Abstract
The selective catalytic reduction (SCR) has been widely used in industrial denitrification owing to its high denitrification efficiency, low operating costs, and simple operating procedures. However, coal containing a large amount of sulfur will produce SO2 during combustion, which makes the catalyst easy to be deactivated, thus limiting the application of this technology. This review summarizes the latest NH3-SCR reaction mechanisms and the deactivation mechanism of catalyst in SO2-containing flue gas. Some strategies are summarized for enhancing the poison-resistance through modification, improvement of support, the preparation of complex oxide catalyst, optimizing the preparation methods, and acidification. The mechanism of improving sulfur resistance of catalysts at low temperatures is summarized, and the further development of the catalyst is also prospected. This paper could provide a reference and guidance for the development of SO2 resistance of the catalyst at low temperatures.
1. Introduction
Nitrogen oxide (NOx) is a general term composed of nitrogen, oxygen, and other compounds. It is one of the major pollutants from the exhaust gas of thermal power plants, industrial furnaces, motor vehicles, ship exhaust emissions, and includes N2O, NO, NO2, etc.—among which NO and NO2 account for the largest proportion [1]. A large amount of NOx emitted into the air will cause a series of environmental concerns. Coal matters a lot in the energy consumption of China, which is the largest source of NOx, accounting for 67% of the total NOx emissions in China. Among all coal-fired industries, thermal power plants have the largest NOx emission, the NOx emission standard of which is 100 mg/Nm3 [2]. Therefore, exploring and developing efficient exhaust gas deNOx technology has been an area of intense investigation.
Among all flue gas denitrification technologies, selective catalytic reduction (SCR) is an extensively applied technology due to its low reaction temperature and high denitrification efficiency [3,4]. Selective catalytic reduction (SCR) mainly refers to the reaction of NOx using NH3 as a reducing agent in the presence of O2 to produce pollution-free N2 and H2O, whose core is the catalyst. The main reaction equations are (1)–(3) [5].
V2O5/TiO2 catalyst is a mature and typical catalyst which has a high denitrification efficiency and has been commercialized for a long time. However, this catalyst still has some problems, such as low selectivity of N2 at high temperatures and a narrow reaction temperature window 300 °C–400 °C. SO2 can be effortlessly converted to SO3 in the smoke, resulting in catalyst deactivation [6]. Additionally, vanadium oxide is toxic and easy to cause secondary pollution and other environmental problems. On the basis of original catalyst, the new V2O5/TiO2 catalyst—adding WO3 and MoO2 as the promoter—has solved some problems, but there are still some pivotal challenges such as low catalytic activity at low temperatures and poor performance for the improved catalyst [7]. Besides, there is no room for a denitration device between the air preheater and economizer in the built thermal power plants in China, which makes the flue gas temperature lower after treatment. In order to meet the use conditions of conventional catalysts, the flue gas must be heated, which increases the cost of flue gas treatment. As another main source of flue gas emission, industrial combustion boilers and industrial furnaces have lower flue gas emission temperatures, which makes the existing SCR catalyst difficult to meet the use requirements. The working temperature of the low-temperature SCR catalyst is 150 °C–300 °C or lower. The position of the reactor is behind the dust removal and desulfurization device, which can effectively avoid the toxic effect of dust and high concentration SO2 on the catalyst. Therefore, the research and development of SCR catalysts at low temperatures have been brought to the fore by data as the rapid rise of increasingly severe NOx emission standards.
2. SO2 Poisoning Mechanism of Low-Temperature Catalyst
At present, some power plants adopt wet desulfurization to remove SO2 with lower flue gas temperature, failing to meet the reaction requirements of V2O5/TiO2 catalyst. Therefore, there are many drawbacks such as low denitrification efficiency and catalyst waste. After desulfurization, tiny amounts of SO2 still exist in the exhaust gas, bringing about the deactivation of SCR catalyst. Therefore, developing a vanadium-free catalyst with great denitrification performance and sulfur and water resistance at low temperatures is extremely necessary [8]. To solve the sulfur poisoning of catalysts, many scholars have done a lot of research and elaborated on the poisoning mechanism in detail. Pan et al. [9] studied the deactivation mechanism of MnOx/MWCNTs catalyst at low temperatures. The MnSO4 was formed by the reaction between the active components Mn and SO2 under the condition with O2, which not only decreased the content of the active site but also slowed down the reaction process. On the other hand, the deposition such as (NH4)2SO4 and NH4HSO4 formed by the reaction between SO2 and NH3 would block the active sites and reduce the specific surface area. In addition, the intermediate substances such as N2O4 and nitrosyl (NO–) would be produced by the adsorbed NO species and further participate in the reaction. However, SO2 could compete for adsorption sites with NOx on the catalyst surface sharply and had the momentous inhibition influence on the formation of N2O4 and nitroso, leading to the reduction of NOx conversion rate. These two substances were the intermediate medium and there was a major role to play in the SCR reaction. Jiang et al. [10] synthesized Fe–Mn/TiO2 catalyst and explored its SCR performance systematically. NO complexes, monodentate nitrate, and bidentate nitrate would be found after NO and O2 reacted for 30 min without SO2, but these substances were replaced by sulfate when SO2 was introduced, which also indicated that SO2 could compete for adsorption sites with NO on the catalyst surface, leading to the disappearance of intermediates and reducing reaction efficiency. A similar SO2 poisoning mechanism was also obtained in the study of Xu et al. [11] on Ce/TiO2 catalyst and Zhang et al. [12] on Cu-SAPO-34 catalyst.
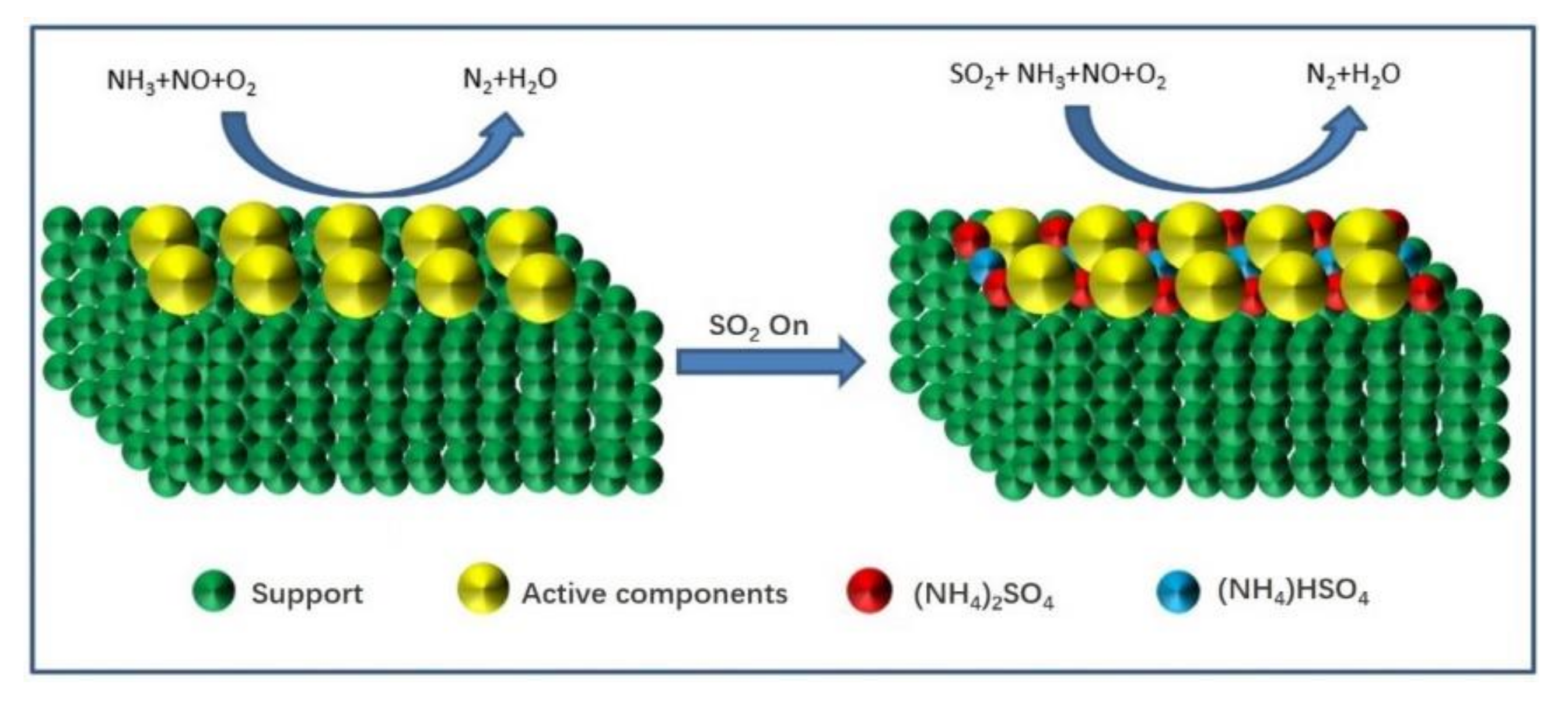
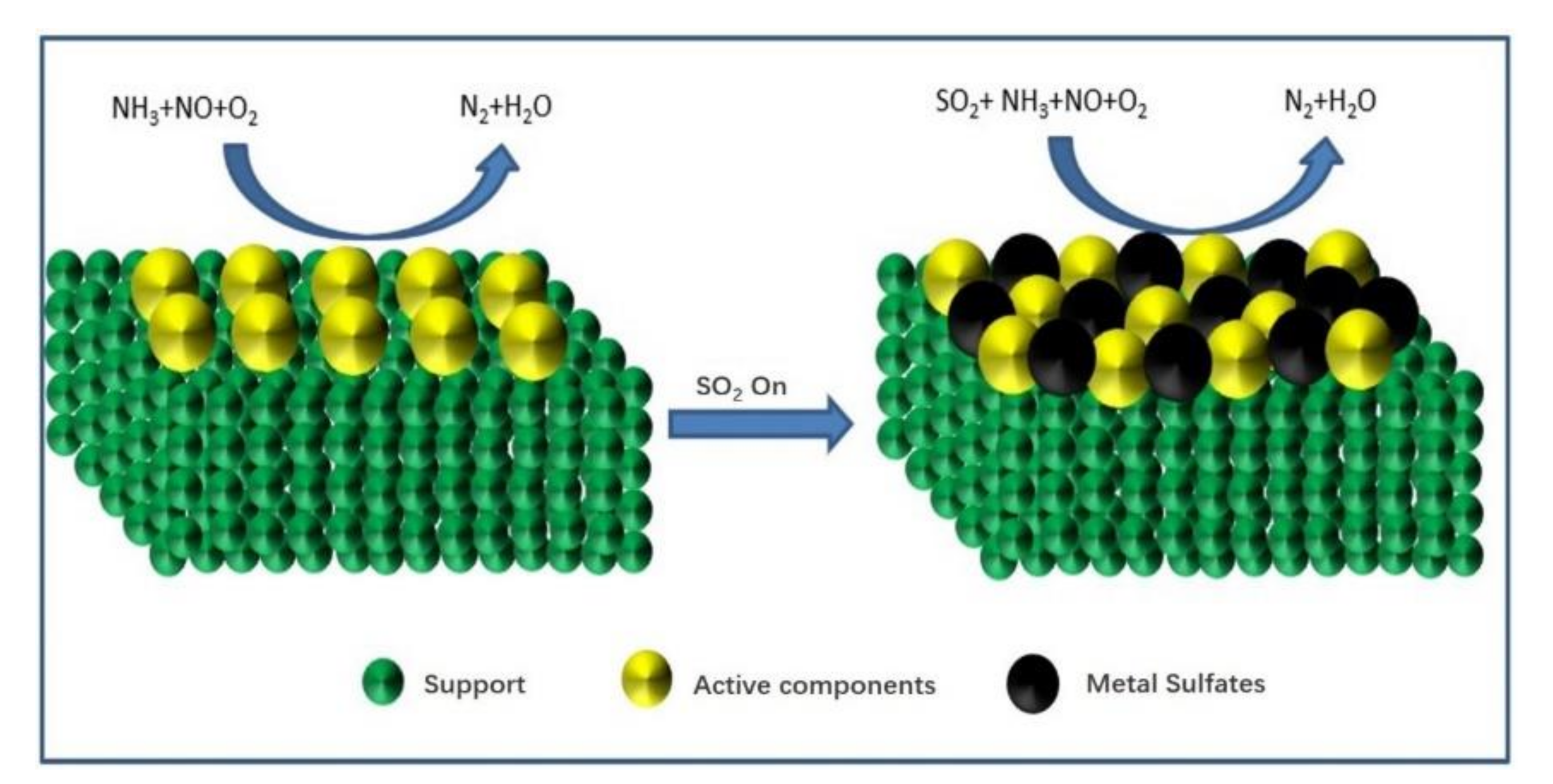
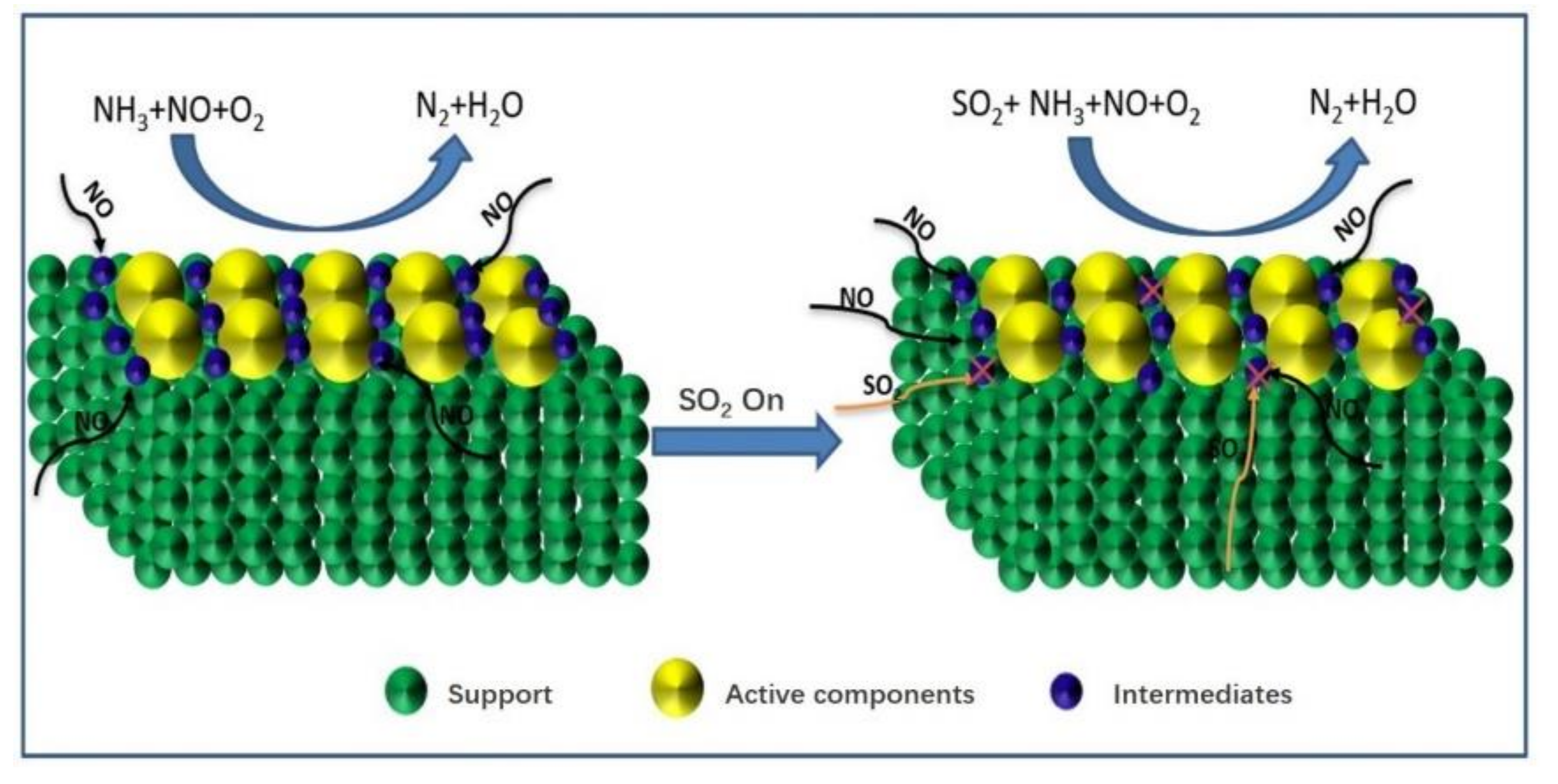
From the above studies, the SO2 deactivation mechanism on the catalyst at low temperatures can be observed mainly in the following three aspects. (1) The ammonium sulfate and ammonium bisulfate are formed by the reaction of SO2 and NH3 in the presence of O2 and attach to the catalyst surface, which can decrease the surface area, pore volume, and pore size of the catalyst, and then reduce the reaction rate. However, ammonium sulfate and ammonium bicarbonate will self-decompose when the NH3-SCR reaction is carried out above 280 °C and 350 °C, respectively, so the catalytic activity is able to be restored by the washing method at low temperatures [13]. (2) In the presence of O2, SO2 will react with the active component (mainly transition metal) on the catalyst surface to generate metal sulfate salt, which will cause irreversible deactivation of the catalyst. (3) SO2 will compete with NO at the adsorption sites on the catalyst surface when these acidic gases are present in the reaction system, which would reduce the formation of SCR intermediate products and the catalytic efficiency of catalyst. Figure 1, Figure 2 and Figure 3 show the mechanism of catalyst sulfur poisoning.
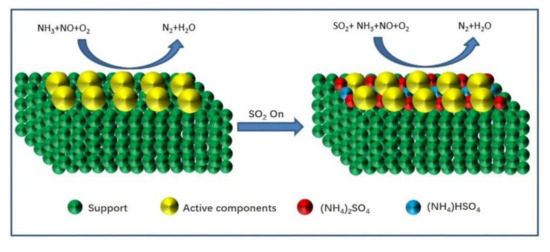
Figure 1.
The formation process of (NH4)2SO4 and NH4HSO4.
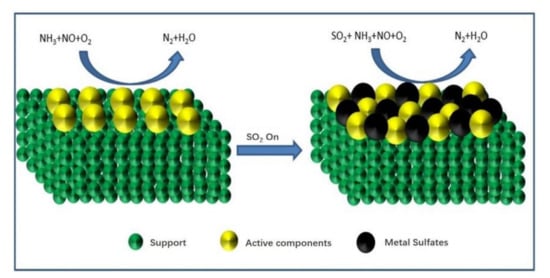
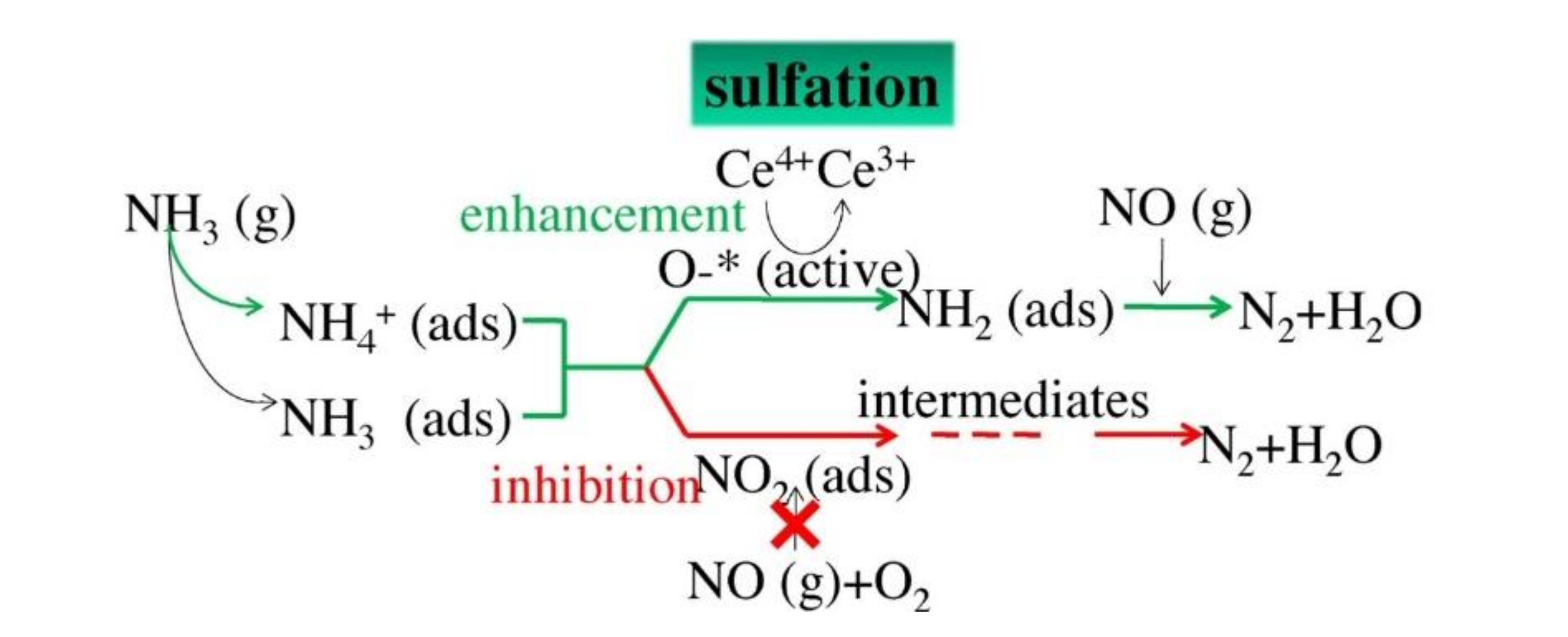
Figure 2.
Sulfation of active components.
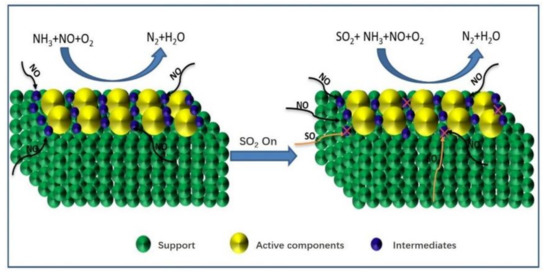
Figure 3.
Competitive adsorption of NO and SO2.
3. Research Progress of SO2 Resistance Catalyst at Low Temperatures
Catalyst is usually composed of active component and support, and there are other forms of catalysts such as composite oxide catalysts. To enhance the low-temperature sulfur resistance of catalysts, many scholars have focused their attention on the improvement of active components and supports. In addition, some scholars have found that the preparation method—the handling catalyst by acidification and reaction conditions—can make a difference in the SO2 resistance of catalyst.
3.1. Effects of Active Components
The active component, which is composed of one or more substances, is the main unit of catalyst and affects the NH3-SCR reaction significantly. Using rare earth metals as well as transition metal oxides to improve active components is one of the most effective methods to improve sulfur resistance at low temperatures.
3.1.1. Ce-Modified Catalysts
Cerium has an excellent ability to store and release O2. The electron transfer between Ce4+ and Ce3+ is favorable for catalyst to form reactive oxygen species, promoting the conversion of NO into NO2. Besides, the catalyst modified with Ce can increase the content of acidic sites and reduce the oxidizability of catalyst in some extent [14,15]. SO2 would preferentially react with CeO2 on the surface of catalyst to form Ce2(SO4)3 in the presence of O2 and H2O, which can reduce the reaction between NH3 and SO2 to produce ammonium sulfate and ammonium bisulfate, and inhibit the catalyst deactivation [16].
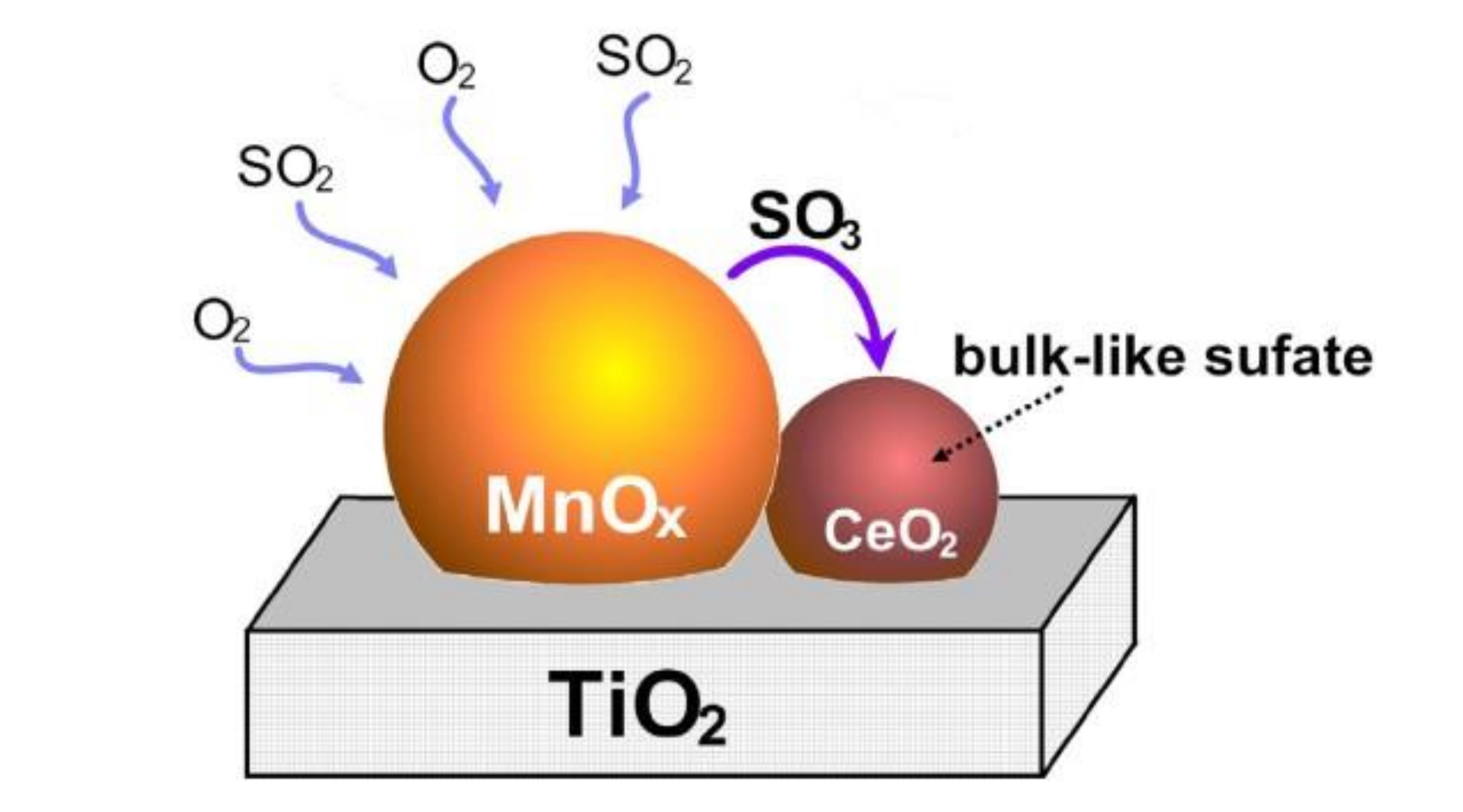
Wei et al. [17] studied the Mn/TiO2 catalyst modified by Ce and the sulfur resistance was tested. The results demonstrated that the surface of Ce-doped catalyst retained the Lewis acid sites and produced new Brønsted acid sites. After doping with Ce, SO2 was preferentially adsorbed on Ce in the form of sulfate instead of the Lewis acid sites and Brønsted acid sites on MnOx, which were the active sites of Mn–Ce/TiO2 catalyst. Ammonium sulfate, ammonium hydrogen sulfate, and titanium sulfate on the surface of the catalyst were also inhibited to form, which improved the sulfur resistance of catalyst. Jin et al. [18] also explored the CeO2-modified Mn–Ce/TiO2 catalyst. It was found that SO2 would react with O2 to produce SO3, which reacted with CeO2 preferentially and reduced the sulfation of active component MnOx. It meant that CeO2 can act as a catalyst SO2 collector, which can limit the sulfation of the active component (Figure 4).
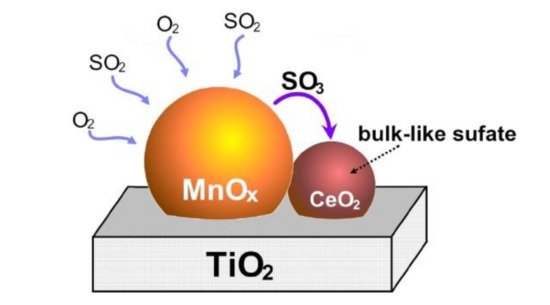
Figure 4.
The sulfation mechanism of Ce-modified Mn–Ce/TiO2 catalyst [18].
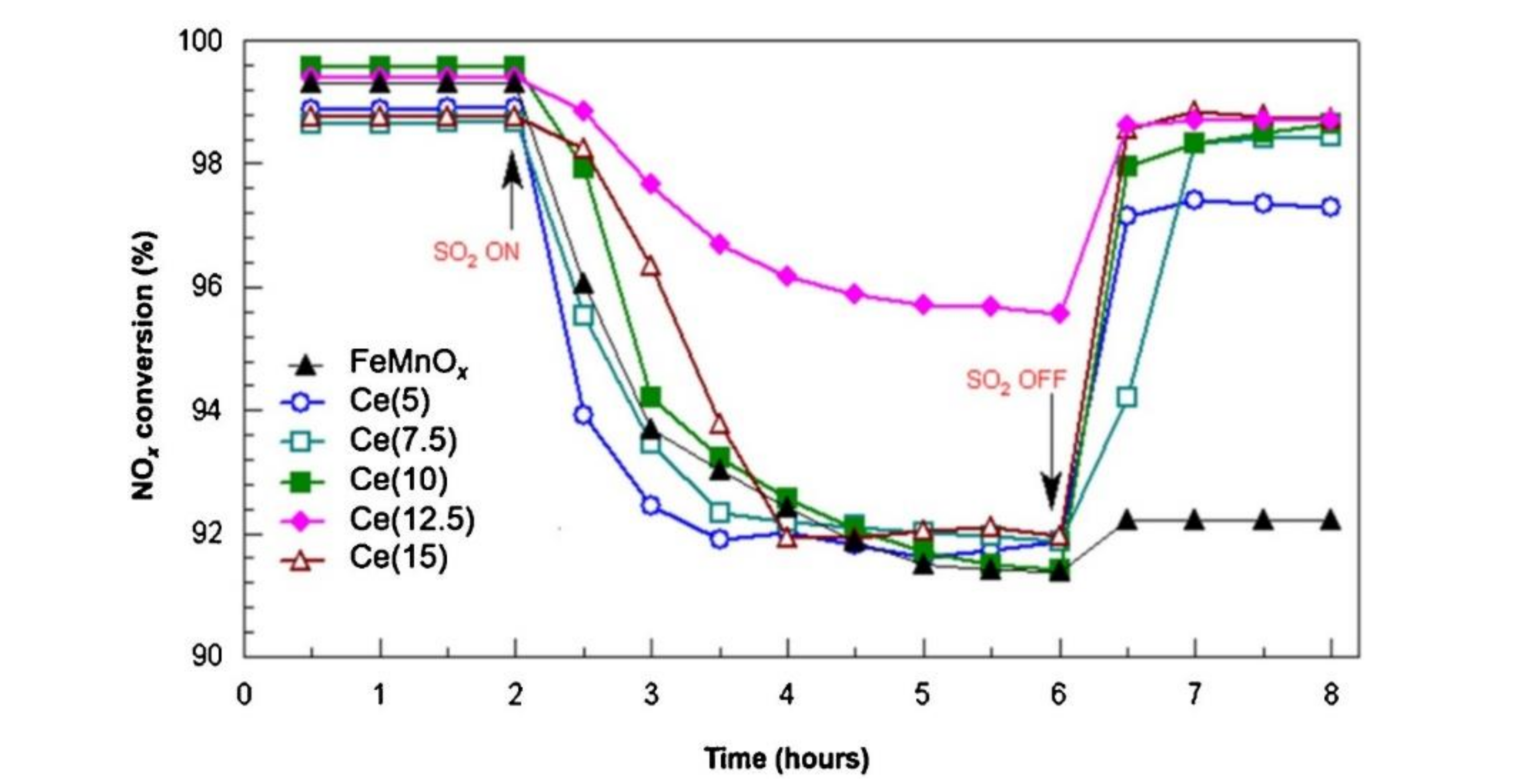
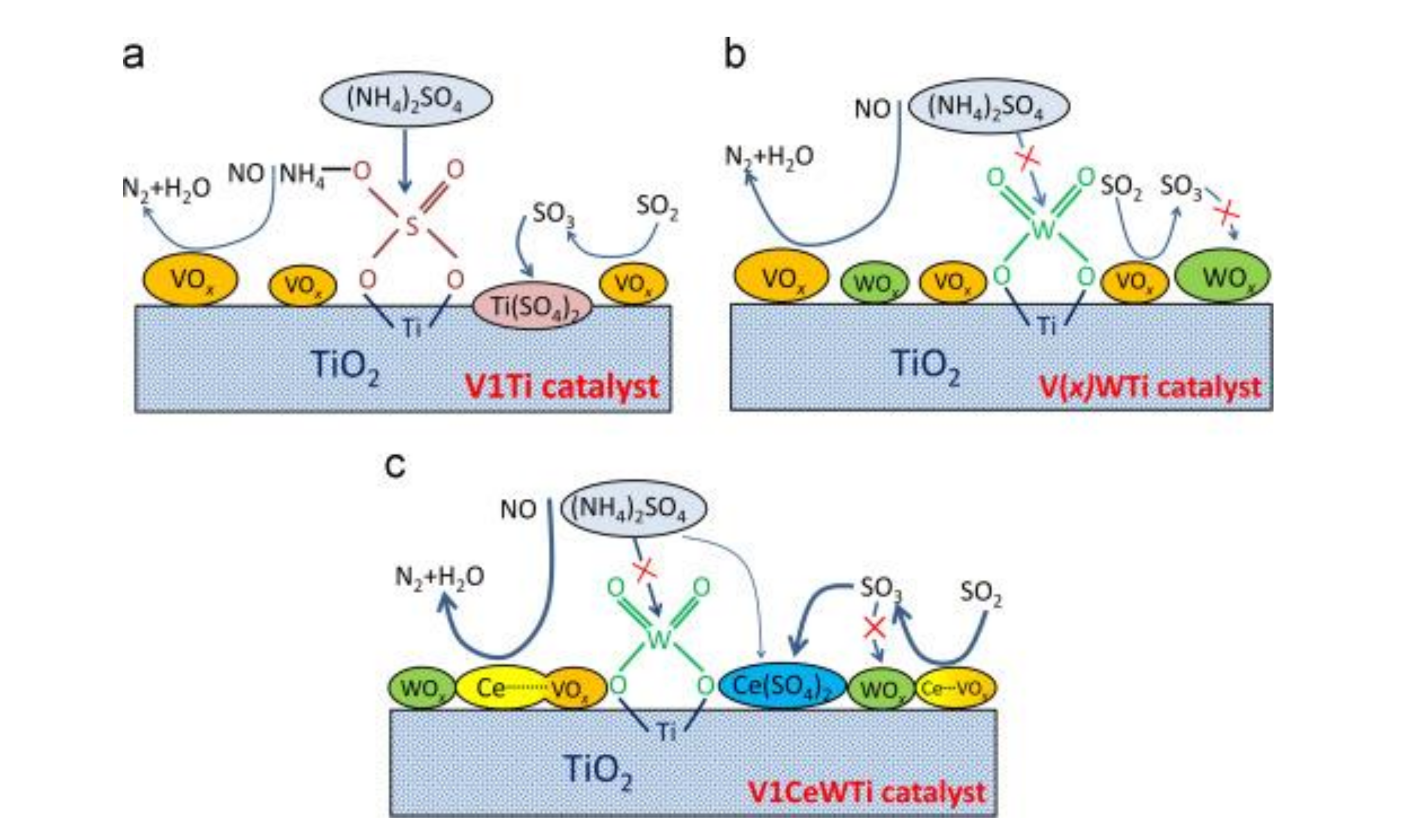
The V2O5–WO3/CeO2–TiO2 catalyst prepared by coprecipitation method showed the best low-temperature catalytic efficiency and sulfur tolerance when the ratio of Ce/Ti was 0.1. Ce modification could not only increase the surface area and adsorbed oxygen but also strengthen the interaction between Ce and Ti [19]. The research of Lee et al. [20] revealed that the Sb–V2O5/TiO2 catalyst with 10% CeO2 could markedly strengthen the catalytic performance and sulfur resistance of catalyst at 220 °C–550 °C. XRD test showed that active components were more evenly distributed on Sb-V2O5/TiO2 catalyst than those on CeO2/TiO2 catalyst. Adding CeO2 not only enhanced the acidity of the catalyst but also reduced SO2 adsorption due to the rejection to SO2, further slowing down the effect of SO2 poisoning. The research of France et al. [15] indicated that compared with the original catalyst, the catalyst modified by the CeO2 could increase the amount of the chemical adsorption of oxygen on the surface, which could increase the rate of NO oxidation to NO2 and facilitate the rapid response of NH3-SCR. In the presence of SO2, the catalytic efficiency of catalyst decreased slightly, while the catalyst could nearly return to the initial catalytic state after SO2 was stopped (Figure 5). Based on the V2O5/TiO2 catalyst, Ma et al. [21] added WO3 and CeO2 to it and considered the SO2 poisoning of the catalysts at low temperatures. CeO2 doping improved the redox ability while inhibiting the formation and deposition of (NH4)2SO4 on the catalyst surface. Figure 6 showed the low-temperature deactivation mechanism of the catalyst under the condition with SO2.
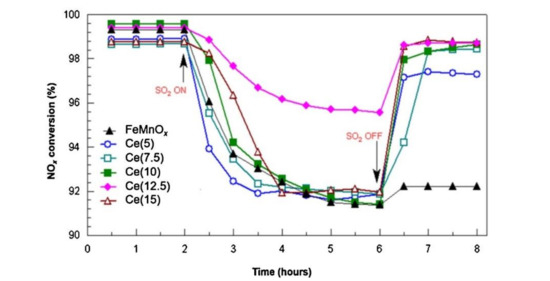
Figure 5.
Influence of SO2 on NOx conversion of FeMnOx and Ce(y) catalysts. Reaction conditions: [NO] = [NH3] = 0.1%; [SO2] = 100 ppm; [O2] = 3%; N2 balance, GHSV = 30,000 h−1; reaction temperature = 120 °C [15].
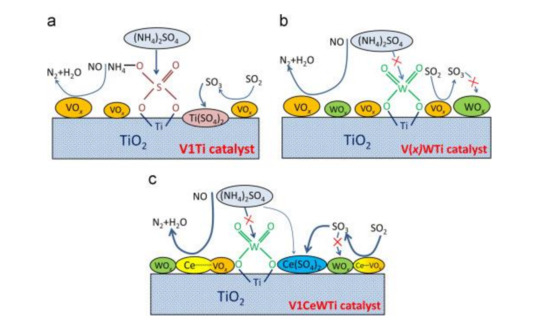
Figure 6.
The mechanism of sulfur poisoning at low temperatures of catalysts (a) V1Ti; (b) V(x)WTi; (c) V1CeWTi [21].
3.1.2. Fe-Modified Catalysts
Fe is also a common and efficient modifier for low-temperature SCR catalyst [22]. Due to its low price, excellent reduction performance, and various chemical values, Fe can be used to improve the active components and support of catalysts, which has also been widely concerned by scholars.
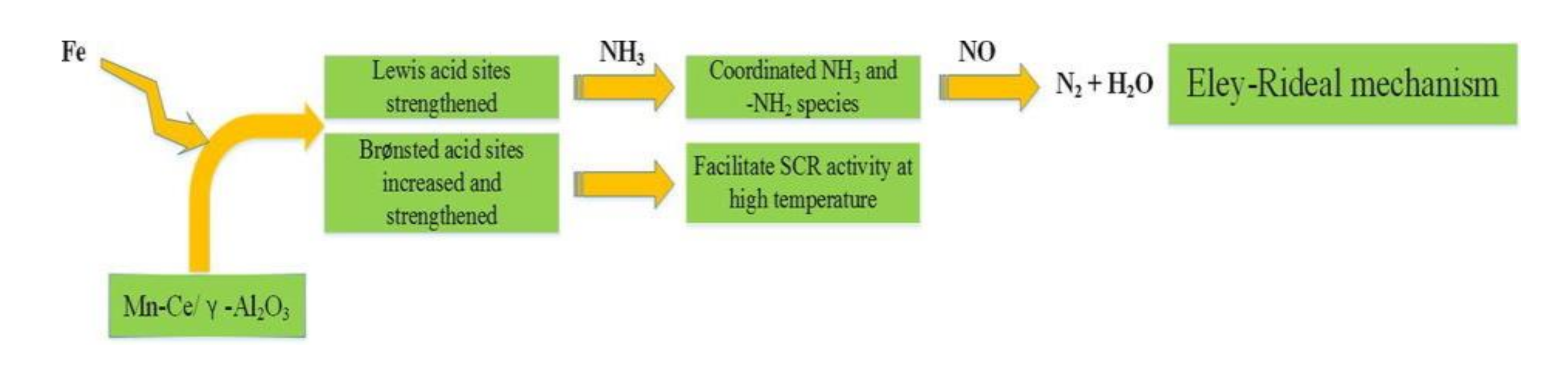
Cao et al. [23] used sol-gel method to synthesize the Fe-doped Mn–Ce/γ-Al2O3 catalyst. Compared with the original catalyst, Fe-modified catalyst could reach 95% conversion efficiency of NOX at 250 °C–350 °C and had excellent sulfur and water resistance. The experiment results demonstrated that the surface area, acid sites, and adsorption capacity of NO, as well as the pore size of the catalyst were increased after Fe was doped, which could enhance the catalytic efficiency and sulfur resistance of catalyst at low temperatures. Lewis acid sites accounted for more than Brønsted acid sites, although the number of Brønsted sites increased with the addition of Fe. According to some research [24], the SCR reaction of the catalyst mainly follows the Eley-Rideal (E-R) mechanism at high temperatures. It means that the adsorbed NH3 and NH4+ species can react with NO to generate N2 and H2O finally. Among them, the NH4+ species is formed by NH3 adsorbed on Brønsted acid sites. Therefore, Brønsted acid sites are favorable for the process of E-R mechanism and further promote the SCR reaction. Figure 7 was a flow chart of the reaction of the Fe-modified enhanced catalyst.
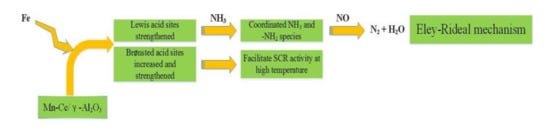
Figure 7.
The reaction flow chart of Fe–Mn–Ce/γ-Al2O3 catalyst [23].
Based on Mn-Ce/TiO2 catalyst, Shen et al. [25] synthesized Fe–Mn–Ce/TiO2 catalyst modified with Fe. It was found that when Fe/Ti = 0.1, Fe–Mn–Ce/TiO2 catalyst could reach 96.8% NOx conversion efficiency at 180 °C as well as wonderful sulfur resistance over the Mn–Ce/TiO2 catalyst on account of the increase of acid sites. Wu et al. [26] studied the V2O5/TiO2 catalyst doped with Fe and found that the Fe–V2O5/TiO2 catalyst could achieve almost 100% conversion efficiency at 270 °C. The addition of Fe promoted the dispersion of active components, which is related to the special surface area, the synergistic effect, etc., of catalyst. With the increase of Fe doping, the BET (Brunauer-Emmett-Teller) surface area also increased. Besides, the synergistic effects between Fe, V2O5, and TiO2 are beneficial to improve the dispersion of active components. Meanwhile, the content of adsorbed oxygen and acid sites increased, improving the activity and sulfur resistance at low temperatures.
3.1.3. Cu-Modified Catalysts
Cu has many advantages such as good thermal conductivity, corrosion resistance, strong chemical stability, and low toxicity. In the field of NH3-SCR, Cu has been studied for its high catalytic activity, multiple recycling, mild temperature, and simple ligand [27].
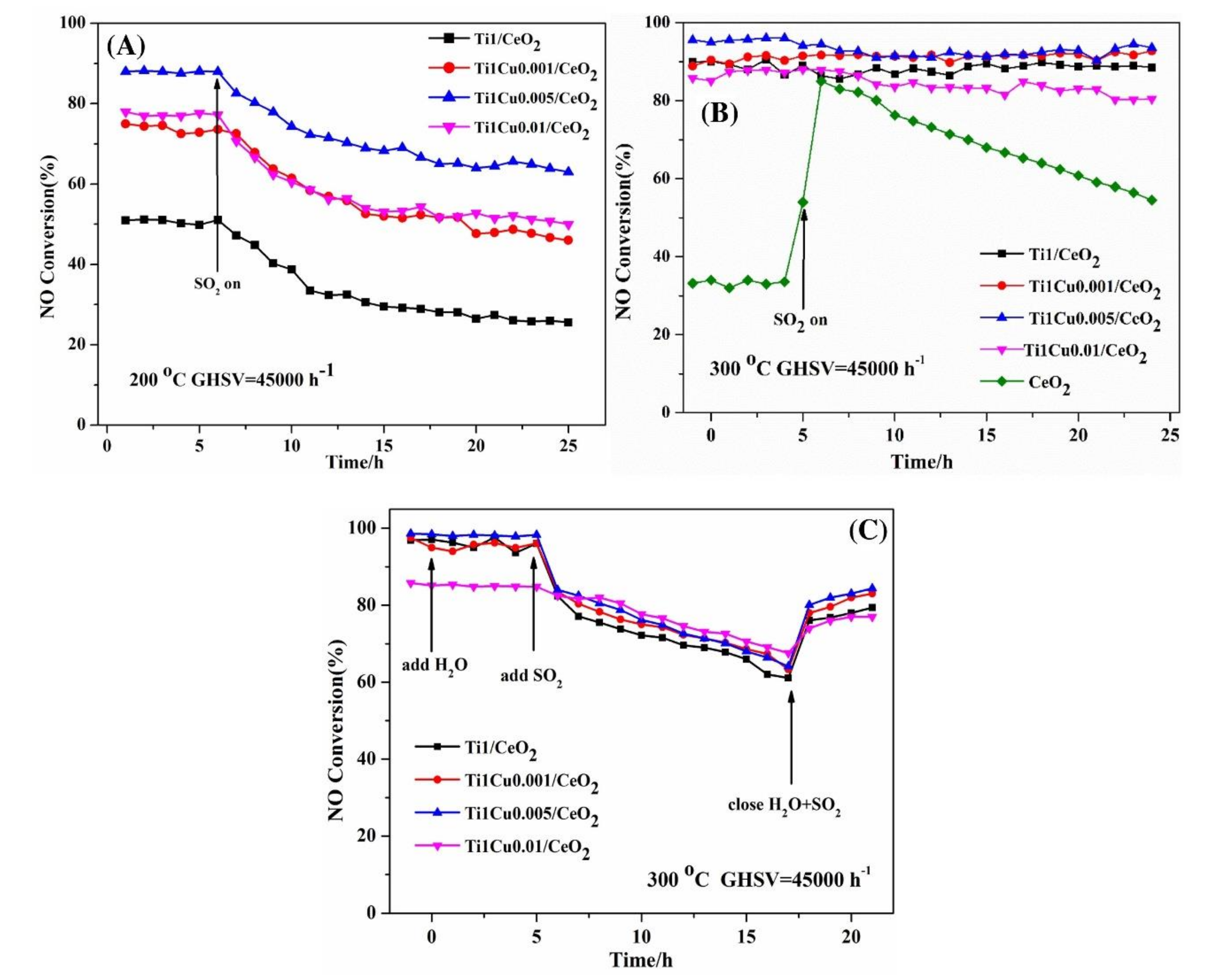
Zhao et al. [28] added Cu, Fe, Mn, and Co to the V2O5/TiO2 catalyst to prepare the modified catalysts by impregnation method. Among all the modified catalysts, the Cu–V2O5/TiO2 catalyst exactly exhibited the best catalytic performance and can reach 90% conversion efficiency of NOx at 225 °C–375 °C. After introducing SO2 and H2O, the catalytic efficiency of catalyst decreased slightly for a long time. Xu et al. [29] explored the low-temperature catalytic performance and sulfur resistance of Cu–Fe/Beta catalyst. Compared with Cu/Beta catalyst and Fe/Beta catalyst, Cu–Fe/Beta catalyst broadened the temperature window and could achieve more than 80% catalytic efficiency at 125 °C–500 °C. Besides the interaction between Cu and Fe, the increasing of Cu2+/Cu+ and Fe3+/Fe2+ was also the main reason for the improvement of activity and sulfur resistance. Li et al. [30] doped light CuO into TiO2/CeO2 catalyst and found the catalytic performance of the TiO2–CuO/TiO2 catalyst was increased obviously. When Cu/Ce = 0.005, TiO2–CuO/TiO2 catalyst exhibited the most excellent catalytic performance and sulfur tolerance, as shown in Figure 8. The addition of CuO produced more adsorbed oxygen and Ce3+ species, which enhanced the reduction performance and surface acidity of TiO2–CuO/TiO2 catalyst.
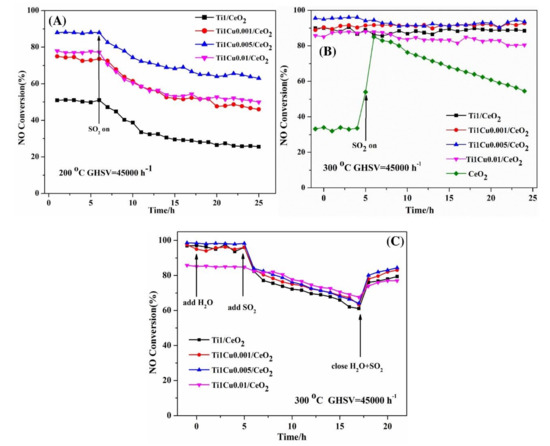
Figure 8.
The H2O and SO2 durability of catalysts (A) SO2 durability over Ti1/CeO2 and Ti1Cuy/CeO2 catalysts at 200 °C (B) SO2 durability over Ti1/CeO2 and Ti1Cuy/CeO2 catalysts at 300 °C. (C) H2O/H2O + SO2 durability over Ti1/CeO2 and Ti1Cuy/CeO2 at 300 °C [30].
3.1.4. W-Modified Catalysts
W has been widely used in conventional commercial catalysts. The addition of WO3 can widen the reaction temperature window of catalyst, increase the content of active substances and acid sites, and effectively promote the SCR activity and SO2 tolerance of catalyst at low temperatures [31].
Chen et al. successfully used impregnation method to synthesize W-modified CeO2/TiO2 catalyst. The experimental results showed that when Ce and W were impregnated together and W content is 6%, CeO2–WO3/TiO2 catalyst can achieve over 90% of the catalytic efficiency at 200 °C–450 °C with excellent sulfur resistance. The addition of W significantly increased the content of Ce3+ and was propitious to the conversion of NO to NO2, thus promoting the rapid reaction of SCR and greatly improving the activity and sulfur resistance at low temperatures [32]. Shan et al. [33] prepared Ce–W–TiO2 catalyst by coprecipitation method. The experiment showed that Ce0.2W0.2TiOx catalyst could achieve over 90% catalytic efficiency at 275 °C–450 °C and had excellent sulfur and water resistance. Besides, Ce0.2W0.2TiOx catalyst could still maintain considerable catalytic activity at high space velocity. The addition of W in Ce0.2W0.2TiOx catalyst produced more acid sites, oxygen vacancies, and active substances, which were conducive to improving the catalytic activity and sulfur resistance at low temperatures. On the basis of Ce/TiO2 catalyst, Jiang et al. [34] used sol-gel method to prepare CeO2–MoO3–WO3/TiO2 catalyst by adding WO3 and MoO3. The results demonstrated that adding W and Mo greatly would produce more active sites and acid sites, which made CMWT catalyst achieve 93.8% catalytic efficiency and showed excellent sulfur and water resistance at 275 °C–450 °C under large space velocity.
3.1.5. Catalysts Modified with Other Metal Elements
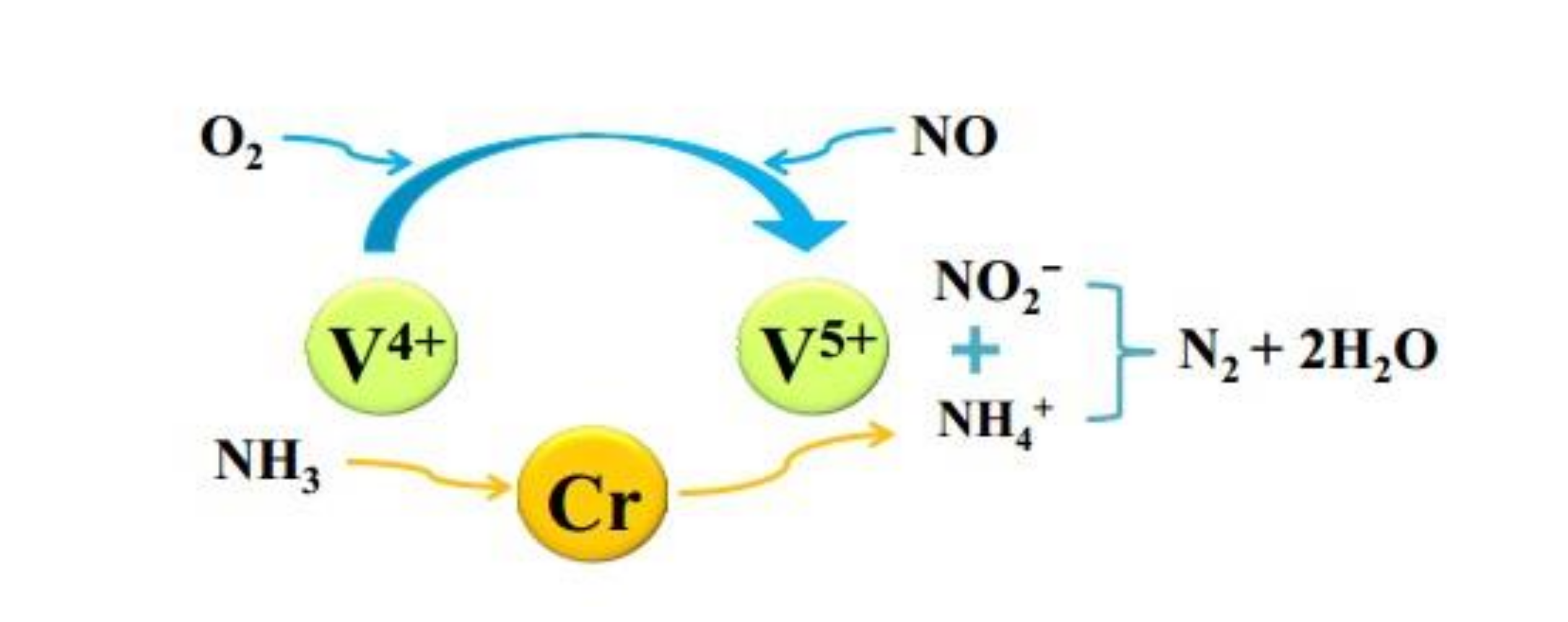
Xu et al. [35] explored the NH3-SCR performance of V2O5–Sb2O3/TiO2 catalyst systematically, which widened the temperature window and showed better sulfur tolerance in comparison to the conventional catalyst. The results demonstrated that adding Sb could slow down the SO2 oxidation in exhaust gas, making the catalyst produce less ammonium sulfate and ammonium bisulfate on the surface when the temperature was low. Yang et al. [36] demonstrated that Cr–V/TiO2 catalyst doped with Cr not only improved the catalytic performance but also improved the low-temperature sulfur tolerance of catalyst. Cr doping could increase the number of acid sites, which inhibited the adsorption of SO2 and promoted the NH3 adsorbed on the catalyst surface. In addition, the surfactant dispersion and the ratio of V4+/V5+ were also improved. Figure 9 was the mechanism of Cr doping improving sulfur resistance of Cr–V/TiO2 catalyst.
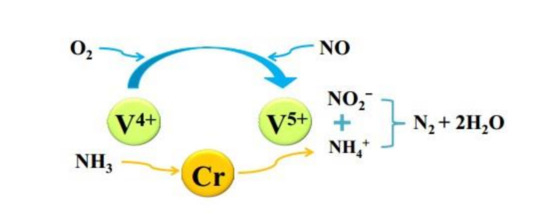
Figure 9.
Mechanism of Cr doping improving sulfur resistance of Cr–V/TiO2 catalyst [36].
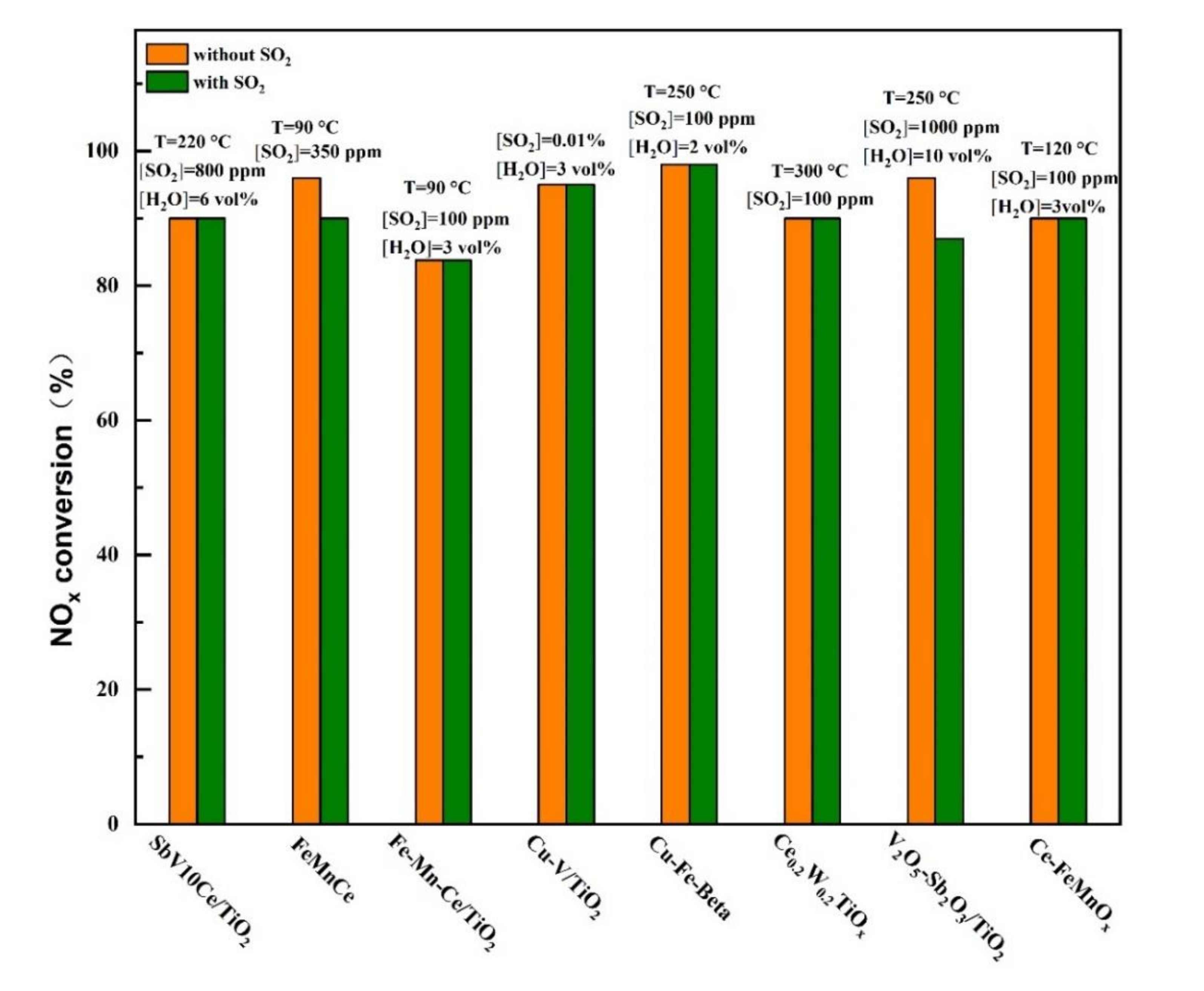
Tian et al. [37] studied the Ca-doped Mn/TiO2 catalyst. It was found that after SO2 was introduced into the catalyst, CaSO4 was preferentially generated by reaction with Ca, which could avoid the sulfation of active components and improve the catalytic activity and sulfur resistance at low temperatures. Figure 10 shows the typical SO2-tolerant modified catalyst at low temperatures for selective catalytic reduction (SCR) reaction.
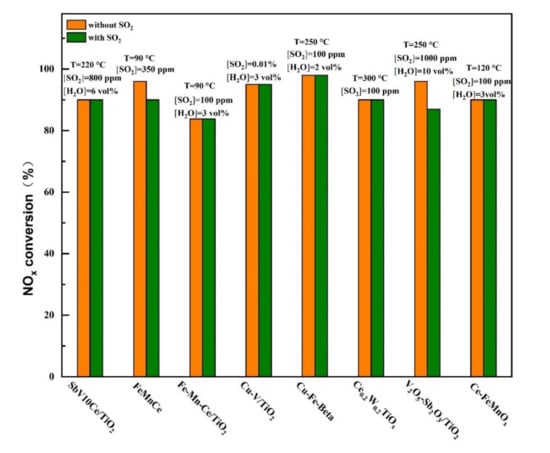
Figure 10.
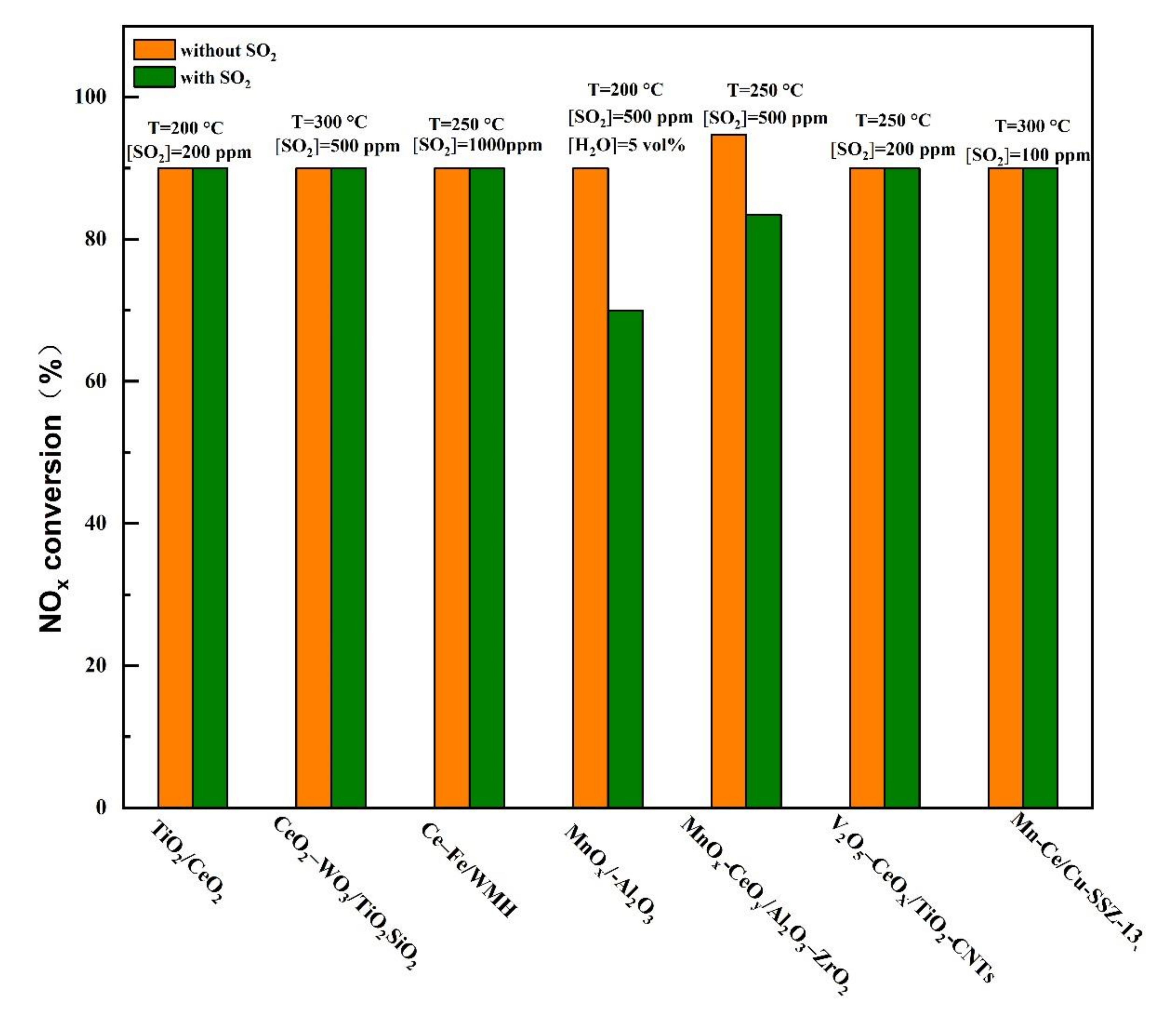
Typical SO2-tolerant modified catalyst at low temperatures for selective catalytic reduction (SCR) reaction [15,20,23,25,28,29,33,35].
Above all, the content of oxygen adsorbed on the active material and catalyst surface—and even the number of acid sites—could be increased by adding Ce, Fe, Cu, W, and others to the active components, which would accelerate the rapid reaction of SCR and increase the catalytic performance of catalyst. Under the reaction conditions containing SO2, these additives can preferentially react with SO2 as the SO2 catchers to avoid the sulfation of active substances. Besides, the stability of NH4HSO4 and (NH4)2SO4 on the surface was reduced, and the low-temperature sulfur tolerance of catalyst was effectively improved. It is believed that transition metals and rare earth elements will have promising applications in improving sulfur resistance of catalyst at low temperatures.
3.2. Effects of Supports
The support of catalyst is of vital importance in the catalytic activity. Loading the active components onto the support contributes to improving the specific surface area, thermal resistance, and mechanical strength of catalyst. Especially, the supports can slow down SO2 poisoning on the catalytic activity of catalyst. As of now, there are TiO2, Al2O3, activated carbon and zeolites, and other conventional supports in practical application. However, different supports show different catalytic activity and sulfur resistance, which makes the research and improvement of the support become a part of the emphasis of research to improve the low-temperature SO2 resistance of catalyst.
3.2.1. Effects of TiO2 Support
TiO2 is one of the most common supports and has great catalytic efficiency. Compared with other supports, TiO2 can provide more meaningful acid sites with the characteristics of large surface area, high dispersion of active components, and strong chemical stability [24,38]. Besides, the protection by TiO2 support on active components and the interaction between them also play significant roles in SO2 tolerance.
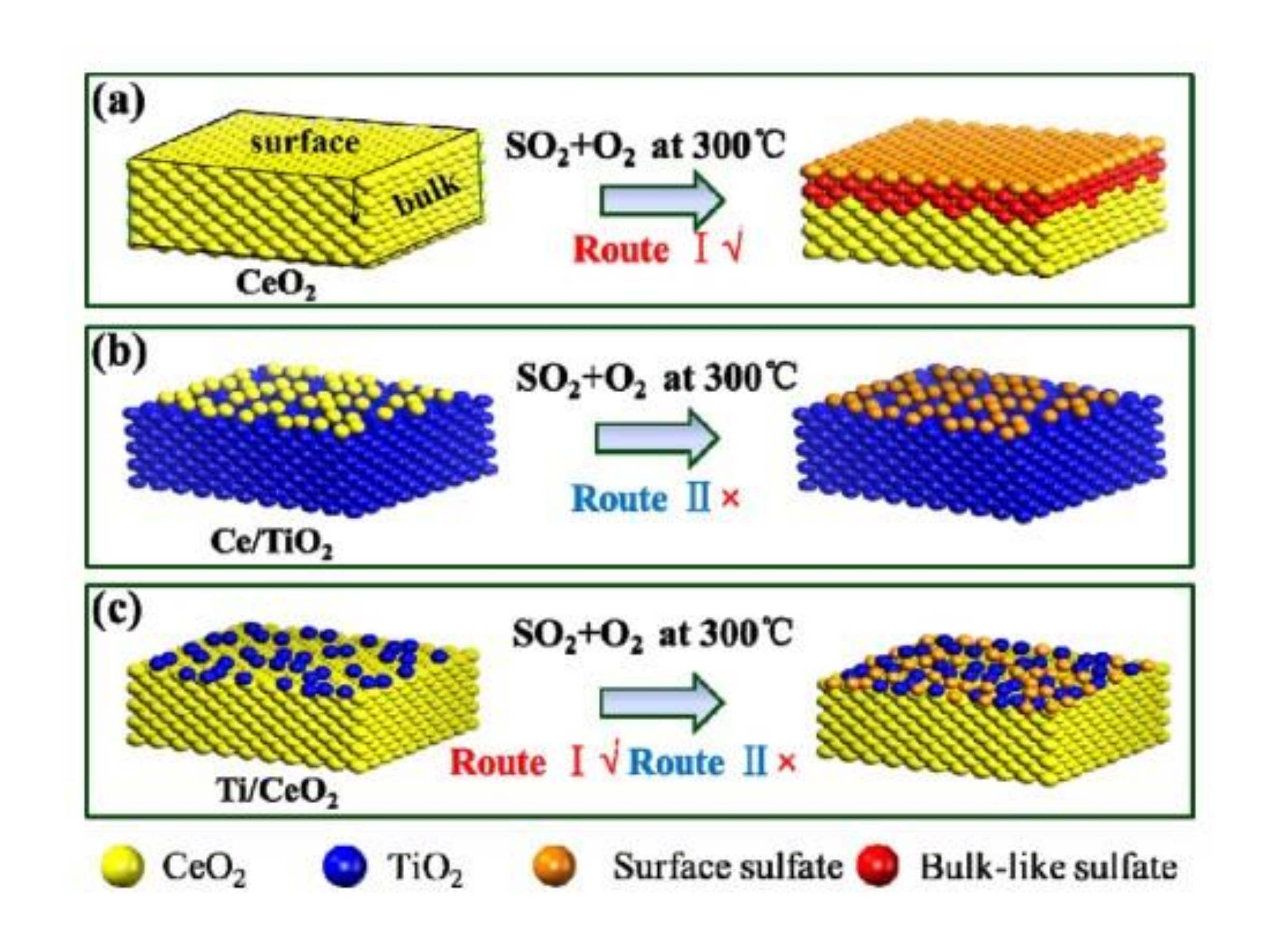
Zhang et al. [39] used impregnation method to synthesize the TiO2/CeO2 catalyst by reversing the support and active components of the most common CeO2/TiO2 catalyst. They found that the TiO2/CeO2 catalyst with changed structure had better catalytic activity and sulfur resistance due to the protection of TiO2, which could maintain the 90% conversion efficiency of NOx at 300 °C after introducing SO2. Although the active sites of TiO2/CeO2 catalyst were also covered by sulfate, the NH3-SCR reaction could still be carried out by synergistic action between surface sulfate and CeO2. Figure 11 showed the sulfur resistance mechanism of Ti/Ce catalyst.
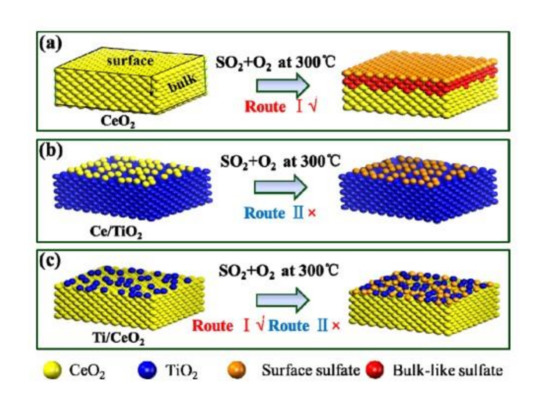
Figure 11.
Sulfur resistance mechanism of (a) CeO2, (b) Ce/Ti and (c) Ti/Ce catalyst [39].
Peng et al. [40] used impregnation to synthesize the SiO2–CeO2–WO3/TiO2 catalyst. It was reported that when Ti/Si = 3, CeO2–WO3/TiO2–SiO2 catalyst could achieve more than 80% catalytic efficiency at 250 °C–500 °C and maintain good sulfur and water resistance. SiO2 doping significantly improved the specific surface area and showed more Brønsted acid sites, which would play a key role in excellent SCR performance of catalyst at low temperatures. Shu et al. [41] improved the Ce–Fe/TiO2 catalyst by adding Al2O3 and honeycomb wire into the support, and synthesized the Ce–Fe/WMH catalyst by immersion method. It was reported that in the presence of SO2, the sulfation of Ce was prior to Ce–Fe/WMH in SCR reaction. At the same time, more strong acid sites provided by surface hydroxyls adsorbed more NH3 in the form of NH4+ instead of SO2. Excellent SO2 durability of Ce–Fe/WMH depended on these two key factors. Figure 12 was the reaction mechanism of Ce–Fe/WMH catalyst.
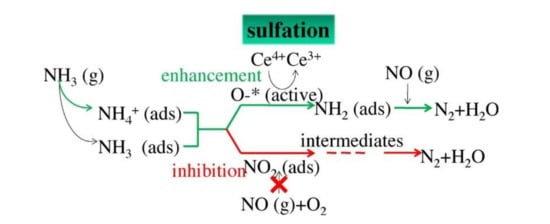
Figure 12.
Reaction mechanism of Ce–Fe/WMH catalyst [41].
3.2.2. Effects of Al2O3 Support
The high-thermal-stabilized Al2O3 support exists plenty of hydroxyl groups and acidic sites on the surface, which matter a lot in good SO2 tolerance. Additionally, Al2O3 can promote NO oxidation, NH3 absorption, and the rapid reaction of SCR, attracting a lot of attention and application in the field of the NH3-SCR [42].
Yao et al. [43] systematically explored the NH3-SCR performance of Mn/Al2O3, Mn/TiO2, Mn/CeO2, and Mn/SiO2 catalysts. The active components were found to have better dispersion on the surface of Al2O3. Mn/Al2O3 catalyst had more acidic sites and stronger adsorption capacity for NOx, which made its catalytic performance and sulfur tolerance superior to the other three supports in the whole temperature range. Qu et al. [44] found that the ZrO2-modified MnOx–CeOy/Al2O3–ZrO2 catalyst increased the ratio of Ce4+/Ce3+ and acid sites, and SCR intermediates. After introducing SO2, the formed bidentate sulfates increased the Lewis acid sites, further promoting the adsorption of NH3 and improving the SO2 tolerance of catalyst.
3.2.3. Effects of Activated Carbon Support
Activated carbon material is amorphous carbon obtained by processing, and includes activated carbon and activated carbon fiber. It has a larger specific surface area, prominent pore structure, strong adsorption capacity, and low price, therefore receiving plenty of attention [45]. Besides, the special structure of activated carbon material is responsible for good sulfur tolerance. Li et al. [46] prepared Ce-, Co-, Cr-, Mo-, and Ni-modified V2O5/TiO2–CNTs catalysts. Among all the modified catalysts, the content of chemically adsorbed oxygen could be increased with the addition of Ce and TiO2. The special support structure reduced the oxidizability of catalyst and strengthened acidity, which would relatively cut off the process of SO2 to SO3 and contribute to NH3 adsorption, leading to the excellent low-temperature catalytic performance and sulfur tolerance of V2O5/TiO2 carbon nanotube catalyst.
3.2.4. Effects of Zeolite Support
Zeolite is generally composed of crystalline porous aluminosilicates, which refer to microporous materials with skeletal structures. It has attracted much attention due to its high surface area, strong thermal stability, and nontoxicity. However, it is easy to be poisoned in the poor reaction environment and lead to the deactivation of catalyst [47,48], while some kinds of zeolite show good SO2 tolerance. Liu et al. [49] found that compared with Mn–Ce and Cu-SSZ-13 catalyst, the Mn–Ce/Cu-SSZ-13 catalyst could maintain 90% NOx conversion at 300 °C after introducing SO2, which resulted from the synergistic effect between the Cu-SSZ-13, MnOx, and CeO2 species. Figure 13 shows the typical SO2-tolerant catalysts with different supports at low temperatures for SCR reaction.
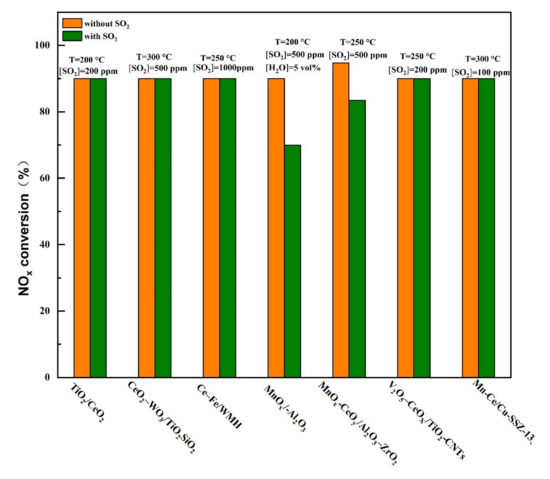
Figure 13.
Typical SO2-tolerant catalysts with different supports at low temperatures for SCR reaction [39,40,41,43,44,46,49].
3.3. Composite Oxide Catalysts
In recent years, extensive work has been done in the area of composite oxide catalyst. Compared with the supported catalysts, these catalysts all use metal oxides and have no clear support or active components. Many achievements have been made in the research of the catalytic performance and sulfur tolerance of catalyst.
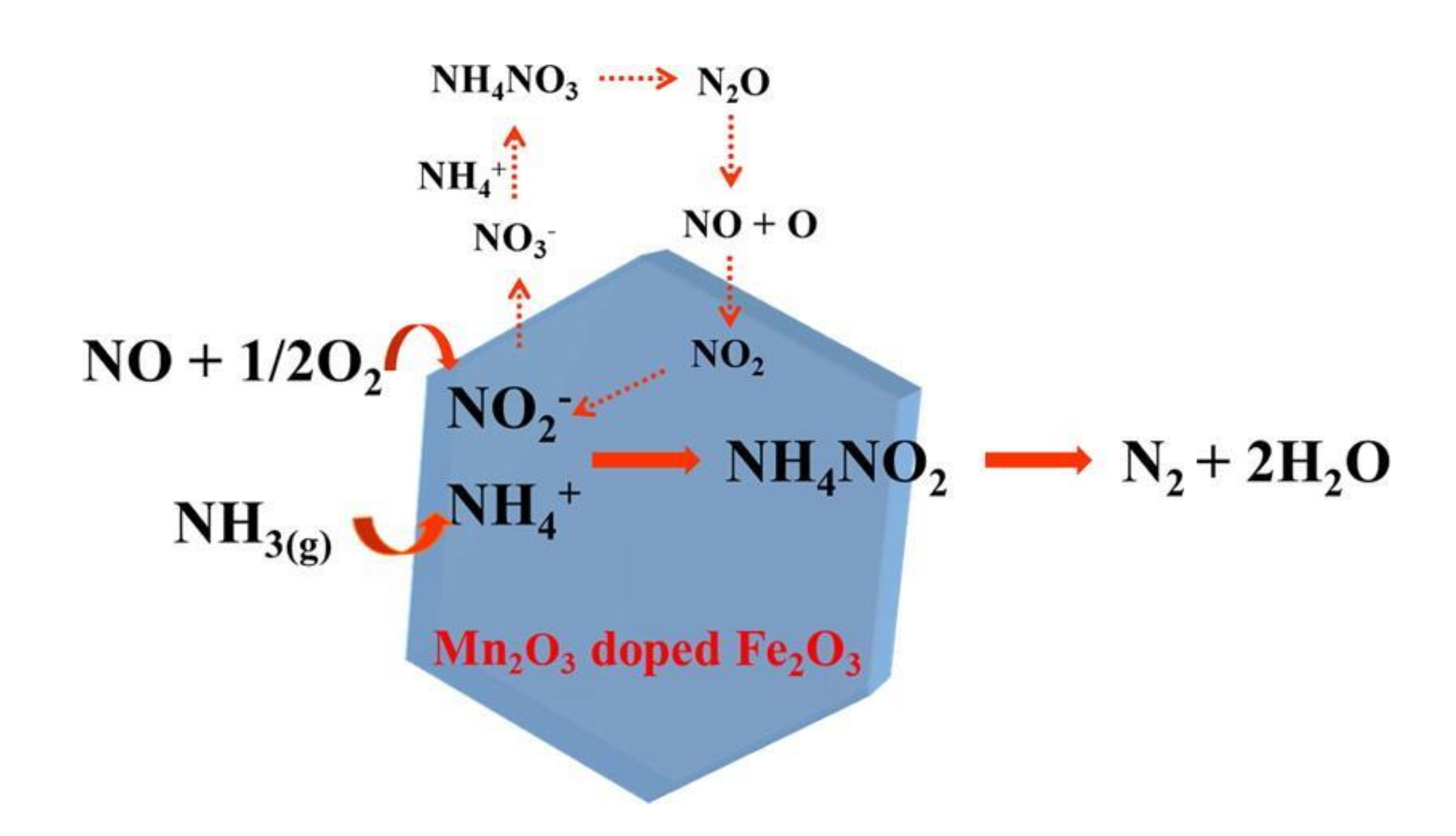
Fan et al. [50] modified MnO2 with a proper amount of Gd. It was reported that adding Gd could have a positive impact on the surface area of catalyst, acid sites, and chemisorption oxygen, which inhibited the sulfation of active substance. The catalyst would reach 100% conversion efficiency within 120 °C–330 °C and had excellent sulfur resistance. Li et al. [51] doped Mn2O3 on the surface of Fe2O3 nanocrystals and prepared MnFeOx catalysts. The experiment indicated that the MnFeOx catalyst reached 98% catalytic efficiency at 200 °C. The doping of Mn2O3 improved the content of active components and the adsorption of NH3, which made MnFeOx catalyst have a good resistance to sulfur and water. Furthermore, they also studied the mechanism of the catalyst reaction (Figure 14).
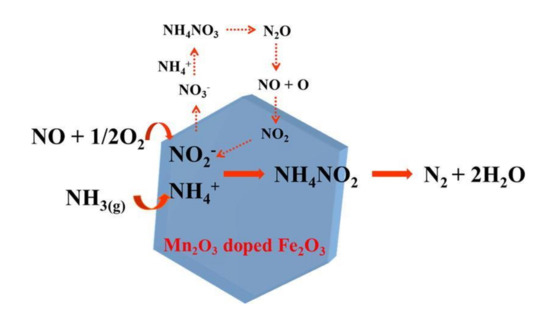
Figure 14.
The reaction mechanism of the MnFeOx catalyst [51].
Sun et al. [52] prepared FeCeOx catalyst by impregnation method and found the FeCeOx catalyst can reach more than 94% catalytic efficiency at 200 °C–300 °C when Fe:Ce = 1:6. The catalytic efficiency was only slightly decreased when adding 100 ppm SO2. The NiCeLaOx catalyst studied by Zhang et al. [53], the Cu modified CeCuTiOx catalyst studied by Yang et al. [54], the Co-modified and Ni-modified CoMnCeOx, and NiMnCeOx catalysts studied by Gao et al. [55] all exhibited good SCR catalytic efficiency and sulfur resistance at low temperatures.
3.4. Other Strategies to Improve the SO2 Resistance
3.4.1. Effects of Preparation Methods
So far, the impregnation method, precipitation method, citric acid method, ion exchange method, and sol-gel method have been the main preparation methods, which have been used for a long time. Besides, many scholars have done a lot of research on how the preparation methods affect the activity and sulfur tolerance of catalyst. Preparation methods of catalyst mainly have an influence on the physical aspect, including the surface area, active components, supports, and surface oxygen vacancy [56]. However, due to the diversity of catalysts, the influence of preparation methods on low-temperature catalytic performance and sulfur tolerance of catalyst will have a difference.
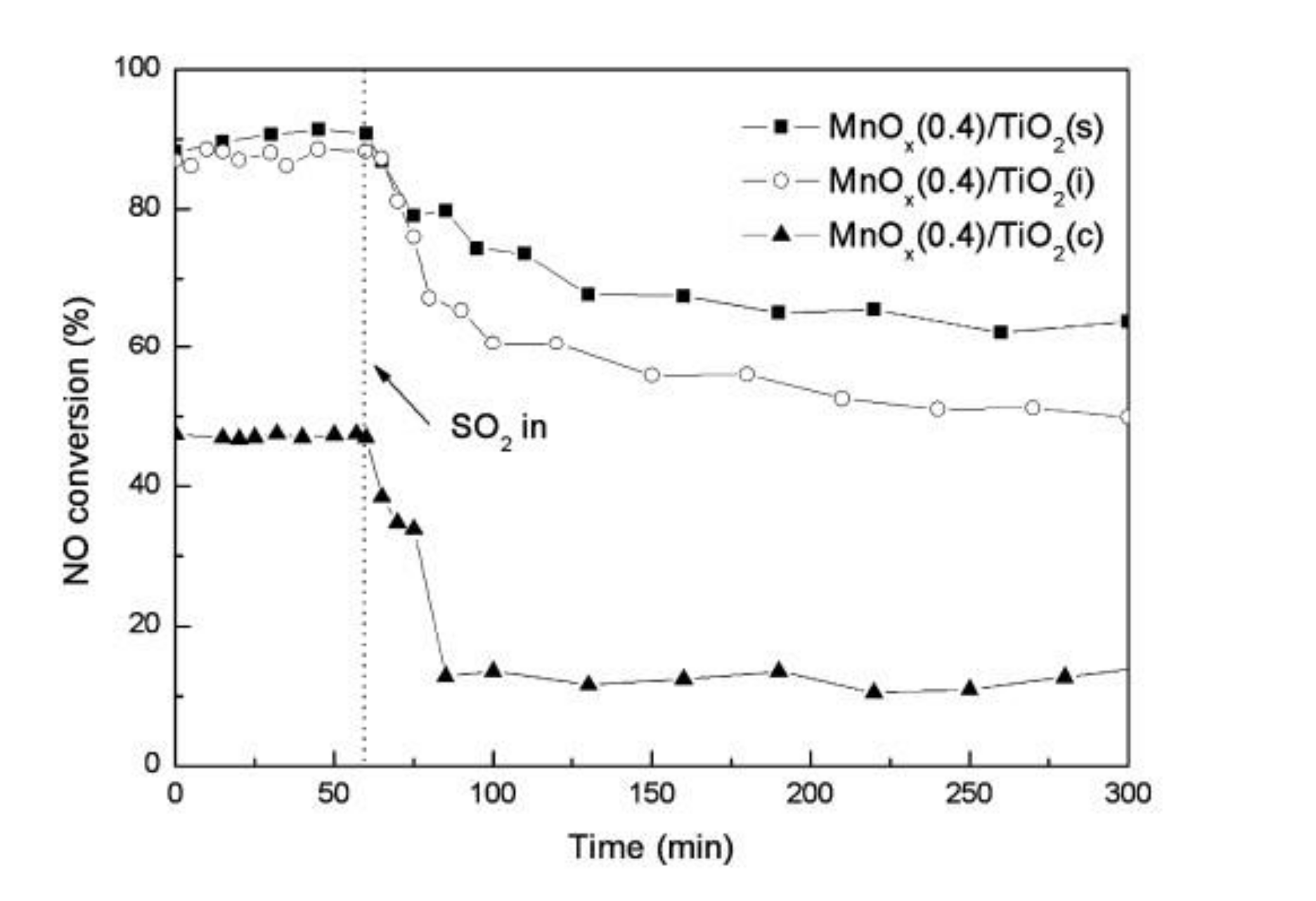
Jiang et al. [57] synthesized Mn/TiO2 catalyst by different methods. It was reported that Mn/TiO2 catalyst synthesized by sol-gel method exhibited the highest content of Mn, the largest specific surface area, and the lowest crystallinity; its catalytic performance and sulfur resistance were more outstanding than the other two methods (Figure 15). After preparing the CeO2/TiO2 catalyst by different methods, Gao et al. [58] explored its catalytic efficiency for SCR reaction and obtained similar conclusions with Jiang.
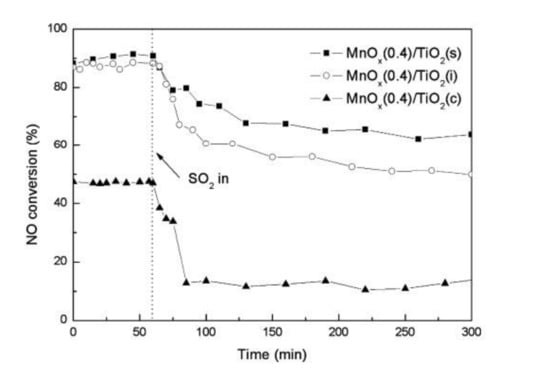
Figure 15.
Sulfur resistance of Mn/TiO2 catalyst synthesized by impregnation, coprecipitation and sol-gel method. Reaction conditions: [NO] = [NH3] = 1000 ppm; [O2] = 3%; N2 balance, total flow rate = 2000 mL/min [57].
Liu et al. [59] prepared CeWTiOx catalyst by coprecipitation method and the Ce–W/TiO2 catalyst by the water-phase method. Compared with the CeWTiOx catalyst, the Ce–W/TiO2 catalyst prepared could reach 90% conversion efficiency at 205 °C–515 °C and could keep a higher degree of catalytic efficiency after the introduction of SO2. Vuong et al. [60] found that compared with the V/CeO2 catalyst synthesized by citric acid method, the catalyst synthesized by precipitation method showed the characteristics of larger specific surface area, increased oxygen vacancy, and higher Ce3+ concentration, leading to a greater catalytic performance and sulfur resistance under low temperatures.
3.4.2. Roles of Acidification
Among all the measures to enhance the sulfur resistance of the catalyst, some researchers have tried to form sulfate on the surface of catalysts by acidification or modification with sulfate, promoting the acidity and catalytic efficiency of catalyst [61,62].
Qiu et al. [63] explored the catalytic performance of the SO42–-modified Mn–Co–Ce/TiO2–SiO2 catalyst. The results illustrated that the catalyst treated with 0.1 mol/l of sulfuric acid exhibited the 99.5% conversion efficiency of NOx at 250 °C when introducing 50 of ppm SO2. The catalytic efficiency failed to decrease in a certain temperature range. Zhang et al. [64] used ammonium sulfate as the source of sulfate ion to prepare the SO42–/CeO2 catalyst. It was reported that the conversion efficiency of NOx was improved obviously after sulfation, whose catalytic efficiency could reach more than 90% at 200 °C–480 °C when doping 2.5% sulfate ion. The increase of acid sites was also the key to promote the SO2 resistance of catalyst. Du et al. [65] found that Ce–TiOx catalyst modified with ferric sulfate and copper sulfate had the highest catalytic activity and sulfur resistance than V–W–Ti catalyst at 150 °C–350 °C.
From these studies, the major factors for the improvement of low-temperature catalytic efficiency and SO2 resistance of the acidification catalyst can be summarized as follows: (1) It can react with active components and promote the conversion between active component ions, increasing the reaction rate. (2) The improvement of catalytic performance and sulfur tolerance of sulfated catalyst is mainly affected by the increase of acid sites. (3) It reacts with other substances of catalyst to produce sulfate, making sulfate ions occupy the adsorption position of SO2 and indirectly reducing the reduction of SCR intermediate.
3.4.3. Effects of Preparation and Reaction Conditions
In addition to the above factors, the preparation conditions of the catalyst and the reaction conditions for NH3-SCR have a bearing on the catalytic performance and sulfur tolerance of catalyst.
Meng et al. [66] synthesized a series of SmMnOx catalysts under different calcination temperatures. The data demonstrated that the low-temperature catalytic activity of SmMnOx catalyst improved obviously from 350 °C to 450 °C, while the specific surface area decreased above 550 °C. Besides, the reduction of Mn4+ species and adsorbed oxygen resulted in a decrease of NO oxidation rate and intermediate content, which further brought down the low-temperature catalytic performance and SO2 tolerance of catalyst. Liu et al. [67] used coprecipitation to prepare Ce3W2SbOx catalyst. The results evidenced that Ce3W2SbOx catalyst showed great catalytic performance, sulfur, and water resistance when the temperature was low. At the same time, the higher the space velocity was, the lower the catalytic activity was, while the space velocity failed to greatly affect the catalytic efficiency of the catalyst at high temperatures. Qi et al. [68] explored the catalytic performance and sulfur tolerance of the Mn–Ce/TiO2 catalysts which were calcined under N2, O2, and air atmosphere. The research data indicated that the NOx conversion efficiencies of the catalysts were 94%, 75.6%, and 85.6%, respectively, when the catalysts were calcined under N2, O2, and air atmosphere. It was reported that the catalyst calcined under the N2 atmosphere could reduce the oxidation of MnOx and the formation of crystals, and increase dispersion of active components and acidic sites, having a positive impact on catalytic performance and sulfur tolerance of Mn–Ce/TiO2 catalyst.
4. Conclusions and Perspectives
Facing progressively strict legislation and policies to control NOx emission, the research and design of low-temperature catalysts for NH3-SCR have received a great deal of attention. Although the poisoning mechanism of catalyst at low temperatures has been studied thoroughly, how to maintain the high catalytic efficiency of catalyst at low temperatures and promote the SO2-resistance-poisoning ability of catalyst to achieve practical application is still an urgent problem.
In the presence of O2, SO3 is easily formed on the catalyst due to the oxidation reaction of SO2, and further combined with NH3 to produce NH4HSO4 and/or (NH4)2SO4, which can deposit on the surface of catalyst and inhibit the reaction gas to be adsorbed on the catalyst to participate in the SCR reaction. Besides, sulfate of active components can be formed and cause irreversible deactivation of catalyst. Therefore, it is effective to adopt several measurements to improve the SO2 tolerance. Firstly, it is to reduce the adsorption of SO2 on the catalyst. The highly acidic catalysts are effective to prevent the SO2 adsorbing. Secondly, preventing the oxidation of SO2 to SO3 plays a significant role in high SO2 tolerance by reducing the redox ability of catalyst, which can cut off the oxidation of SO2 to some extent. Furthermore, the synergistic effect between catalyst components can also improve the sulfur resistance of catalyst, such as the construction of sacrificial sites, which is responsible for the reduction of active components sulfation. Along with these existing excellent sulfur resistant catalysts, it is expected that future studies will focus on optimizing the supports and preparation methods and concentrating on the application of new structures and technology, which are effective strategies to improve the low-temperature SO2 tolerance of SCR catalysts.
Author Contributions
Writing—original draft preparation, C.L.; writing—review and editing, H.W. and Q.L.; supervision, Z.Z. and Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (2016YFC0205302 and 2016YFC0205300), the National Youth Natural Science Foundation of China (No. 21507100) and Science and Technology Project of Hebei Province (NO.206Z3702G).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Jia, W. The effect of SO2 on NH3-SCO and SCR properties over Cu/SCR catalyst. Appl. Surf. Sci. 2020, 507. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, B.; Li, J.; Wang, X.; Crocker, M.; Shi, C. Insights into the structure-activity relationships of highly efficient CoMn oxides for the low temperature NH3-SCR of NOx. Appl. Catal. B 2020, 277. [Google Scholar] [CrossRef]
- Meng, D.; Xu, Q.; Jiao, Y.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G.; Zhan, W. Spinel structured CoaMnbOx mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2018, 221, 652–663. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, Z.; Huang, S.; Zhang, W. Cu-SSZ-13 zeolite–metal oxide hybrid catalysts with enhanced SO2-tolerance in the NH3-SCR of NOx. Appl. Catal. B 2020, 269, 118825. [Google Scholar] [CrossRef]
- Gan, L.; Guo, F.; Yu, J.; Xu, G. Improved Low-Temperature Activity of V2O5-WO3/TiO2 for Denitration Using Different Vanadium Precursors. Catalysts 2016, 6, 25. [Google Scholar] [CrossRef]
- Tang, X.; Shi, Y.; Gao, F.; Zhao, S.; Yi, H.; Xie, Z. Promotional role of Mo on Ce0.3FeOx catalyst towards enhanced NH3-SCR catalytic performance and SO2 resistance. Chem. Eng. J. 2020, 398, 125619. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B.-C. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT study of the SO2 effect on low-temperature SCR reaction over Fe-Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Yu, Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Shi, Y.J.; Shu, H.; Zhang, Y.-H.; Fan, H.-M.; Zhang, Y.-P.; Yang, L.-J. Formation and decomposition of NH4HSO4 during selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Fuel Process. Technol. 2016, 150, 141–147. [Google Scholar] [CrossRef]
- Yan, Q.; Gao, Y.; Li, Y.; Vasiliades, M.A.; Chen, S.; Zhang, C.; Gui, R.; Wang, Q.; Zhu, T.; Efstathiou, A.M. Promotional effect of Ce doping in Cu4Al1Ox—LDO catalyst for low-T practical NH3-SCR: Steady-state and transient kinetics studies. Appl. Catal. B 2019, 255. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx-Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B 2015, 166, 37–44. [Google Scholar] [CrossRef]
- Wei, L.; Cui, S.; Guo, H.; Ma, X.; Zhang, L. DRIFT and DFT study of cerium addition on SO2 of manganese-based catalysts for low temperature SCR. J. Mol. Catal. A Chem. 2016, 421, 102–108. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Zhang, T.; Li, J.M.; Zhao, Z.; Wei, Y.C.; Jiang, G.Y.; Duan, A.J. Effect of Ce doping of TiO2 support on NH3-SCR activity over V2O5-WO3/CeO2-TiO2 catalyst. J. Environ. Sci. 2014, 26, 2106–2113. [Google Scholar] [CrossRef]
- Lee, K.J.; Kumar, P.A.; Maqbool, M.S.; Rao, K.N.; Song, K.H.; Ha, H.P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal. B 2013, 142, 705–717. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D.; Shi, L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5-WO3/TiO2 catalyst. Prog. Nat. Sci. 2015, 25, 342–352. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Yang, J.; Zhang, J.; Zheng, C. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas. Chem. Eng. J. 2018, 348, 618–629. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.; Hu, S.; Sun, L.; Zhang, A. The activity and mechanism study of Fe–Mn–Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, L.; Li, J.H.; Ma, L.; Arandiyan, H.; Du, Y.; Xu, J.Y.; Hao, J.M. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3. Environ. Sci. Technol. 2012, 46, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, T.; Zhao, N.; Yang, X.; Deng, L. Iron-doped Mn–Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2010, 22, 1447–1454. [Google Scholar] [CrossRef]
- Wu, L.-C.; Wang, Q.; Zhao, W.; Payne, E.K. Study of Fe-doped V2O5/TiO2 catalyst for an enhanced NH3-SCR in diesel exhaust aftertreatment. Chem. Pap. 2018, 72, 1981–1989. [Google Scholar] [CrossRef]
- Du, X.-S.; Gao, X.; Cui, L.-W.; Fu, Y.-C.; Luo, Z.-Y.; Cen, K.-F. Investigation of the effect of Cu addition on the SO2-resistance of a Ce–Ti oxide catalyst for selective catalytic reduction of NO with NH3. Fuel 2012, 92, 49–55. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Han, J.; Shi, L.; Zhang, D. Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Xu, L.; Shi, C.; Chen, B.; Zhao, Q.; Zhu, Y.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; et al. Improvement of catalytic activity over Cu–Fe modified Al-rich Beta catalyst for the selective catalytic reduction of NOx with NH3. Microporous Mesoporous Mater. 2016, 236, 211–217. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Ma, K.; Zou, W.; Cao, Y.; Xiong, Y.; Tang, C.; Dong, L. Ultra-low loading of copper modified TiO2 /CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 207, 366–375. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Liu, S.; Ren, X.; Ma, J.; Su, W.; Peng, Y. Ultra hydrothermal stability of CeO2-WO3/TiO2 for NH3-SCR of NO compared to traditional V2O5-WO3/TiO2 catalyst. Catal. Today 2015, 258, 11–16. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M.; Zhu, R. Enhanced activity of tungsten modified CeO2/TiO2 for selective catalytic reduction of NOx with ammonia. Catal. Today 2010, 153, 77–83. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. A superior Ce–W–Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2012, 115, 100–106. [Google Scholar] [CrossRef]
- Jiang, Y.; Bao, C.Z.; Liu, Q.Y.; Liang, G.T.; Lu, M.Y.; Ma, S.Y. A novel CeO2-MoO3-WO3/TiO2 catalyst for selective catalytic reduction of NO with NH3. Catal. Commun. 2018, 103, 96–100. [Google Scholar] [CrossRef]
- Xu, T.F.; Wu, X.D.; Gao, Y.X.; Lin, Q.W.; Hu, J.F.; Weng, D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 monolithic catalysts for low-temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Yang, R.; Huang, H.F.; Chen, Y.J.; Zhang, X.X.; Lu, H.F. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2015, 36, 1256–1262. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, H.; Yao, W.; Wang, Y.; Liu, Y.; Wu, Z.; Wang, H.; Weng, X. SO2 poisoning behaviors of Ca–Mn/TiO2 Catalysts for selective catalytic reduction of NO with NH3 at low temperature. J. Nanomater. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Yao, X.J.; Zhao, R.D.; Chen, L.; Du, J.; Tao, C.Y.; Yang, F.M.; Dong, L. Selective catalytic reduction of NOx by NH3 over CeO2 supported on TiO2: Comparison of anatase, brookite, and rutile. Appl. Catal. B 2017, 208, 82–93. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.L.; Cao, Y.; Yao, X.J.; Ge, C.Y.; Gao, F.; Deng, Y.; Tang, C.J.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, C.X.; Zhang, X.Y.; Li, J.H. The effect of SiO2 on a novel CeO2-WO3/TiO2 catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B 2013, 140, 276–282. [Google Scholar] [CrossRef]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H.T. Selective catalytic reaction of NOx with NH3 over Ce–Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B 2014, 150, 630–635. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Lu, P.; Wen, Q.; Zhao, Y.; Zhang, X.; Fan, C.; Tao, S. Iron, lanthanum and manganese oxides loaded on gamma-Al2O3 for selective catalytic reduction of NO with NH3 at low temperature. Environ. Technol. 2013, 34, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Kong, T.; Yu, S.; Li, L.; Yang, F.; Dong, L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 402, 208–217. [Google Scholar] [CrossRef]
- Qu, L.; Li, C.; Zeng, G.; Zhang, M.; Fu, M.; Ma, J.; Zhan, F.; Luo, D. Support modification for improving the performance of MnOx-CeOy/gamma-Al2O3 in selective catalytic reduction of NO by NH3. Chem. Eng. J. 2014, 242, 76–85. [Google Scholar] [CrossRef]
- Tian, W.; Yang, H.; Fan, X.; Zhang, X. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature. J. Hazard. Mater. 2011, 188, 105–109. [Google Scholar] [CrossRef]
- Li, Q.; Hou, X.; Yang, H.; Ma, Z.; Zheng, J.; Liu, F.; Zhang, X.; Yuan, Z. Promotional effect of CeOx for NO reduction over V2O5/TiO2-carbon nanotube composites. J. Mol. Catal. A Chem. 2012, 356, 121–127. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.Q.; Yu, T.; Shen, M.Q.; Wang, W.L.; Li, W. The effect of sulfate species on the activity of NH3-SCR over Cu/SAPO-34. Appl. Catal. B 2017, 204, 239–249. [Google Scholar] [CrossRef]
- Joshi, S.Y.; Kumar, A.; Luo, J.Y.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. New insights into the mechanism of NH3-SCR over Cu- and Fe-zeolite catalyst: Apparent negative activation energy at high temperature and catalyst unit design consequences. Appl. Catal. B 2018, 226, 565–574. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, Z.; Ma, L.; Niu, H.; Liu, C.; Li, J.; Zhang, Z. MnO-CeO2 supported on Cu-SSZ-13: A novel SCR catalyst in a wide temperature range. Appl. Catal. A Gen. 2017, 547, 146–154. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.R.; Wang, Y.; He, C.; Niu, C.M. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance. Chem. Eng. J. 2018, 348, 820–830. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Li, Y.P.; Zhan, S.H.; Guan, Q.X.; Tian, Y. Low-temperature selective catalytic reduction of NO with NH3 over Mn2O3-doped Fe2O3 hexagonal microsheets. ACS Appl. Mater. Interfaces 2016, 8, 5224–5233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, Y.; Su, W.; Wei, Y. Low-temperature selective catalytic reduction of NO with NH3 over Fe–Ce–Ox catalysts. Trans. Tianjin Univ. 2016, 23, 35–42. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, H.; Du, T.; Ma, W.; Zhong, Q. H2O and SO2 tolerance, activity and reaction mechanism of sulfated Ni–Ce–La composite oxide nanocrystals in NH3-SCR. Chem. Eng. J. 2016, 296, 122–131. [Google Scholar] [CrossRef]
- Yang, B.; Shen, Y.; Zeng, Y.; Shen, S.; Zhu, S. Promotional effects of copper doping on Ti-Ce-Ox for selective catalytic reduction of NO by NH3 at low temperature. J. Rare Earths 2016, 34, 268–275. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Yao, X.; Ma, K.; Zou, W.; He, S.; An, J.; Yang, F.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Wu, Z. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Fu, Y.; Zhong, Y.; Luo, Z.; Cen, K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 11, 465–469. [Google Scholar] [CrossRef]
- Liu, X.; Ning, P.; Li, H.; Song, Z.; Wang, Y.; Zhang, J.; Tang, X.; Wang, M.; Zhang, Q. Probing NH3-SCR catalytic activity and SO2 resistance over aqueous-phase synthesized Ce-W@TiO2 catalyst. J. Fuel. Chem. Technol. 2016, 44, 225–231. [Google Scholar] [CrossRef]
- Thanh Huyen, V.; Radnik, J.; Schneider, M.; Atia, H.; Armbruster, U.; Brueckner, A. Effect of support synthesis methods on structure and performance of VOx/CeO2 catalysts in low-temperature NH3-SCR of NO. Catal. Commun. 2016, 84, 171–174. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, J.; Wang, J.; Chen, Y. Performances of CuSO4/TiO2 catalysts in selective catalytic reduction of NOx by NH3. Chin. J. Catal. 2016, 37, 281–287. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Nahata, M.; Chen, X.; Li, J.; Schwank, J.W. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. B 2018, 232, 246–259. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Y.; Pang, D.; Ouyang, F.; Zhang, C. SO42-–Mn–Co–Ce supported on TiO2/SiO2 with high sulfur durability for low-temperature SCR of NO with NH3. Catal. Commun. 2016, 78, 22–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Song, Z.; Ning, P.; Li, H.; Liu, X. A novel and environmentally friendly SO42-/CeO2 catalyst for the selective catalytic reduction of NO with NH3. J. Ind. Eng. Chem. 2016, 34, 165–171. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Chen, Y.; Gao, X.; Zhang, L. Supported metal sulfates on Ce-TiOx as catalysts for NH3-SCR of NO: High resistances to SO2 and potassium. J. Ind. Eng. Chem. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm–Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: Effect of the calcination temperature. J. Mol. Catal. A Chem. 2016, 420, 272–281. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.-Q.; Zhang, Y.-F.; Liu, X.-Q.; Wang, Y.; Li, Y. Novel Ce–W–Sb mixed oxide catalyst for selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2017, 401, 7–16. [Google Scholar] [CrossRef]
- Qi, K.; Xie, J.; Fang, D.; Li, F.; He, F. Performance enhancement mechanism of Mn-based catalysts prepared under N2 for NOx removal: Evidence of the poor crystallization and oxidation of MnOx. Chin. J. Catal. 2017, 38, 845–852. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).