Comparison of Ag and AgI-Modified ZnO as Heterogeneous Photocatalysts for Simulated Sunlight Driven Photodegradation of Metronidazole
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Composition of Ag and AgI-Modified ZnO Photocatalysts
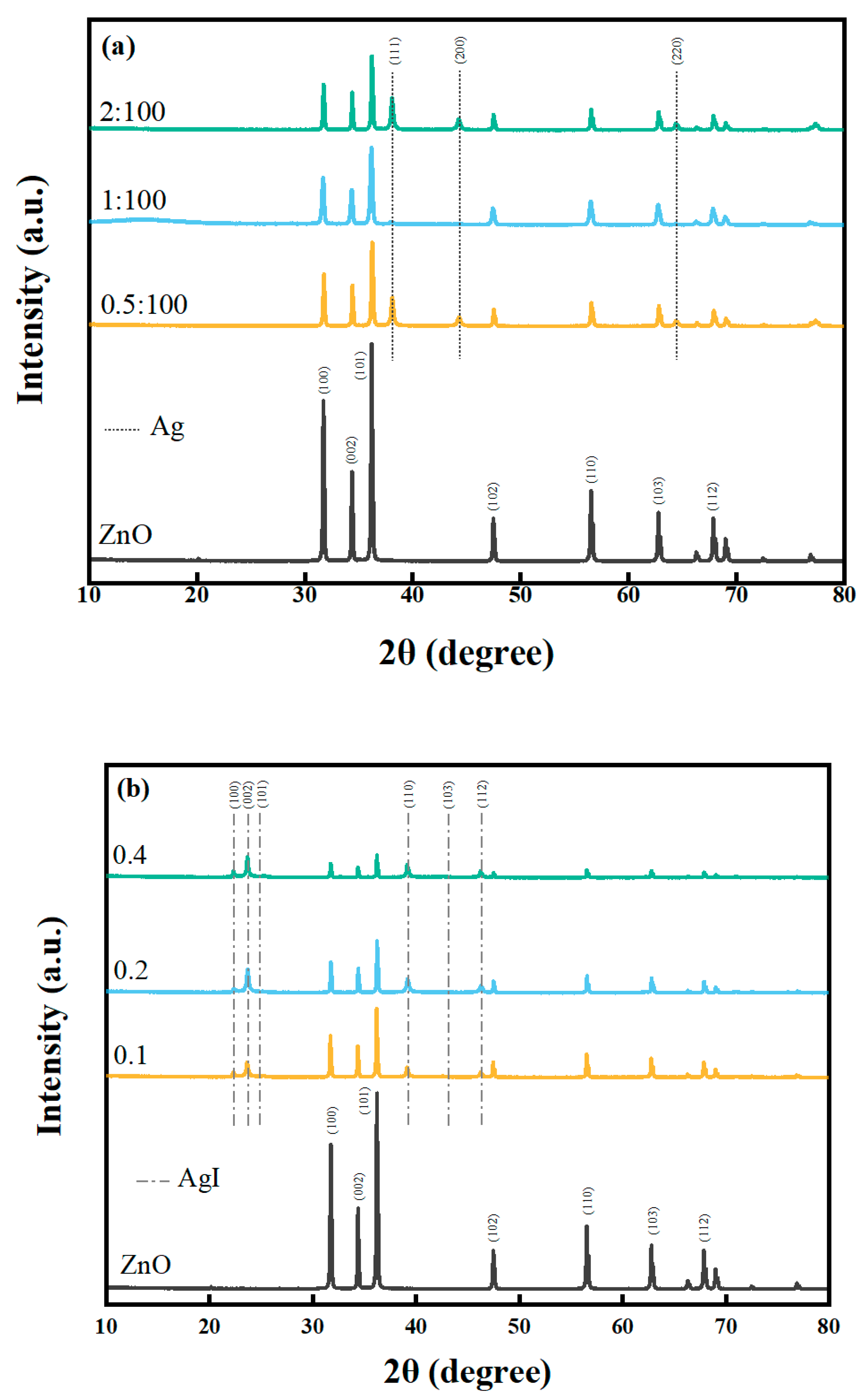
2.1.1. XRD
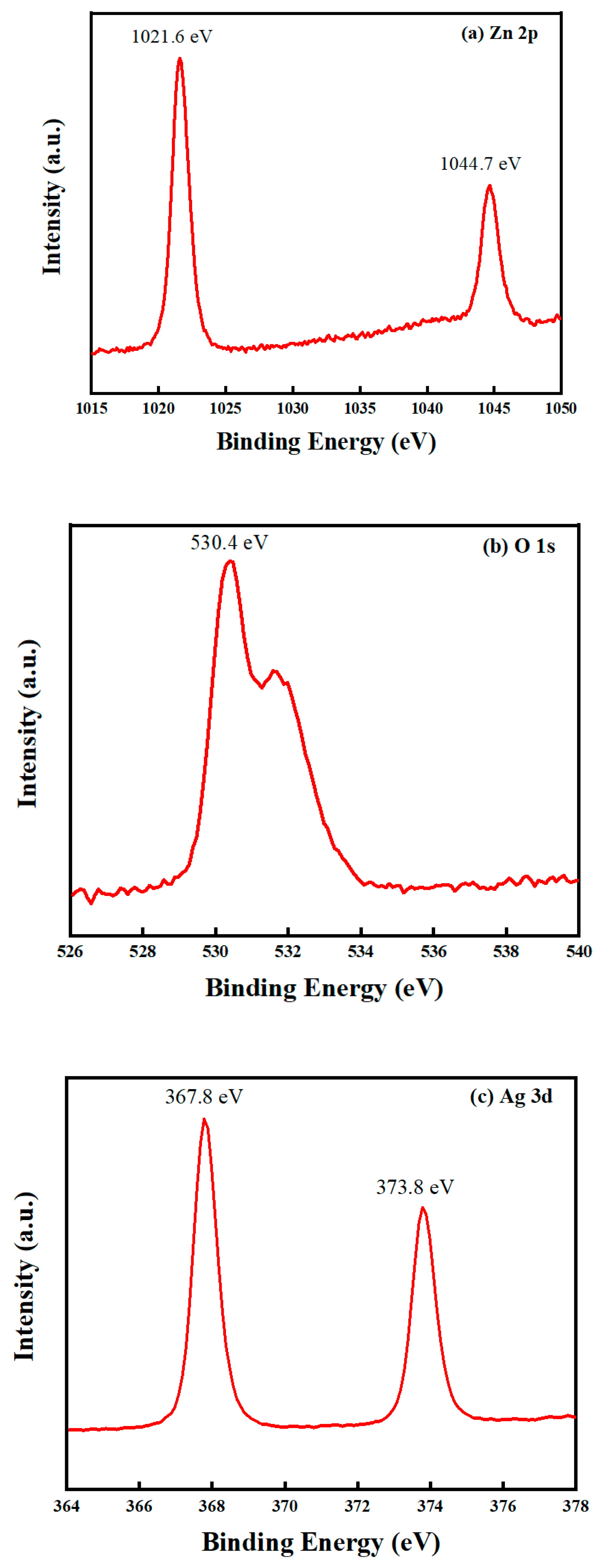
2.1.2. XPS
2.2. Optical Properties of Ag and AgI-Modified ZnO Photocatalysts
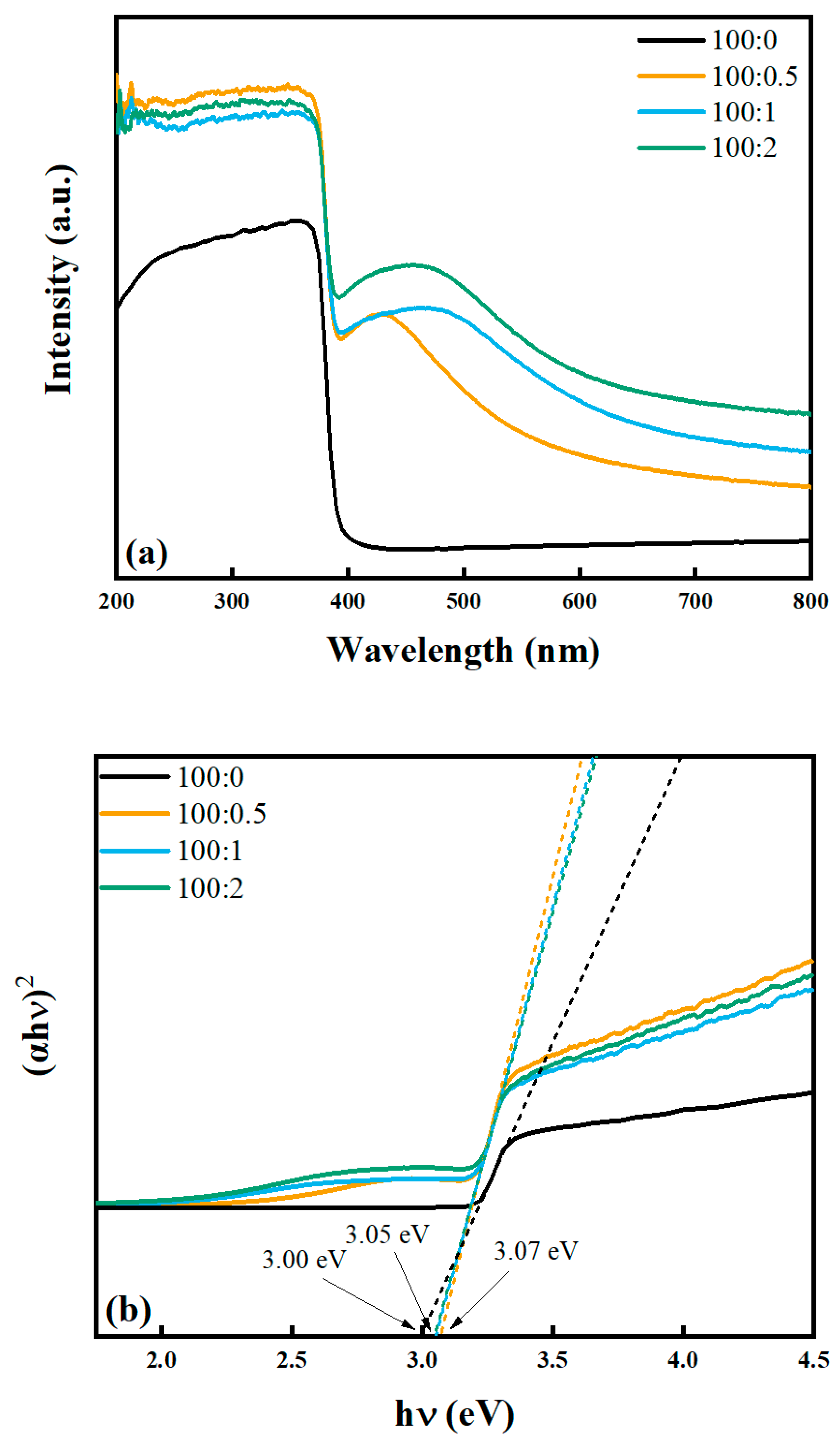
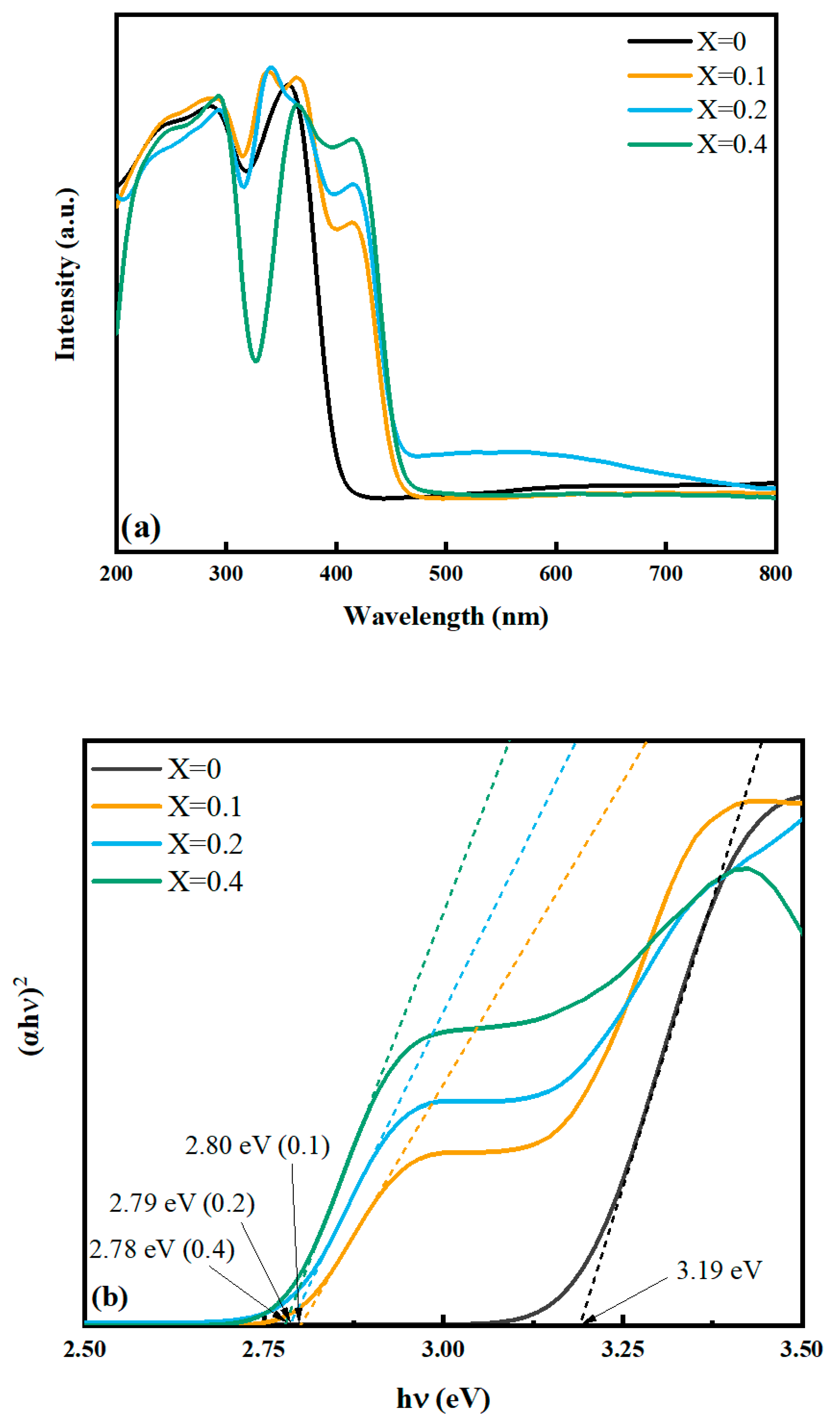
2.2.1. UV-Vis Diffuse Reflectance Spectra
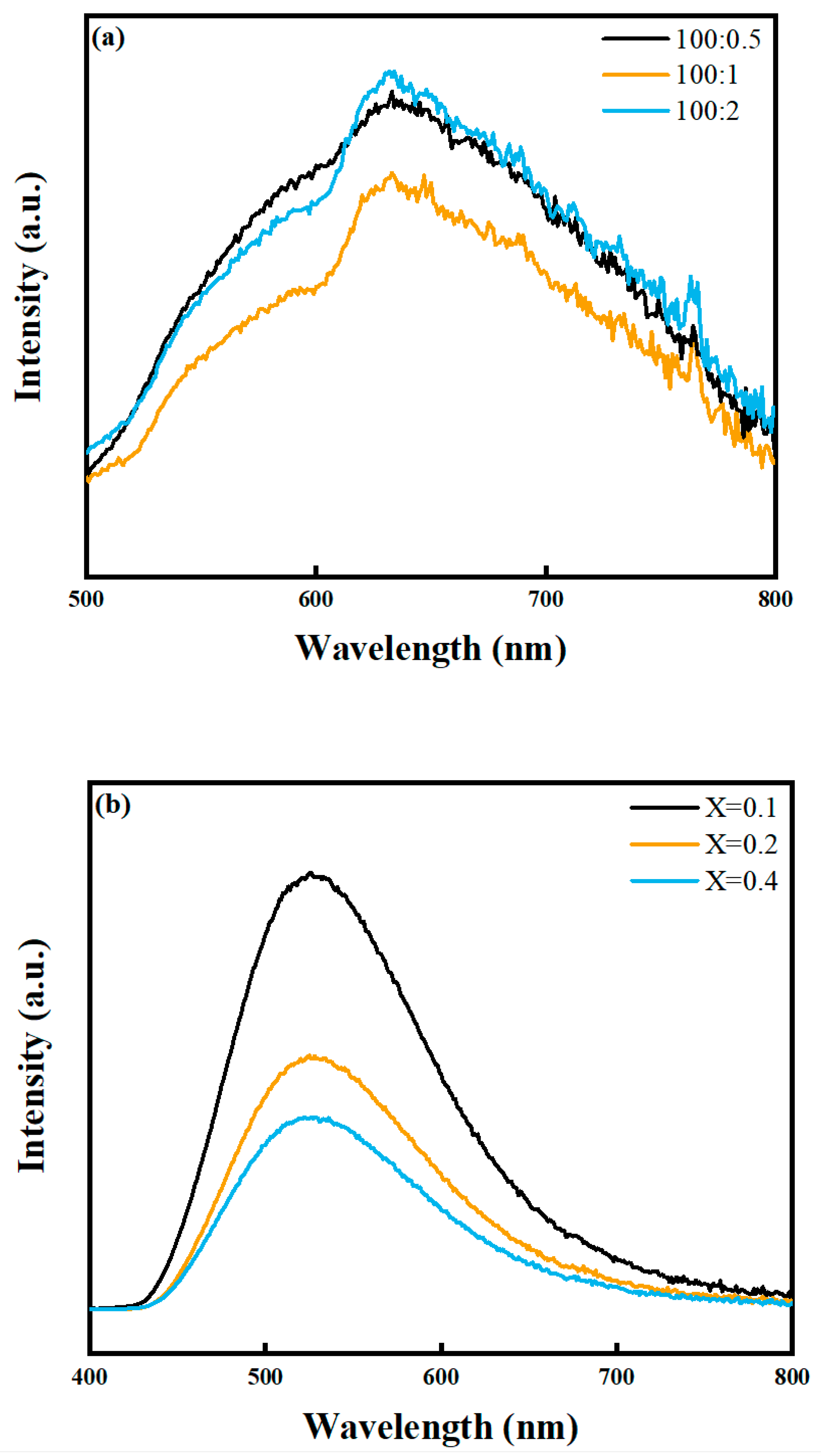
2.2.2. PL Spectra
2.3. Morphology of Ag and AgI-Modified ZnO Photocatalysts
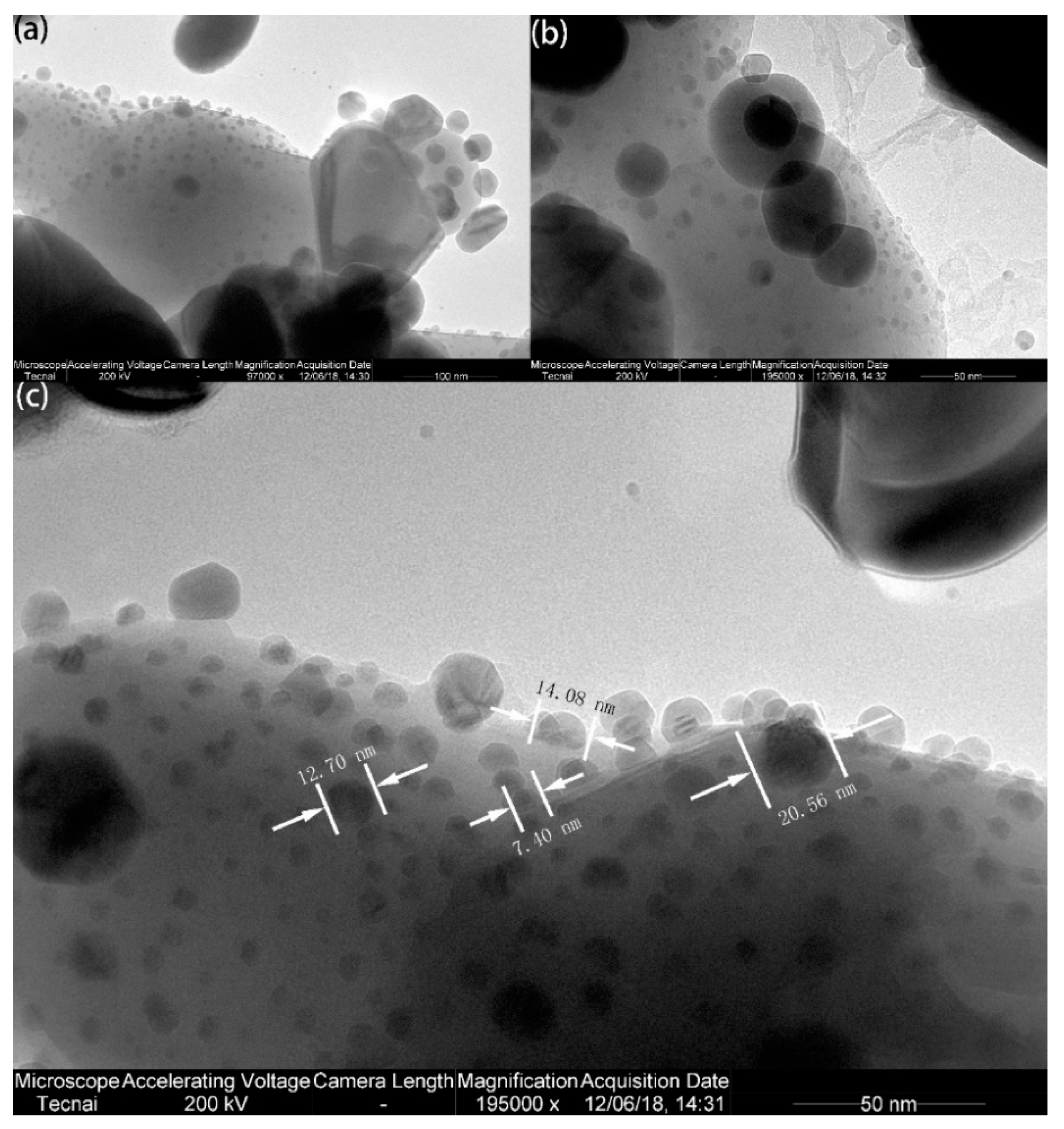
2.3.1. TEM
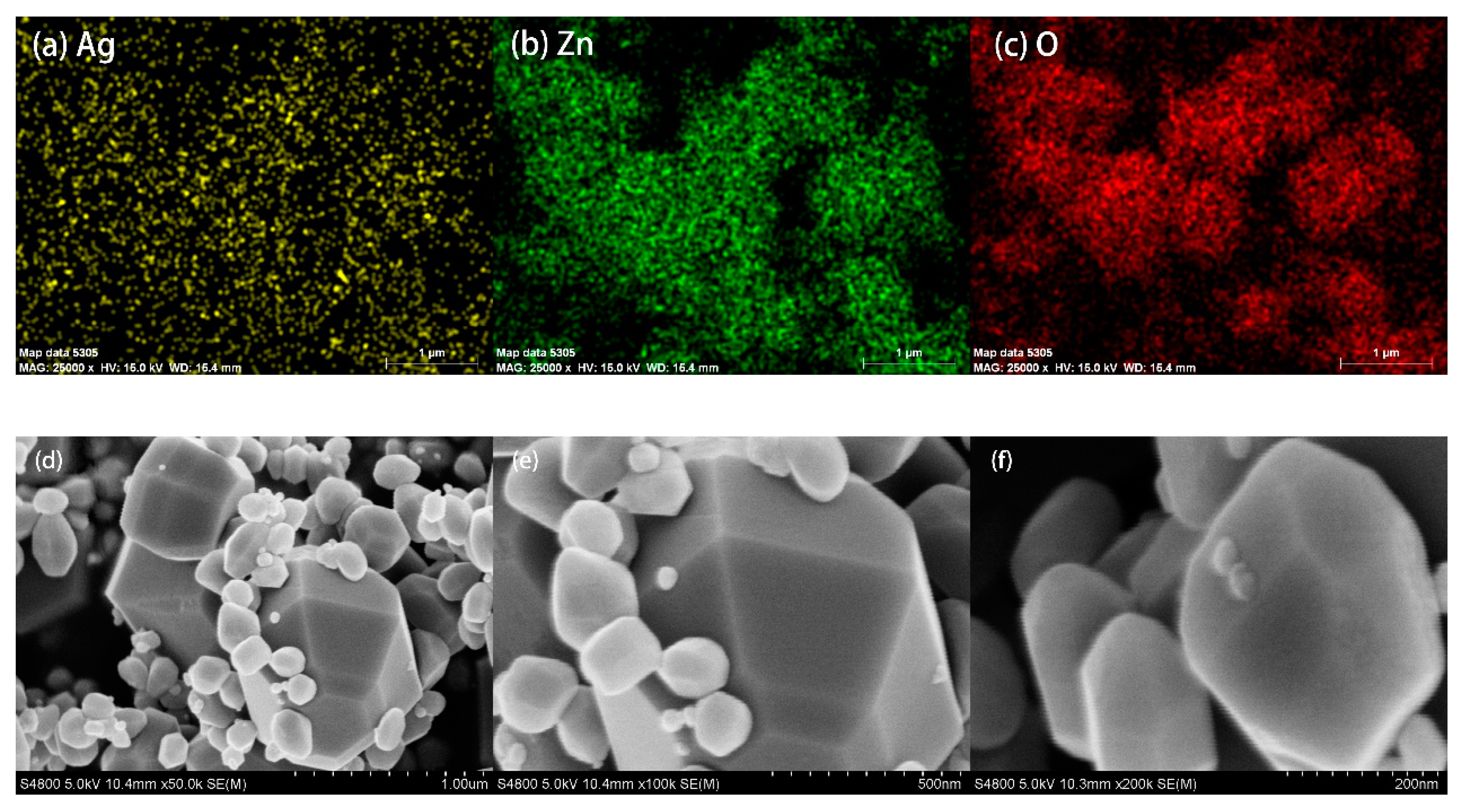
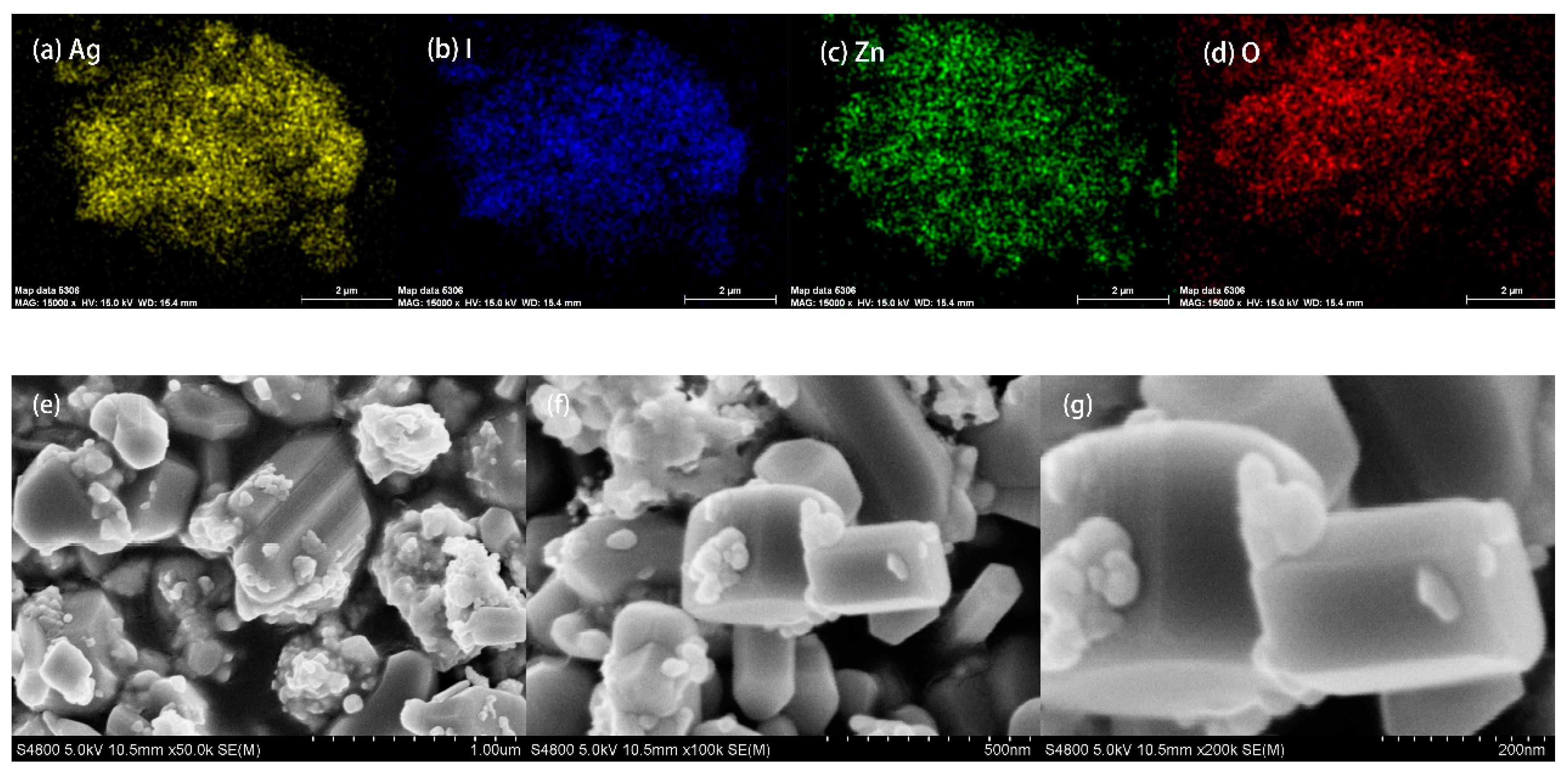
2.3.2. SEM and EDS Mapping
2.4. The Photodegradation of Metronidazole under Simulated Sunlight Irradiation
2.4.1. The Effect of Ag and AgI Loading
2.4.2. Initial Concentration of MNZ
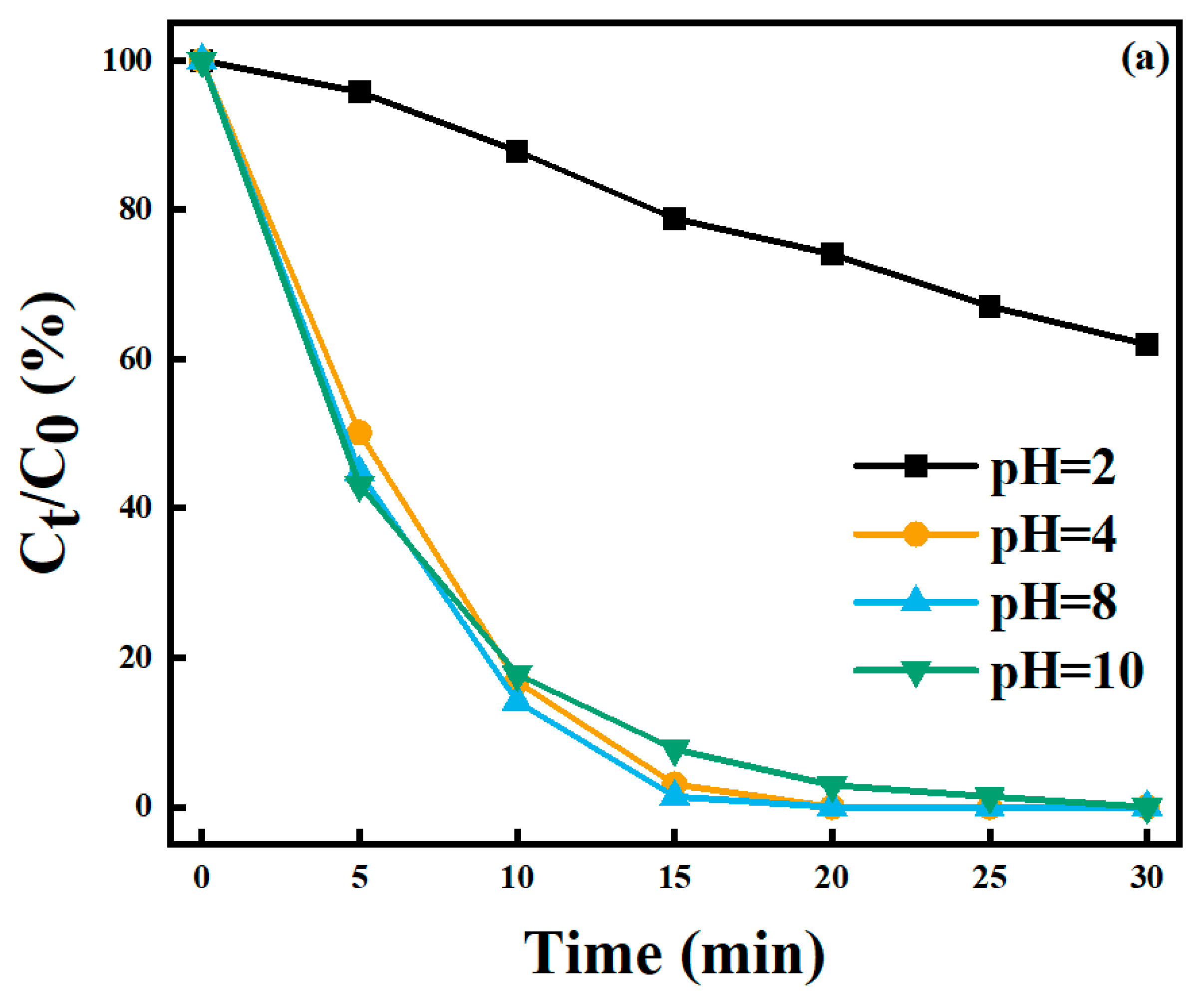
2.4.3. pH
2.5. Mechanism of Photocatalytic Reactions
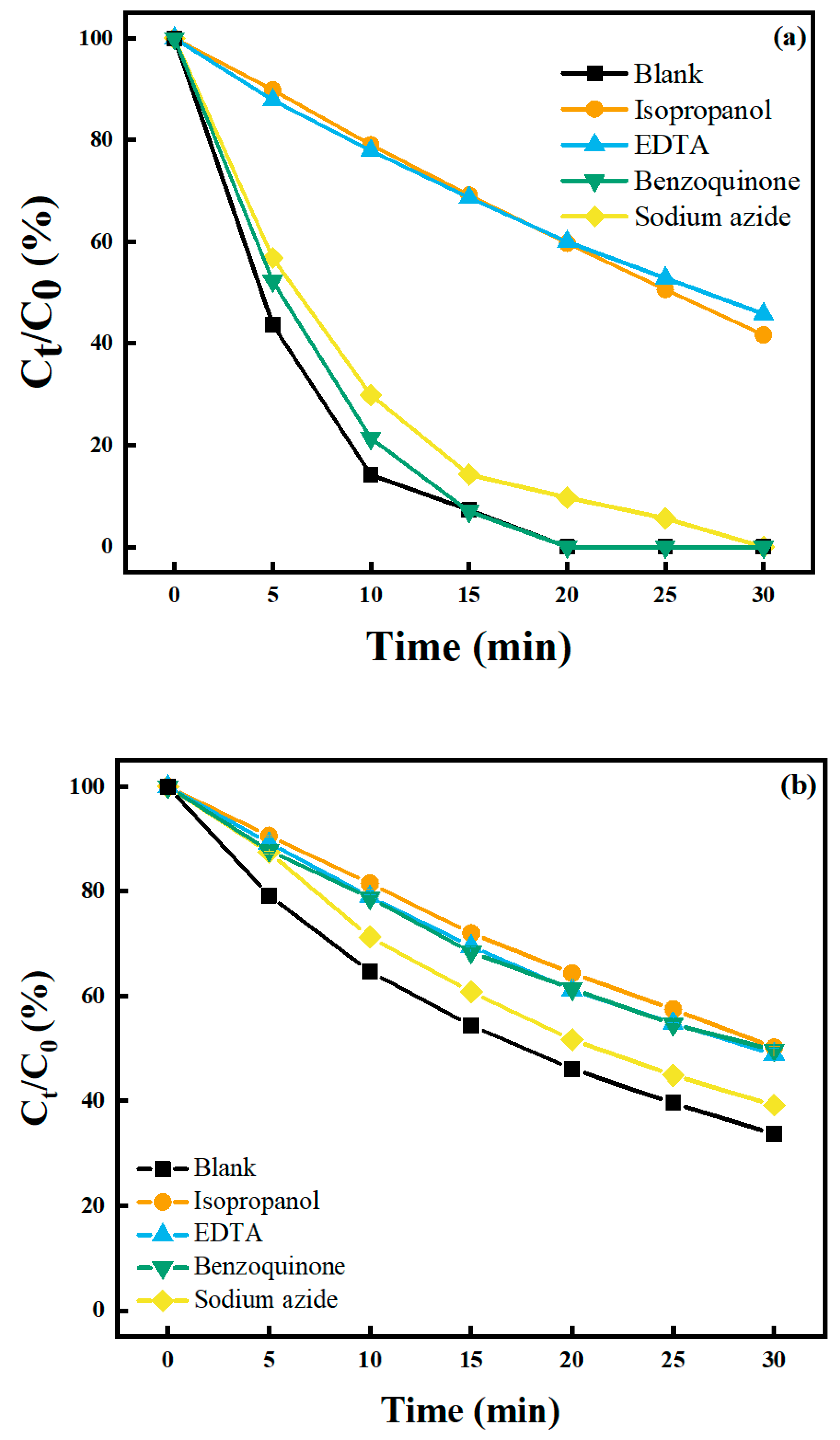
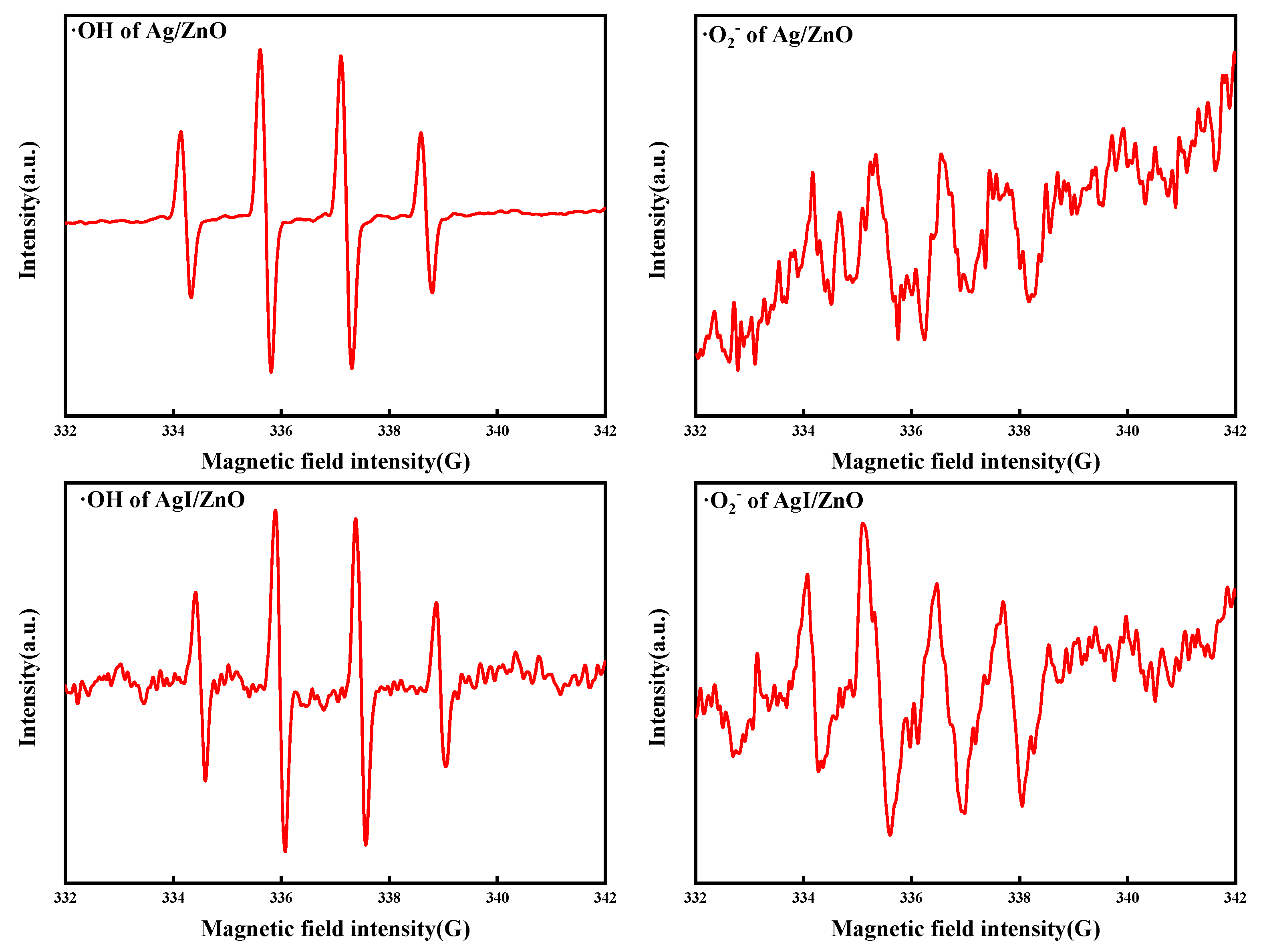
2.5.1. Validating the Presence of Reactive Oxygen Species
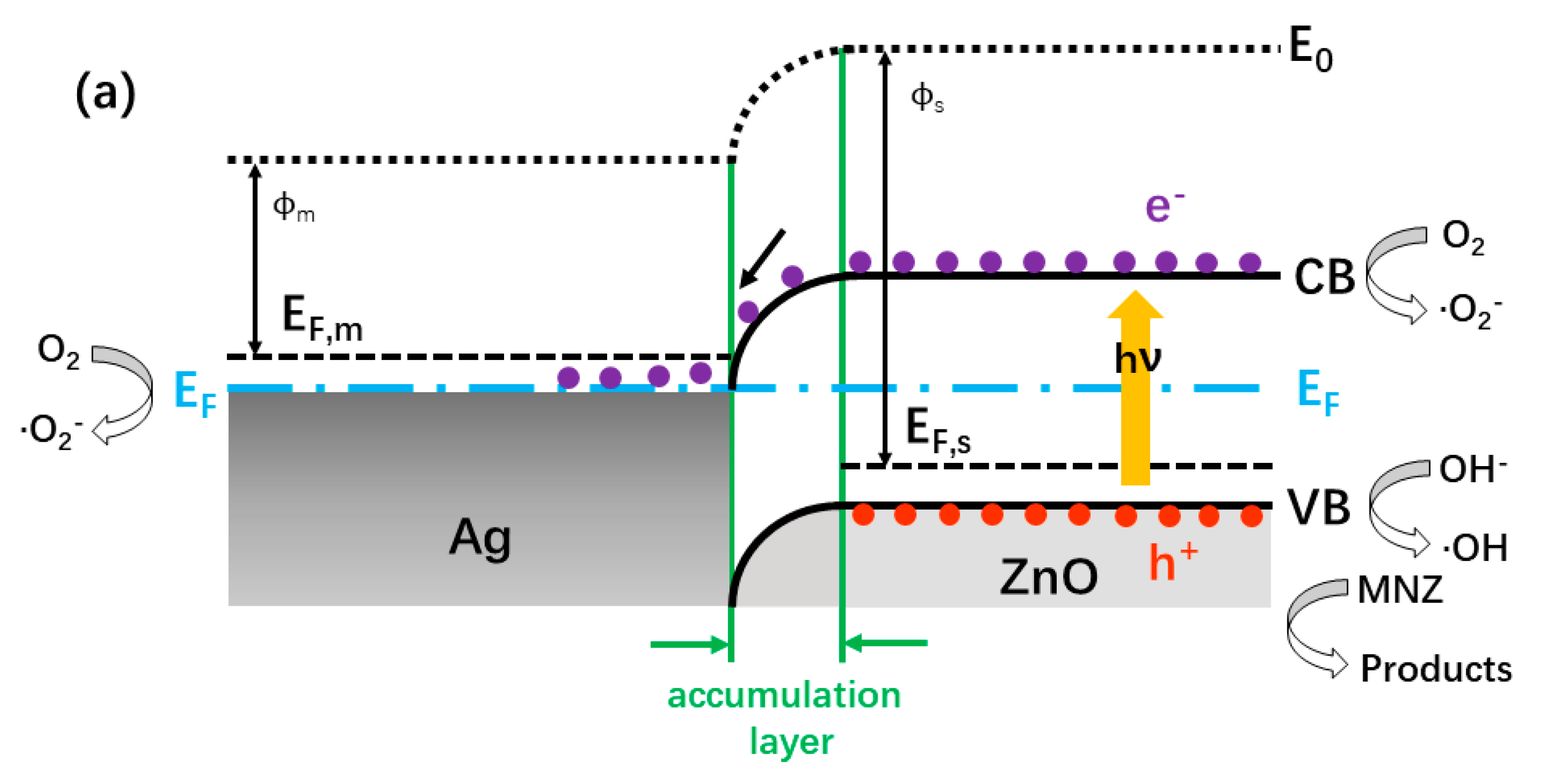
2.5.2. Proposed Photocatalytic Mechanism
2.6. Identification of Possible Intermediates during Photocatalytic Degradation
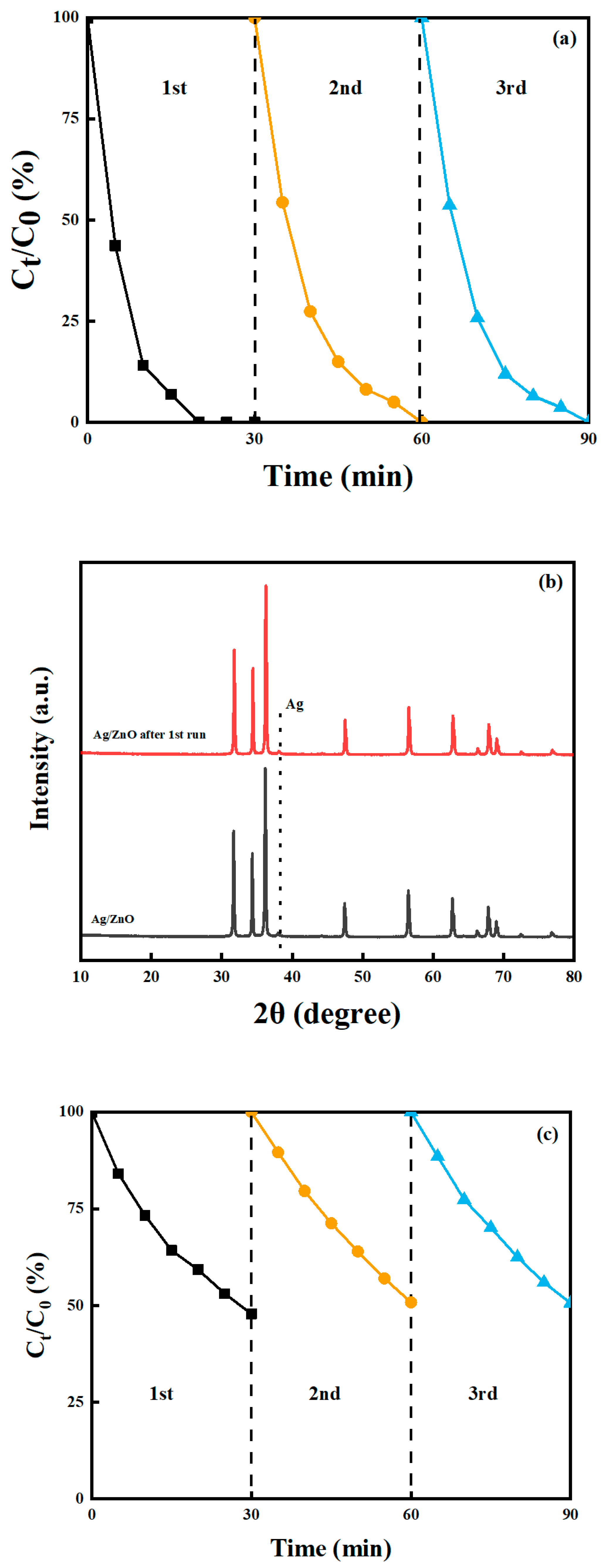
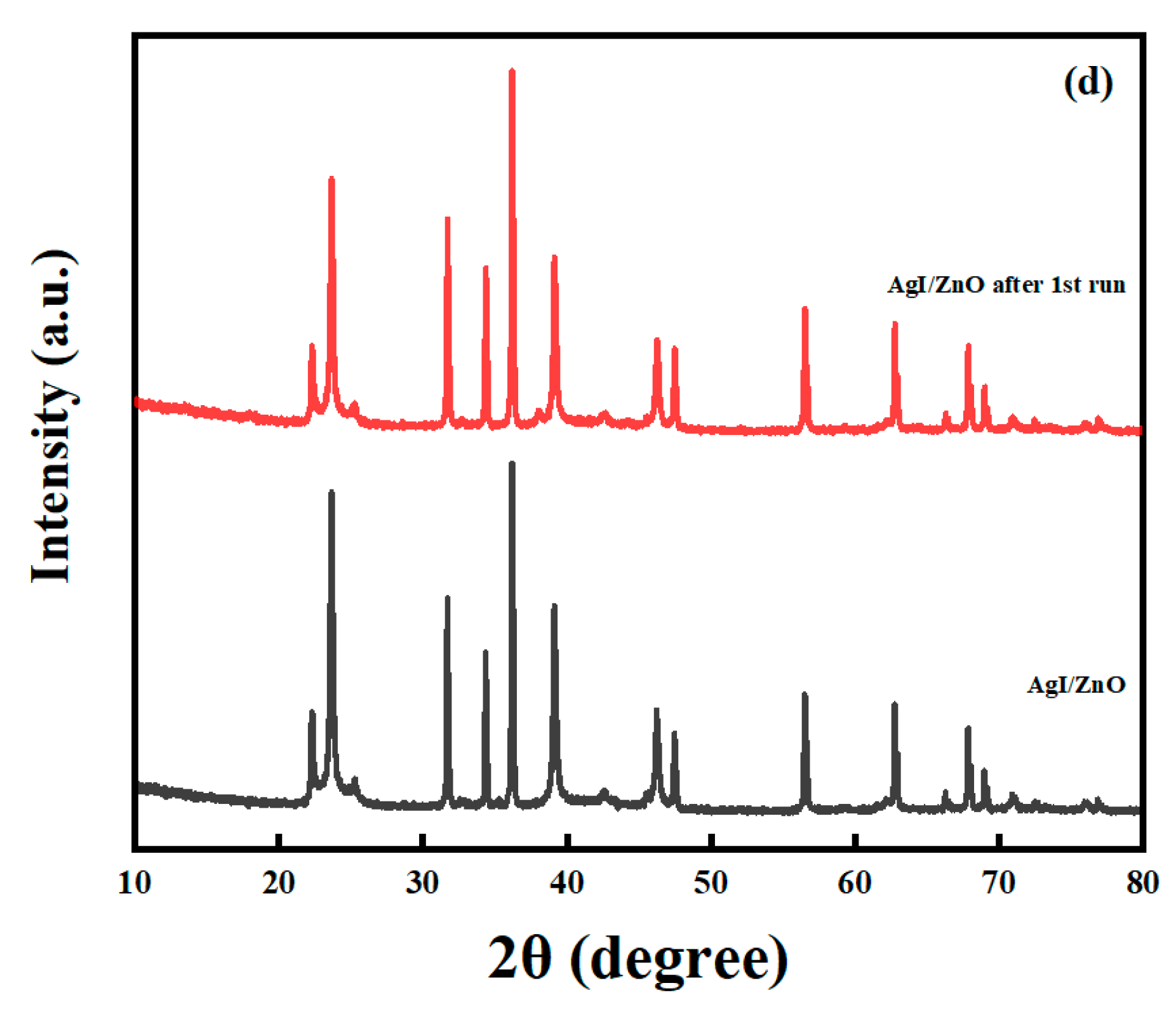
2.7. Stability of the Photocatalysts
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Catalysts
3.3. Characterization
3.4. Evaluation of Photocatalytic Activity
3.5. Evaluation of Reactive Oxygen Species
3.6. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al, A.E.; Elasala, G.S.; Ibrahim, R.S. Synthesis, Characterization, spectral, thermal analysis and Biological Activity studies of metronidazole complexes. J. Mol. Struct. 2018, 1176, 673–684. [Google Scholar] [CrossRef]
- Santana, D.R.; Espino-Estévez, M.R.; Santiago, D.E.; Méndez, J.A.O.; González-Díaz, O.; Doña-Rodríguez, J.M. Treatment of aquaculture wastewater contaminated with metronidazole by advanced oxidation techniques. Environ. Nanotechnol. Monit. Manag. 2017, 8, 11–24. [Google Scholar] [CrossRef]
- Aboudalle, A.; Djelal, H.; Fourcade, F.; Domergue, L.; Assadi, A.A.; Lendormi, T.; Taha, S.; Amrane, A. Metronidazole removal by means of a combined system coupling an electro-Fenton process and a conventional biological treatment: By-products monitoring and performance enhancement. J. Hazard. Mater. 2018, 359, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Gao, L.; Zhao, Y.; Peng, W.; Chen, Z. Simultaneous determination of metronidazole, chloramphenicol and 10 sulfonamide residues in honey by LC–MS/MS. Anal. Methods 2013, 5, 1283–1288. [Google Scholar] [CrossRef]
- Gomez, M.J.; Malato, O.; Ferrer, I.; Aguera, A.; Fernandez-Alba, A.R. Solid-phase extraction followed by liquid chromatography-time-of-flight-mass spectrometry to evaluate pharmaceuticals in effluents. A pilot monitoring study. J. Environ. Monit. 2007, 9, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodriguez, A.; Perdigon-Melon, J.A.; Petre, A.; Garcia-Calvo, E.; Gomez, M.J.; Aguera, A.; Fernandez-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Hilla, S.K.; Kunukcu, Y.K.; Linden, K.G. Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 2006, 63, 269–276. [Google Scholar]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. B Environ. 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Dantas, R.F.; Rossiter, O.; Teixeira, A.K.R.; Simões, A.S.M.; Da Silva, V.L.D. Direct UV photolysis of propranolol and metronidazole in aqueous solution. Chem. Eng. J. 2010, 158, 143–147. [Google Scholar] [CrossRef]
- Aboudalle, A.; Fourcade, F.; Assadi, A.A.; Domergue, L.; Djelal, H.; Lendormi, T.; Taha, S.; Amrane, A. Reactive oxygen and iron species monitoring to investigate the electro-Fenton performances. Impact of the electrochemical process on the biodegradability of metronidazole and its by-products. Chemosphere 2018, 199, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, M.; Tian, X.; Nie, Y.; Yang, C.; Lin, H.-M.; Luo, W.; Wang, Y. Safe and efficient degradation of metronidazole using highly dispersed beta-FeOOH on palygorskite as heterogeneous Fenton-like activator of hydrogen peroxide. Chemosphere 2019, 236, 124367. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.Q.; Tu, G.Q.; Zhao, D.Y.; Tsang, P.E.; Fang, Z.Q. Pyrolysis of different biomass pre-impregnated with steel pickling waste liquor to prepare magnetic biochars and their use for the degradation of metronidazole. Bioresour. Technol. 2019, 289, 121613. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, X.; Fu, K.; Deng, H.; Shi, J. Degradation of metronidazole by UV/chlorine treatment: Efficiency, mechanism, pathways and DBPs formation. Chemosphere 2019, 224, 228–236. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, G.C.; Wu, J.; Lu, J.; Zhang, Z.H. Simultaneous catalytic ozonation degradation of metronidazole and removal of heavy metal from aqueous solution using nano-magnesium hydroxide. Environ. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Esrafili, A.; Baghapour, M.A.; Shahamat, Y.D.; Okhovat, N. Degradation of metronidazole in aqueous solution by nano-ZnO/UV photocatalytic process. Desalin. Water Treat. 2014, 52, 4947–4952. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Gonçalves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.; Juang, R. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Tran, M.L.; Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Hybridizing Ag-Doped ZnO nanoparticles with graphite as potential photocatalysts for enhanced removal of metronidazole antibiotic from water. J. Environ. Manag. 2019, 252, 109611. [Google Scholar] [CrossRef]
- Bahareh, K.; Habibi, M.H. High photocatalytic activity of light-driven Fe2TiO5 nanoheterostructure toward degradation of antibiotic metronidazole. J. Ind. Eng. Chem. 2019, 80, 292–300. [Google Scholar] [CrossRef]
- Pawar, R.C.; Lee, C.S. Single-step sensitization of reduced graphene oxide sheets and CdS nanoparticles on ZnO nanorods as visible-light photocatalysts. Appl. Catal. B Environ. 2014, 144, 57–65. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.; Rojas, H.; Navío, J.A.; Hidalgo, M.C. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Sun, F.Z.; Qiao, X.L.; Tan, F.T.; Wang, W.; Qiu, X.L. One-step microwave synthesis of Ag/ZnO nanocomposites with enhanced photocatalytic performance. J. Mater. Sci. 2012, 47, 7262–7268. [Google Scholar] [CrossRef]
- Fei, J.B.; Li, J.B. Controlled Preparation of Porous TiO2-Ag Nanostructures through Supramolecular Assembly for Plasmon-Enhanced Photocatalysis. Adv. Mater. 2014, 27, 314–319. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Sau, T.K.; Pal, T. Growing small silver particle as redox catalyst. J. Phys. Chem. B 1999, 103, 115–121. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Zhang, L. New insight into daylight photocatalysis of AgBr@Ag: Synergistic effect between semiconductor photocatalysis and plasmonic photocatalysis. Chemistry 2012, 18, 6360–6369. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. One-step synthesis of the nanostructured AgI/BiOI composites with highly enhanced visible-light photocatalytic performances. Langmuir 2010, 26, 6618–6624. [Google Scholar] [CrossRef]
- Victora, R.H. Calculated electronic structure of silver halide crystals. Phys. Rev. B 1997, 56, 4417–4421. [Google Scholar] [CrossRef]
- Wang, X.; Wan, X.; Xu, X.; Chen, X. Facile fabrication of highly efficient AgI/ZnO heterojunction and its application of methylene blue and rhodamine B solutions degradation under natural sunlight. Appl. Surf. Sci. 2014, 321, 10–18. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. One pot synthesis and characterization of Ag-ZnO/g-C 3 N 4 photocatalyst with improved photoactivity and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 477–484. [Google Scholar] [CrossRef]
- Shaker-Agjekandy, S.; Habibi-Yangjeh, A. Facile one-pot method for preparation of AgI/ZnO nanocomposites as visible-light-driven photocatalysts with enhanced activities. Mater. Sci. Semicond. Process. 2015, 34, 74–81. [Google Scholar] [CrossRef]
- Jaramillo-Paez, C.; Navio, J.A.; Hidalgo, M.C.; Macias, M. High UV-photocatalytic activity of ZnO and Ag/ZnO synthesized by a facile method. Catal. Today 2017, 284, 121–128. [Google Scholar] [CrossRef]
- Gao, L.K.; Gan, W.T.; Xiao, S.L.; Zhan, X.X.; Li, J. A robust superhydrophobic antibacterial Ag-TiO2 composite film immobilized on wood substrate for photodegradation of phenol under visible-light illumination. Ceram. Int. 2016, 42, 2170–2179. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Palominos, R.; Freer, J.; Mondaca, M.A.; Mansilla, H.D. Evidence for hole participation during the photocatalytic oxidation of the antibiotic flumequine. J. Photochem. Photobiol. A-Chem. 2008, 193, 139–145. [Google Scholar] [CrossRef]
- Chamjangali, M.A.; Bagherian, G.; Javid, A.; Boroumand, S.; Farzaneh, N. Synthesis of Ag-ZnO with multiple rods (multipods) morphology and its application in the simultaneous photo-catalytic degradation of methyl orange and methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 230–237. [Google Scholar] [CrossRef]
- Sun, L.; Shao, R.; Tang, L.; Chen, Z. Synthesis of ZnFe2O4/ZnO nanocomposites immobilized on graphene with enhanced photocatalytic activity under solar light irradiation. J. Alloys Compd. 2013, 564, 55–62. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Deng, H. Ag modified g-C 3 N 4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Mol. Catal. A Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Shu, J.X.; Wang, Z.H.; Xia, G.Q.; Zheng, Y.Y.; Yang, L.H.; Zhang, W. One-pot synthesis of AgCl@Ag hybrid photocatalyst with high photocatalytic activity and photostability under visible light and sunlight irradiation. Chem. Eng. J. 2014, 252, 374–381. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Kim, T.K. Controlled synthesis of heterostructured Ag@AgI/ZnS microspheres with enhanced photocatalytic activity and selective separation of methylene blue from mixture dyes. J. Taiwan Inst. Chem. Eng. 2016, 66, 200–209. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Enhanced Raman scattering and photocatalytic activity of Ag/ZnO heterojunction nanocrystals. Dalton Trans. 2011, 40, 9566–9570. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Lee, J.; Cho, M.H. Biogenic Synthesis, Photocatalytic, and Photoelectrochemical Performance of Ag–ZnO Nanocomposite. J. Phys. Chem. C 2013, 117, 27023–27030. [Google Scholar] [CrossRef]
- Sarma, B.; Sarma, B.K. Fabrication of Ag/ZnO heterostructure and the role of surface coverage of ZnO microrods by Ag nanoparticles on the photophysical and photocatalytic properties of the metal-semiconductor system. Appl. Surf. Sci. 2017, 410, 557–565. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Liu, H.R.; Shao, G.X.; Zhao, J.F.; Zhang, Z.X.; Zhang, Y.; Liang, J.; Liu, X.G.; Jia, H.S.; Xu, B.S. Worm-Like Ag/ZnO Core–Shell Heterostructural Composites: Fabrication, Characterization, and Photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. [Google Scholar] [CrossRef]
- LiQiang, J.; Dejun, W.; Baiqi, W.; Shudan, L.; Baifu, X.; Honggang, F.; Jiazhong, S. Effects of noble metal modification on surface oxygen composition, charge separation and photocatalytic activity of ZnO nanoparticles. J. Mol. Catal. A Chem. 2006, 244, 193–200. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 Surfaces—Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Huang, H.; Huang, N.; Wang, Z.; Xia, G.; Chen, M.; He, L.; Tong, Z.; Ren, C. Room-temperature synthesis of carnation-like ZnO@AgI hierarchical nanostructures assembled by AgI nanoparticles-decorated ZnO nanosheets with enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 502, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Xiao, X.H.; Xu, J.X.; Cai, G.X.; Ren, F.; Jiang, C.Z. Mechanism of the enhancement and quenching of ZnO photoluminescence by ZnO-Ag coupling. EPL (Europhys. Lett.) 2011, 93, 57009. [Google Scholar] [CrossRef]
- Lin, D.; Wu, H.; Zhang, R.; Pan, W. Enhanced Photocatalysis of Electrospun Ag−ZnO Heterostructured Nanofibers. Chem. Mater. 2009, 21, 3479–3484. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Shaker-Agjekandy, S.; Habibi-Yangjeh, A. Ultrasonic-assisted preparation of novel ternary ZnO/AgI/Ag2CrO4 nanocomposites as visible-light-driven photocatalysts with excellent activity. Mater. Sci. Semicond. Process. 2016, 44, 48–56. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an Environmental Photocatalyst that Generates OH Radicals under Visible Light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Chen, Y.T.; Ren, F.F.; Gu, S.L.; Tan, H.H.; Jagadish, C.; Ye, J.D. Band alignment and band bending at α-Ga2O3/ZnO n-n isotype hetero-interface. Appl. Phys. Lett. 2019, 115, 202101. [Google Scholar] [CrossRef]
- Wu, D.; Long, M. Realizing Visible-Light-Induced Self-Cleaning Property of Cotton through Coating N-TiO2 Film and Loading AgI Particles. ACS Appl. Mater. Interfaces 2011, 3, 4770–4774. [Google Scholar] [CrossRef]
- Pérez, T.; Garcia-Segura, S.; El-Ghenymy, A.; Nava, J.L.; Brillas, E. Solar photoelectro-Fenton degradation of the antibiotic metronidazole using a flow plant with a Pt/air-diffusion cell and a CPC photoreactor. Electrochim. Acta 2015, 165, 173–181. [Google Scholar] [CrossRef]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Green electrochemical process for metronidazole degradation at BDD anode in aqueous solutions via direct and indirect oxidation. Sep. Purif. Technol. 2016, 157, 9–16. [Google Scholar] [CrossRef]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Enhanced degradation of metronidazole by sunlight via photo-Fenton process under gradual addition of hydrogen peroxide. J. Mol. Catal. A Chem. 2016, 420, 222–227. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Fu, K.; Pan, Y.; Liu, J.; Deng, H.; Shi, J. Comparison of Ag and AgI-Modified ZnO as Heterogeneous Photocatalysts for Simulated Sunlight Driven Photodegradation of Metronidazole. Catalysts 2020, 10, 1097. https://doi.org/10.3390/catal10091097
Ding C, Fu K, Pan Y, Liu J, Deng H, Shi J. Comparison of Ag and AgI-Modified ZnO as Heterogeneous Photocatalysts for Simulated Sunlight Driven Photodegradation of Metronidazole. Catalysts. 2020; 10(9):1097. https://doi.org/10.3390/catal10091097
Chicago/Turabian StyleDing, Chao, Kun Fu, Yishuai Pan, Jia Liu, Huiping Deng, and Jun Shi. 2020. "Comparison of Ag and AgI-Modified ZnO as Heterogeneous Photocatalysts for Simulated Sunlight Driven Photodegradation of Metronidazole" Catalysts 10, no. 9: 1097. https://doi.org/10.3390/catal10091097
APA StyleDing, C., Fu, K., Pan, Y., Liu, J., Deng, H., & Shi, J. (2020). Comparison of Ag and AgI-Modified ZnO as Heterogeneous Photocatalysts for Simulated Sunlight Driven Photodegradation of Metronidazole. Catalysts, 10(9), 1097. https://doi.org/10.3390/catal10091097



