Monolith Metal-Oxide-Supported Catalysts: Sorbent for Environmental Application
Abstract
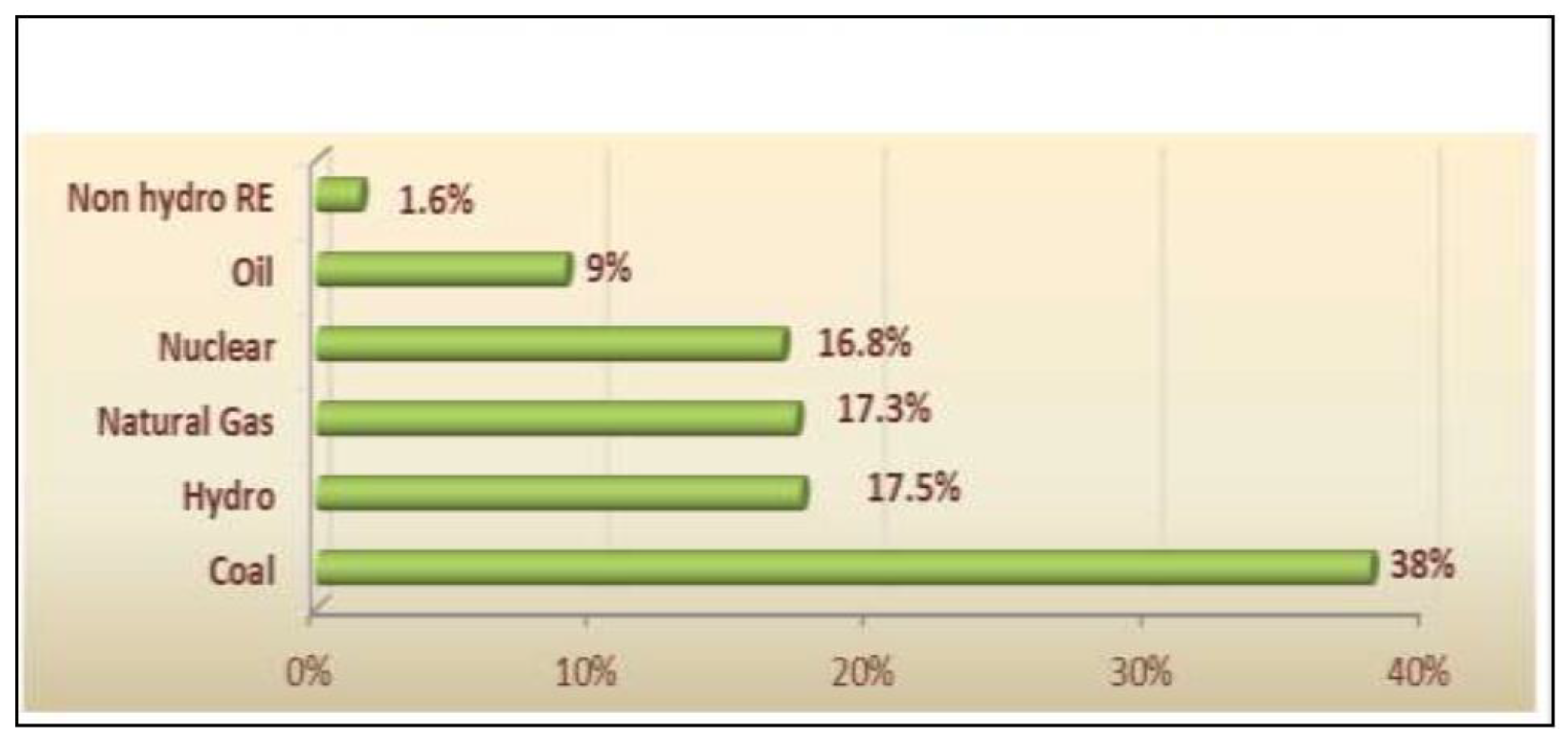
1. Introduction
2. Monolith Structure
3. Catalysts
3.1. Metal-Oxide-Supported Catalysts
3.2. Catalyst Loading
3.3. Catalyst Deactivation
3.4. Surface Area and Mechanism
- micropores (d < 2 nm).
- mesopores (2 nm < d < 50 nm).
- macropores (50 nm < d < 200 nm).
4. Adsorption Kinetics
- External diffusion; molecules diffuses towards the interface space from the bulk phase.
- Internal diffusion; molecules diffuses inside the pores.
- Surface diffusion; molecules diffuses in the surface phase.
- Adsorption-desorption elementary processes.
4.1. The Freundlich Isotherm Model
4.2. The Langmuir Model
4.3. Pressure Drop, Mass Transfer and Heat Transfer in Monolith
- Pore diffusion occurs as ordinary diffusion when the pore diameter of the adsorbent is large compared to the mean free path of the adsorbed molecule or as Knudsen diffusion when the pores are much smaller than the mean free path of the gas. The diffusion can also occur in the adsorption process as both ordinary and Knudsen diffusions.
- Surface diffusion: molecules are adsorbed and transported from one site to another along the pore wall in the direction of decreasing concentration, however surface diffusion may be ignored as it contributes little to the overall transportation.
5. Catalyst Application
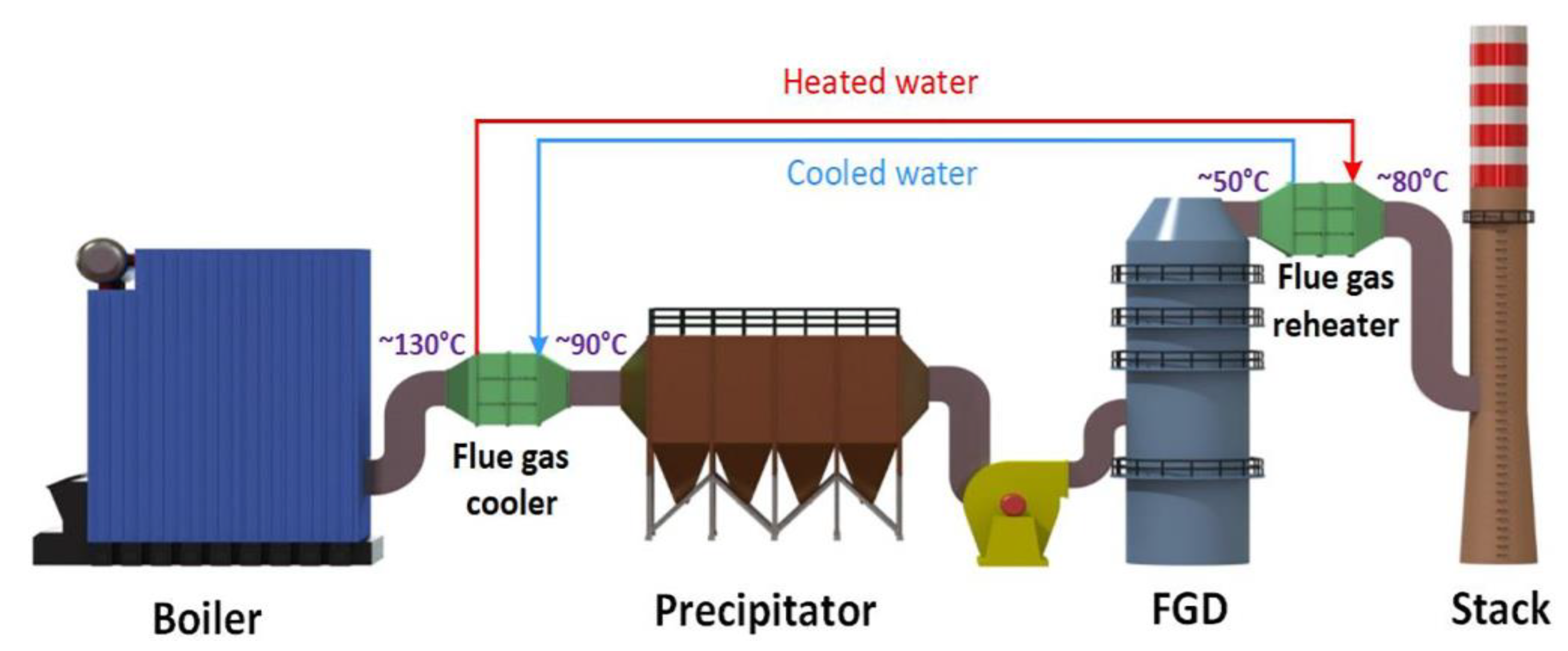
5.1. SO2 Removal from Flue Gas
5.2. NOx Removal from Flue Gas
5.3. Simultaneous SO2/NOx Removal from Flue Gas
6. Regeneration
Overview on Fluidized-Bed/Fixed-Bed Reactors for Activity Test
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ACM | Activated Carbon Monolith |
| AC | Activated Carbon |
| CO2 | Carbon Dioxide |
| MO | Metal Oxide |
| NOx | Nitrogen Oxide |
| q | Adsorption Capacity |
| RE | Regeneration Efficiency |
| SCR | Selective Catalytic Reduction |
| SO2 | Sulfur dioxide |
| WFGD | Wet Flue Gas Desulphurization |
References
- Cheng, G.; Zhang, C. Desulfurization and denitrification technologies of coal-fired flue gas. Pol. J. Environ. Stud. 2018, 272, 481–489. [Google Scholar] [CrossRef]
- Koplitz, S.N.; Jacob, D.J.; Sulprizio, M.P.; Myllyvirta, L.; Reid, C. Burden of disease from rising coal-fired power plant emissions in Southeast Asia. Environ. Sci. Technol. 2016, 51, 1–10. [Google Scholar] [CrossRef]
- Kiman, S.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NOx removal from flue gas. Fuel Process. Technol. 2018, 180, 155–165. [Google Scholar]
- Lee, D.W.; Yoo, B.R. Metal oxide (supported) catalysts: Synthesis and applications. J. Ind. Eng. Chem. Adv. 2014, 20, 3947–3959. [Google Scholar] [CrossRef]
- Rau, J.Y.; Tseng, H.H.; Chiang, B.C.; Wey, M.Y.; Lin, M.D. Evaluation of SO2 oxidation and fly ash filtration by an activated carbon fluidized-bed reactor: The effects of acid modification, copper addition and operating condition. Fuel 2010, 89, 732–742. [Google Scholar] [CrossRef]
- Hu, H.; Wang, S.; Zhang, X.; Zhao, Q.; Li, J. Study on simultaneous catalytic reduction of sulfur dioxide and nitric oxide on rare earth mixed compounds. J. Rare Earths 2006, 24, 24695–24698. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, R.; Han, X.; Huang, Z.; Cui, Y. The mechanism of CO regeneration on V2O5/AC catalyst and sulfur recovery. Chem. Eng. J. 2017, 316, 744–750. [Google Scholar] [CrossRef]
- Liu, Z.S. Adsorption of SO2 and NO from incineration flue gas onto activated carbon fibers. Waste Manag. 2008, 28, 2329–2335. [Google Scholar] [CrossRef]
- Hajari, A.; Atanga, M.; Hartvigsen, J.L.; Rownaghi, A.A.; Rezaei, F. Combined flue gas cleanup process for simultaneous removal of SOx, NOx, and CO2: A techno-economic analysis. Energ. Fuels 2017, 31, 4165–4172. [Google Scholar] [CrossRef]
- Liu, Y.; Bisson, T.M.; Yang, H.; Xu, Z. Recent developments in novel sorbents for flue gas clean up. Fuel Process. Technol. 2010, 91, 1175–1197. [Google Scholar] [CrossRef]
- Sinha, S.; Chattopadhyay, S. A study on application of renewable energy technologies for mitigatting the adverse environmental impacts generated from power generation units in Himalayan region. Int. J. Innov. Res. Sci. Technol. 2016, 3, 212–232. [Google Scholar]
- Hosseini, S.; Marahel, E.; Bayesti, I.; Abbasi, A.; Abdullah Choong, C.T.S.Y. CO2 adsorption on modified carbon coated monolith: Effect of surface modification by using alkaline solutions. Appl. Surf. Sci. 2015, 324, 569–575. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Yang, J.; Zhang, S.; Zhang, J.; Zheng, C. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energ. Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Lin, Y.S.; Deng, S.G. Removal of trace sulfur dioxide from gas stream by regenerative sorption processes. Sep. Purif. Technol. 1998, 13, 65–77. [Google Scholar] [CrossRef]
- Gupta, A.; Gaur, V.; Verma, N. Breakthrough analysis for adsorption of sulfur-dioxide over zeolites. Chem. Eng. Process. Process Intensif. 2004, 43, 9–22. [Google Scholar] [CrossRef]
- Trimm, D.L. The regeneration or disposal of deactivated heterogeneous catalysts. Appl. Catal. A Gen. 2001, 212, 153–160. [Google Scholar] [CrossRef]
- Jia, L.Y.; AlFarouha, L.; Pinard, S.; Hedan, J.D.; Comparot, A.; Dufour, K.; Ben, T.H.; Vezin, C.; Batiot, D. Environmental new routes for complete regeneration of coked zeolite. Appl. Catal. B Environ. 2017, 219, 82–91. [Google Scholar] [CrossRef]
- Tsybulevski, A.M.; Tkachenko, O.P.; Rode, E.J.; Weston, K.C.; Kustov, L.M.; Sulman, E.M.; Doluda, V.Y.; Greish, A.A. Reactive adsorption of sulfur compounds by transition metal polycation exchanged zeolites and desulfurization of hydrocarbon streams. Energ. Technol. 2017, 5, 1627–1637. [Google Scholar] [CrossRef]
- Ju, F.; Liu, C.; Li, K.; Meng, C.; Gao, S.; Ling, H. Reactive adsorption desulfurization of FCC gasoline over a Ca-Doped Ni-ZnO/Al2O3-SiO2. Energ. Fuels 2016, 30, 6688–6697. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Li, K.; Yu, X.; Wei, Y.; Liu, J.; Peng, Y.; Li, Y.; Chen, Y. Highly active monolith catalysts of LaKCoO3 perovskite-type complex oxide on alumina-washcoateddiesel particulate filter and the catalytic performances for the combustion of soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Chen, B.; Shi, C.; Li, Y.; Wang, C. Exceptional activity for formaldehyde combustion using siliceous beta zeolite as a catalyst support. Catal. Today 2020, 339, 174–180. [Google Scholar] [CrossRef]
- Davó-quiñonero, A.; Sorolla-rosario, D.; Bailón-garcía, E.; Lozano-castelló, D. Improved asymmetrical honeycomb monolith catalyst prepared using a 3D printed template. J. Hazard. Mater. 2019, 368, 638–643. [Google Scholar] [CrossRef] [PubMed]
- José, M.; Gatica, J.; Castiglioni, C.; Santos, M.; Pilar, Y.; Gustavo, C.; Martín, T.; Hilario, V. Use of pillared clays in the preparation of washcoatedclay honeycomb monoliths as support of manganese catalysts for the total oxidation of VOCs. Catal. Today 2017, 296, 84–94. [Google Scholar]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation. Appl. Catal. A Gen. 2018, 554, 44–53. [Google Scholar] [CrossRef]
- Kiman, S.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Regeneration/optimization of activated carbon monolith in simultaneous SO2/NOx removal from flu gas. Chem. Eng. Technol. 2019, 9, 1928–1940. [Google Scholar]
- Sharma, M.; Choudhury, D.; Hazra, S.; Basu, S. Effective removal of metal ions from aqueous solution by mesoporous MnO2 and TiO2 monoliths: Kinetic and equilibrium modelling. J. Alloys Compd. 2017, 720, 221–229. [Google Scholar] [CrossRef]
- Darunte, L.A.; Terada, Y.; Murdock, C.R.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Monolith supported amine functionalized Mg adsorbents for CO2 capture. ACS Appl. Mater. Interfaces 2017, 2, 17042–17050. [Google Scholar] [CrossRef]
- Zhu, L.; Tan, C.F.; Gao, M.; Ho, G.W. Design of a metal oxide-organic framework (MoOF) foam microreactor: Solar-induced direct pollutant degradation and hydrogen generation. Adv. Mater. 2015, 27, 7713–7719. [Google Scholar] [CrossRef]
- Shen, F.; Liu, J.; Dong, Y.; Wu, D.; Gu, C.; Zhang, Z. Elemental mercury removal from syngas by porous carbon-supported CuCl2 sorbents. Fuel 2019, 239, 138–144. [Google Scholar] [CrossRef]
- Abubakar, U.C.; Alhooshani, K.R.; Adamu, S.; Al Thagfi, J.; Saleh, T.A. The effect of calcination temperature on the activity of hydrodesulfurization catalysts supported on mesoporous activated carbon. J. Clean. Prod. 2019, 211, 1567–1575. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Xu, Z.; Xiong, J.; Zhu, T. Transformation of functional groups in the reduction of NO with NH3 over nitrogen-enriched activated carbons. Fuel 2018, 223, 312–323. [Google Scholar] [CrossRef]
- Shiung, S.; Rock, L.; Liew, K.; Mun, Y.; Elfina, W. Activated carbon for catalyst support from microwave pyrolysis of orange peel. Waste Biomass Valor. 2017, 8, 2109–2119. [Google Scholar]
- Kiman, S.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Carbonaceous materials modified catalysts for simultaneous SO2/NOx removal from flue gas: A review. Catal. Rev. Sci. Eng. 2019, 61, 134–161. [Google Scholar]
- Patton, A.; Crittenden, B.D.; Perera, S.P. Use of the linear driving force approximation to guide the design of monolithic adsorbents. Chem. Eng. Res. Des. 2004, 82, 999–1009. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Du, P.; Heiszwolf, J.J.; Kapteijn, F.; Moulijn, J.A. Mass transfer characteristics of three-phase monolith reactors. Chem. Eng. Sci. 2001, 56, 6015–6023. [Google Scholar] [CrossRef]
- Heiszwolf, J.J.; Engelvaart, L.B.; Van den Eijnden, M.G.; Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A. Hydrodynamic aspects of the monolith loop reactor. Chem. Eng. Sci. 2001, 56, 805–812. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Pérez-Cadenas, A.F. Carbon-based honeycomb monoliths for environmental gas-phase applications. Materials 2010, 3, 1203–1227. [Google Scholar] [CrossRef]
- Boger, T.; Heibel, A.K.; Sorensen, C.M. Monolithic catalysts for the chemical industry. Ind. Eng. Chem. Res. 2004, 43, 4602–4611. [Google Scholar] [CrossRef]
- Roy, S.; Bauer, T.; Al-Dahhan, M.; Lehner, P.; Turek, T. Monoliths as multiphase reactors: A review. AIChE J. 2004, 50, 2918–2938. [Google Scholar] [CrossRef]
- Hosseini, S.; Rashid, S.A.; Abbasi, A.; Babadi, F.E.; Abdullah, L.C.; Choong, T.S.Y. Effect of catalyst and substrate on growth characteristics of carbon nanofiber onto honeycomb monolith. Rev. Mex. Urol. 2016, 76, 440–449. [Google Scholar] [CrossRef]
- Lei, Z.; Long, A.; Jia, M.; Liu, X. Experimental and kinetic study of selective catalytic reduction of NO with NH3 over CuO/Al2O3/cordierite catalyst. Chin. J. Chem. Eng. 2010, 18, 721–729. [Google Scholar] [CrossRef]
- Boyano, A.; Lázaro, M.J.; Cristiani, C.; Maldonado-hodar, F.J.; Forzatti, P.; Moliner, R. A comparative study of V2O5/AC and V2O5/Al2O3 catalysts for the selective catalytic reduction of NO by NH3. Chem. Eng. J. 2009, 149, 173–182. [Google Scholar] [CrossRef]
- Campanati, M.; Fornasari, G.; Vaccari, A. Fundamental in the preparation of heterogeneous catalysts. Catal. Today 2003, 77, 299–314. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, P.; Li, K.; Tang, L.; Hao, J.; Song, X.; Zhang, G.; Wang, C. Simultaneous removal of NOx and SO2 by low-temperature selective catalytic reduction over modified activated carbon catalysts. Russ. J. Phys. Chem. A 2017, 91, 490–499. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Hasegawa, J.; Li, S.; Shen, Y.; Li, H.; Shi, L.; Zhang, D. SO2-tolerant selective catalytic reduction of NOx over meso-TiO2@Fe2O3@Al2O3 metal-based monolith catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef]
- Mendes, N.; Ozhan, C.; Da Costa, P.; Bacariza, M.C.; Henriques, C. Optimizing washcoatingconditions for the preparation of zeolite-based cordierite monoliths for NOx CH4-SCR: A required step for real application. Ind. Eng. Chem. Res. 2019, 58, 11799–11810. [Google Scholar]
- Li, X.; Zhang, X.; Zhong, L.; Zhang, C.; Fang, Q.; Chen, G. Enhancement of SCR performance of monolithic Mn–Ce/Al2O3/cordierite catalysts by using modified deposition precipitation method. Asia-Pac. J. Chem. Eng. 2019, 14, e2318. [Google Scholar] [CrossRef]
- Qi, K.; Xie, J.; Li, F.; He, F. Experimental study on preparation and operating conditions over a promising monolithic catalyst for NOx removal: MnOx/TiO2/cordierite. Mater. Sci. Forum 2017, 898, 1905–1915. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2014, 40, 948–979. [Google Scholar] [CrossRef]
- Teoh, Y.P.; Khan, M.A.; Choong, T.S.Y. Kinetic and isotherm studies for lead adsorption from aqueous phase on carbon coated monolith. Chem. Eng. J. 2013, 217, 248–255. [Google Scholar] [CrossRef]
- Ma, Z.; Zaera, F. Heterogeneous catalysis by metals. Encycl. Inorg. Bioinorg. Chem. 2011, 1–17. [Google Scholar]
- Zhang, F.; Tian, X.; Shah, M.; Yang, W. Synthesis of magnetic carbonaceous acids derived from hydrolysates of Jatropha hulls for catalytic biodiesel production. RSC Adv. 2017, 7, 11403–11413. [Google Scholar] [CrossRef]
- Guo, P.; Huang, F.; Zheng, M. Magnetic solid base catalysts for the production of biodiesel. J. Am. Oil Chem. Soc. 2012, 89, 925–933. [Google Scholar] [CrossRef]
- Xue, B.; Luo, J.; Zhang, F.; Fang, Z. Biodiesel production from soybean and Jatropha oils by magnetic. Energy 2014, 68, 584–591. [Google Scholar] [CrossRef]
- Feyzi, M.; Norouzi, L. Preparation and kinetic study of magnetic Ca/Fe3O4@SiO2 nanocatalysts for biodiesel production. Renew. Energ. 2016, 94, 579–586. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, Z.; Wang, Y. Biodiesel production directly from oils with high acid value by magnetic Na2SiO@Fe3O4/C catalyst and ultrasound. Fuel 2015, 150, 370–377. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Al-tamer, M.H. Biodiesel production from Silybummarianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energ. Convers. Manag. 2016, 108, 255–265. [Google Scholar] [CrossRef]
- Cao, M.O.; Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic. Fuel 2016, 164, 314–321. [Google Scholar]
- Stankiewicz, A. Process Intensity, cation in-line monolithic reactor. Chem. Eng. Sci. 2001, 56, 359–364. [Google Scholar] [CrossRef]
- Li, J.; Liang, X. Magnetic solid acid catalyst for biodiesel synthesis from waste oil. Energ. Conver. Manag. 2017, 141, 126–132. [Google Scholar] [CrossRef]
- Dai, H. Environmental catalysis: A solution for the removal of atmospheric pollutants. Sci. Bull. 2015, 60, 1708–1710. [Google Scholar] [CrossRef]
- Ruiz-Martínez, E.; Sánchez-Hervás, J.M.; Otero-Ruiz, J. Effect of operating conditions on the reduction of nitrous oxide by propane over a Fe-Zeolite monolith. Appl. Catal. B Environ. 2005, 61, 306–315. [Google Scholar]
- Radwan, N.R.E.; El-Shall, M.S.; Hassan, H.M.A. Synthesis and characterization of nanoparticle Co3O4, CuO and NiOcatalysts prepared by physical and chemical methods to minimize air pollution. Appl. Catal. A Gen. 2007, 331, 8–18. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, D.; Tong, Z.; Zhang, J.; Luo, H. Oxidation of NO over cobalt oxide supported on mesoporous silica. J. Nat. Gas Chem. 2009, 18, 421–428. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, J.; Zou, R.; Liu, L.; Zhang, Z.; Wang, X. Hydrothermal synthesis of CeO2 nanoparticles on activated carbon with enhanced desulfurization activity. Energ. Fuels 2012, 26, 5879–5886. [Google Scholar] [CrossRef]
- Haakana, T.; Kolehmainen, E.; Turunen, I.; Mikkola, J.P.; Salmi, T. The development of monolith reactors: General strategy with a case study. Chem. Eng. Sci. 2004, 59, 5629–5635. [Google Scholar] [CrossRef]
- Kiman, S.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Activated carbon monolith Co3O4 based catalyst: Synthesis, characterization and adsorption Studies. Environ. Technol. Innov. 2018, 12, 273–285. [Google Scholar]
- Liu, Y.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts. CuihuaXuebao/Chin. J. Catal. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Neyestanaki, A.K.; Klingstedt, F.; Salmi, T.; Murzin, D.Y. Deactivation of postcombustioncatalysts. A review. Fuel 2004, 83, 395–408. [Google Scholar] [CrossRef]
- Kiman, S.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Optimization of activated carbon monolith Co3O4-based catalyst for simultaneous SO2/NOx removal from flue gas using response surface methodology. Combust. Sci. Technol. 2020, 192, 786–803. [Google Scholar]
- Boyano, A.; Lombardo, N.; Moliner, R. Vanadium-loaded carbon-based monoliths for the on-board NO reduction: Experimental study of operating conditions. Chem. Eng. J. 2008, 144, 343–351. [Google Scholar] [CrossRef]
- Boyano, A.; Herrera, C.; Larrubia, M.A.; Alemany, L.J.; Moliner, R.; Lázaro, M.J. Vanadium loaded carbon-based monoliths for the on-board no reduction: Influence of temperature and period of the oxidation treatment. Chem. Eng. J. 2010, 160, 623–633. [Google Scholar] [CrossRef]
- Couck, S.; Lefevere, J.; Mullens, S.; Protasova, L.; Meyen, V.; Desmet, G.; Baron, G.V.; Denayer, J.F. CO2, CH4 and N2 separation with a 3DFD-printed ZSM-5 monolith. Chem. Eng. J. 2017, 308, 719–726. [Google Scholar] [CrossRef]
- Kiel, J.H.A.; Edelaar, A.C.S.; Prins, W.; Van Swaaij, W.P.M. Performance of silica-supported copper oxide sorbents for SO2/NOx-removal from flue gas. Appl. Catal. B Environ. 1992, 141–160. [Google Scholar]
- Bin, H.; Fang, J.T.; Zhao, Y.T.; Fang, J.J.; Huang, Y. Selective oxidation of hydrogen sulfide to sulfur over activated carbon-supported metal oxides. Fuel 2013, 108, 143–148. [Google Scholar]
- Nijhuis, T.A.; Kreutzer, M.T.; Romijn, C.J.; Kapteijn, F.; Moulijn, J.A. Monolithic catalysts as efficient three-phase reactors. Chem. Eng. Sci. 2001, 56, 823–829. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanism of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Argyle, M.D. Advances in catalyst deactivation and regeneration. Catalysts 2015, 5, 949–954. [Google Scholar] [CrossRef]
- Worstell, J. Catalyst deactivation. Adiabatic Fixed Bed React. 2014, 52, 35–65. [Google Scholar]
- Moulijn, J.A.; Van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Kiełtyka, M.; Dias, A.P.S.; Kubiczek, H.; Sarapata, B.; Grzybek, T. The influence of poisoning on the deactivation of Denoxcatalysts. C. R. Chim. 2015, 18, 1036–1048. [Google Scholar] [CrossRef]
- Rusu, A.O.; Dumitriu, E. Destruction of volatile organic compounds by catalytic oxidation. Environ. Eng. Manag. J. 2003, 2, 273–302. [Google Scholar] [CrossRef]
- Rudyak, V.; Minakov, A. Modeling and optimization of Y-type of micromixer. Micromachines 2009, 33, 75–88. [Google Scholar] [CrossRef]
- Marsh, H. Adsorption methods to study microporpsity of coals and carbons. Carbon 1987, 25, 49–58. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 2001, 39, 877–886. [Google Scholar] [CrossRef]
- Hinkov, I.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Carbon dioxide capture by adsorption. J. Chem. Technol. Metall. 2016, 41, 609–626. [Google Scholar]
- Marsh, H.; Rand, B. The characterization of microporous carbons by means of the Dubinin-Radushkevichequation. J. Colloid Interface Sci. 1970, 33, 101–116. [Google Scholar] [CrossRef]
- Heigl, N.; Greider, A.; Petter, C.H.; Kolomiets, O.; Sieser, H.W.; Ulbricht, M.; Bonn, G.K.; Huck, C.W. Simultaneous determination of the micro-, meso-, and macropore size fractions of porous polymers by a combined use of fouriertransform hear-infrared diffuse reflection spectroscopy and multivariate techniques. Anal. Chem. 2008, 80, 8493–8500. [Google Scholar] [CrossRef]
- Choma, J.; Górka, J.; Jaroniec, M.; Zawislak, A. Development of microporosity in mesoporous carbons. Top. Catal. 2010, 53, 283–290. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Spent catalyst waste management:A review. Part I-Developments in hydroprocessingcatalyst waste reduction and use. Resour. Conserv. Recycl. 2008, 52, 859–873. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Gálvez, M.T.; Izquierdo, E.; García-Bordejé, C.; Ruiz, R.; Juan, R.; Moliner, J. Novel carbon-based catalysts for the reduction of NO: Influence of support precursors and active phase loading. Catal. Today 2008, 137, 215–221. [Google Scholar] [CrossRef]
- Wu, C.; Liang, Y.; Yang, K.; Min, Y.; Liang, Z.; Zhang, L.; Zhang, Y. Clickable periodic mesoporous organosilica monolith for highly efficient capillary chromatographic separation. Anal. Chem. 2016, 88, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Long, M.; Bai, X.; Wang, C.; Cai, C.; Fu, J. Monolithic cobalt-doped carbon aerogel for efficient catalytic activation of peroxymonosulfate in water. J. Hazard. Mater. 2017, 332, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X.J. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(χ)-CeO2 at low temperatures. Appl. Catal. B Environ. 2017, 203, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-reinoso, F. The role of carbon materials in heterogeneouscatalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Su, S.; An, H.; Yu, X.X. Post combustion CO2 capture by carbon fibremonolithic adsorbents. Prog. Energ. Combust. Sci. 2009, 35, 438–455. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Deactivation and regeneration of Hopcalitecatalyst for carbon monoxide oxidation: A review. Mater. Today Chem. 2019, 14, 100180. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, H.; Tang, X.; Deng, H.; Liu, H. Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber. Chem. Eng. J. 2012, 200–202, 399–404. [Google Scholar] [CrossRef]
- Sircar, S.; Myers, A.L. Gas adsorption operations: Equilibrium, kinetics, column dynamics and design. Adsorpt. Sci. Technol. 1985, 2, 69–87. [Google Scholar] [CrossRef]
- Dabrowski, A. Adsorption from theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E.A. Comparative studies on CO2 adsorption kinetics by solid adsorbents. Energ. Procedia 2015, 90, 316–325. [Google Scholar] [CrossRef]
- Rezaei, F.; Webley, P. Structured adsorbents in gas separation processes. Sep. Purif. Technol. 2010, 70, 243–256. [Google Scholar] [CrossRef]
- Podkościelny, P.; Nieszporek, K. Adsorption of phenols from aqueous solutions: Equilibria, calorimetry and kinetics of adsorption. J. Colloid Interface Sci. 2011, 354, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Balathanigaimani, M.S.; Shim, W.G.; Lee, J.W.; Moon, H. Adsorption characteristics of phenol on novel corn grain-based activated carbons. Microp. Mesop. Mater. 2010, 127, 1–8. [Google Scholar] [CrossRef]
- Kalavathy, H.; Regupathi, M.I.; Pillai, M.G.; Miranda, L.R. Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Colloids Surf. B Biointerfaces 2009, 70, 35–45. [Google Scholar] [CrossRef]
- Chaudhary, N.; Balomajumder, C. Optimization study of adsorption parameters for removal of phenol on aluminum impregnated fly ash using response surface methodology. J. Taiwan Inst. Chem. Eng. 2014, 45, 852–859. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Kenneth, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1–19. [Google Scholar] [CrossRef]
- Ravennia, G.; Elhamib, O.H.; Ahrenfeldta, J.; Henriksena, U.B.; Neubauerb, Y. Adsorption and decomposition of tar model compounds over the surface of gasification char and active carbon within the temperature range 250–800 °C. Appl. Energ. 2019, 241, 139–151. [Google Scholar] [CrossRef]
- Sumathi, S.; Bhatia, S.; Lee, K.T.; Mohamed, A.R. Performance of shell activated carbon impregnated with CeO2 and V2O5 catalyst in simultaneous removal of SO2 and NO. J. Appl. Sci. 2010, 12, 1052–1059. [Google Scholar]
- Sircar, S. Comments on practical use of Langmuir gas adsorption isotherm model. Adsorption 2017, 23, 121–130. [Google Scholar] [CrossRef]
- Ghorbani, F.; Younesi, H.; Ghasempouri, S.M.; Zinatizadeh, A.A.; Amini, M.; Daneshi, A. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces Cerevisiae. Chem. Eng. J. 2008, 145, 267–275. [Google Scholar] [CrossRef]
- Abdedayem, A.; Guiza, M.; Ouederni, A. Copper supported on porous activated carbon obtained by wetness impregnation: Effect of preparation conditions on the ozonation catalyst’s characteristics. C. R. Chim. 2015, 18, 100–109. [Google Scholar] [CrossRef]
- Boger, T.; Heibel, A.K. Heat transfer in conductive monolith structures. Chem. Eng. Sci. 2005, 60, 1823–1835. [Google Scholar] [CrossRef]
- Lua, A.C.; Guo, J. Adsorption of sulfur dioxide on activated carbon from oil-palm waste. J. Environ. Eng. 2001, 127, 895–901. [Google Scholar] [CrossRef]
- Andrigo, P.; Bagatin, R.; Pagani, G.; Hugo, A. Fixed bed reactors. Catal. Today 2011, 52, 197–221. [Google Scholar] [CrossRef]
- Yavuz, R. Sulfur dioxide adsorption by activated carbons having different textural and chemical properties. Fuel 2008, 87, 3207–3215. [Google Scholar]
- Gu, T.; Balakotaiah, V. Impact of heat and mass dispersion and thermal effects on the scale-up of monolith reactors. Chem. Eng. J. 2016, 284, 513–535. [Google Scholar] [CrossRef]
- Visconti, C.G.; Groppi, G.; Tronconi, E. Accurate prediction of the effective radial conductivity of highly conductive honeycomb monoliths with square channels. Chem. Eng. J. 2013, 223, 224–230. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Honeycomb supports with high thermal conductivity for gas/solid chemical processes. Catal. Today 2005, 105, 297–304. [Google Scholar] [CrossRef]
- Tronconi, E.; Groppi, G.; Visconti, C.G. Structured catalysts for non-adiabatic applications. Curr. Opin. Chem. Eng. 2014, 5, 55–67. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E.; Cortelli, C.; Leanza, R. Conductive monolithic catalysts: Development and industrial pilot tests for the oxidation of O-xylene to phthalic anhydride. Ind. Eng. Chem. Res. 2012, 51, 7590–7596. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Ismagilov, Z.R.; Koptyug, I.V.; Andrievskaya, I.P. Formation of textural and mechanical properties of extruded ceramic honeycomb monoliths: An 1H-NMR imaging study. Catal. Today 2005, 105, 507–515. [Google Scholar] [CrossRef]
- Liu, Y.X.; Ziyang, L.; Yan, W.; Yanshan, Y.; Jian, F.P.; Jun, Z.; Qian, W. Simultaneous absorption of SO2 and NO from flue gas using ultrasound/Fe2+/heat coactivated persulfate system. J. Hazard. Mater. 2018, 342, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zwolińska, E.; Chmielewski, A.G. Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 119–142. [Google Scholar] [CrossRef]
- Jing, W.; Hou, Y.; Guo, Q.; Huang, Z.; Han, X.; Ma, G. Using vanadyl sulfate to prepare carbon-supported vanadium catalyst for flue gas desulfurization. J. Fuel Chem. Technol. 2013, 41, 1223–1233. [Google Scholar] [CrossRef]
- Pan, P.; Chen, H.; Liang, Z.; Zhao, Q. Deposition and corrosion characteristics of liquid-solid droplets on tubular corrosion probes in desulfurized flue gas. Eng. Fail. Anal. 2018, 90, 129–140. [Google Scholar] [CrossRef]
- Gingerich, D.B.; Grol, E.; Mauter, M.S. Fundamental challenges and engineering opportunities in flue gas desulfurization wastewater treatment at coal fired power. Environ. Sci. Water Res. Technol. 2018, 4, 909–925. [Google Scholar] [CrossRef]
- Liu, X.; Khinast, J.G.; Glasser, B.J. A parametric investigation of impregnation and drying of supported catalysts. Chem. Eng. Sci. 2008, 63, 4517–4530. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Nova, I.; Beretta, A.; Groppi, G.; Lietti, L.; Tronconi, E.; Forzatti, P. Monolithic catalysts for NOx removal from stationary sources. Struct. Catal. React. 2005, 171–214. [Google Scholar]
- Liu, Q.; Liu, Z. Carbon supported vanadiafor multi-pollutants removal from flue gas. Fuel 2013, 108, 149–158. [Google Scholar] [CrossRef]
- Kasaoka, S.; Sasaoka, E.; Iwasaki, H. Vanadium oxides (V2Ox) catalysts for dry-type and simultaneous removal of sulfur oxides and nitrogen oxides with ammonia at low temperature. Bull. Chem. Soc. Jpn. 1989, 62, 1226–1232. [Google Scholar] [CrossRef]
- Athappan, A.; Sattler, M.L.; Sethupathi, S. Selective catalytic reduction of nitric oxide over cerium-doped activated carbons. J. Environ. Chem. Eng. 2005, 3, 2502–2513. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Feng, Y.; Mingyuan, Z.Y.; Jianming, D.; Jinli, Z.; Peng, C.; Bin, D. Micro spherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Mat, R.; Somderam, S.; Aziz, A.S.A.; Mohamed, A. Hydrogen sulfide adsorption by zinc oxide-impregnated zeolite (synthesized from Malaysian Kaolin) for biogas desulfurization. J. Ind. Eng. Chem. 2018, 65, 334–342. [Google Scholar] [CrossRef]
- Hao, R.; Yaoyu, Z.; Zhaoyue, W.; Yuanpeng, L.; Bo, Y.; Xingzhou, M.; Yi, Z. An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent. Chem. Eng. J. 2017, 307, 562–571. [Google Scholar] [CrossRef]
- Yang, B.; Chen, L.; Sun, F. Recovery of sulfur dioxide from gas mixture in packed bed column. Int. J. Energ. Environ. 2011, 2, 211–218. [Google Scholar]
- Liu, Q.; Liu, Z.; Wu, W. Effect of V2O5additive on simultaneous SO2 and NO removal from flue gas over a monolithic cordierite-based CuO/Al2O3 catalyst. J. Fuel Chem. Technol. 2009, 35, 285–289. [Google Scholar]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of carbonaceous adsorbents. Part II: Chemical, microbiological and vacuum regeneration. Microp. Mesopor. Mater. 2015, 202, 277–296. [Google Scholar] [CrossRef]
- Piotrowski, K.; Wiltowski, T.; Szyma, T.; Mondal, K. Cycling effects on the methane regeneration kinetics of CuO/Al2O3 sorbent. Chem. Eng. J. 2005, 108, 227–237. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Z.Y.; Zhao, Y. Kinetics of SO2 removal from flue gas on CuO/Al2O3 sorbent catalyst. Chem. Eng. Technol. 2007, 30, 1221–1227. [Google Scholar] [CrossRef]
- Edelaar, A.C.S.; Prms, W.; Van, W.P.M. Performance of silica-supported copper oxide sorbents for SO2/NO, Removal from flue gas II. Selective catalytic reduction of nitric oxide by ammonia. App. Catal. B Environ. 1992, 1, 41–60. [Google Scholar]
- Mathieu, Y.; Tzanis, L.; Soulard, M.; Patarin, J.; Vierling, M.; Molière, M. Adsorption of SOx by oxide materials: A review. Fuel Process. Technol. 2013, 114, 81–100. [Google Scholar] [CrossRef]
- Afonso, J.C.; Aranda, D.A.G.; Schmal, M.; Frety, R. Regeneration of a Pt-Sri/Al2O3 catalyst: Influence of heating rate, temperature and time of Regeneration. Fuel Process. Technol. 1997, 50, 35–48. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, J.; Sun, K.; Chen, C. Oxidation of formic acid over palladium catalyst supported on activated carbon derived from polyaniline and modified lignosulfonate composite. J. Fuel Chem. Technol. 2017, 45, 100–105. [Google Scholar] [CrossRef]
- Schobing, J.; Tschamber, V.; Brilhac, J.F.; Auclaire, A.; Hohl, Y. Simultaneous soot combustion and NOx reduction over a vanadia-based selective catalytic reduction catalyst. C. R. Chim. 2018, 21, 221–231. [Google Scholar] [CrossRef]
- Liu, W.; He, X.; Pang, S.; Zhang, Y. Effect of relative humidity on O3 and NO2 oxidation of SO2 on α-Al2O3 particles. Atmos. Environ. 2017, 167, 245–253. [Google Scholar] [CrossRef]
- Shang, X.; Hu, G.; He, C.; Zhao, J.; Zhang, F.; Xu, Y.; Zhang, Y.; Li, J.; Chen, J. Regeneration of full-scale commercial honeycomb monolith catalyst (V2O5-WO3/TiO2) used in coal-fired power plant. J. Ind. Eng. Chem. 2012, 18, 513–519. [Google Scholar] [CrossRef]
- Yu, Y.; Meng, X.; Chen, J.; Yin, L.; Qiu, T.; He, C. Deactivation mechanism and feasible regeneration approaches for the used commercial NH3-SCR catalysts. Environ. Technol. 2016, 37, 828–836. [Google Scholar] [CrossRef]
- Federica, R.; Michela, A.; Gargiulo, R.; Paola, A. Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem. Eng. J. 2019, 372, 526–535. [Google Scholar]
- Rodrigues, C.P.; Kraleva, V.; Ehrich, H.; Noronha, F.B. Structured reactors as an alternative to fixed-bed reactors: Influence of catalyst preparation methodology on the partial oxidation of ethanol. Catal. Today 2016, 273, 12–24. [Google Scholar] [CrossRef]
- Zafer, S.; Oana, M.; Merve, K.; Louise, O.; Emrah, O. Trade-off between NOx storage capacity and sulfur tolerance on Al2O3/ZrO2/TiO2–based DeNOx catalysts. Catal. Today 2019, 320, 152–164. [Google Scholar]
- Kolb, G.; Hessel, V. Micro-structured reactors for gas phase reactions. Chem. Eng. J. 2004, 98, 1–38. [Google Scholar] [CrossRef]
- Lin, H.; Gao, X.; Luo, Z.; Cen, K.; Huang, Z. Removal of NO. Fuel 2004, 83, 1349–1355. [Google Scholar] [CrossRef]
- Yeh, J.T.; Demski, R.J.; Strakey, J.P.; Joubert, J.I. Combined SO2/NOx removal from flue gas. Environ. Prog. 1985, 4, 223–228. [Google Scholar] [CrossRef]
- Warnecke, R. Gasification of biomass: Comparison of fixed bed and fluidized bed gasifier. Biomass Bioenergy 2000, 18, 489–497. [Google Scholar] [CrossRef]
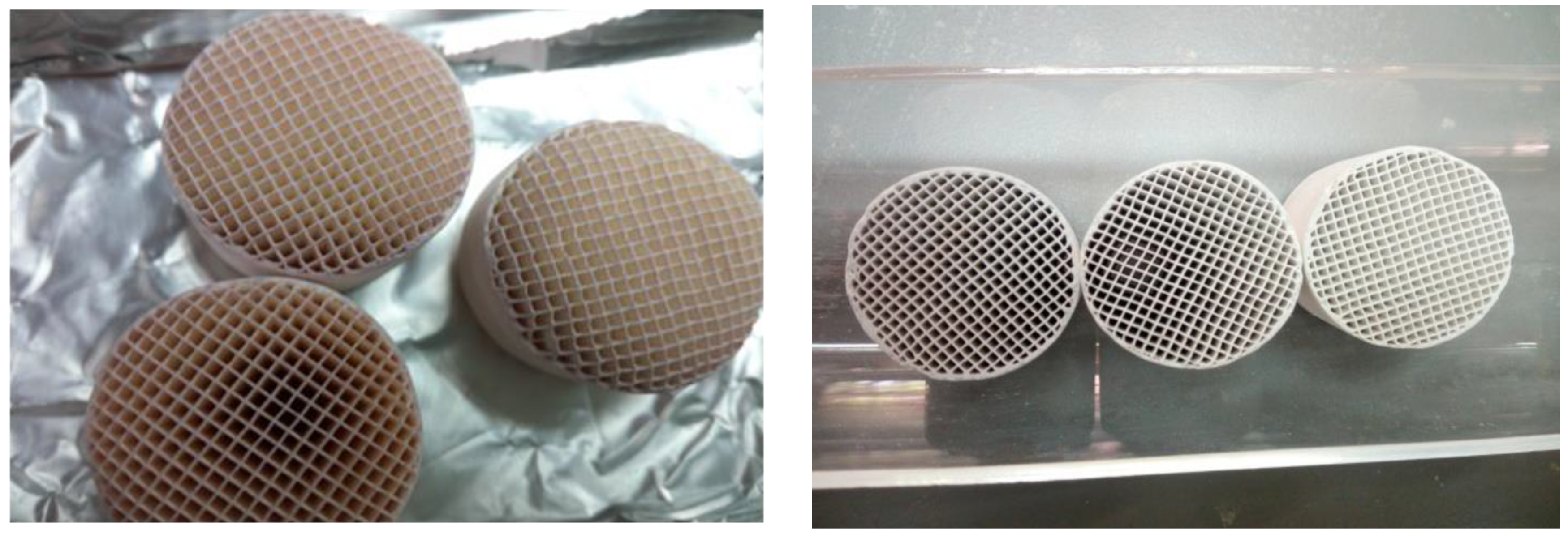
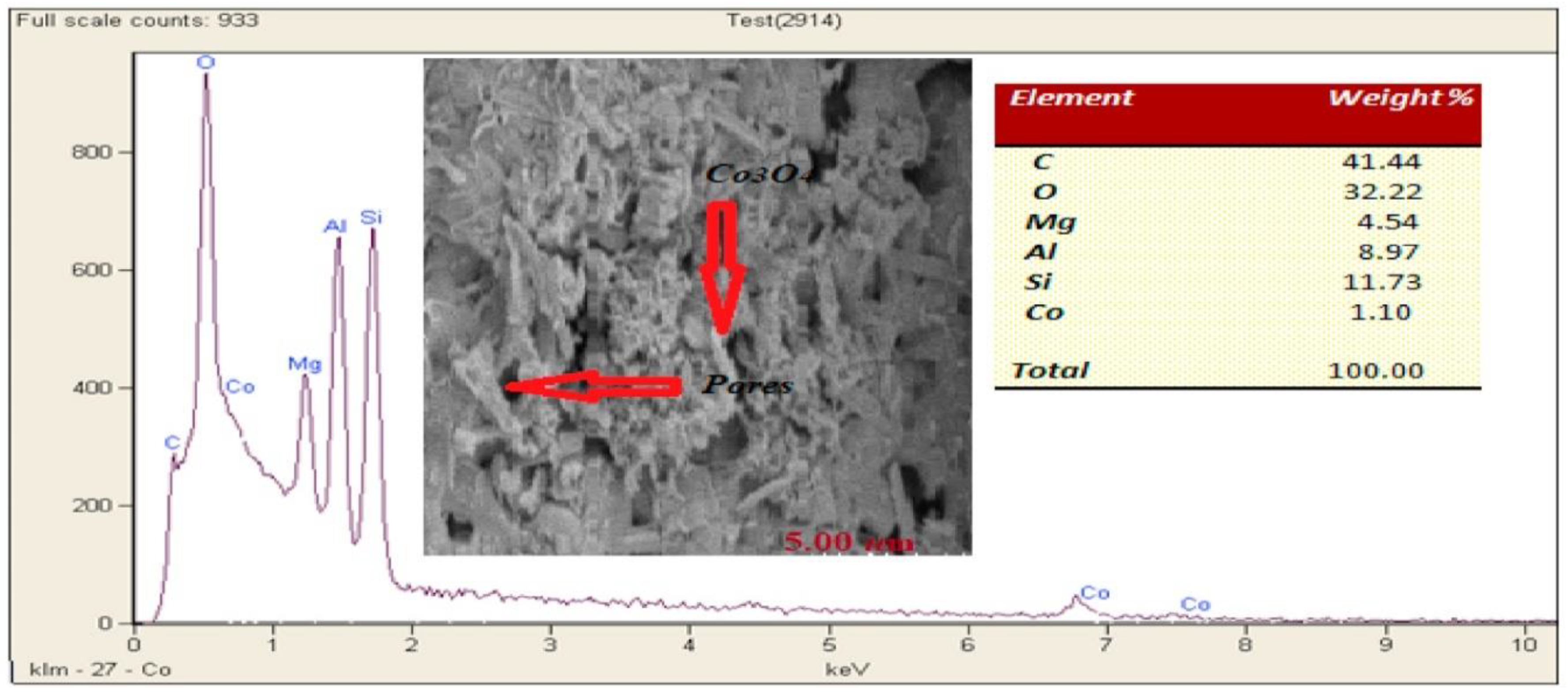





| Monolith | Cells | Chemical Compositions |
|---|---|---|
| Cross section circular | Channel square | SiO2 50.9 ± 1.0% |
| Surface area 1 cm2/g | Wall thickness 0.25 ± 0.02 mm | Al2O3 35.2 ± 1.0% |
| Length 2.50 ± 0.02 mm | Width 1.02 ± 0.02 mm | MgO 13.9 ± 0.5% |
| Diameter 2.50 ± 0.02 mm | Cells 400 (cpsi) | Others < 1% |
| Adsorbent/s | Catalyst/s Supported | Method of Doping | Application | Ref. |
|---|---|---|---|---|
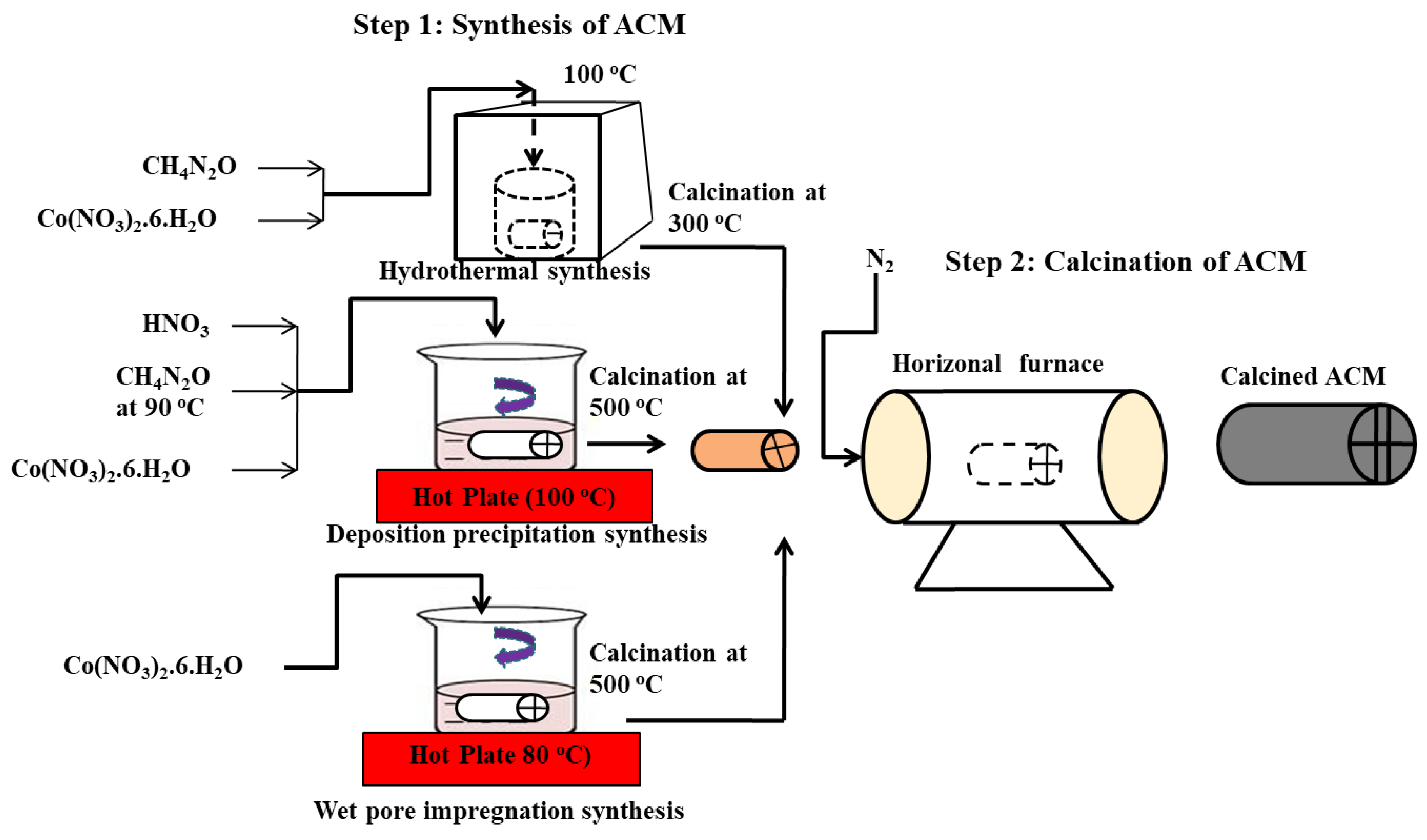
| Monolith | Co3O4 | Pore impregnation, deposition precipitation, hydrothermal methods | Simultaneous SO2/NOx removal from flue gas | [3] |
| Monolith | TiO2, Fe2O3 | One-pot self-assembly method | Low-temperature SO2-tolerant monolithic selective catalytic reduction SCR catalysts with high N2 selectivity | [45] |
| Zeolite-based cordierite monoliths | Pd−Ce−Zeolite powder catalyst | Impregnation | NOx CH4 selective catalytic reduction | [46] |
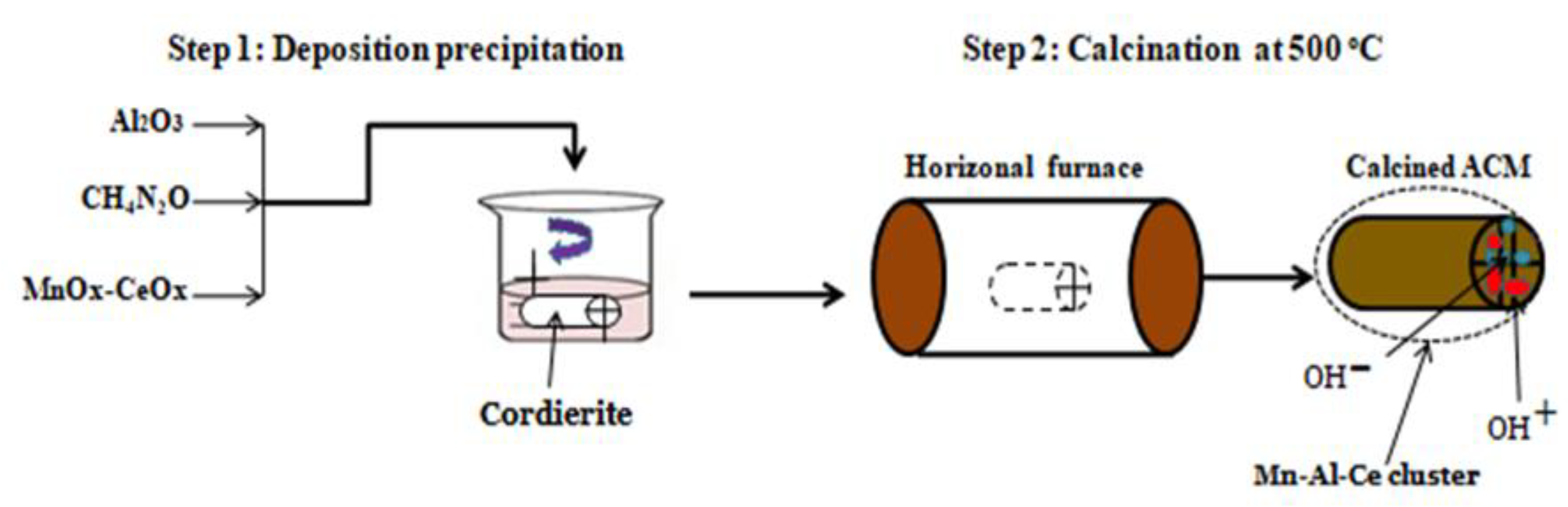
| Cordierite | Mn–Ce/Al2O3 | Impregnation, deposition precipitation, modified deposition precipitation, | SO2 and NO conversion | [47] |
| Cordierite | MnOx/TiO2 | Sol-gel-impregnation method, | Low-temperature selective catalytic reduction (SCR) of NOx with NH3 | [48] |
| Adsorbent | Catalyst | Catalyst Loading | Synthesis Method | Application | Ref. |
|---|---|---|---|---|---|
| AC | Fe(NO3)3.9H2O Mn(NO3)2 Co(NO3)2.6H2O Ce(NO3)3.6H2O (V,NH4VO3H2C2O4) Cu(NO3)2.3H2O | 1.79 × 10−2 1.82 × 10−2 1.70 × 10−2 7.3 × 10−3 1.96 × 10−2 1.57 × 10−2 Mmol | Pore volume wetness impregnation | Oxidation of H2S to elemental sulfur. | [75] |
| Monolith | Ni(NO3)2 | 1 wt% | Deposition precipitation | Comparison of monolith and a trickle-bed catalyst system | [76] |
| AC | V2O5 | 1 wt%. | Pore volume impregnation | Desulfurization and regeneration | [7] |
| Cordierite | Cu/Cu(NO3)2.3H2O | 1.5% | Incipient impregnation | Modelling of monolithic SCR reactor | [41] |
| Deactivation Type | Cause of Deactivation | Prevention/Treatment | Ref. |
|---|---|---|---|
| Chemical poisoning | Sulfur, chlorine, heavy metals, halogens, silicones, phosphorus, acid catalysts are poisoned by basic materials, oxide catalysts are poisoned by Pb, Hg, As, Cd. | Metal oxide-based catalysts exhibit good resistant to deactivation by poisoning, oxidation can reduce the effect of some poisons/regeneration. | [16,79,83] |
| Thermal sintering | Catalyst exposure to high temperature, high partial pressure of water, crystallite growth on the catalytic phase resulting in loss of catalytic surface area, calcinations process, reduction (fresh or passivated catalyst), reaction (hot spots, maldistribution), or regeneration. | Stabilizers are used to fill vacancies in the lattice, employing chemical vapor deposition (CVD)-type treatment to restore the active sites/it is better to avoid sintering from occurring. | [16,77,80,83] |
| Fouling | Carbon deposit, ash, soot, rust, and scale | Regeneration/rejuvenation | [16,80] |
| Objective/Comment | Catalytic Activity | Ref. |
|---|---|---|
| Simultaneous removal of NO and SO2 from flue gas catalysts/uses simulated flue gas. | 0.8 g of catalyst was used with reaction gas flow rate of 500 cm3 min−1 corresponding to gas hourly space velocity (GHSV) of 30,000 h−1. N2-based gas mixture containing 500 ppm NO, 600 ppm NH3, and 1000 ppm SO2 and 0–5% O2. | [44] |
| Vanadia based SCR catalyst study/uses simulated flue gas. | The reactive gas flow, containing 1000 ppm of NOx (100 ppm NO2 and 900 ppm NO), 1000 ppm of NH3, 15% of O2, and 8% of H2O at flow rate of GHSV = 55,000 h−1. | [147] |
| Ammonium sulfate salt deactivation in SCR study/uses simulated flue gas. | A simulated flue gas of 620 ppm NO, 620 ppm NH3, 3 vol% H2O, 5.5 vol% O2, and balance Ar. | [54] |
| Simultaneous removal of SO2/NO2 from simulated flue gas. | Simulated flue gas of 2000 ppm for SO2, 200 ppm for NO2, and about 5% for O2. The flow rate was controlled at 0.15 m3h−1 with a rotameter. | [148] |
| Fluidized-Bed Reactors (+Merit, -Demerit) | Fixed-Bed Reactors (+Merit, -Demerit) | Ref. |
|---|---|---|
| (+) Fly ash passes through the reactor without plugging | (-) Plugging can occur (monolith adsorbent/catalyst reactor remedy) | [5,80,155,156,157] |
| (-) Higher pressure drop | (+) Simple and robust construction | |
| (+) Shutdown for replacement of catalyst is not needed. | (-) Process must be shut down for reloading the reactor with fresh catalyst | |
| (-) Possible attrition of the bed material | (+) Pressure drop is low | |
| (+) Continuous operation and regeneration | (-) Bad temperature distribution, Low specific capacity | |
| (+) Lots of processes for different applications in operation | ||
| (+) High thermal/mass transfer coefficient, high gas/solid, and solid/solid contact areas are continuous | (-) Long period to heat-up | |
| (+) No valve problems | (-) Valves are required to isolate the regenerator’s absorber | |
| (+) Less space requirement because of great scale-up | (+) Can operate at partial load (20 ± 110%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silas, K.; Wan Ab Karim Ghani, W.A.; Choong, T.S.Y.; Rashid, U. Monolith Metal-Oxide-Supported Catalysts: Sorbent for Environmental Application. Catalysts 2020, 10, 1018. https://doi.org/10.3390/catal10091018
Silas K, Wan Ab Karim Ghani WA, Choong TSY, Rashid U. Monolith Metal-Oxide-Supported Catalysts: Sorbent for Environmental Application. Catalysts. 2020; 10(9):1018. https://doi.org/10.3390/catal10091018
Chicago/Turabian StyleSilas, Kiman, Wan Azlina Wan Ab Karim Ghani, Thomas Shean Yaw Choong, and Umer Rashid. 2020. "Monolith Metal-Oxide-Supported Catalysts: Sorbent for Environmental Application" Catalysts 10, no. 9: 1018. https://doi.org/10.3390/catal10091018
APA StyleSilas, K., Wan Ab Karim Ghani, W. A., Choong, T. S. Y., & Rashid, U. (2020). Monolith Metal-Oxide-Supported Catalysts: Sorbent for Environmental Application. Catalysts, 10(9), 1018. https://doi.org/10.3390/catal10091018







