Efficient Sorbitol Producing Process through Glucose Hydrogenation Catalyzed by Ru Supported Amino Poly (Styrene-co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3
Abstract
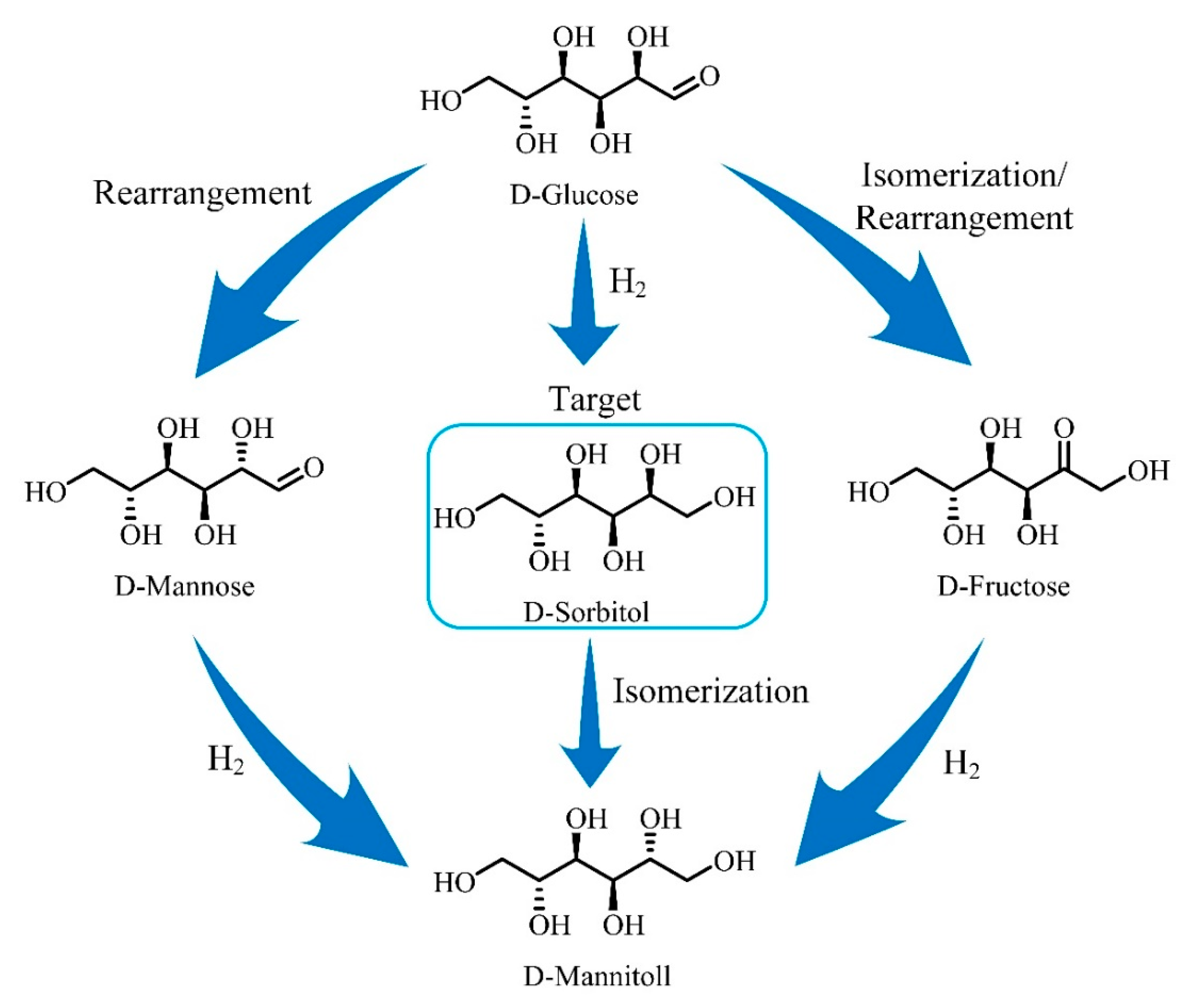
:1. Introduction
2. Results and Discussion
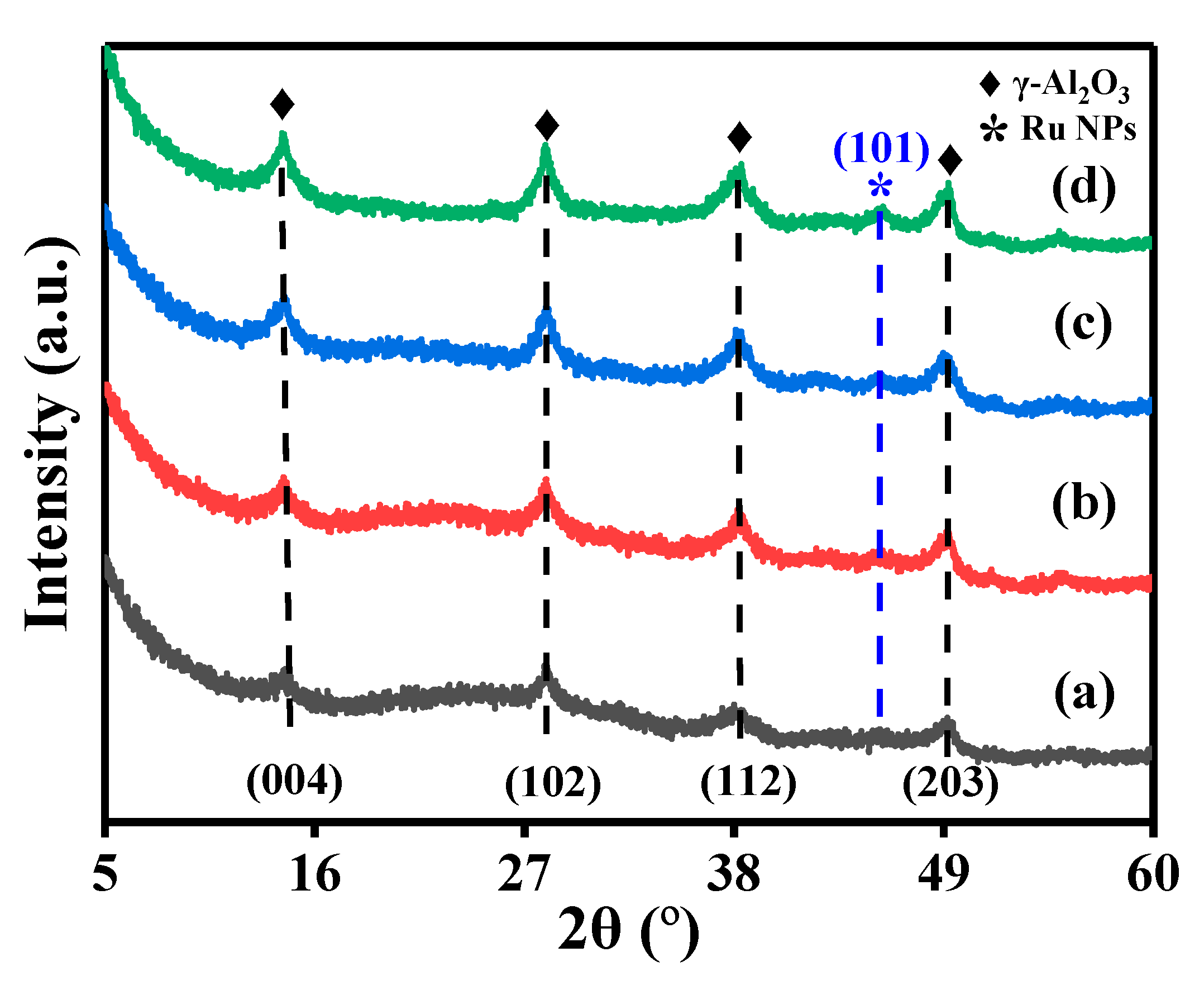
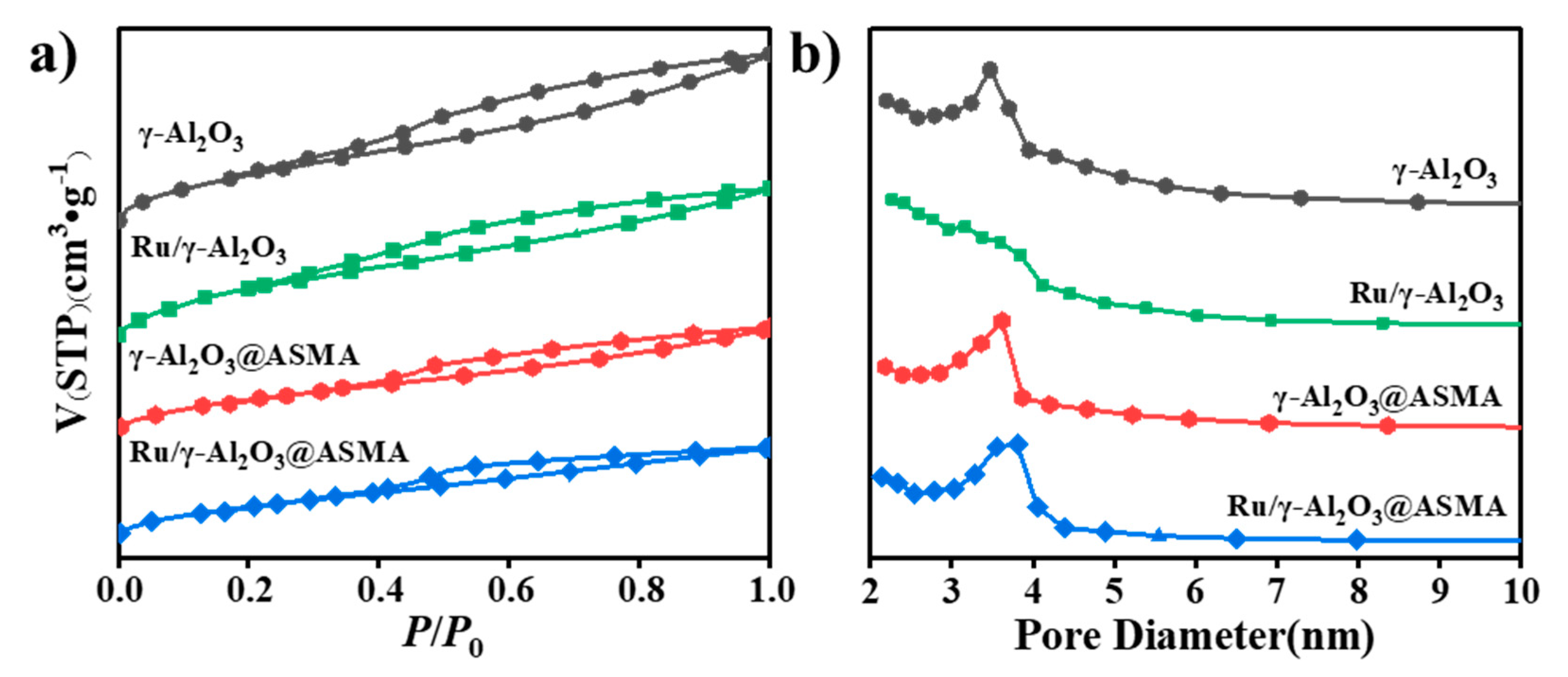
2.1. Crystallinity and Textural Properties
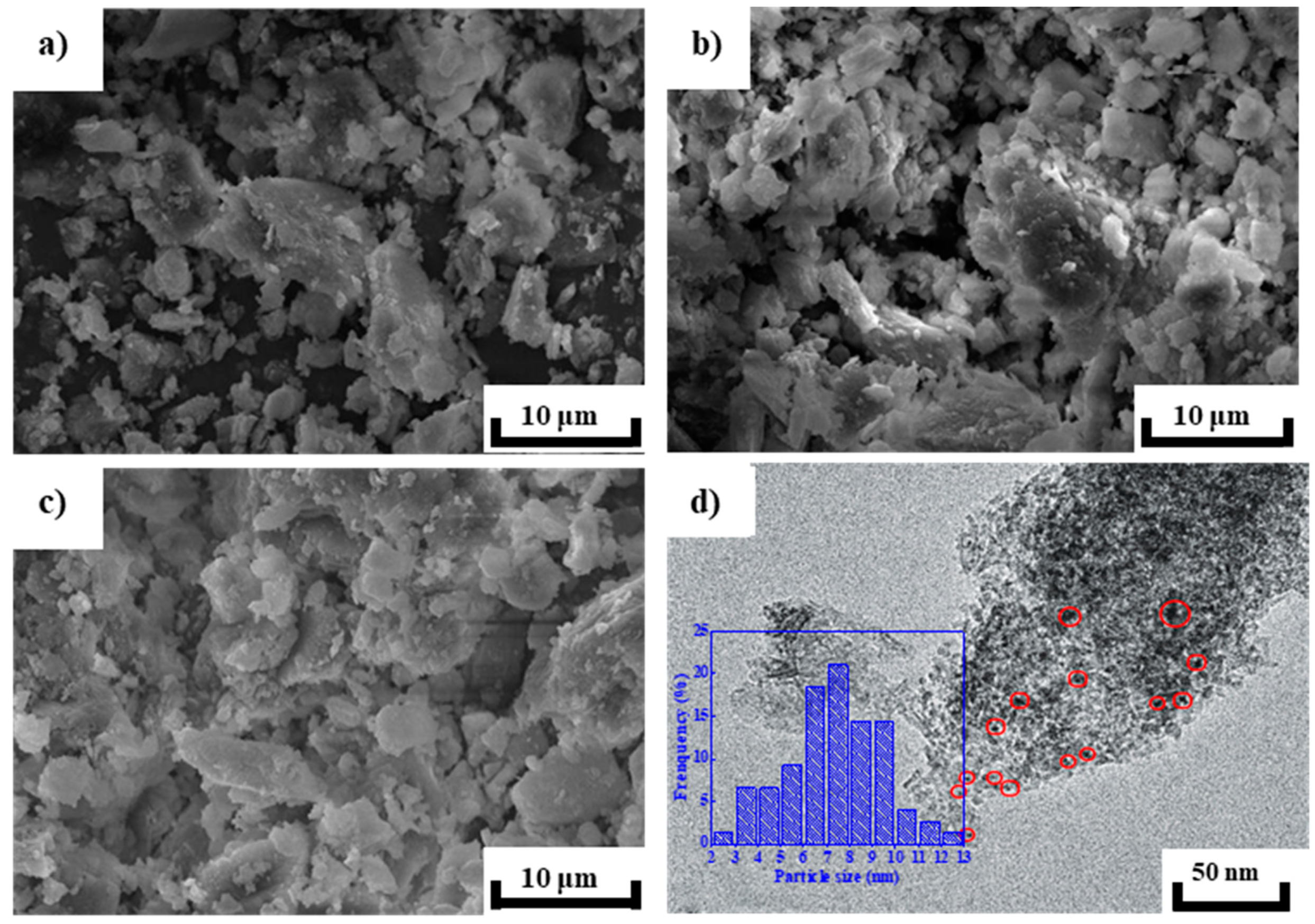
2.2. Morphology and Microstructure
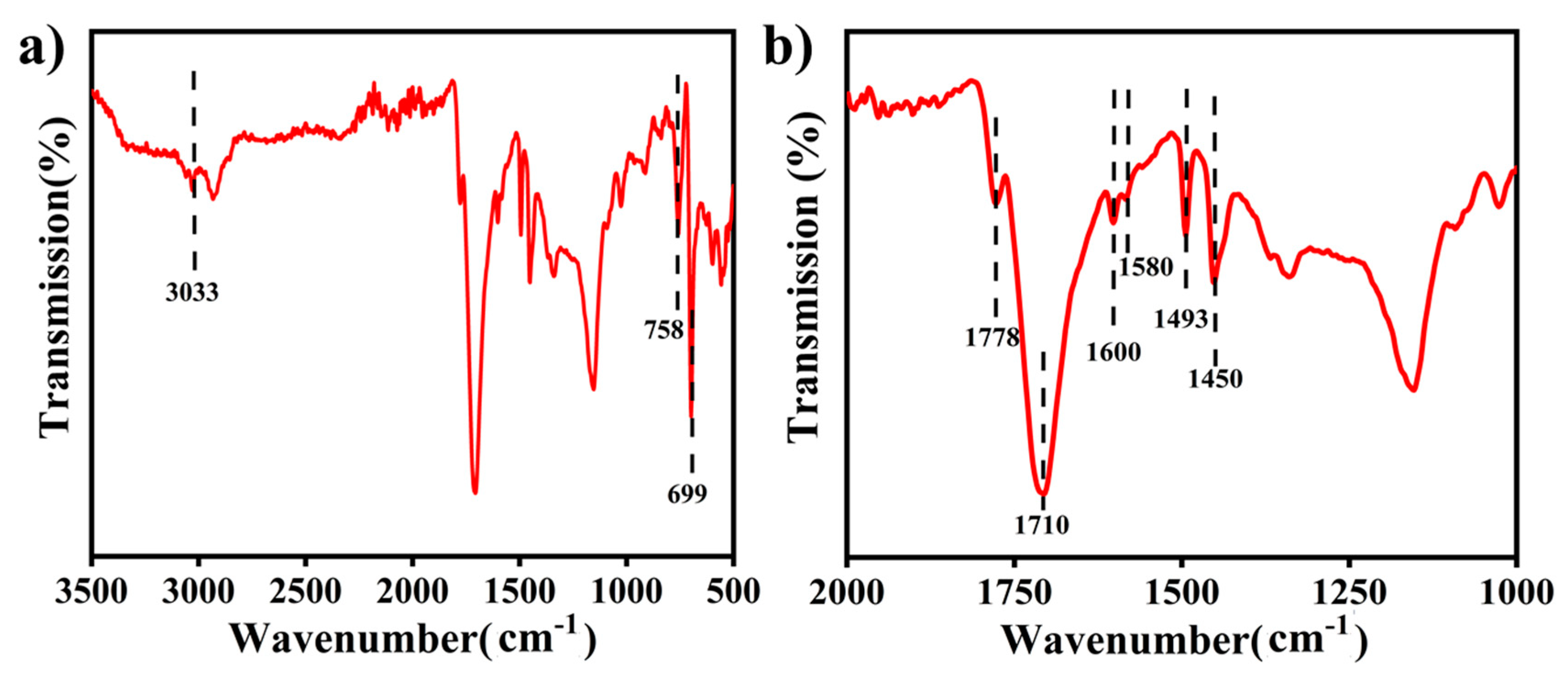
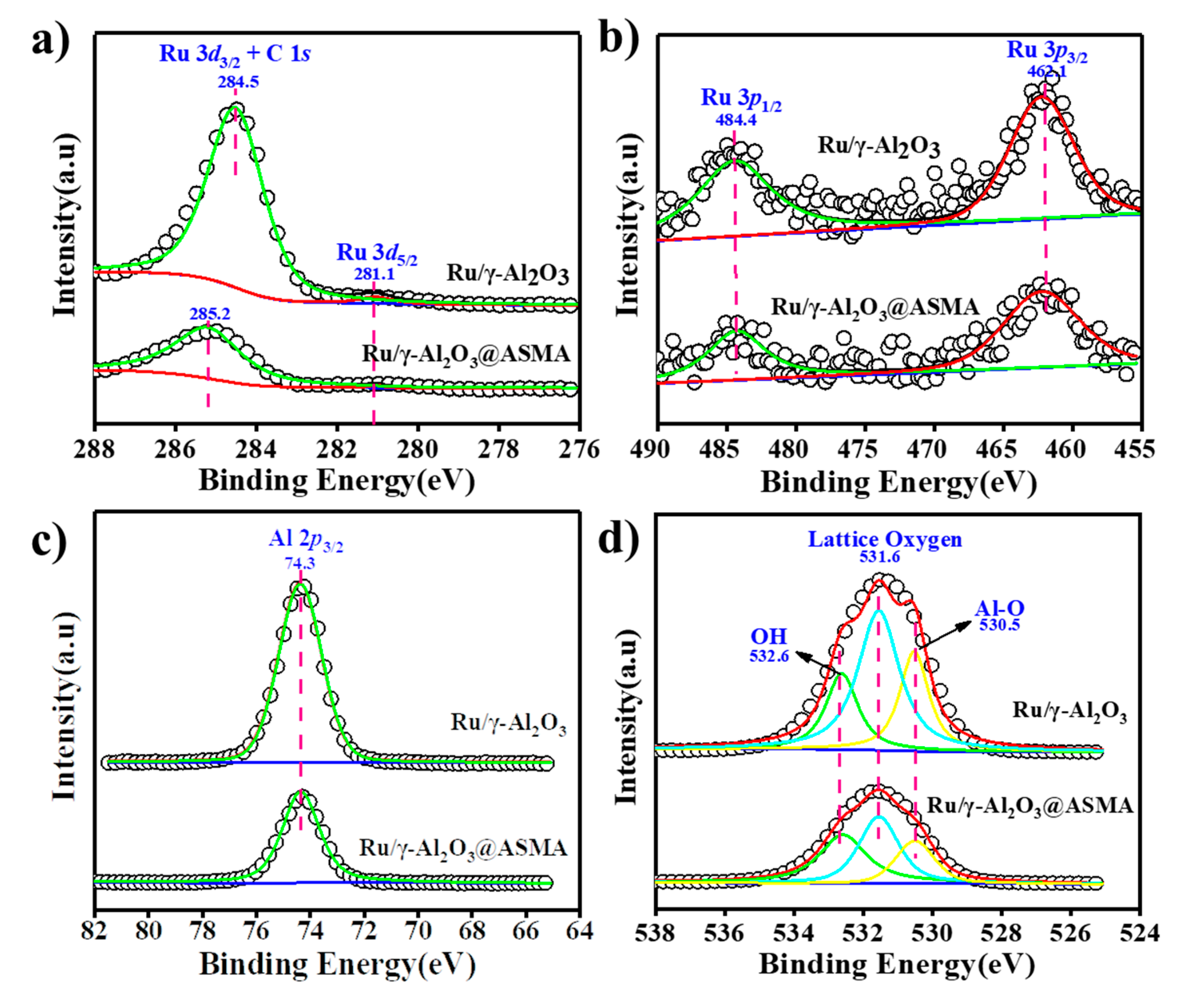
2.3. Surface Characterization of the Ru Catalyst
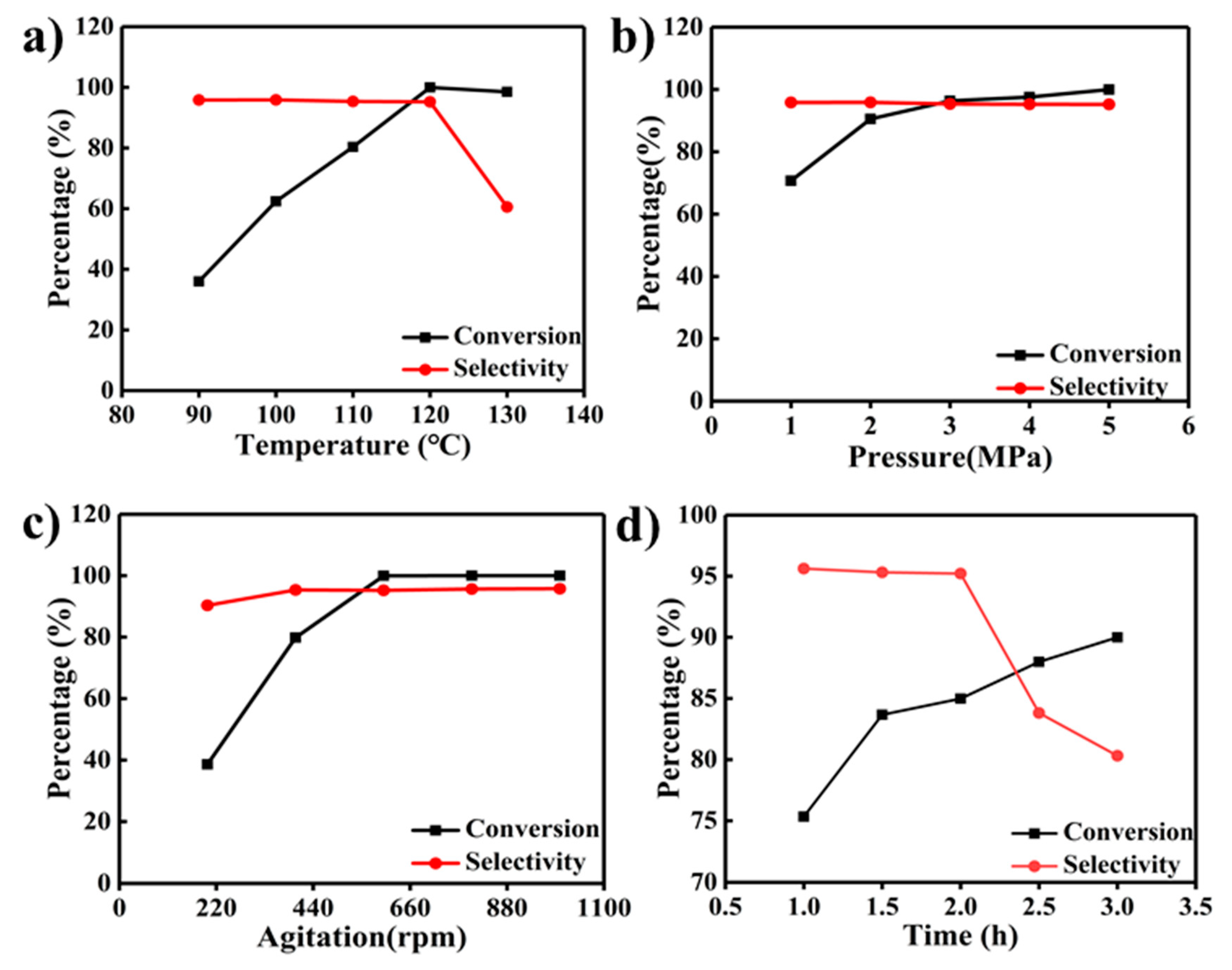
2.4. Catalytic Activity
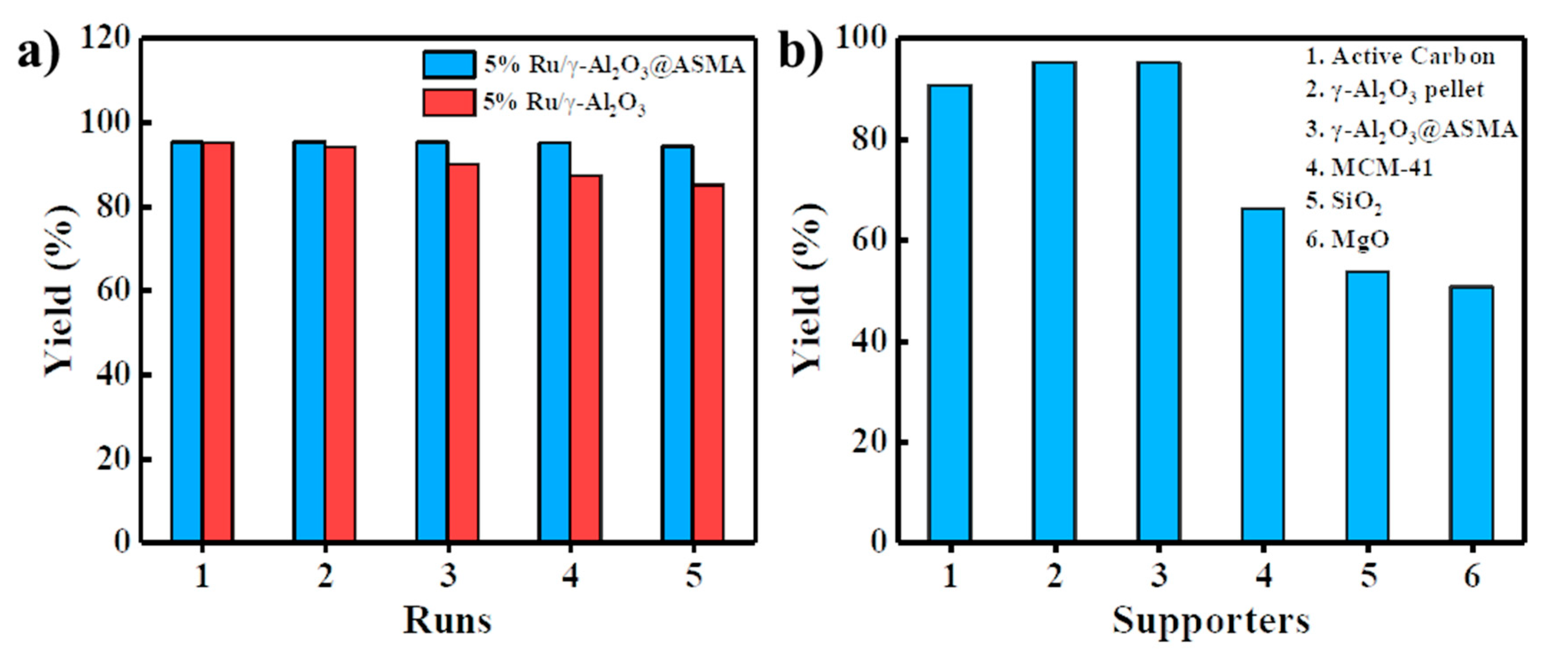
2.5. Reusability and Peer Comparison
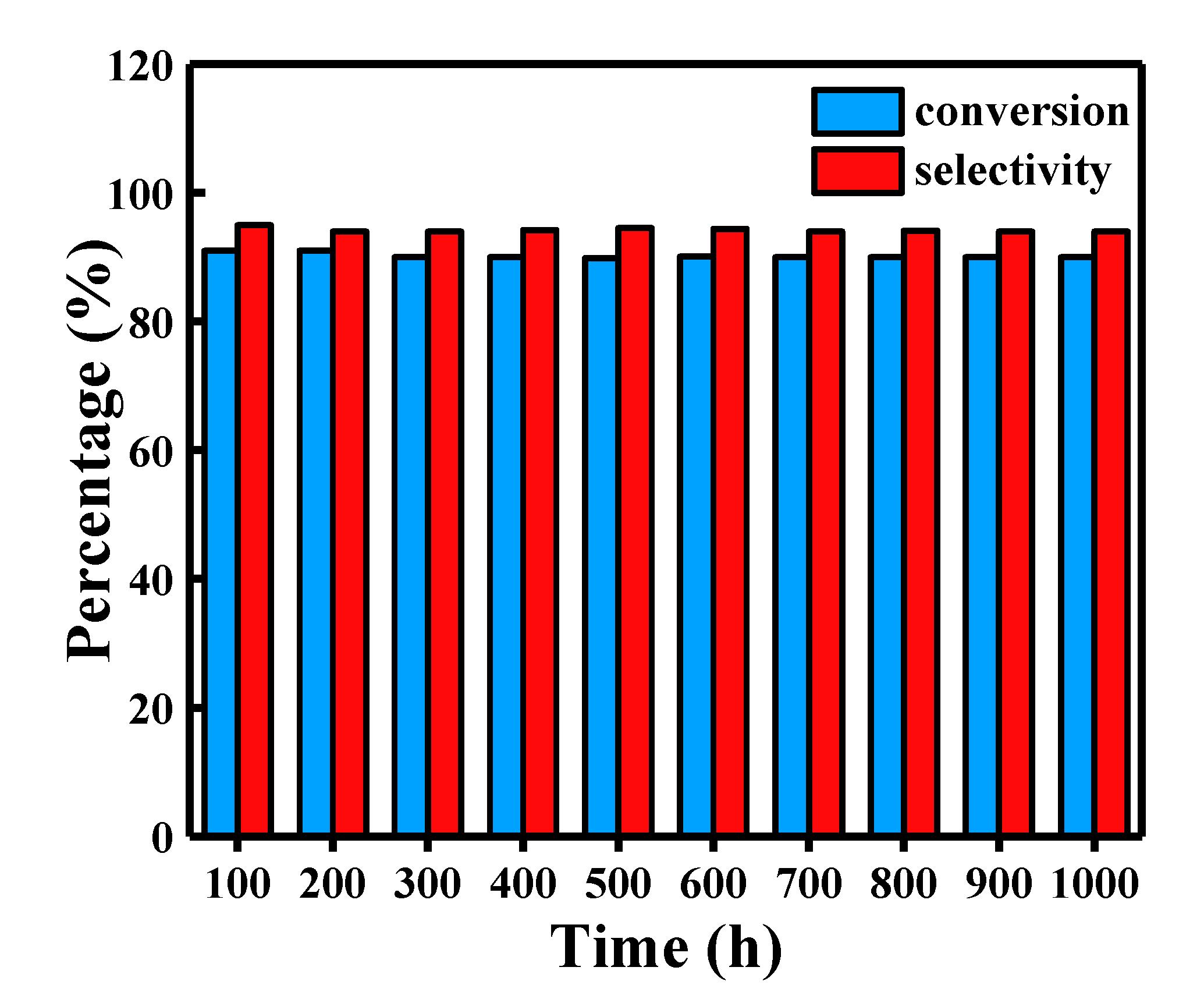
2.6. Continuous Test
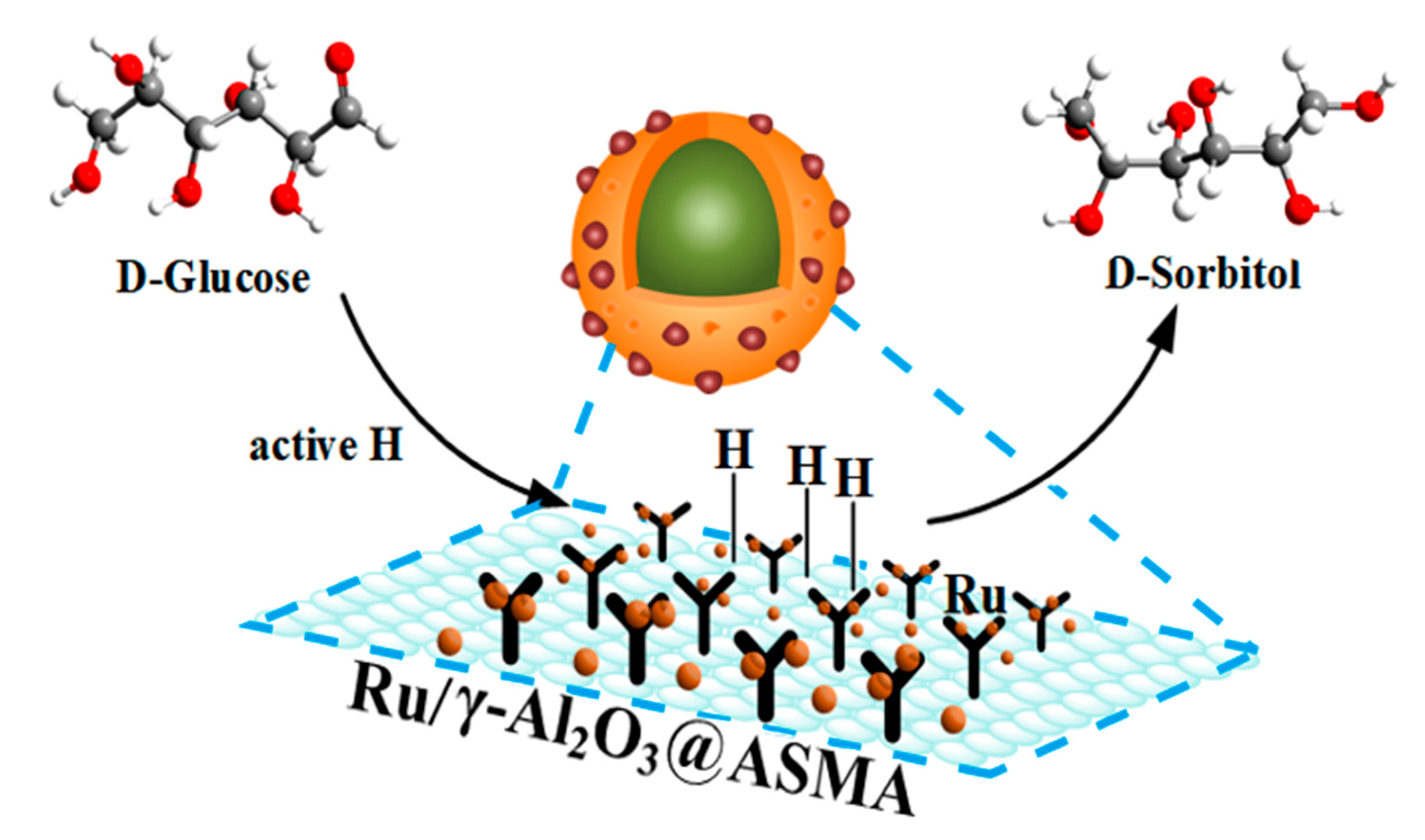
3. Catalytic Mechanism Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Catalytic Hydrogenation of Glucose to Sorbitol
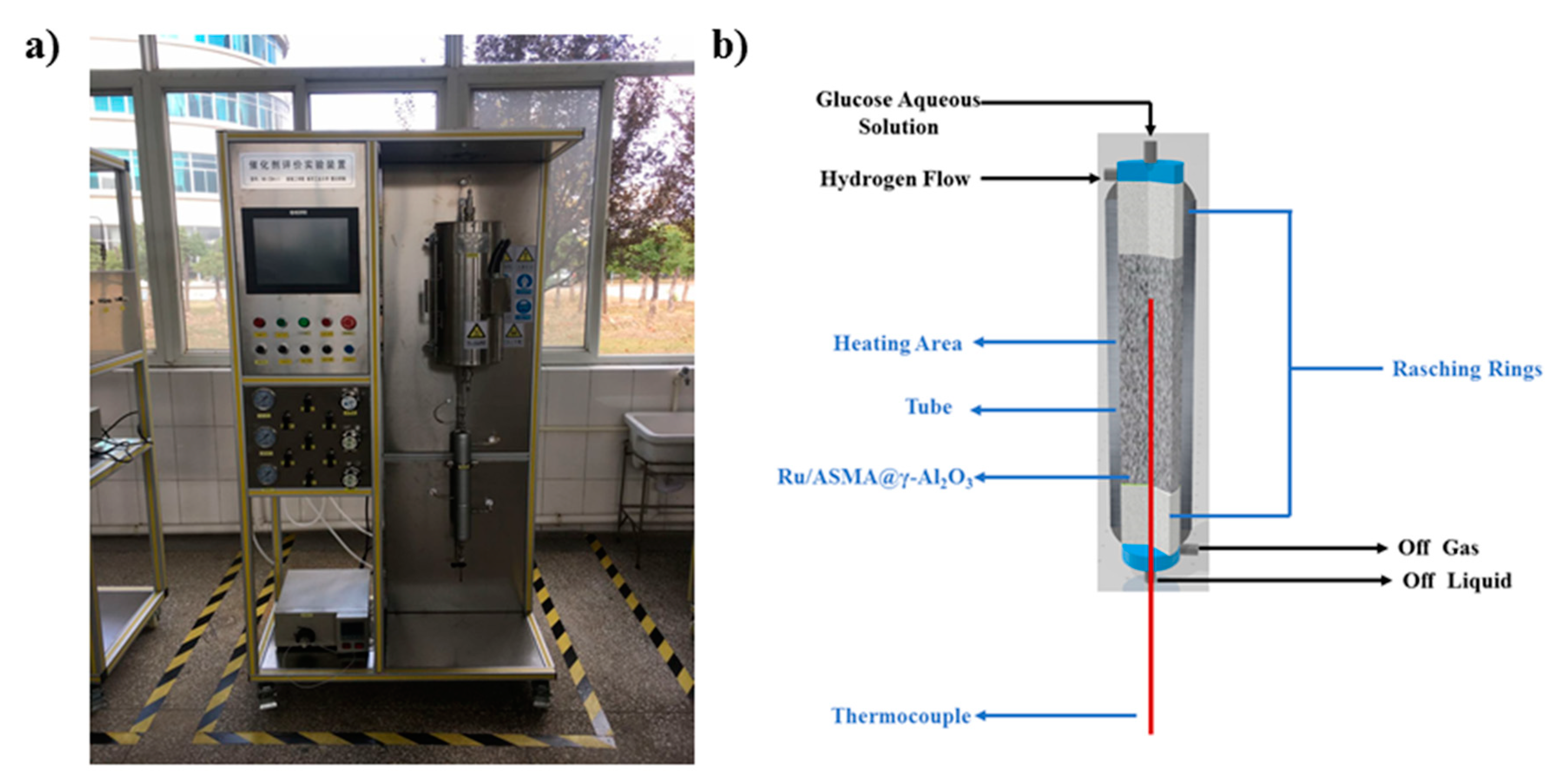
4.5. Continuous Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silvester, L.; Ramos, F.; Thuriot-Roukos, J.; Heyte, S.; Araque, M.; Paul, S.; Wojcieszak, R. Fully Integrated High-Throughput Methodology for the Study of Ni- and Cu-Supported Catalysts for Glucose Hydrogenation. Catal. Today 2019, 338, 72–80. [Google Scholar] [CrossRef]
- Zhang, X.; Wilson, K.; Lee, A.F. Heterogeneously Catalyzed Hydrothermal Processing of C5-C6 Sugars. Chem. Rev. 2016, 116, 12328–12368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, M.Y.; Park, D.-W.; Lee, M.S. Effect of Sodium Propionate as a Stabilizer on the Catalytic Activity of Pt/C Catalysts for D-Glucose Hydrogenation. Catal Today 2020, 352, 88–94. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Y.; Huang, B.; Li, X.; Guo, L.; Zheng, A.; Luque, R.; Sun, Y. Functionalized CeO2/SBA-15 Materials as Efficient Catalysts for Aqueous Room Temperature Mono-dehydration of Sugar Alcohols. ACS Sustain. Chem. Eng. 2020, 8, 6371–6380. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, H.; Zhang, Q.; Li, H.; Li, H. Ordered Mesoporous Ni-P Amorphous Alloy Nanowire Arrays: High-Efficiency Catalyst for Production of Polyol from Sugar. ACS Appl. Mater. Interfaces 2020, 12, 26101–26112. [Google Scholar] [CrossRef] [PubMed]
- García, B.; Moreno, J.; Iglesias, J.; Melero, J.; Morales, G. Transformation of Glucose into Sorbitol on Raney Nickel Catalysts in the Absence of Molecular Hydrogen: Sugar Disproportionation vs. Catalytic Hydrogen Transfer. Catalysis 2019, 62, 570–578. [Google Scholar] [CrossRef]
- Azadi, P.; Khodadadi, A.A.; Mortazavi, Y.; Farnood, R. Hydrothermal Gasification of Glucose using Raney Nickel and Homogeneous Organometallic Catalysts. Fuel Process. Technol. 2009, 90, 145–151. [Google Scholar] [CrossRef]
- Melero, J.A.; Moreno, J.; Iglesias, J.; Morales, G.; Fierro, J.L.G.; Sánchez-Vázquez, R.; Cubo, A.; García, B. Ru-ZrO2-SBA-15 as Efficient and Robust Catalyst for the Aqueous Phase Hydrogenation of Glucose to Sorbitol. Mol. Catal. 2020, 484, 110802. [Google Scholar] [CrossRef]
- Ban, C.; Yang, S.; Kim, H.; Kim, D.H. Catalytic Hydrogenation of Alginic Acid into Sugar Alcohols over Ruthenium Supported on Nitrogen-Doped Mesoporous Carbons. Catal. Today 2020, 352, 66–72. [Google Scholar] [CrossRef]
- Romero, A.; Nieto-Márquez, A.; Essayem, N.; Alonso, E.; Pinel, G. Improving Conversion of D-Glucose into Short-Chain Alkanes over Ru/MCM-48 based Catalysts. Microporous Microporous Mater. 2019, 286, 25–35. [Google Scholar] [CrossRef]
- Miguel Sanz-Moral, L.; Aho, A.; Kumar, N.; Eränen, K.; Peurla, M.; Peltonen, J.; Murzin, D.Y.; Salmi, T. Synthesis and Characterization Ru-C/SiO2 Aerogel Catalysts for Sugar Hydrogenation Reactions. Catal. Lett. 2018, 148, 3514–3523. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wu, S. Efficient Conversion of D-Glucose into D-Sorbitol over Carbonized Cassava Dregs-Supported Ruthenium Nanoparticles Catalyst. Bioresources 2018, 13, 1278–1288. [Google Scholar] [CrossRef]
- Romero, A.; Nieto-Márquez, A.; Alonso, E. Bimetallic Ru:Ni/MCM-48 Catalysts for the Effective Hydrogenation of D-Glucose into Sorbitol. Appl. Catal. A Gen. 2017, 529, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.; Yan, L.; Chen, M.; Deng, J.; Fu, Y. Preparation of D-Sorbitol by Catalytic Hydrogenation of D-Glucose with Semi Sandwich Iridium Catalyst. Chin. J. Org. Chem. 2017, 37, 3170–3176. [Google Scholar] [CrossRef]
- Zhang, X.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Platinum-Catalyzed Aqueous-Phase Hydrogenation of D-Glucose to D-Sorbitol. ACS Catal. 2016, 6, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Alonso, E.; Sastre, A.; Nieto-Márquez, A. Conversion of Biomass into Sorbitol: Cellulose Hydrolysis on MCM-48 and D-Glucose Hydrogenation on Ru/MCM-48. Microporous Microporous Mater. 2016, 224, 1–8. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Mishra, D.K.; Hwang, J.-S. Selective Hydrogenation of D-Glucose Using Amine Functionalized Nanoporous Polymer Supported Ru Nanoparticles based Catalyst. Catal. Today 2016, 265, 163–173. [Google Scholar] [CrossRef]
- Wang, S.; Wei, W.; Zhao, Y.; Li, H.; Li, H. Ru-B Amorphous Alloy Deposited on Mesoporous Silica Nanospheres: An Efficient Catalyst for D-Glucose Hydrogenation to D-Sorbitol. Catal. Today 2015, 258, 327–336. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Karakoulia, S.; Delimitis, A.; Coman, S.M.; Parvulescu, V.I.; Triantafyllidis, K.S. D-Glucose Hydrogenation/Hydrogenolysis Reactions on Noble Metal (Ru, Pt)/Activated Carbon Supported Catalysts. Catal. Today 2015, 257, 281–290. [Google Scholar] [CrossRef]
- Koklin, A.E.; Klimenko, T.A.; Kondratyuk, A.V.; Lunin, V.V.; Bogdan, V.I. Transformation of Aqueous Solutions of Glucose over the Pt/C Catalyst. Kinet. Catal. 2015, 56, 84–88. [Google Scholar] [CrossRef]
- Aho, A.; Roggan, S.; Simakova, O.A.; Salmi, T.; Murzin, D. Structure Sensitivity in Catalytic Hydrogenation of Glucose over Ruthenium. Catal. Today 2015, 241, 195–199. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Guan, J.; Chen, X.; Qin, Z.; Mu, X.; Xian, M. Selective Hydrogenation of D-Glucose to D-Sorbitol over Ru/ZSM-5 Catalysts. Chin. J. Catal. 2014, 35, 733–740. [Google Scholar] [CrossRef]
- Garcia, B.; Moreno, J.; Morales, G.; Melero, J.; Iglesias, J. Production of Sorbitol via Catalytic Transfer Hydrogenation of Glucose. Appl. Sci. 2020, 10, 1843. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.S.; Chauhan, K.; Pandey, A.; Larroche, C. Biocatalytic Strategies for the Production of High Fructose Syrup from Inulin. Bioresour. Technol. 2018, 260, 395–403. [Google Scholar] [CrossRef]
- Putra, M.D.; Sulieman, A.K.; Abasaeed, A.E.; Gaily, M.H.; Al-Zahrani, S.M.; Zeinelabdeen, M.A.; Atiyeh, H.K. A Green Process for Simultaneous Production of Fructose and Ethanol via Selective Fermentation. J. Clean. Prod. 2017, 162, 420–426. [Google Scholar] [CrossRef]
- Graça, I.; Bacariza, M.C.; Fernandes, A.; Chadwick, D. Desilicated NaY Zeolites Impregnated with Magnesium as Catalysts for Glucose Isomerisation into Fructose. Appl. Catal. B Environ. 2018, 224, 660–670. [Google Scholar] [CrossRef]
- Sebastian, J.; Zheng, M.; Li, X.; Pang, J.; Wang, C.; Zhang, T. Catalytic Conversion of Glucose to Small Polyols over a Binary Catalyst of Vanadium Modified Beta Zeolite and Ru/C. J. Energy Chem. 2019, 34, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.J.; Hameed, B.H. Hydrogenation of Glucose and Fructose into Hexitols over Heterogeneous Catalysts: A Review. J. Taiwan Inst. Chem. E 2019, 96, 341–352. [Google Scholar] [CrossRef]
- Perrard, A.; Gallezot, P.; Joly, J.-P.; Durand, R.; Baljou, C.; Coq, B.; Trens, P. Highly Efficient Metal Catalysts Supported on Activated Carbon Cloths: A Catalytic Application for the Hydrogenation of D-Glucose to D-Sorbitol. Appl. Catal. A Gen. 2007, 331, 100–104. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, P.; Ma, J.; Fan, H.; Wang, W.; Jiang, T.; Han, B. Catalytic Activity of Immobilized Ru Nanoparticles in a Porous Metal-Organic Framework using Supercritical Fluid. Chin. J. Catal. 2013, 34, 167–175. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Huang, J.; Chen, L.; Ma, L.; Huang, X. Conversion of Cellulose and Cellobiose into Sorbitol Catalyzed by Ruthenium Supported on a Polyoxometalate/Metal-Organic Framework Hybrid. ChemSusChem 2013, 6, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, W.; Thapa, K.; Yang, X.; Liang, J.; Zhu, L.; Zhu, J. Morpholine-Modified Pd/γ-Al2O3@ASMA Pellet Catalyst with Excellent Catalytic Selectivity in the Hydrogenation of p-Chloronitrobenzene to p-Chloroaniline. Catalysts 2017, 7, 292. [Google Scholar] [CrossRef] [Green Version]
- Dellacasa, E.; Forouharshad, M.; Rolandi, R.; Pastorino, L.; Monticelli, O. Poly (Styrene-co-Maleic Anhydride) Nanoparticles as Protein Carriers. Mater. Lett. 2018, 220, 241–244. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E.; Beheshtiha, Y.S.; Ahmadi, S.; Hosseinnejad, T. PdCl2 on Modified Poly(Styrene-co-Maleic Anhydride): A Highly Active and Recyclable Catalyst for the Suzuki–Miyaura and Sonogashira Reactions. J. Mol. Catal. A Chem. 2014, 394, 74–82. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dabbawala, A.A.; Hwang, J.-S. Poly (styrene-co-divinylbenzene) Amine Functionalized Polymer Supported Ruthenium Nanoparticles Catalyst Active in Hydrogenation of Xylose. Catal. Commun. 2013, 41, 52–55. [Google Scholar] [CrossRef]
- Sapunoy, V.N.; Grigoryey, M.Y.; Sulman, E.M.; Konyaeva, M.B.; Matveeva, V.G. D-Glucose Hydrogenation over Ru Nanoparticles Embedded in Mesoporous Hypercrosslinked Polystyrene. J. Phys. Chem. A 2013, 117, 4073–4083. [Google Scholar] [CrossRef]
- Edubilli, S.; Gumma, S. A systematic evaluation of UiO-66 metal organic framework for CO2/N2 separation. Sep. Purif. Technol. 2019, 224, 85–94. [Google Scholar] [CrossRef]
- Aho, A.; Roggan, S.; Eranen, K.; Salmi, T.; Murzin, D.Y. Continuous Hydrogenation of Glucose with Ruthenium on Carbon Nanotube Catalysts. Catal. Sci. Technol. 2015, 5, 953–959. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, C.; Lu, M.; Shi, Y.; Qian, G.; Zhou, X.; Duan, X. Coupling Non-Isothermal Trickle-Bed Reactor with Catalyst Pellet Models to Understand the Reaction and Diffusion in Gas Oil Hydrodesulfurization. Chin. J. Chem. Eng. 2020, 28, 1095–1106. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Chu, G.-W.; Li, Y.-B.; Luo, Y.; Fan, T.X.; Shao, L.; Chen, J.F. Liquid-Solid Mass Transfer in a Rotating Trickle-Bed Reactor: Mathematical Modeling and Experimental Verification. Chem. Eng. Sci. 2020, 220. [Google Scholar] [CrossRef]
- An, N.; Wang, H.; Zhang, M.; Xie, J.; Dai, Y.; Yuan, X.; Geng, L. Ultrafine Ru Species within Confined Space: An Efficient Adsorbent for Ultra-Deep Desulfurization of Benzene. Chemosphere 2020, 256, 127077. [Google Scholar] [CrossRef] [PubMed]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Malpartida, I.; Alemany, L.J. Transient Study of the Dry Reforming of Methane over Pt supported on Different γ-Al2O3. Catal. Today 2010, 149, 380–387. [Google Scholar] [CrossRef]
- Lee, S.; Zaborenko, N.; Varma, A. Acetophenone Hydrogenation on Rh/Al2O3 Catalyst: Flow Regime Effect and Trickle Bed Reactor Modeling. Chem. Eng. J. 2017, 317, 42–50. [Google Scholar] [CrossRef]
- Brandi, F.; Baeumel, M.; Molinari, V.; Shekova, I.; Lauermann, I.; Heil, T.; Antonietti, M.; Al-Naji, M. Nickel on Nitrogen-Doped Carbon Pellets for Continuous-Flow Hydrogenation of Biomass-Derived Compounds in Water. Green Chem. 2020, 22, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, C.; Liu, K.; Wang, Y.; Fan, G.; Sun, S.; Xu, J.; Zhu, Y.; Li, Y. Aqueous-Phase Hydrogenolysis of Glucose to Value-Added Chemicals and Biofuels: A Comparative Study of Active Metals. Biomass Bioenerg 2015, 72, 189–199. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, C.; Jin, F.; Chen, C.; Jiang, L. Ru/TiO2 Catalyst for Selective Hydrogenation of Benzene: Effect of Surface Hydroxyl Groups and Spillover Hydrogen. Appl. Surf. Sci. 2020, 525, 146627. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, H.; Liu, W.; Yin, J.; Xiao, L.; Lu, J.; Zhuang, L. Extraordinary Activity of Mesoporous Carbon Supported Ru toward the Hydrogen Oxidation Reaction in Alkaline Media. J. Power Sources 2020, 461, 228147. [Google Scholar] [CrossRef]
- Amirilargani, M.; Merlet, R.B.; Chu, L.; Nijmeijer, A.; Winnubst, L.; de Smet, L.C.P.M.; Sudhölter, E.J.R. Molecular Separation using Poly (Styrene-co-Maleic Anhydride) Grafted to γ-Alumina: Surface versus Pore Modification. J. Membr. Sci. 2019, 582, 298–306. [Google Scholar] [CrossRef]
- Samadi, N.; Ansari, R.; Khodavirdilo, B. Synthesized Nano Particle Derivation of Poly (Styrene -co-Maleic Anhydride) and Sour Cherry Rock for Removing Nickel(II) Ion from Aqueous Solutions. Toxicol. Rep. 2019, 6, 590–597. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Liu, T.; Wu, W. Recovery of Uranium(VI) from Aqueous Solution by 2-Picolylamine Functionalized Poly (Styrene-co-Maleic Anhydride) Resin. J. Colloid Interface Sci. 2017, 497, 385–392. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Wang, K.; Tang, Z.; Chen, S. High-Performance Ru-Based Electrocatalyst Composed of Ru Nanoparticles and Ru Single Atoms for Hydrogen Evolution Reaction in Alkaline Solution. Int. J. Hydrog. Energy 2020, 45, 18840–18849. [Google Scholar] [CrossRef]
- Li, L.; Cai, J.; Liu, Y.; Ni, J.; Lin, B.; Wang, X.; Au, C.; Jiang, L. Zeolite-Seed-Directed Ru Nanoparticles Highly Resistant against Sintering for Efficient Nitrogen Activation to Ammonia. Sci. Bull. 2020, 65, 1085–1093. [Google Scholar] [CrossRef]
- Zhao, F.; Yi, L.; Deng, R.; You, K.; Song, J.; Jian, J.; Liu, P.; Ai, Q.; Luo, H. Supported WO3/γ-Al2O3 as Bifunctional Catalyst for Liquid-Phase Highly Selective Oxidation of Cyclohexylamine to Cyclohexanone Oxime under Solvent-Free Conditions. Mol. Catal. 2019, 475, 110494. [Google Scholar] [CrossRef]
- Zhao, J.P.; Hernández, W.Y.; Zhou, W.J.; Yang, Y.; Vovk, E.I.; Wu, M.; Naghavi, N.; Capron, M.; Ordomsky, V. Nanocell type Ru@Quinone Core-Shell Catalyst for Selective Oxidation of Alcohols to Carbonyl Compounds. Appl Catal. A Gen. 2020, 602, 117693. [Google Scholar] [CrossRef]
- Lv, L.; Wang, S.; Ding, Y.; Zhang, L.; Gao, Y.; Wang, S. Deactivation Mechanism and Anti-Deactivation Modification of Ru/TiO2 Catalysts for CH3Br Oxidation. Chemosphere 2020, 257, 127249. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Ribeiro, L.S.; Órfão, J.J.M.; Figueiredo, J.L.; Pereira, M.F.R. Catalytic Conversion of Cellulose to Sorbitol over Ru Supported on Biomass-Derived Carbon-Based Materials. Appl. Catal. B Environ. 2019, 256. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, T.; Fang, Y. High-Efficient Preparation of Gasoline-Ranged C5–C6 Alkanes from Biomass-Derived Sugar Polyols of Sorbitol over Ru-MoO3−x/C catalyst. Fuel Process. Technol. 2019, 183, 19–26. [Google Scholar] [CrossRef]
- Dosso, L.A.; Vera, C.R.; Grau, J.M. Aqueous Phase Reforming of Polyols from Glucose Degradation by Reaction over Pt/Alumina Catalysts Modified by Ni or Co. Int J. Hydrog. Energy 2017, 42, 18853–18864. [Google Scholar] [CrossRef]
- Schimpf, S.; Louis, C.; Claus, P. Ni/SiO2 Catalysts Prepared with Ethylenediamine Nickel Precursors: Influence of the Pretreatment on the Catalytic Properties in Glucose Hydrogenation. Appl. Catal. A Gen. 2007, 318, 45–53. [Google Scholar] [CrossRef]
- Zada, B.; Yan, L.; Fu, Y. Effective Conversion of Cellobiose and Glucose to Sorbitol using Non-Noble Bimetallic NiCo/HZSM-5 Catalyst. Sci. China Chem. 2018, 61, 1167–1174. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yang, X.; Wang, W.; Liang, J.; Orooji, Y.; Dai, C.; Fu, X.; Yang, Y.; Xu, W.; Zhu, J. Efficient Sorbitol Producing Process through Glucose Hydrogenation Catalyzed by Ru Supported Amino Poly (Styrene-co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3. Catalysts 2020, 10, 1068. https://doi.org/10.3390/catal10091068
Zhao J, Yang X, Wang W, Liang J, Orooji Y, Dai C, Fu X, Yang Y, Xu W, Zhu J. Efficient Sorbitol Producing Process through Glucose Hydrogenation Catalyzed by Ru Supported Amino Poly (Styrene-co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3. Catalysts. 2020; 10(9):1068. https://doi.org/10.3390/catal10091068
Chicago/Turabian StyleZhao, Jing, Xiaorui Yang, Wei Wang, Jinhua Liang, Yasin Orooji, Chaowen Dai, Xiaomin Fu, Yunsong Yang, Wenlong Xu, and Jianliang Zhu. 2020. "Efficient Sorbitol Producing Process through Glucose Hydrogenation Catalyzed by Ru Supported Amino Poly (Styrene-co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3" Catalysts 10, no. 9: 1068. https://doi.org/10.3390/catal10091068







