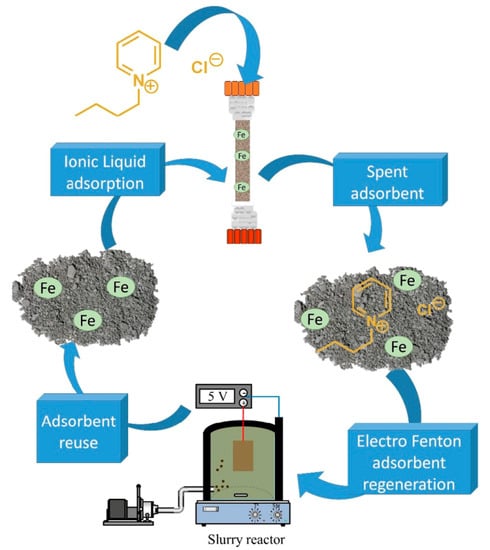
Iron-Loaded Catalytic Silicate Adsorbents: Synthesis, Characterization, Electroregeneration and Application for Continuous Removal of 1-Butylpyridinium Chloride
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorbents Screening
2.2. Desorption Tests
2.3. Catalytic Adsorbent Preparation and Characterization
2.4. Kinetic and Isotherm Adsorption Studies
2.4.1. Kinetics
2.4.2. Isotherms
2.5. Fixed-Bed Adsorption and Breakthrough Curve
2.6. AOPs Adsorbent Regeneration Tests
2.6.1. AOPs Preliminary Assays
2.6.2. EF Treatment
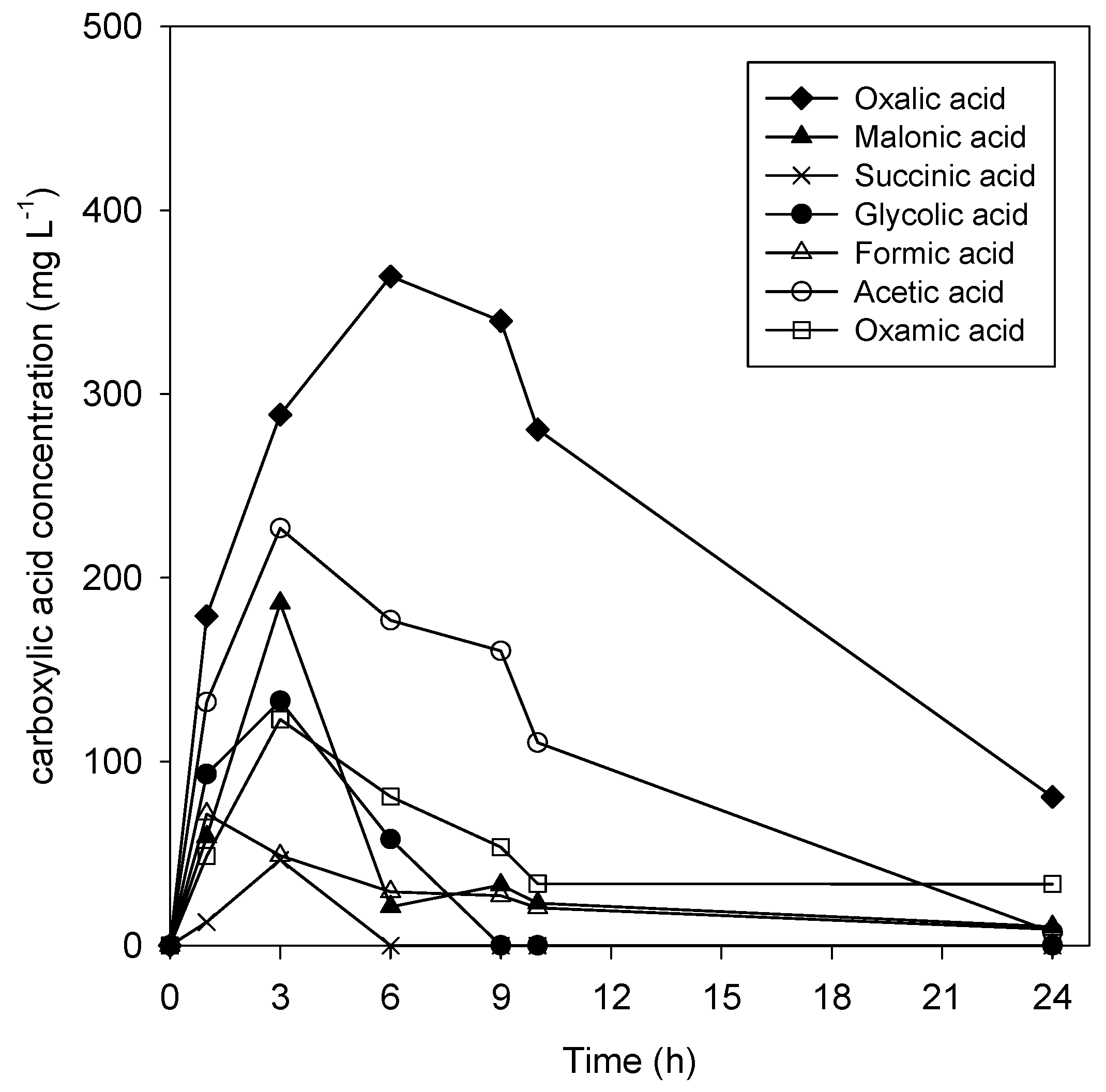
2.7. TOC Measurement and Carboxylic Acids Determination
2.8. Adsorbent Stability and Reuse
2.9. Future Uses
3. Materials and Methods
3.1. Reagents
3.2. Experimental Set-up
3.2.1. Adsorbents Screening
3.2.2. Desorption
3.2.3. Catalytic Adsorbent Preparation
3.2.4. Kinetics and Isotherms
Kinetics
Isotherms
3.2.5. Fixed-Bed Adsorption
3.2.6. AOPs S-Fe Regeneration Studies
Fenton Trials
AO and EF Treatment
3.3. Analytical Procedures
3.3.1. Adsorbent Characterization
Buffering Capacity
Point of Zero Charge (PZC)
Conductivity and pH
Structure and Mineralogical Composition
3.3.2. Iron Content Evaluation
3.3.3. High-Performance Liquid Chromatography (HPLC) Measurements
3.3.4. Total Organic Carbon (TOC) Measurements
3.4. Energy Consumption
3.5. Remediation Process Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stepnowski, P.; Zaleska, A. Comparison of different advanced oxidation processes for the degradation of room temperature ionic liquids. J. Photochem. Photobiol. A Chem. 2005, 170, 45–50. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Halambek, J.; Srček, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef]
- Peziak-Kowalska, D.; Fourcade, F.; Niemczak, M.; Amrane, A.; Chrzanowski, L.; Lota, G. Removal of herbicidal ionic liquids by electrochemical advanced oxidation processes combined with biological treatment. Environ. Technol. 2017, 38, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Picchioni, F. Prediction of toxicity of Ionic Liquids based on GC-COSMO method. J. Hazard. Mater. 2020, 398, 122964–122976. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, M.; Moniruzzaman, M.; Goto, M. An overview on the toxicological properties of ionic liquids toward microorganisms. Biotechnol. J. 2020, 15, 1900073. [Google Scholar] [CrossRef]
- Gomez-Herrero, E.; Tobajas, M.; Polo, A.; Rodriguez, J.J.; Mohedano, A.F. Removal of imidazolium-based ionic liquid by coupling Fenton and biological oxidation. J. Hazard. Mater. 2019, 365, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Díez, A.M.; Sanromán, M.A.; Pazos, M. Fenton-based processes for the regeneration of catalytic adsorbents. Catal. Today 2018, 313, 122–127. [Google Scholar] [CrossRef]
- Wang, M.; Wen, B.; Fan, B.; Zhang, H. Study on adsorption mechanism of silicate adsorbents with different morphologies and pore structures towards formaldehyde in water. Colloids Surf. A 2020, 599, 124887. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Y.S.; Lin, J. Advanced tertiary treatment of municipal wastewater using raw and modified diatomite. J. Hazard. Mater. 2005, 127, 196–203. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of carbonaceous adsorbents. Part I: Thermal regeneration. Microporous Mesoporous Mater. 2015, 202, 259–276. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Gonzalez-Olmos, R.; Martin, M.J. Regeneration of siloxane-exhausted activated carbon by advanced oxidation processes. J. Hazard. Mater. 2015, 285, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: A critical review. Desalin. Water Treat. 2014, 57, 518–544. [Google Scholar] [CrossRef]
- Iglesias, O.; Fernández de Dios, M.A.; Pazos, M.; Sanromán, M.A. Using iron-loaded sepiolite obtained by adsorption as a catalyst in the electro-Fenton oxidation of Reactive Black 5. Environ. Sci. Pollut. Res. 2013, 20, 5983–5993. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, R.; Anbia, M. Zeolites for adsorptive desulfurization from fuels: A review. Fuel Process Technol. 2017, 167, 99–116. [Google Scholar] [CrossRef]
- Bensalah, H.; Younssib, S.A.; Ouammoub, M.; Gurloa, A.; Bekheeta, M.F. Azo dye adsorption on an industrial waste-transformed hydroxyapatite adsorbent: Kinetics, isotherms, mechanism and regeneration studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- Ouiriemmi, I.; Díez, A.M.; Rosales, E.; Pazos, M.; Sanromán, M.A. Pre-concentration by natural adsorbent as plausible tool for effective electro-Fenton removal of micropollutants. Sep. Purif. Technol. 2020, 241, 116676. [Google Scholar] [CrossRef]
- Ouiriemmi, I.; Rosales, E.; Pazos, M.; Gadri, A.; Ammar, S.; Sanromn, M.A. Towards sustainable removal of methylthioninium chloride by using adsorption-electroradical regeneration. Chemosphere 2018, 210, 476–485. [Google Scholar] [CrossRef]
- Vale-Júnior, E.D.; Silva, D.R.D.; Fajardo, A.S.; Martínez-Huitle, C.A. Treatment of an azo dye effluent by peroxi-coagulation and its comparison to traditional electrochemical advanced processes. Chemosphere 2018, 204, 548–555. [Google Scholar] [CrossRef]
- Chen, C.; Wang, F.; Liang, J.; Tang, Q.; Chen, Y. Zeta potential of sepiolite in aqueoussystem. Adv. Mater. Res. 2012, 427, 208–211. [Google Scholar] [CrossRef]
- Sabah, E.; Ouki, S. Sepiolite and sepiolite-bound humic acid interactions in alkaline media and the mechanism of the formation of sepiolite-humic acid complexes. Int. J. Miner. Process. 2017, 162, 69–80. [Google Scholar] [CrossRef]
- Sandu, C.; Popescu, M.; Rosales, E.; Pazos, M.; Lazar, G.; Sanromán, M.A. Electrokinetic oxidant soil flushing: A solution for in situ remediation of hydrocarbons polluted soils. J. Electroanal. Chem. 2017, 799, 1–8. [Google Scholar] [CrossRef]
- Xiao, Y.; Hill, J.M. Impact of pore size on Fenton oxidation of methyl orange adsorbed on magnetic carbon materials: Trade-off between capacity and regenerability. Environ. Sci. Technol. 2017, 51, 4567–4575. [Google Scholar] [CrossRef] [PubMed]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Razmi, F.A.; Ngadi, N.; Wong, S.; Inuwa, I.M.; Opotu, L.A. Kinetics, thermodynamics, isotherm and regeneration analysis of chitosan modified pandan adsorbent. J. Clean. Prod. 2019, 231, 98–109. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Wu, Z.; Gao, Y.; Li, X. Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions. Sci. Total Environ. 2020, 708, 134409. [Google Scholar] [CrossRef]
- Huling, S.G.; Jones, P.K.; Ela, W.P.; Arnold, R.G. Fenton-driven chemical regeneration of MTBE-spent GAC. Water Res. 2005, 39, 2145–2153. [Google Scholar] [CrossRef]
- Ho, Y. Review of second-order models for adsorption systems. J. Hazard. Mat. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Elmoubarki, R.; Mahjoubi, F.Z.; Tounsadi, H.; Moustadraf, J.; Abdennouri, M.; Zouhri, A.; El Albani, A.; Barka, N. Adsorption of textile dyes on raw and decanted Moroccan clays: Kinetics, equilibrium and thermodynamics. Water Resour. Ind. 2015, 9, 16–29. [Google Scholar] [CrossRef]
- Gautam, R.K.; Rawat, V.; Banerjee, S.; Sanromán, M.A.; Soni, S.; Singh, S.K.; Chattopadhyaya, M.C. Synthesis of bimetallic Fe-Zn nanoparticles and its application towards adsorptive removal of carcinogenic dye malachite green and Congo red in water. J. Mol. Liq. 2015, 212, 227–236. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater—A meta-analysis of pilot- and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef]
- Aksu, Z.; Gonen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Deng, H.; Li, Y.; Wu, L.; Ma, X. The novel composite mechanism of ammonium molybdophosphate loaded on silica matrix and its ion exchange breakthrough curves for cesium. J. Hazard. Mater. 2017, 324, 348–356. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, X. Experiment and simulation on adsorption of 3,5,6-trichloro-2-pyridinol in typical farmland of purple soil, Southwestern China. Soil Sediment Contam. 2017, 26, 345–363. [Google Scholar] [CrossRef]
- Bouafia-Chergui, S.; Oturan, N.; Khalaf, H.; Oturan, M.A. Parametric study on the effect of the ratios [H2O2]/[Fe3+] and [H2O2]/[substrate] on the photo-Fenton degradation of cationic azo dye Basic Blue 41. J. Environ. Sci. Health A 2010, 45, 622–629. [Google Scholar] [CrossRef]
- Banić, N.; Abramović, B.; Šibul, F.; Orčić, D.; Watson, M.; Vraneš, M.; Gadžurić, S. Advanced oxidation processes for the removal of [bmim][Sal] third generation ionic liquids: Effect of water matrices and intermediates identification. RSC Adv. 2016, 6, 52826–52837. [Google Scholar] [CrossRef]
- Lin, H.; Oturan, N.; Wu, J.; Zhang, H.; Oturan, M.A. Cold incineration of sucralose in aqueous solution by electro-Fenton process. Sep. Purif. Technol. 2017, 173, 218–225. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Garrido, J.A.; Rodrίguez, R.M.; Brillas, E.; Oturan, N.; Oturan, M.A. Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene. Appl. Catal. B Environ. 2007, 72, 382–394. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Yazirdagi, M.; Kilinc, Z.; Konstantinou, I.; Katsaounis, A.; Mantzavinos, D. Boron-doped diamond electrooxidation of ethyl paraben: The effect of electrolyte on by-products distribution and mechanisms. J. Environ. Manag. 2017, 195, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, N.; Ormad, M.P.; Miguel, N.; Kokkinos, P.; Ioannidi, A.; Mantzavinos, D.; Frontistis, Z. Electrochemical oxidation of butyl paraben on boron doped diamond in environmental matrices and comparison with sulfate radical-AOP. J. Environ. Manag. 2020, 269, 110783–110792. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chu, W. Degradation of 2,4,5-trichlorophenoxyacetic acid by a novel Electro-Fe(II)/Oxone process using iron sheet as the sacrificial anode. Water Res. 2011, 45, 3883–3889. [Google Scholar]
- Midassi, S.; Bedoui, A.; Bensalah, N. Efficient degradation of chloroquine drug by electro-Fenton oxidation: Effects of operating conditions and degradation mechanism. Chemosphere 2020, 260, 127558–127569. [Google Scholar] [CrossRef]
- Acevedo-García, V.; Rosales, E.; Puga, A.; Pazos, M.; Sanromán, M.A. Synthesis and use of efficient adsorbents under the principles of circular economy: Waste valorisation and electroadvanced oxidation process regeneration. Sep. Purif. Technol. 2020, 242, 116796–116808. [Google Scholar] [CrossRef]
- Bocos, E.; Pazos, M.; Sanromán, M.A. Electro-Fenton treatment of imidazolium-based ionic liquids: Kinetics and degradation pathways. RSC Adv. 2016, 6, 1958–1965. [Google Scholar] [CrossRef]
- Meijide, J.; Rodríguez, S.; Sanromán, M.A.; Pazos, M. Comprehensive solution for acetamiprid degradation: Combined electro-Fenton and adsorption process. J. Electroanal. Chem. 2018, 808, 446–454. [Google Scholar] [CrossRef]
- Pimentel, M.; Oturan, N.; Dezotti, M.; Oturan, M.A. Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl. Catal. B Environ. 2008, 83, 140–149. [Google Scholar] [CrossRef]
- Hamza, M.; Abdelhedi, R.; Brillas, E.; Sirés, I. Comparative electrochemical degradation of the triphenylmethane dye Methyl Violet with boron-doped diamond and Pt anodes. J. Electroanal. Chem. 2009, 627, 41–50. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S.; Skoumal, M.; Arias, C. Electrochemical incineration of diclofenac in neutral aqueous medium by anodic oxidation using Pt and boron doped diamond anodes. Chemosphere 2010, 79, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Anasie, D.; Pazos, M.; Lazar, J.; Sanromán, M.A. Kaolinite adsorption-regeneration system for dyestuff treatment by Fenton based processes. Sci. Total Environ. 2018, 622, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.H.; Lei, L.C. Electrochemical regeneration of activated carbon loaded with p-nitrophenol in a fluidized electrochemical reactor. Electrochim. Acta 2006, 51, 4489–4496. [Google Scholar] [CrossRef]
- Gaied, F.; Louhichi, B.; Jeday, M.R. Tertiary treatment of waste water by Electro-Fenton process: Economical study. In Proceedings of the 2017 International Conference on Green Energy Conversion Systems (GECS), Hammamet, Tunisia, 23–25 March 2017. [Google Scholar]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Priyantha, N.; Usman, A. Artocarpusodoratissimus leaves as an eco-friendly adsorbent for the removal of toxic rhodamine B dye in aqueous solution: Equilibrium isotherm, kinetics, thermodynamics and regeneration studies. Arab. J. Sci. Eng. 2018, 43, 6011–6020. [Google Scholar] [CrossRef]
- Reddy, K.; Donahue, M.; Saichek, R.; Sasaoka, R. Preliminary assessment of electrokinetic remediation of soil and sludge contaminated with mixed waste. J. Air Waste Manag. Assoc. 1999, 49, 823–830. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Driscoll, C.T.; Bhanot, A.K. The zero point of charge of silica-alumina oxide suspensions. J. Colloid Int. Sci. 1984, 97, 55–61. [Google Scholar] [CrossRef]
- Fortune, W.B.; Mellon, M.G. Determination of iron with o-phenanthroline: A spectrophotometric study. Ind. Eng. Chem. Anal. Ed. 1938, 10, 60–64. [Google Scholar] [CrossRef]





| Adsorbent | Q (mg g−1) | R (%) |
|---|---|---|
| Perlite beads | 0 | 0 |
| Charred attapulgite | 0.287 | 61.18 |
| NaY Zeolite | 0.485 | 95.98 |
| Zeolite 13X | 0.282 | 57.85 |
| Attapulgite | 0.484 | 94.78 |
| Sepiolite | 0.486 | 100 |
| Eluent | D% |
|---|---|
| H2SO4 (0.1 M) | 5.9 |
| NaOH (0.1 M) | 7.3 |
| HCl (0.1 M) | 10.7 |
| Na2SO4 (0.1 M) | 8.3 |
| H2O | 0 |
| H2O/acetonitrile (20/80) with NH4Cl (0.2M) | 62.6 |
| H2O/acetonitrile (20/80) | 7.2 |
| NH4Cl (0.2M) | 21.3 |
| Adsorbent | Kinetic Models | Isotherms Models | ||||
|---|---|---|---|---|---|---|
| Model | Parameters | Values | Model | Parameters | Values | |
| Sepiolite | Pseudo-first order model | k1 (min−1) | 0.415 | Freundlich | KF (mg1−1/n L1/n g−1) | 24.397 |
| Qe (mg g−1) | 30.095 | n | 22.694 | |||
| R2 | 0.965 | R2 | 0.834 | |||
| SEE | 2.113 | SEE | 1.048 | |||
| Pseudo-second order model | k2 (g mg−1 min−1) | 0.02 | Langmuir | KL (L mg−1) | 0.298 | |
| Qe (mg g−1) | 32.25 | Qmax (mg g−1) | 31.923 | |||
| R2 | 0.995 | R2 | 0.973 | |||
| SEE | 0.773 | SEE | 2.496 | |||
| Intra-particle diffusion model | Kid (mg g−1 min−1) | 1.509 | Temkin | kT (L mg−1) | 41.979 | |
| Id (mg g−1) | 19.189 | bT (J mol−1) | 723.143 | |||
| R2 | 0.612 | R2 | 0.956 | |||
| SEE | 4.417 | SEE | 3.169 | |||
| S-Fe | Pseudo-first order model | k1 (min−1) | 0.824 | Freundlich | KF (mg1−1/n L1/n g−1) | 8.586 |
| Qe (mg g−1) | 21.768 | n | 6.536 | |||
| R2 | 0.97 | R2 | 0.942 | |||
| SEE | 1.272 | SEE | 2.584 | |||
| Pseudo-second order model | k2 (g mg−1 min−1) | 0.062 | Langmuir | KL (L mg−1) | 0.042 | |
| Qe (mg g−1) | 22.72 | Qmax (mg g−1) | 23.71 | |||
| R2 | 0.998 | R2 | 0.962 | |||
| SEE | 0.348 | SEE | 2.096 | |||
| Intra-particle diffusion model | Kid (mg g−1 min−1) | 0.695 | Temkin | kT (L mg−1) | 3.011 | |
| Id (mg g−1) | 17.106 | bT (J mol−1) | 805.97 | |||
| R2 | 0.544 | R2 | 0.949 | |||
| SEE | 2.344 | SEE | 2.408 | |||
| Model | Parameters | Values |
|---|---|---|
| Thomas | q0 (mg g−1) | 22.77 |
| kT (L mg−1 min−1) | 1.617 · 10−5 | |
| R2 | 0.998 | |
| SEE | 0.02 | |
| Yoon–Nelson | kY (min−1) | 3.234 · 10−3 |
| τ (min) | 2245.56 | |
| R2 | 0.998 | |
| SEE | 0.02 | |
| Adams–Bohart | KA (L mg−1 min−1) | 162.348 · 10−6 |
| N0 (mg L−1) | 899.935 | |
| R2 | 0.805 | |
| SEE | 0.188 |
| Trial Number | AOP Treatment | [H2O2] (mM) | I (mA) | pH | [Na2SO4] (M) | Treatment Time (h) | TOC Removal (%) |
|---|---|---|---|---|---|---|---|
| 1 | Fenton | 3 | - | 3 | - | 24 | 4.58 |
| 2 | Fenton | 30 | - | 3 | - | 24 | 19.01 |
| 3 | Fenton | 300 | - | 3 | - | 24 | 26.47 |
| 4 | AO | - | 100 | 8.6 | 0.1 | 9 | 13.36 |
| 5 | AO | - | 300 | 8.6 | 0.1 | 9 | 31.01 |
| 6 | AO | - | 500 | 8.6 | 0.1 | 9 | 34.42 |
| 7 | AO | - | 300 | 3 | 0.1 | 9 | 31.65 |
| 8 | EF | - | 300 | 8.6 | 0.1 | 9 | 43.23 |
| 9 | EF | - | 300 | 3 | 0.1 | 9 | 56.98 |
| 10 | EF | - | 300 | 3 | 0.2 | 9 | 82.79 |
| 11 | EF | - | 300 | 3 | 0.3 | 9 | 93.70 |
| Cycle Number | Pseudo-Second Order Kinetic | nr (%) | Surface Area (m2 g−1) | |
|---|---|---|---|---|
| Parameters | Values | |||
| 1 | k2 (g mg−1 min−1) | 0.062 | 100 | 144.54 |
| Qe (mg g−1) | 22.72 | |||
| R2 | 0.998 | |||
| SEE | 0.348 | |||
| 2 | k2 (g mg−1 min−1) | 0.045 | 100 | 126.6 |
| Qe (mg g−1) | 22.72 | |||
| R2 | 0.999 | |||
| SEE | 0.151 | |||
| 3 | k2 (g mg−1 min−1) | 0.04 | 102.33 | 153.04 |
| Qe (mg g−1) | 23.25 | |||
| R2 | 0.998 | |||
| SEE | 0.338 | |||
| 4 | k2 (g mg−1 min−1) | 0.041 | 102.33 | 141.53 |
| Qe (mg g−1) | 23.25 | |||
| R2 | 0.998 | |||
| SEE | 0.319 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouiriemmi, I.; Díez, A.M.; Pazos, M.; Sanromán, M.Á. Iron-Loaded Catalytic Silicate Adsorbents: Synthesis, Characterization, Electroregeneration and Application for Continuous Removal of 1-Butylpyridinium Chloride. Catalysts 2020, 10, 950. https://doi.org/10.3390/catal10090950
Ouiriemmi I, Díez AM, Pazos M, Sanromán MÁ. Iron-Loaded Catalytic Silicate Adsorbents: Synthesis, Characterization, Electroregeneration and Application for Continuous Removal of 1-Butylpyridinium Chloride. Catalysts. 2020; 10(9):950. https://doi.org/10.3390/catal10090950
Chicago/Turabian StyleOuiriemmi, Imen, Aida M. Díez, Marta Pazos, and María Ángeles Sanromán. 2020. "Iron-Loaded Catalytic Silicate Adsorbents: Synthesis, Characterization, Electroregeneration and Application for Continuous Removal of 1-Butylpyridinium Chloride" Catalysts 10, no. 9: 950. https://doi.org/10.3390/catal10090950
APA StyleOuiriemmi, I., Díez, A. M., Pazos, M., & Sanromán, M. Á. (2020). Iron-Loaded Catalytic Silicate Adsorbents: Synthesis, Characterization, Electroregeneration and Application for Continuous Removal of 1-Butylpyridinium Chloride. Catalysts, 10(9), 950. https://doi.org/10.3390/catal10090950