2020, 207, 263–277. [Google Scholar] [CrossRef]
- Patel, S.; Majumder, S.K.; Das, P.; Ghosh, P. Ozone microbubble-aided intensification of degradation of naproxen in a plant prototype. J. Environ. Chem. Eng. 2019, 7, 103102. [Google Scholar] [CrossRef]
- Liu, J.; Ke, L.; Liu, J.; Sun, L.; Yuan, X.; Li, Y.; Xia, D. Enhanced catalytic ozonation towards oxalic acid degradation over novel copper doped manganese oxide octahedral molecular sieves nanorods. J. Hazard. Mater. 2019, 371, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, Y.; Xu, Z.; Luo, M.; Zhu, C.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Elghniji, K.; Hentati, O.; Ribeiro, A.R.; Silva, A.M.; Ksibi, M. UV and solar photo-degradation of naproxen: TiO2 catalyst effect, reaction kinetics, products identification and toxicity assessment. J. Hazard. Mater. 2016, 304, 329–336. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, F.; Wu, H.; Wei, C. The mineralization of oxalic acid and bio-treated coking wastewater by catalytic ozonation using nickel oxide. Environ. Sci. Pollut. Res. 2018, 25, 2389–2400. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Y.; Li, S.; Xu, S. A weighted auto regressive LSTM based approach for chemical processes modeling. Neurocomputing 2019, 367, 64–74. [Google Scholar] [CrossRef]
- Salgado, I.; Chairez, I.; Bandyopadhyay, B.; Fridman, L.; Camacho, O. Discrete-time non-linear state observer based on a super twisting-like algorithm. IET Control Theory Appl. 2014, 8, 803–812. [Google Scholar] [CrossRef]
- Levant, A. Robust exact differentiation via sliding mode technique. Automatica 1998, 34, 379–384. [Google Scholar] [CrossRef]


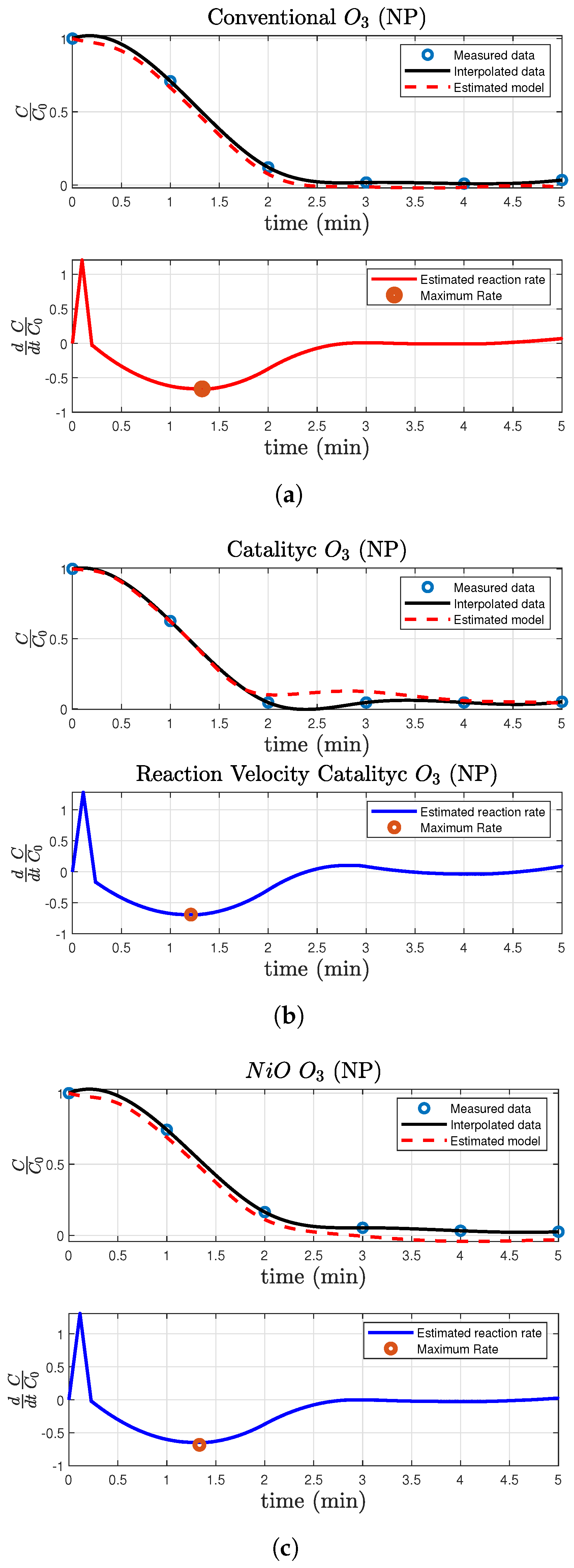
| Parameter | -conv | - | - |
| , [min−1] | 0.6605 | 0.6914 | 0.6784 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Melo, C.M.; Rodríguez, J.L.; Chairez, I.; Salgado, I.; Andraca Adame, J.A.; Galaviz-Pérez, J.A.; Vazquez-Arenas, J.; Poznyak, T. Enhanced Naproxen Elimination in Water by Catalytic Ozonation Based on NiO Films. Catalysts 2020, 10, 884. https://doi.org/10.3390/catal10080884
Aguilar-Melo CM, Rodríguez JL, Chairez I, Salgado I, Andraca Adame JA, Galaviz-Pérez JA, Vazquez-Arenas J, Poznyak T. Enhanced Naproxen Elimination in Water by Catalytic Ozonation Based on NiO Films. Catalysts. 2020; 10(8):884. https://doi.org/10.3390/catal10080884
Chicago/Turabian StyleAguilar-Melo, Claudia M., Julia L. Rodríguez, Isaac Chairez, Iván Salgado, J. A. Andraca Adame, J. A. Galaviz-Pérez, Jorge Vazquez-Arenas, and Tatyana Poznyak. 2020. "Enhanced Naproxen Elimination in Water by Catalytic Ozonation Based on NiO Films" Catalysts 10, no. 8: 884. https://doi.org/10.3390/catal10080884
APA StyleAguilar-Melo, C. M., Rodríguez, J. L., Chairez, I., Salgado, I., Andraca Adame, J. A., Galaviz-Pérez, J. A., Vazquez-Arenas, J., & Poznyak, T. (2020). Enhanced Naproxen Elimination in Water by Catalytic Ozonation Based on NiO Films. Catalysts, 10(8), 884. https://doi.org/10.3390/catal10080884





