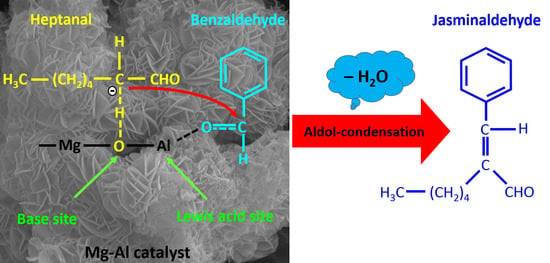
Solvent-Free Synthesis of Jasminaldehyde in a Fixed-Bed Flow Reactor over Mg-Al Mixed Oxide
Abstract
:1. Introduction
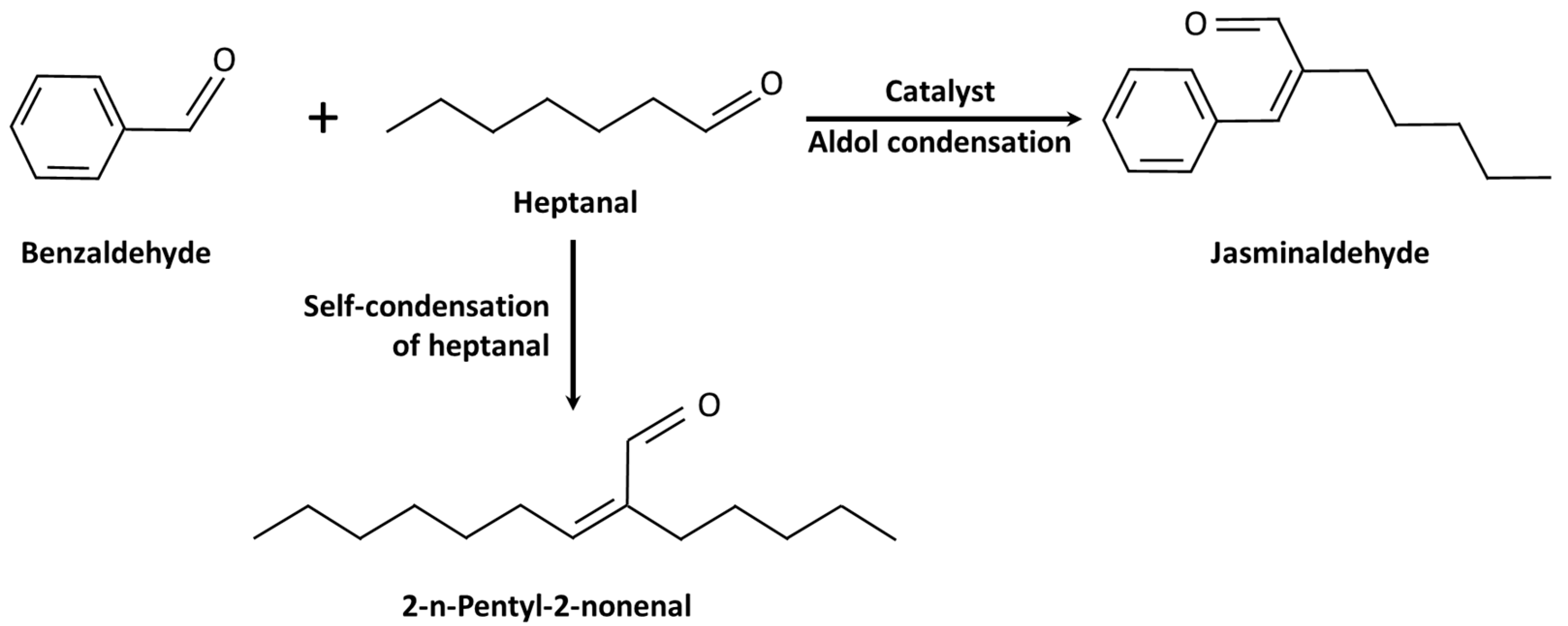
2. Results and Discussion
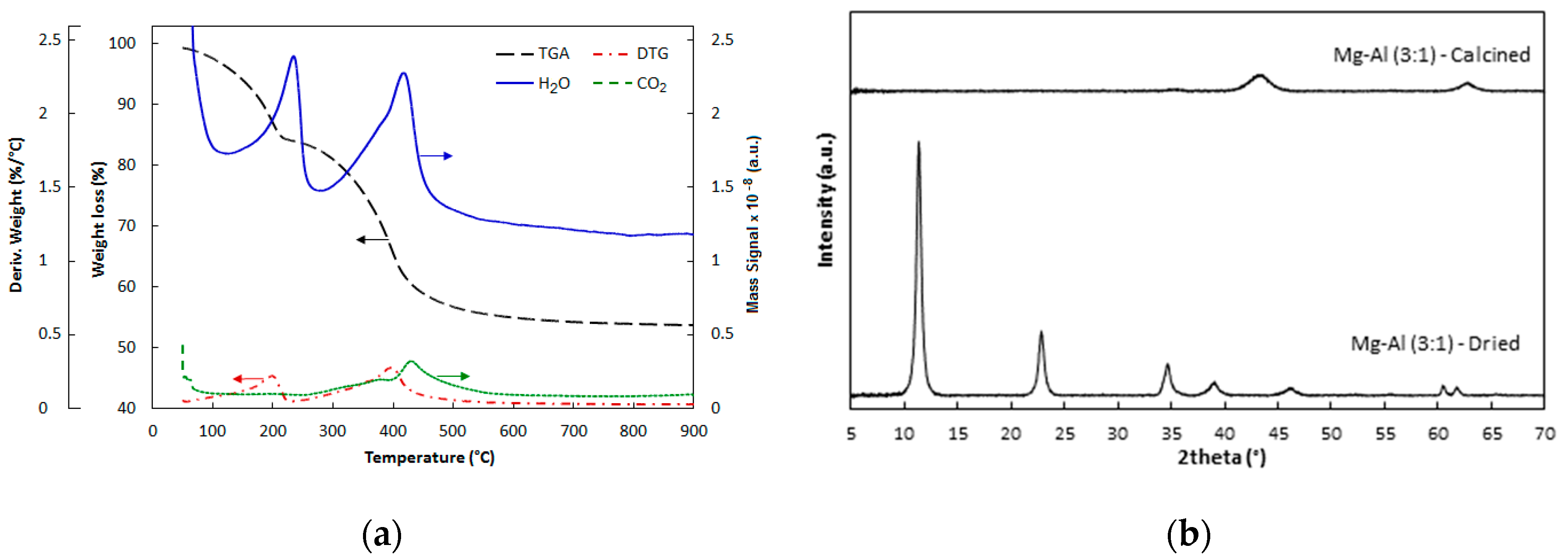
2.1. Catalyst Characterization
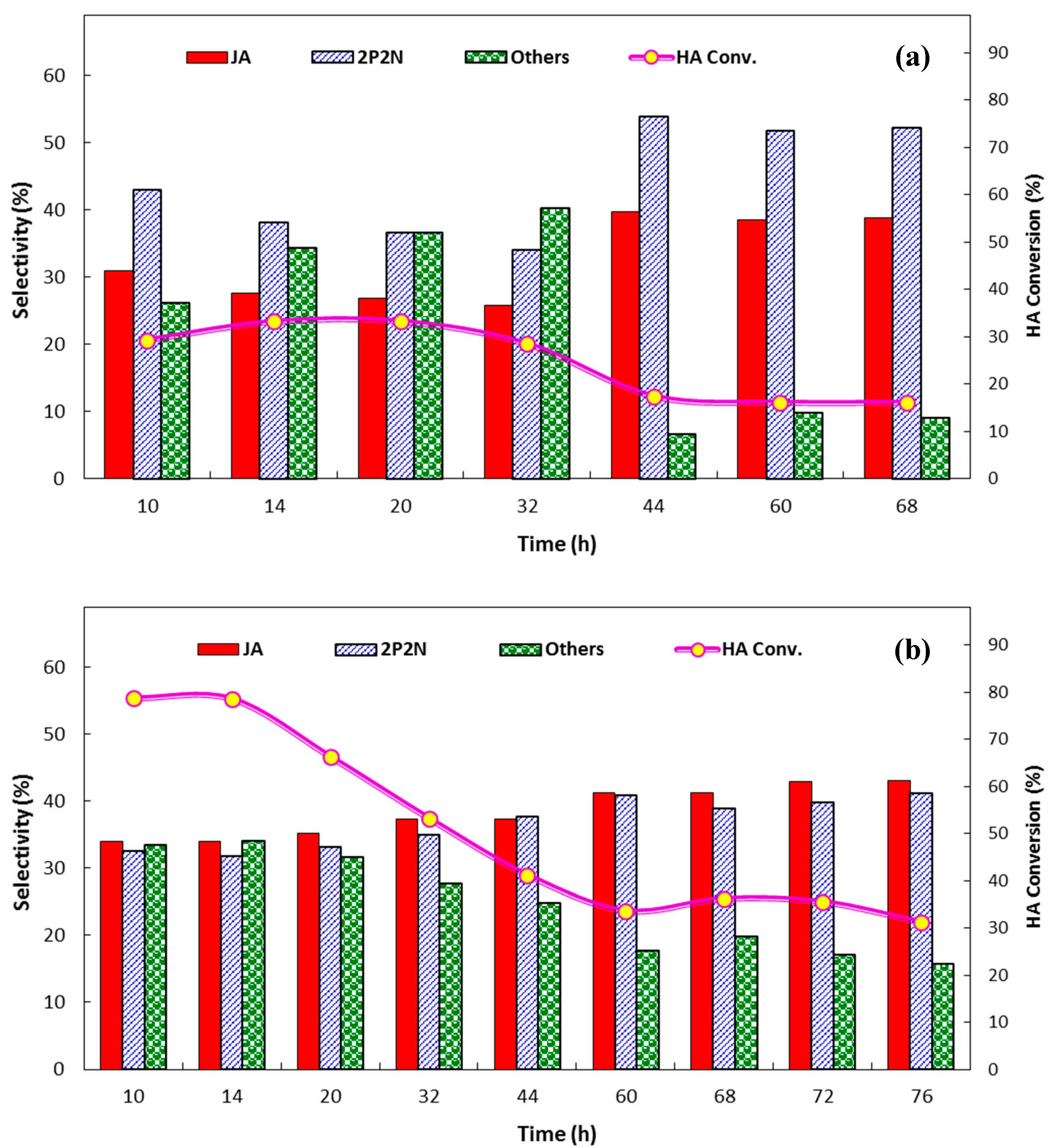
2.2. Catalytic Performance for the Aldol Condensation Reaction
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Evaluation of Catalyst
3.3. Characterization of Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Srinivasan, K.; Manayil, J.C.; Antonyraj, C.A. Chapter 11—Heterogeneous catalysis for perfumery chemicals. In Industrial Catalytic Processes for Fine and Specialty Chemicals; Joshi, S.S., Ranade, V.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 463–508. [Google Scholar] [CrossRef]
- Fortineau, A.-D. Chemistry perfumes your daily life. J. Chem. Educ. 2004, 81, 45. [Google Scholar] [CrossRef]
- Fan, A. Acid–base bifunctional magnesium oxide catalyst prepared from a simple hydrogen peroxide treatment for highly selective synthesis of jasminaldehyde. Energy Source Part A 2019, 42, 2501–2515. [Google Scholar] [CrossRef]
- Vrbková, E.; Tišler, Z.; Vyskočilová, E.; Kadlec, D.; Červený, L. Aldol condensation of benzaldehyde and heptanal: A comparative study of laboratory and industrially prepared Mg-Al mixed oxides. J. Chem. Technol. Biotechnol. 2018, 93, 166–173. [Google Scholar] [CrossRef]
- Tišler, Z.; Vrbková, E.; Kocík, J.; Kadlec, D.; Vyskočilová, E.; Červený, L. Aldol condensation of benzaldehyde and heptanal over zinc modified mixed Mg/Al oxides. Catal. Lett. 2018, 148, 2042–2057. [Google Scholar] [CrossRef]
- Vrbková, E.; Vyskočilová, E.; Červený, L. Potassium modified alumina as a catalyst for the aldol condensation of benzaldehyde with linear C3–C8 aldehydes. React. Kinet. Mech. Catal. 2017, 121, 307–316. [Google Scholar] [CrossRef]
- Hamza, A.; Nagaraju, N. Amorphous metal-aluminophosphate catalysts for aldol condensation of n-heptanal and benzaldehyde to jasminaldehyde. Chin. J. Catal. 2015, 36, 209–215. [Google Scholar] [CrossRef]
- Sharma, S.K.; Parikh, P.A.; Jasra, R.V. Eco-friendly synthesis of jasminaldehyde by condensation of 1-heptanal with benzaldehyde using hydrotalcite as a solid base catalyst. J. Mol. Catal. A Chem. 2008, 286, 55–62. [Google Scholar] [CrossRef]
- Heynderickx, P.M. Activity coefficients for liquid organic reactions: Towards a better understanding of true kinetics with the synthesis of jasmin aldehyde as showcase. Int. J. Mol. Sci. 2019, 20, 3819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adwani, J.H.; Khan, N.-H.; Shukla, R.S. An elegant synthesis of chitosan grafted hydrotalcite nano-bio composite material and its effective catalysis for solvent-free synthesis of jasminaldehyde. RSC Adv. 2015, 5, 94562–94570. [Google Scholar] [CrossRef]
- Sharma, S.K.; Patel, H.A.; Jasra, R.V. Synthesis of jasminaldehyde using magnesium organo silicate as a solid base catalyst. J. Mol. Catal. A Chem. 2008, 280, 61–67. [Google Scholar] [CrossRef]
- Sharma, S.K.; Parikh, P.A.; Jasra, R.V. Reconstructed Mg/Al hydrotalcite as a solid base catalyst for synthesis of jasminaldehyde. Appl. Catal. A Gen. 2010, 386, 34–42. [Google Scholar] [CrossRef]
- Yadav, G.D.; Aduri, P. Aldol condensation of benzaldehyde with heptanal to jasminaldehyde over novel Mg–Al mixed oxide on hexagonal mesoporous silica. J. Mol. Catal. A Chem. 2012, 355, 142–154. [Google Scholar] [CrossRef]
- Abbaspourrad, A.; Kalbasi, R.J.; Xiao, Q. Highly selective aldol condensation using amine-functionalized SiO2-Al2O3 mixed-oxide under solvent-free condition. Chin. J. Chem. 2010, 28, 2074–2082. [Google Scholar] [CrossRef]
- Wong, S.; Deekamwong, K.; Wittakayun, J.; Ling, T.; Muzara, O.; Lee, H.; Adam, F.; Ng, E. Nanocrystalline KF zeolite from rice husk silica as an eco-friendly solid base catalyst for the synthesis of jasminaldehyde under microwave irradiation. Sains Malays. 2018, 47, 337–345. [Google Scholar] [CrossRef]
- Kadlec, D.; Tišler, Z.; Velvarská, R.; Pelíšková, L.; Akhmetzyanova, U. Comparison of the properties and catalytic activity of commercially and laboratory prepared Mg/Al mixed oxides in aldol condensation of cyclohexanone with furfural. React. Kinet. Mech. Cat. 2019, 126, 219–235. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Lauwaert, J.; Van Der Voort, P.; Verberckmoes, A. Recent advances on the utilization of layered double hydroxides (LDHs) and related heterogeneous catalysts in a lignocellulosic-feedstock biorefinery scheme. Green Chem. 2017, 19, 5269–5302. [Google Scholar] [CrossRef] [Green Version]
- Kuśtrowski, P.; Chmielarz, L.; Bożek, E.; Sawalha, M.; Roessner, F. Acidity and basicity of hydrotalcite derived mixed Mg-Al oxides studied by test reaction of MBOH conversion and temperature programmed desorption of NH3 and CO2. Mater. Res. Bull. 2004, 39, 263–281. [Google Scholar] [CrossRef]
- Kloproggea, J.T.; Kristófb, J.; Frosta, R.L. Thermogravimetric analysis-mass spectrometry (TGA-MS) of hydrotalcites containing CO32−, NO3−, Cl−, SO42− or ClO4. Clay Odyssey 2001, 1, 451. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, S.G.; Lee, K.B. High-temperature CO2 sorption on hydrotalcite having a high Mg/Al molar ratio. ACS Appl. Mater. Int. 2016, 8, 5763–5767. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, W.; Wang, Y.; Tao, N.; Cai, H.; Liu, J.; Lv, J. Mg-Al mixed oxide derived from hydrotalcites prepared using the solvent-free method: A stable acid–base bifunctional catalyst for continuous-flow transesterification of dimethyl carbonate and ethanol. Ind. Eng. Chem. Res. 2020, 59, 5591–5600. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Ni, H.; Liu, D.; Sun, Z.; Li, P.; Yu, J. Enhancement of CO2 adsorption performance on hydrotalcites impregnated with alkali metal nitrate salts and carbonate salts. Ind. Eng. Chem. Res. 2020, 59, 6043–6052. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Zhou, F.; Yang, B.; Alshammari, A.S.; Deng, Y. Catalytic alcoholysis of urea to diethyl carbonate over calcined Mg-Zn-Al hydrotalcite. RSC Adv. 2015, 5, 19534–19540. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Duraczyska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni-Mg-Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Aliç, F.; Verberckmoes, A.; Van Der Voort, P. Tuning the acidic–basic properties by Zn-substitution in Mg-Al hydrotalcites as optimal catalysts for the aldol condensation reaction. J. Mater. Sci. 2017, 52, 628–642. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, K.K.; Gray, M.; Job, H.; Santosa, D.; Li, X.S.; Devaraj, A.; Karkamkar, A.; Wang, Y. Role of calcination temperature on the hydrotalcite derived MgO–Al2O3 in converting ethanol to butanol. Top. Catal. 2016, 59, 46–54. [Google Scholar] [CrossRef]
- Vrbková, E.; Vyskočilová, E.; Krupka, J.; Červený, L. Aldol condensation of benzaldehyde with heptanal using solid-supported caesium and potassium catalysts. Prog. React. Kinet. Mech. 2016, 41, 289–300. [Google Scholar] [CrossRef]
- Vashishtha, M.; Mishra, M.; Shah, D.O. A novel approach for selective cross aldol condensation using reusable NaOH-cationic micellar systems. Appl. Catal. A Gen. 2013, 466, 38–44. [Google Scholar] [CrossRef]

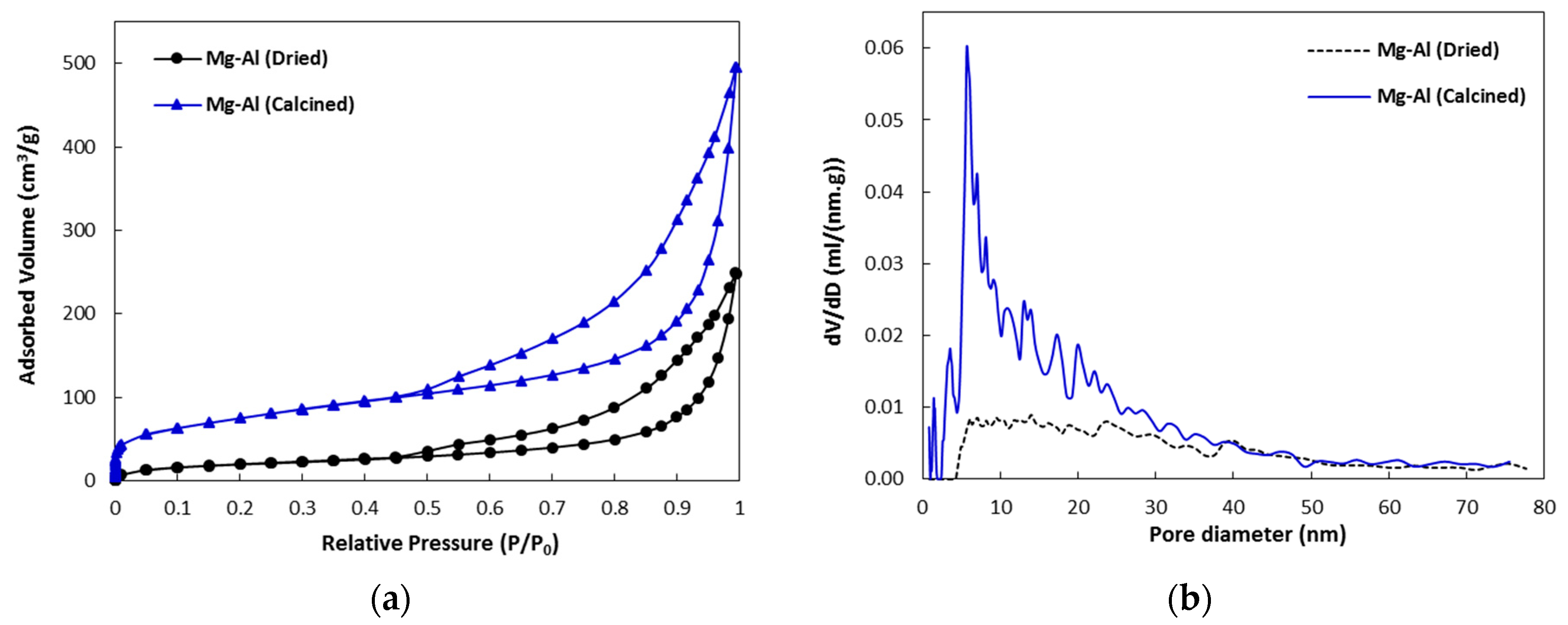


| Sample | Isotherm Type | Hysteresis | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|
| Mg-Al (Dried) | IV | H3 | 73.3 | 0.308 |
| Mg-Al (Calcined) | IV | H3 | 241.9 | 0.654 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, Z.; Tišler, Z.; Vondrová, P.; Velvarská, R.; Štěpánek, K. Solvent-Free Synthesis of Jasminaldehyde in a Fixed-Bed Flow Reactor over Mg-Al Mixed Oxide. Catalysts 2020, 10, 1033. https://doi.org/10.3390/catal10091033
Gholami Z, Tišler Z, Vondrová P, Velvarská R, Štěpánek K. Solvent-Free Synthesis of Jasminaldehyde in a Fixed-Bed Flow Reactor over Mg-Al Mixed Oxide. Catalysts. 2020; 10(9):1033. https://doi.org/10.3390/catal10091033
Chicago/Turabian StyleGholami, Zahra, Zdeněk Tišler, Pavla Vondrová, Romana Velvarská, and Kamil Štěpánek. 2020. "Solvent-Free Synthesis of Jasminaldehyde in a Fixed-Bed Flow Reactor over Mg-Al Mixed Oxide" Catalysts 10, no. 9: 1033. https://doi.org/10.3390/catal10091033
APA StyleGholami, Z., Tišler, Z., Vondrová, P., Velvarská, R., & Štěpánek, K. (2020). Solvent-Free Synthesis of Jasminaldehyde in a Fixed-Bed Flow Reactor over Mg-Al Mixed Oxide. Catalysts, 10(9), 1033. https://doi.org/10.3390/catal10091033