The Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in the Presence of Silver–Titanium Based Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Silver Titanium Oxide Samples (AgTi and AgTiY)
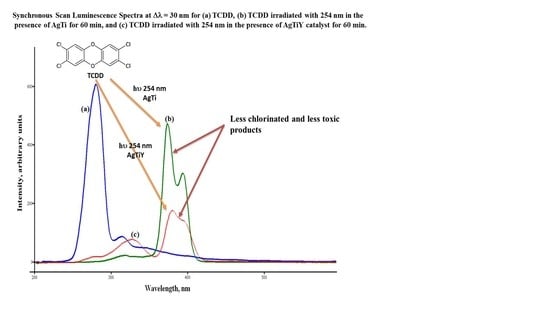
2.2. Photodegradation Experiments
2.3. Evaluation of the Efficiency of Degradation
2.3.1. Degradation Assessment Using GC–MS
2.3.2. Degradation Assessment Relative to Other Studies
2.4. Toxicity Comparison of the Degraded Sample Using ELISA
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Silver Titanium and Silver Titanium-Zeolite Preparation and Characterization
3.3. Irradiation of 2,3,7,8-TCDD
3.4. GC–MS Analysis
3.5. Toxicity Assessment of the Irradiation Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samara, F.; Elsayed, Y.; Soghomonian, B.; Knuteson, S.L. Chemical and biological assessment of sediments and water of Khalid Khor, Sharjah, United Arab Emirates. Mar. Pollut. Bull. 2016, 111, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; Román, M.F.S.; Ortiz, I.; Irabien, A. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (AOPs) to wastewater treatment. Chemosphere 2015, 118, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Marinković, N.; Pašalić, D.; Ferenčak, G.; Grskovic, B.; Rukavina, A. Dioxins and Human Toxicity. Arch. Ind. Hyg. Toxicol. 2010, 61, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorito, F.; Santamaria, R.; Irace, C.; De Martino, L.; Iovane, G. 2,3,7,8-tetrachlorodibenzo-p-dioxin and the viral infection. Environ. Res. 2017, 153, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K. Dioxin: A review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B 2005, 175, 221–230. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monogr. Eval. Carcinog. Risk Hum. 1997, 69, 228–238. [Google Scholar]
- Steenland, K.; Bertazzi, P.A.; Baccarelli, A.A.; Kogevinas, M. Dioxin Revisited: Developments since the 1997 IARC Classification of Dioxin as a Human Carcinogen. Environ. Health Perspect. 2004, 112, 1265–1268. [Google Scholar] [CrossRef] [Green Version]
- US EPA, O. Persistent Organic Pollutants: A Global Issue, a Global Response. Available online: https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response (accessed on 1 June 2020).
- Samara, F.; Jermani, E.; Kanan, S. Photocatalytic UV-degradation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the presence of silver doped zeolite. Arab. J. Chem. 2019, 12, 1870–1878. [Google Scholar] [CrossRef] [Green Version]
- Samara, F.; Al Shamsi, M.; Kanaan, F.; Kanan, S. Photocatalytic UV degradation of 2,3,7,8-tetrachlorodibenzofuran in the presence of silver zeolite. Res. Chem. Intermed. 2017, 46, 1017–1032. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang-Chien, G.-P.; Lee, W.-S. Photodegradation of polychlorinated dibenzo-p-dioxins: Comparison of photocatalysts. J. Hazard. Mater. 2004, 114, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Kumata, K.; Fukunishi, K.; Tanaka, N.; Patterson, N.G.; Alexander, L.R.; Barnhart, E.R.; Barr, J. Photodecomposition of 1,2,3,4-and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in water-alcohol media on a solid support. Chemosphere 1995, 31, 3687–3698. [Google Scholar] [CrossRef]
- Kieatiwong, S.; Nguyen, L.V.; Hebert, V.R.; Hackett, M.; Miller, G.C.; Miille, M.J.; Mitzel, R. Photolysis of chlorinated dioxins in organic solvents and on soils. Environ. Sci. Technol. 1990, 24, 1575–1580. [Google Scholar] [CrossRef]
- Torimoto, T.; Ito, S.; Kuwabata, S.; Yoneyama, H. Effects of Adsorbents Used as Supports for Titanium Dioxide Loading on Photocatalytic Degradation of Propyzamide. Environ. Sci. Technol. 1996, 30, 1275–1281. [Google Scholar] [CrossRef]
- Wodka, D.; Bielanńska, E.; Socha, R.P.; Wodka, M.E.; Gurgul, J.; Nowak, P.; Warszynński, P.; Kumakiri, I. Photocatalytic Activity of Titanium Dioxide Modified by Silver Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1945–1953. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Monteserín, C.; Angulo, A.; Perez-Marquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymer 2019, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Chen, F.; Zhang, J.; Anpo, M. A novel deposition precipitation method for preparation of Ag-loaded titanium dioxide. Catal. Lett. 2005, 102, 247–250. [Google Scholar] [CrossRef]
- Hou, X.; Wu, X.; Liu, A. Studies on photocatalytic activity of Ag/TiO2 films. Front. Chem. China 2006, 1, 402–407. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Droubay, T.C.; Chambers, S.A.; Bagus, P.S. Spectroscopic Evidence for Ag(III) in Highly Oxidized Silver Films by X-ray Photoelectron Spectroscopy. J. Phys. Chem. C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- X-Ray Photoelectron Spectroscopy (XPS) Reference Pages: Titanium. Available online: http://www.xpsfitting.com/2008/09/titanium.html (accessed on 25 June 2020).
- Zhao, C.; Cai, X.; Ma, Z.; Shi, J.; Xu, L.; Wang, H. Excimer formation from particially overlapped anthracene dimer based on saddle-shaped cyclooctatetrathiophene as spacer. J. Photochem. Photobiol. A Chem. 2018, 355, 318–325. [Google Scholar] [CrossRef]
- Mondal, P.; Banerjee, S.; Rath, S.P. Controlling the Photophysics of Aromatic Guests Using a Cyclic Porphyrin Dimer: Synthesis, Structure, and Encapsulation-Mediated “ON-OFF” Switch. Eur. J. Inorg. Chem. 2019, 2019, 3629–3637. [Google Scholar] [CrossRef]
- Ahern, J.C.; Kanan, S.; Sara, Z.; Job, T.; Alnaizy, R.; Abu Farha, N.; Patterson, H.H. Photocatalysis of fenoxycarb over silver-modified zeolites. Environ. Sci. Pollut. Res. 2014, 22, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Samara, F.; Abu-Yousef, I.A.; Abdo, N.; Tobias, D. Silver nanoclusters doped in zeolite to decontaminate water resources from the quinalphos pesticide. Res. Chem. Intermed. 2010, 36, 473–482. [Google Scholar] [CrossRef]
- Kanan, S.; Kanan, M.C.; Patterson, H.H. Silver nanoclusters doped in X and mordenite zeolites as heterogeneous catalysts for the decomposition of carbamate pesticides in solution. Res. Chem. Intermed. 2006, 32, 871–885. [Google Scholar] [CrossRef]
- Kanan, S.; Abdo, N.; Khalil, M.; Li, X.; Abu-Yousef, I.A.; Barilrobert, F.; Patterson, H.H. A study of the effect of microwave treatment on metal zeolites and their use as photocatalysts toward naptalam. Appl. Catal. B Environ. 2011, 106, 350–358. [Google Scholar] [CrossRef]
- Kanan, M.C.; Kanan, S.M.; Austin, R.N.; Patterson, H.H. Photodecomposition of Carbaryl in the Presence of Silver-Doped Zeolite Y and Suwannee River Natural Organic Matter. Environ. Sci. Technol. 2003, 37, 2280–2285. [Google Scholar] [CrossRef]
- Kanan, S.M.; Omary, M.A.; Patterson, H.H.; Matsuoka, M.; Anpo, M. Characterization of the Excited States Responsible for the Action of Silver(I)-Doped ZSM-5 Zeolites as Photocatalysts for Nitric Oxide Decomposition. J. Phys. Chem. B 2000, 104, 3507–3517. [Google Scholar] [CrossRef]
- Husseini, G.A.; Kanan, S.; Al-Sayah, M. Investigating the Fluorescence Quenching of Doxorubicin in Folic Acid Solutions and Its Relation to Ligand-Targeted Nanocarriers. J. Nanosci. Nanotechnol. 2016, 16, 1410–1414. [Google Scholar] [CrossRef]
- Muto, H.; Saitoh, K.; Funayama, H. PCDD/DF formations by the heterogeneous thermal reactions of phenols and their TiO2 photocatalytic degradation by batch-recycle system. Chemosphere 2001, 45, 129–136. [Google Scholar] [CrossRef]
- Choi, W.; Hong, S.J.; Chang, Y.-S.; Cho, Y. Photocatalytic Degradation of Polychlorinated Dibenzo-p-dioxins on TiO2 Film under UV or Solar Light Irradiation. Environ. Sci. Technol. 2000, 34, 4810–4815. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang-Chien, G.-P.; Lee, W.-S. Photodegradation of tetra-and hexachlorodibenzo-p-dioxins. J. Hazard. Mater. 2005, 120, 257–263. [Google Scholar] [CrossRef] [PubMed]
- IN-DF1 Kit Insert. Available online: http://www.cape-tech.com/literature/kits/in_df1a.htm (accessed on 16 May 2020).
- Samara, F.; Gullett, B.; Harrison, R.O.; Chu, A.; Clark, G.C. Determination of relative assay response factors for toxic chlorinated and brominated dioxins/furans using an enzyme immunoassay (EIA) and a chemically-activated luciferase gene expression cell bioassay (CALUX). Environ. Int. 2009, 35, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Stathatos, E.; Lianos, P.; Falaras, P.; Siokou, A. Photocatalytically Deposited Silver Nanoparticles on Mesoporous TiO2 Films. Langmuir 2000, 16, 2398–2400. [Google Scholar] [CrossRef]









| Degradation Products | TCDD (254 nm) | TCDD/AgTi (254 nm) | TCDD/AgTiY (254 nm) | TCDD (302 nm) | TCDD/AgTi (302 nm) | TCDD/AgTiY (302 nm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irradiation Time (min) | ||||||||||||||||||
| 30 | 60 | 300 | 30 | 60 | 300 | 30 | 60 | 300 | 30 | 60 | 300 | 30 | 60 | 300 | 30 | 60 | 300 | |
| 2,7-dichlorodibenzo-p-dioxin (2,7-DCDD) | – | – | X | – | X | X | – | – | – | – | X | – | – | – | – | – | – | – |
| 2,3,7-trichlorodibenzo-p-dioxin (2,3,7-triCDD) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | – | X | X | X |
| 1,3-dixolane | – | – | – | – | – | – | – | – | – | – | – | X | – | – | – | – | – | – |
| docosanoic acid | – | – | – | – | X | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 2-eicosanol | – | – | – | – | – | X | – | – | – | – | – | – | – | – | – | – | – | – |
| methyl-2-ethyhexyl phthalate | – | – | – | – | – | X | – | – | – | – | – | – | – | – | – | – | – | – |
| 1-heptadecence | – | – | – | – | – | – | – | – | – | – | – | – | X | X | – | – | – | – |
| (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide | – | – | – | – | – | – | – | – | – | – | – | – | X | – | X | X | – | – |
| 2,6-di-T-butyl-4-mmethylene-2,5-cyclohexadien-1 | – | – | – | – | – | – | – | X | X | – | – | – | – | – | – | – | X | – |
| 1-dotriacontanol | – | – | – | – | – | – | – | – | – | – | – | – | – | X | – | – | – | – |
| 4-hydroxy-3,5-ditertbutylbenzaldehyde | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | X | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samara, F.; Ghalayini, T.; Abu Farha, N.; Kanan, S. The Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in the Presence of Silver–Titanium Based Catalysts. Catalysts 2020, 10, 957. https://doi.org/10.3390/catal10090957
Samara F, Ghalayini T, Abu Farha N, Kanan S. The Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in the Presence of Silver–Titanium Based Catalysts. Catalysts. 2020; 10(9):957. https://doi.org/10.3390/catal10090957
Chicago/Turabian StyleSamara, Fatin, Thouraya Ghalayini, Nedal Abu Farha, and Sofian Kanan. 2020. "The Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in the Presence of Silver–Titanium Based Catalysts" Catalysts 10, no. 9: 957. https://doi.org/10.3390/catal10090957
APA StyleSamara, F., Ghalayini, T., Abu Farha, N., & Kanan, S. (2020). The Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in the Presence of Silver–Titanium Based Catalysts. Catalysts, 10(9), 957. https://doi.org/10.3390/catal10090957