NHC Ligand Effects on Ru-Catalyzed Cross-Metathesis of Renewable Materials
Abstract
1. Introduction
2. Results and Discussion
2.1. Cross-Metathesis of Ethyl Oleate with Cis-1,4-Diacetoxy-2-Butene
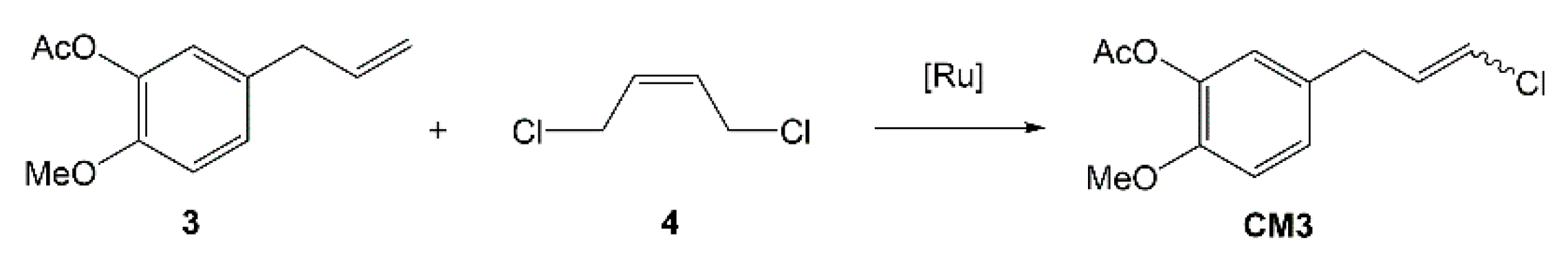
2.2. Cross-Metathesis of Eugenol Acetate with Cis-1,4-Dichloro-2-Butene
3. Materials and Methods
3.1. General Considerations
3.2. Procedures for the CM of Ethyl oleate (1) and Cis-2,4-Diacetoxy-2-Butene (2)
3.3. Procedures for the CM of Eugenol Oleate (3) and Cis-1,4-Dichloro-2-Butene (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grela, K. Olefin Metathesis: Theory and Practice; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-20794-9. [Google Scholar]
- Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-33424-7. [Google Scholar]
- Higman, C.S.; Lummiss, J.A.M.; Fogg, D.E. Olefin Metathesis at the Dawn of Implementation in Pharmaceutical and Specialty-Chemicals Manufacturing. Angew. Chem. Int. Ed. 2016, 55, 3552–3565. [Google Scholar] [CrossRef] [PubMed]
- Report of the United Nations Conference on Environmental Development. Rio de Janeiro, Brazil. Available online: http://www.un.org/esa/sustdev (accessed on 3 June 1992).
- Malacea, R.; Dixneuf, P.H. Alkene Metathesis and Renewable Materials: Selective Transformations of Plants Oils. In Green Metathesis Chemistry. NATO Science for Peace and Security Series A: Chemistry and Biology; Dragutan, V., Demonceau, A., Dragutan, I., Finkelshtein, E.S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 185–206. ISBN 978-90-481-3431-1. [Google Scholar]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- de Espinosa, L.M.; Meier, M.A.R. Organometallics and Renewables; Meier, M.A.R., Weckjusen, B.M., Bruijnincx, P.C.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nickel, A.; Pederson, R.L. Commercial Potential of Olefin Metathesis of Renewable Feedstocks. In Olefin Metathesis: Theory and Practice; Grela, K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 335–348. ISBN 978-1-118-20794-9. [Google Scholar]
- Phillips, J.H. Biorenewable polymers. In Handbook of Metathesis, 2nd ed.; Grubbs, R.H., Wenzel, A.G., O’Leary, D.J., Khosravi, E., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 3, pp. 357–374. ISBN 978-3-527-33424-7. [Google Scholar]
- Meier, M.A.R. Metathesis with Oleochemicals: New Approaches for the Utilization of Plant Oils as Renewable Resources in Polymer Science. Macromol. Chem. Phys. 2009, 210, 1073–1079. [Google Scholar] [CrossRef]
- Behr, A.; Gomes, J.P. The refinement of renewable resources: New important derivatives of fatty acids and glycerol. Eur. J. Lipid Sci. Technol. 2010, 112, 31–50. [Google Scholar] [CrossRef]
- Bidange, J.; Fischmeister, C.; Bruneau, C. Ethenolysis: A Green Catalytic Tool to Cleave Carbon–Carbon Double Bonds. Chem.-Eur. J. 2016, 22, 12226–12244. [Google Scholar] [CrossRef]
- Spekreijse, J.; Sanders, J.P.M.; Bitter, J.H.; Scott, E.L. The Future of Ethenolysis in Biobased Chemistry. ChemSusChem 2017, 10, 470–482. [Google Scholar] [CrossRef]
- Jacobs, T.; Rybak, A.; Meier, M.A.R. Crossmetathesis reactions of allyl chloride with fatty acid methyl esters: Efficient synthesis of α,ω-difunctional chemical intermediates from renewable raw materials. Appl. Catal. A 2009, 353, 32–35. [Google Scholar] [CrossRef]
- Rybak, A.; Meier, M.A.R. Cross-metathesis of fatty acid derivatives with methyl acrylate: Renewable raw materials for the chemical industry. Green Chem. 2007, 9, 1356–1361. [Google Scholar] [CrossRef]
- Biermann, U.; Meier, M.A.R.; Butte, W.; Metzger, J.O. Cross-metathesis of unsaturated triglycerides with methyl acrylate: Synthesis of a dimeric metathesis product. Eur. J. Lipid Sci. Technol. 2011, 113, 39–45. [Google Scholar] [CrossRef]
- Miao, X.; Malacea, R.; Fischmeister, C.; Bruneau, C.; Dixneuf, P.H. Ruthenium-alkylidene catalysed cross-metathesis of fatty acid derivatives with acrylonitrile and methyl acrylate: A key step toward long-chain bifunctional and amino acid compounds. Green Chem. 2011, 13, 2911–2919. [Google Scholar] [CrossRef]
- Abel, G.A.; Nguyen, K.O.; Viamajala, S.; Varanasi, S.; Yamamoto, K. Cross-metathesis approach to produce precursors of nylon 12 and nylon 13 from microalgae. RSC Adv. 2014, 4, 55622–55628. [Google Scholar] [CrossRef]
- Behr, A.; Pérez Gomes, J. The cross-metathesis of methyl oleate with cis-2-butene-1,4-diyl diacetate and the influence of protecting groups. Beilstein J. Org. Chem. 2011, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Slugovc, C. Optimized reaction conditions for the cross-metathesis of methyl oleate and oleylamine with ethyl acrylate. Monatshefte Chem. 2012, 143, 669–673. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Sytniczuk, A.; Grela, K. Metathesis of renewable raw materials-influence of ligands in the indenylidene type catalysts on self-metathesis of methyl oleate and cross-metathesis of methyl oleate with (Z)-2-butene-1,4-diol diacetate. Green Chem. 2014, 16, 1579–1585. [Google Scholar] [CrossRef]
- Winkler, M.; Meier, M.A.R. Olefin cross-metathesis as a valuable tool for the preparation of renewable polyesters and polyamides from unsaturated fatty acid esters and carbamates. Green Chem. 2014, 16, 3335–3340. [Google Scholar] [CrossRef]
- Awang, N.W.; Tsutsumi, K.; Hustakova, B.; Yusoff, S.F.M.; Nomura, K.; Yamin, B.M. Cross metathesis of methyl oleate (MO) with terminal, internal olefins by ruthenium catalysts: Factors affecting the efficient MO conversion and the selectivity. RSC Adv. 2016, 6, 100925–100930. [Google Scholar] [CrossRef]
- Bilel, H.; Hamdi, N.; Zagrouba, F.; Fischmeister, C.; Bruneau, C. Eugenol as a renewable feedstock for the production of polyfunctional alkenes via olefin cross-metathesis. RSC Adv. 2012, 2, 9584–9589. [Google Scholar] [CrossRef]
- Alexander, K.A.; Paulhus, E.A.; Lazarus, G.M.L.; Leadbeater, N.E. Exploring the reactivity of a ruthenium complex in the metathesis of biorenewable feedstocks to generate value-added chemicals. J. Organomet. Chem. 2016, 812, 74–80. [Google Scholar] [CrossRef]
- Vieille-Petit, L.; Clavier, H.; Linden, A.; Blumentritt, S.; Nolan, S.P.; Dorta, R. Ruthenium Olefin Metathesis Catalysts with N-Heterocyclic Carbene Ligands Bearing N-Naphthyl Side Chains. Organometallics 2010, 29, 775–788. [Google Scholar] [CrossRef]
- Broggi, J.; Urbina-Blanco, C.A.; Clavier, H.; Leitgeb, A.; Slugovc, C.; Slawin, A.M.Z.; Nolan, S.P. The Influence of Phosphane Ligands on the Versatility of Ruthenium−Indenylidene Complexes in Metathesis. Chem.-Eur. J. 2010, 16, 9215–9225. [Google Scholar] [CrossRef]
- Lummiss, J.A.M.; Oliveira, K.C.; Pranckevicius, A.M.T.; Santos, A.; dos Santos, E.N.; Fogg, D.E. Chemical Plants: High-Value Molecules from Essential Oils. J. Am. Chem. Soc. 2012, 134, 18889–18891. [Google Scholar] [CrossRef] [PubMed]
- Sytniczuk, A.; Kajetanowicz, A.; Grela, K. Fishing for the right catalysts for the cross-metathesis reaction of methyl oleate with 2-methyl-2-butene. Catal. Sci. Technol. 2017, 7, 1284–1296. [Google Scholar] [CrossRef]
- Le, D.; Samart, C.; Tsutsumi, K.; Nomura, K.; Kongparakul, S. Efficient Conversion of Renewable Unsaturated Fatty Acid Methyl Esters by Cross-Metathesis with Eugenol. ACS Omega 2018, 3, 11041–11049. [Google Scholar] [CrossRef] [PubMed]
- Małecki, P.; Gajda, K.; Gajda, R.; Woźniak, K.; Trzaskowski, B.; Kajetanowicz, A.; Grela, K. Specialized Ruthenium Olefin Metathesis Catalysts Bearing Bulky Unsymmetrical NHC Ligands: Computations, Synthesis, and Application. ACS Catal. 2019, 9, 587–598. [Google Scholar] [CrossRef]
- Keitz, B.K.; Endo, K.; Patel, P.R.; Herbert, M.B.; Grubbs, R.H. Improved Ruthenium Catalysts for Z-Selective Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 693–699. [Google Scholar] [CrossRef]
- Herbert, M.B.; Grubbs, R.H. Z-Selective Cross Metathesis with Ruthenium Catalysts: Synthetic Applications and Mechanistic Implications. Angew. Chem. Int. Ed. 2015, 54, 5018–5024. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Grisi, F. Novel Olefin Metathesis Ruthenium Catalysts Bearing Backbone-Substituted Unsymmetrical NHC Ligands. Organometallics 2014, 33, 5932–5935. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Costabile, C.; Grisi, F. Ruthenium olefin metathesis catalysts featuring unsymmetrical N-heterocyclic carbenes. Dalton Trans. 2016, 45, 561–571. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Costabile, C.; Caruso, T.; Dąbrowski, M.; Grela, K.; Grisi, F. Expanding the Family of Hoveyda–Grubbs Catalysts Containing Unsymmetrical NHC Ligands. Organometallics 2017, 36, 3692–3708. [Google Scholar] [CrossRef]
- Paradiso, V.; Menta, S.; Pierini, M.; Della Sala, G.; Ciogli, A.; Grisi, F. Enantiopure C1-symmetric N-Heterocyclic Carbene Ligands from Desymmetrized meso-1,2-Diphenylethylenediamine: Application in Ruthenium-Catalyzed Olefin Metathesis. Catalysts 2016, 6, 177. [Google Scholar] [CrossRef]
- Ambrosio, C.; Paradiso, V.; Costabile, C.; Bertolasi, V.; Caruso, T.; Grisi, F. Stable ruthenium olefin metathesis catalysts bearing symmetrical NHC ligands with primary and secondary N-alkyl groups. Dalton Trans. 2018, 47, 6615–6627. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.; Grisi, F. Ruthenium-Catalyzed Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-ene with Cyclic Olefins. Adv. Synth. Catal. 2019, 361, 4133–4139. [Google Scholar] [CrossRef]
- Hillier, A.C.; Sommer, W.J.; Yong, B.S.; Petersen, J.L.; Cavallo, L.; Nolan, S.P. A combined experimental and theoretical study examining the binding of N-heterocyclic carbenes (NHC) to the Cp*RuCl (Cp* = η5-C5Me5) moiety: Insight into stereoelectronic differences between unsaturated and saturated NHC ligands. Organometallics 2003, 22, 4322–4326. [Google Scholar] [CrossRef]
- Poater, A.; Cosenza, B.; Correa, A.; Giudice, S.; Ragone, F.; Scarano, V.; Cavallo, L. SambV ca: A Web Application for the Calculation of the Buried Volume of N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem. 2009, 1759–1766. [Google Scholar] [CrossRef]
- Süßner, M.; Plenio, H. π-Face donor properties of N-heterocyclic carbenes. Chem. Commun. 2005, 43, 5417–5419. [Google Scholar] [CrossRef]
- Elias, H.-G. Makromoleküle II. Technologie. Rohstoffe-Industrielle Synthesen-Polymere-Anwendungen; Wiley-VCH: Weinheim, Germany, 1999; ISBN 3-85739-102-0. [Google Scholar]
- Ivanova, N.M.; Cheskis, B.A.; Rubanova, E.V.; Yatsynin, V.G.; Moiseenkov, A.M.; Nefedov, O.M. Synthesis of 3E-tetradecenol, 3E, 9E-tetradecadienol, and their acetates starting from acetylcyclopropane. Bull. Acad. Sci. USSR Div. Chem. Sci. (Engl. Trans.) 1991, 40, 557–561. [Google Scholar] [CrossRef]


| Catalyst | %Catalyst Left | %VBur | ΔE1/2 (V) | ||
|---|---|---|---|---|---|
| (1 Day) | (2 Days) | (14 Days) | |||
| Ru1-anti | 98 | 98 | 25 | 28.9 | 0.950 |
| Ru1-syn | 79 | 66 | 21 | 28.7 | 0.972 |
| Ru2-anti | 93 | 92 | 33 | 31.0 | 1.00 |
| Ru2-syn | 82 | 73 | 33 | 30.9 | 0.996 |
| Ru3-anti | 100 | 100 | 42 | 31.0 | 0.960 |
| Ru3-syn | 82 | 74 | 32 | 30.5 | 0.947 |
| Ru4-anti | 100 | 100 | 100 | 31.8 | 0.950 |
| Ru4-syn | 100 | 100 | 100 | 31.5 | 0.961 |
| HGII | 86 | 84 | 83 | 32.9 | 0.860 |
| Entry | Catalyst [mol%] | Conversion 2 [%] | Selectivity 2 [%] | Yield 2[%] | |||
|---|---|---|---|---|---|---|---|
| CM1 | CM2 | SM1 | SM2 | ||||
| 1 | Ru1-anti (2.5) | 61 | 87 | 53 | 40 | 3 | 4 |
| 2 | Ru1-syn (2.5) | 90 | 96 | 57 | 40 | 1 | 2 |
| 3 | Ru2-anti (2.5) | 90 | 92 | 51 | 45 | 1 | 3 |
| 4 | Ru2-syn (2.5) | - | - | - | - | - | - |
| 5 | Ru3-anti (2.5) | 89 | 95 | 51 | 47 | 1 | 1 |
| 6 | Ru3-syn (2.5) | - | - | - | - | - | - |
| 7 | Ru4-anti (2.5) | - | - | - | - | - | - |
| 8 | Ru4-syn (2.5) | - | - | - | - | - | - |
| 9 | HGII (2.5) | 90 | 94 | 51 | 46 | 1 | 2 |
| 10 | Ru1-anti (1.0) | - | - | - | - | - | - |
| 11 | Ru1-syn (1.0) | 76 | 94 | 53 | 44 | 1 | 2 |
| 12 | Ru2-anti (1.0) | 67 | 90 | 52 | 43 | 2 | 3 |
| 13 | Ru3-anti (1.0) | 84 | 94 | 51 | 46 | 1 | 2 |
| 14 | HGII (1.0) | 59 | 89 | 52 | 42 | 3 | 3 |
| Entry | Catalyst | Yield 2 [%] | E:Z3 |
|---|---|---|---|
| 1 4 | Ru1-anti | 77 | 5.4 |
| 2 4 | Ru1-syn | 84 | 5.4 |
| 3 4 | Ru2-anti | 70 | 3.2 |
| 4 4 | Ru2-syn | 88 | 2.8 |
| 5 | Ru3-anti | 77 | 9.0 |
| 6 | Ru3-syn | 60 | 2.0 |
| 7 | Ru4-anti | 75 | 7.0 |
| 8 | Ru4-syn | 54 | 3.2 |
| 9 | HGII | 50 | 7.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradiso, V.; Contino, R.; Grisi, F. NHC Ligand Effects on Ru-Catalyzed Cross-Metathesis of Renewable Materials. Catalysts 2020, 10, 904. https://doi.org/10.3390/catal10080904
Paradiso V, Contino R, Grisi F. NHC Ligand Effects on Ru-Catalyzed Cross-Metathesis of Renewable Materials. Catalysts. 2020; 10(8):904. https://doi.org/10.3390/catal10080904
Chicago/Turabian StyleParadiso, Veronica, Raffaele Contino, and Fabia Grisi. 2020. "NHC Ligand Effects on Ru-Catalyzed Cross-Metathesis of Renewable Materials" Catalysts 10, no. 8: 904. https://doi.org/10.3390/catal10080904
APA StyleParadiso, V., Contino, R., & Grisi, F. (2020). NHC Ligand Effects on Ru-Catalyzed Cross-Metathesis of Renewable Materials. Catalysts, 10(8), 904. https://doi.org/10.3390/catal10080904





