Abstract
Electrocatalysts featuring robust structure, excellent catalytic activity and strong stability are highly desirable, but challenging. The rapid development of two-dimensional transition metal chalcogenide (such as WO3, MoS2 and WS2) nanostructures offers a hopeful strategy to increase the active edge sites and expedite the efficiency of electronic transport for hydrogen evolution reaction. Herein, we report a distinctive strategy to construct two-dimensional MoS2@dWO3 heterostructure nanosheets by in situ wet etching. Synthesized oxygen-incorporated MoS2-was loaded on the surface of defective WO3 square nanoframes with abundant oxygen vacancies. The resulting nanocomposite exhibits a low overpotential of 191 mV at 10 mA cm−2 and a very low Tafel slope of 42 mV dec−1 toward hydrogen evolution reaction. The long-term cyclic voltammetry cycling of 5000 cycles and more than 80,000 s chronoamperometry tests promises its outstanding stability. The intimate and large interfacial contact between MoS2 and WO3, favoring the charge transfer and electron–hole separation by the synergy of defective WO3 and oxygen-incorporated MoS2, is believed the decisive factor for improving the electrocatalytic efficiency of the nanocomposite. Moreover, the defective WO3 nanoframes with plentiful oxygen vacancies could serve as an anisotropic substrate to promote charge transport and oxygen incorporation into the interface of MoS2. This work provides a unique methodology for designing and constructing excellently heterostructure electrocatalysts for hydrogen evolution reaction.
1. Introduction
Electrocatalysts water-splitting product hydrogen is reproducible and clean energy [1,2,3]. The development of platinum (Pt)-free electrocatalysts is a crucial problem to improve the generally efficiency for hydrogen evolution reaction (HER), especially when facing resources shortage and energy crisis [4,5,6]. Newly, two-dimensional (2D) transition metal chalcogenides have always been mentioned, such as molybdenum disulfide (MoS2) [7,8], tungsten oxide (WO3) [9,10], molybdenum selenide (MoSe2) [11,12] and can demonstrated to clarify the mechanism for HER in strong acids. For instance, MoS2 has great potential as an alternative to Pt for catalyzing overall water-splitting cost-effectively and efficiently [13,14,15,16]. Theoretical studies have shown that along the edges of 2D MoS2 nanosheets is the source of highly HER, which plays a crucial role in constructing hybrid catalyst [15,17,18]. Therefore, the development of MoS2 based catalysts, featuring rich active edge sites [19] and accessible surface areas [20], is of vital significance to improve the overall efficiency for HER. Though many literatures have been reported the great potential of MoS2 as HER electrocatalyst [3,21,22], the severe stacking of MoS2 nanosheets during the preparation and electrochemical reaction process significantly impact its stability and hinders its application compared with Pt-based electrocatalysts [21,23]. Therefore, unless the electronic structure can be appropriately modulated by varying the chemical constituents [18] to increase the population of active sites [17] or introducing durable supportive materials [5,24] to prevent the aggregation, the 2D layered MoS2 could hardly be employed into practical application without long-term stability [25].
Tungsten trioxide (WO3) is another distinctive 2D layered oxide [26] with faster proton insertion kinetics than other two-dimensional transition metal chalcogenides, attributing to its larger proton diffusion coefficient [9]. Recently, improving the HER performance by designing and constructing various nanostructure components has been considered an available strategy to remarkably increase the active edge sites [25,27] and improve the long-term stability [28]. Indeed, researchers have reported that regulating the interlayer spacing of MoS2 nanosheets by forming hetero-structure can optimize the HER performance [29,30,31]. For example, Lan and coworkers synthesized a molybdenum disulfide/nitrogen-doped reduced graphene oxide (MoS2/N-RGO) hetero-composite and found that the enlarged interlayer spacing of MoS2 is beneficial to improve HER performance [32]. Because of the unique 2D ultra-thin layered morphology, which ensure the ultra-fast electron transfer, the MoS2/N-RGO t exhibited excellent catalytic activity with a lower onset potential, lower Tafel slope, larger current density and good stability over 5000 cycles. Moreover, Wang reported a newly strategy to construct well-defined W17O47–MoS2 heterostructure catalyst with clear interface. By constructing heterostructure, it is possible to create highly accessible anion-deficit sites for precise electronic structures and intimate hetero-interface for spatial charge-flow steering [18]. The W17O47–MoS2 composite shows that the mass activity of MoS2 is 116 times higher than pure MoS2. Therefore, the structural combination of different nanostructures, leaded to more active edge sites [33,34] and enlarged interlayer spacing of MoS2 [35,36], is demonstrated to be an effective approach to improve the HER activity. In this regard, rational design and further synthesis of MoS2-based catalysts with low-dimensional constrains and thermodynamic limitations between two completely different compounds are highly desirable, but also challenging.
Herein, we demonstrate an in situ wet etching method by growing the oxygen-incorporated 2D MoS2 nanosheets on defective WO3 nanoframes (denoted as MoS2@dWO3) to form a unique two-dimensional heterostructure. The defective WO3 nanoframes with abundant oxygen vacancies serve as an anisotropic substrate to promote charge transport and carrier injection into the interface of MoS2 nanosheets [37], while 2D MoS2 nanosheets provide highly active sites on edges for HER. The resulting hetero-catalyst exhibits highly activity with a low overpotential of 191 mV at 10 mA cm−2, with a Tafel slope of 42 mV dec−1 toward hydrogen evolution reaction. The long-term cyclic voltammetry cycling of 5000 cycles and more than 80,000 s chronoamperometric (CA) tests promises its outstanding stability. The success synthesis of MoS2@dWO3 via the construction of 2D heterostructure through defects-engineering (in situ wet etching) will provide unique pathways for pursuing efficient electrocatalysts for energy storage and conversion.
2. Results and Discussion
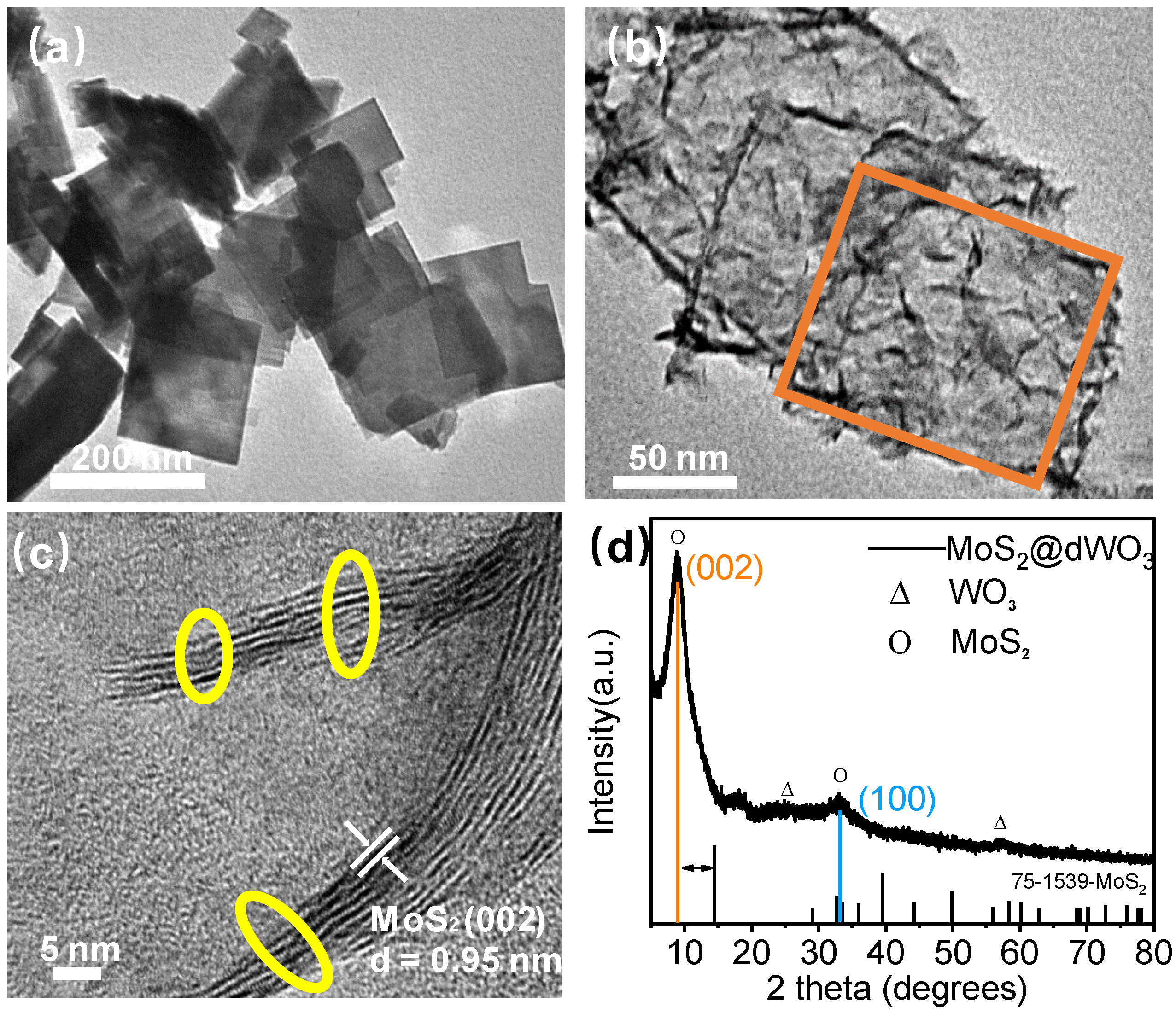
The MoS2@dWO3 heterostructure nanosheets were prepared by in situ wet etching of presynthesized WO3 nanosheets at the presence of Mo-based etching agent. The pure WO3 nanosheets exhibited a uniform square of 100–120-nm lateral size and 8–10-nm-thickness with a relative smooth and regular surface morphology, as shown in Figure 1a. After the in situ etching, transmission electron microscope (TEM) image shown that a heterostructure hollow 2D MoS2@dWO3 nanoframe with uniform shape and size was prepared (Figure 1b). In addition, the MoS2 sheets were grown on the main body of WO3 nanoframe through the thermolysis of (NH4)2MoS4 in DMF at hydrothermal process of 180 ℃ for 10 h. Ultrasmall (10 ± 1 nm) multilayer (3–6 layers) MoS2 sheets were in situ grown up the surface of relatively large WO3 nanoframe to construct MoS2@dWO3 heterostructure. It is worth nothing that the excessive use of glucose and citric acid in the synthesis process can ensure the formation of uniform 2D square nanoframe morphology of WO3 and can also reduce the interface energy to promote the subsequent growth of MoS2. Though delicate control of WO3/(NH4)2MoS4 mass ratio and etching time, the load in this 2D heterostructure was systematically optimized.
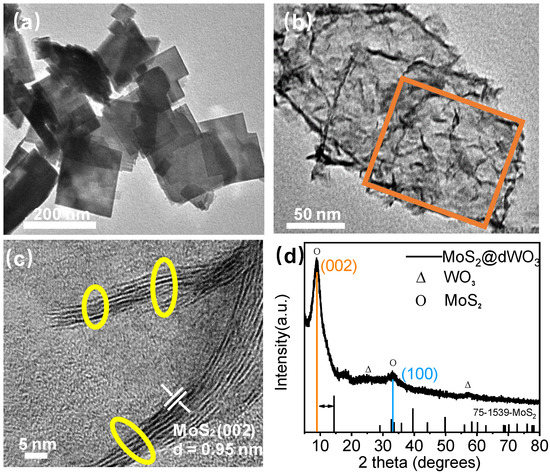
Figure 1.
Synthesis and structure characterization. (a) TEM image of pure tungsten oxide (WO3); (b) TEM image of MoS2@dWO3. At the image, the orange circle indicated frame of WO3; (c) HRTEM image of MoS2@dWO3. At the image, the yellow circle indicates the active edges of MoS2; (d) XRD patterns of MoS2@dWO3 heterostructures nanosheets.
The morphology and subtle structural evolution of the as-prepared MoS2@dWO3 catalyst were also observed by high resolution transmission electron microscopy (HRTEM). Comparing to the direct thermolysis of (NH4)2MoS4 to give relatively large MoS2 nanosheets of ~200 nm, as shown in Figure S2, the introduction of WO3 sheets as template substrate could significantly restrain the lateral growth of MoS2 nanosheets around 10 nm, offering abundant active edge sites as potential catalytic centers for HER. Meanwhile, the high crystalline solid 2D WO3 nanosheets have been simultaneously etched to form hollow square nanoframes accompanied the hydrothermal reaction of (NH4)2MoS4. As shown in Figure 1c, the high magnification lattice fringes of MoS2@dWO3 exhibited an enlarged interlayer spacing of 0.95 nm for (002) MoS2, which is considerably different from pure MoS2 with a conventional interlayer spacing of 0.62 nm. The MoS2@dWO3 heterostructure nanosheets were prepared by in situ wet etching of WO3 nanosheets by (NH4)2MoS4 in the solution of dimethylformamide (DMF), as shown in Equation (1) [38]. The intimate and large interfacial contact between MoS2 and WO3, favoring the promoted charge transfer and electron–hole separation by the synergy of defective WO3 and MoS2, is believed the decisive factor for improving the electrocatalytic efficiency of the nanocomposite. Specifically, the pyrolysis of (NH4)2MoS4 in DMF environment, produces MoS3 with a strong reducibility (Equation (2)), which makes the WO3 nanosheets etched to form a nanoframe, and abundant oxygen vacancies are generated. As an anisotropic substrate, WO3 is conducive to charge transport and carrier injection into the interface of MoS2. Meanwhile, controllable disorder engineering of layered MoS2 by oxygen incorporation to enlarge their interlayer spacing, providing plenty of unsaturated sulfur atoms as active sites for HER [39] and effectively regulating the electronic structure [40,41] of this nanocomposite to further enhance the intrinsic conductivity.
(NH4)2MoS4→MoS3 + 2NH3 + H2S
MoS3 + 2O→MoS2 + SO2
At the same time, the material structure of MoS2@dWO3 was further characterized via XRD. As shown in Figure 1d, the XRD pattern of MoS2@dWO3 is markedly different from that of pure 2H-MoS2 (JCPDS No. 75-1539). After the formation of heterostructure, the nanocomposite showed a prominent (002) peak come out at the low-angle region at 9.3°, corresponding to a d spacing of 9.5 Å, verified the increased interlayer spacing from observed TEM image. In addition, a broadened peak at high angle region (33°) corresponds to the (100) planes of the pristine MoS2, indicating the similarly atomic arrangement along the basal planes. Therefore, it can be inferred from the above characterization that the main reason for the enlarged interlayer spacing of MoS2@dWO3 is due to the oxygen incorporation. It is noted that both theoretical and experimental studies have implied that the MoS2 with an enlarged interlayer spacing can present a more preferable free energy change for hydrogen adsorption (ΔGH) and exhibits more short-range disordering than the general MoS2-with an interlayer spacing of 6.2 Å, favoring the ultrafast kinetics process of HER [15,36,42].
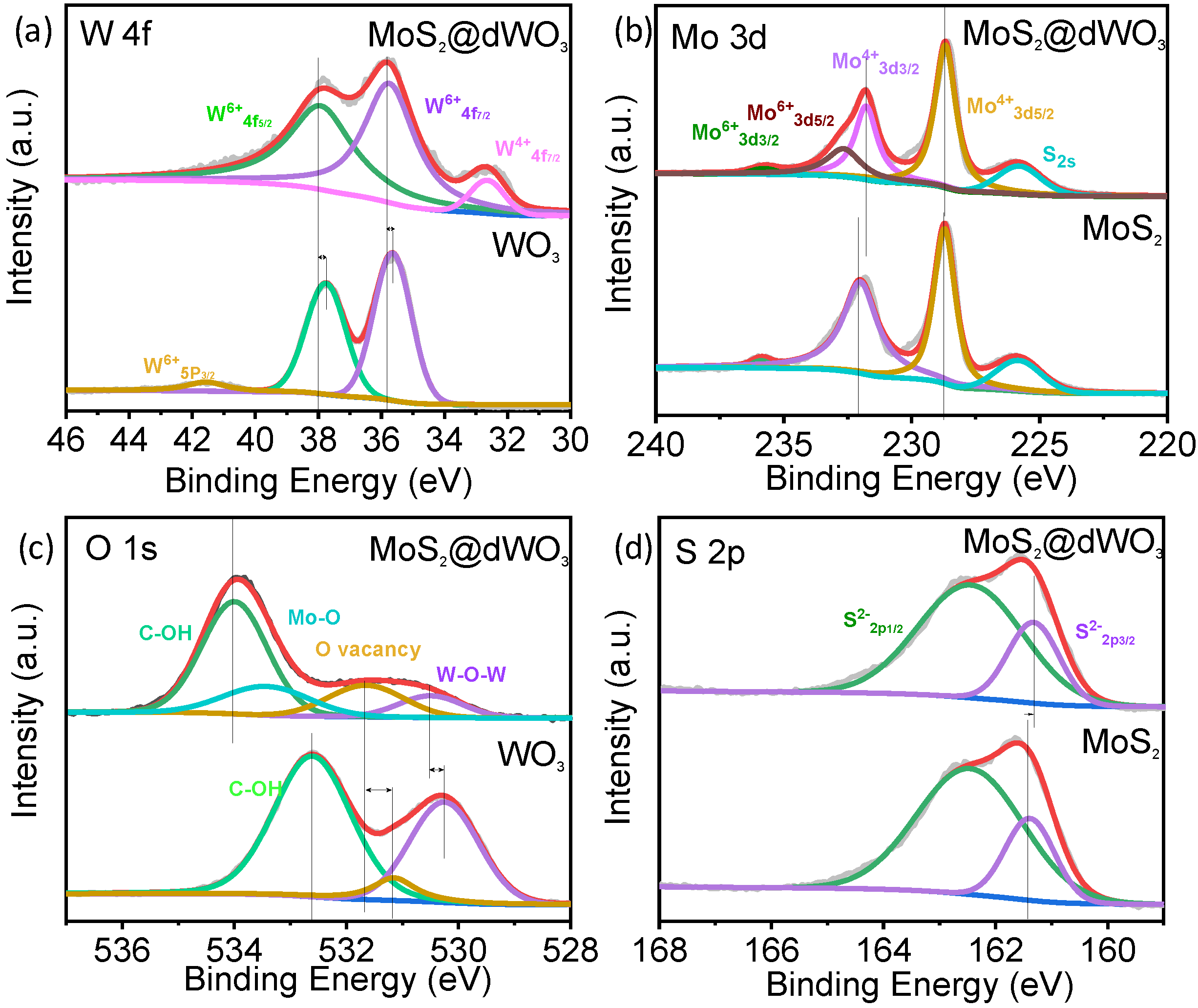
Chemical state and electronic properties of the catalyst surface was further detected by X-ray photoelectron spectrum (XPS). As shown in Figure 2, XPS survey scan confirmed the presence of W, Mo, O and S elements in MoS2@dWO3 heterostructure. Compared with pure WO3, a distinctive new peak assigned to W4+4f7/2 was appeared around 32.5 eV, indicating that the wet etching of pristine WO3 would induce the electron transfer from oxygen vacancies to tungsten cations on the defective WO3 surface (Figure 2a). Moreover, a positive shift and peak broadening of both W6+ 4f7/2 and W6+ 4f5/2 demonstrates the electron modulation and recombination of different compound between defective WO3 and in situ grown MoS2 [43]. In Figure 2b, the Mo 3d XPS spectrum of MoS2@WO3 had two characteristic peaks of Mo4+ 3d3/2 (231.75 eV) and Mo4+ 3d5/2 (228.65 eV), indicating the dominance of MoS2 in the product. However, the enhanced peak of Mo 6+ 3d5/2 (232.63 eV) and Mo 6+ 3d3/2 (235.73 eV) represents the formation of Mo–O bonds [32,44], verifying that the oxygen was incorporated into MoS2-layer to generate the interlayer disorder, which also corresponds to the peak shit of S. As shown in Figure 2c, the O 1s spectrum revealed the existence of W–O–W (530.53 eV) and O-vacancies (531.68 eV) [18] in MoS2@dWO3. The increase of O-vacancies peak area indicates the enhancement of O-vacancies after the formation of heterostructure. Meanwhile, the appearance of new peak at 533.46 eV is ascribed to Mo–O bond, indicating a certain amount of oxygen atoms were incorporated into the layered MoS2 structure. The C–OH peak in the O 1s spectrum mainly comes from the glucose ligand on the surface. All the positions of S 2p1/2, S 2p 3/2 peak were in accordance with the previous literature (Figure 2d) [24]. However, we observed that the peak of S2- 2p3/2 have 0.05 eV shift toward low blinding energy, which indicated that S became easier to obtain electrons after forming heterostructure [45] and also confirmed peak of Mo 6+ 3d5/2 (232.63 eV) and Mo 6+ 3d3/2 (235.73 eV) were further enhanced because of the conservation of gain and loss electrons [5]. In summary, formation of heterostructure caused the increase of O-vacancies on the defective WO3 surface and the generation of Mo–O bond by oxygen incorporation into MoS2 layer.
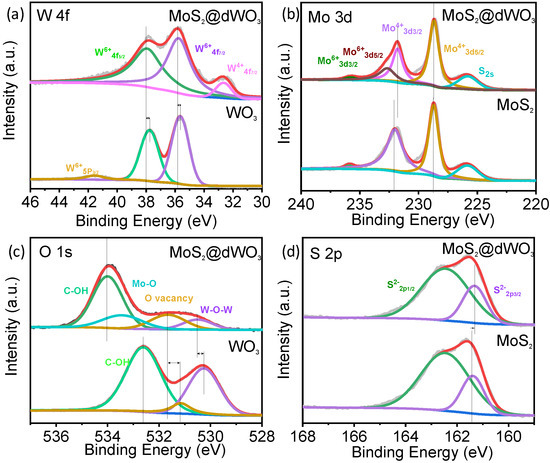
Figure 2.
X-ray photoelectron spectrum of MoS2@dWO3 heterostructures nanosheets. XPS spectrum of (a) W 4f, (b) Mo 3d, (c) O 1s and (d) S 2p from MoS2, WO3 and MoS2@dWO3.
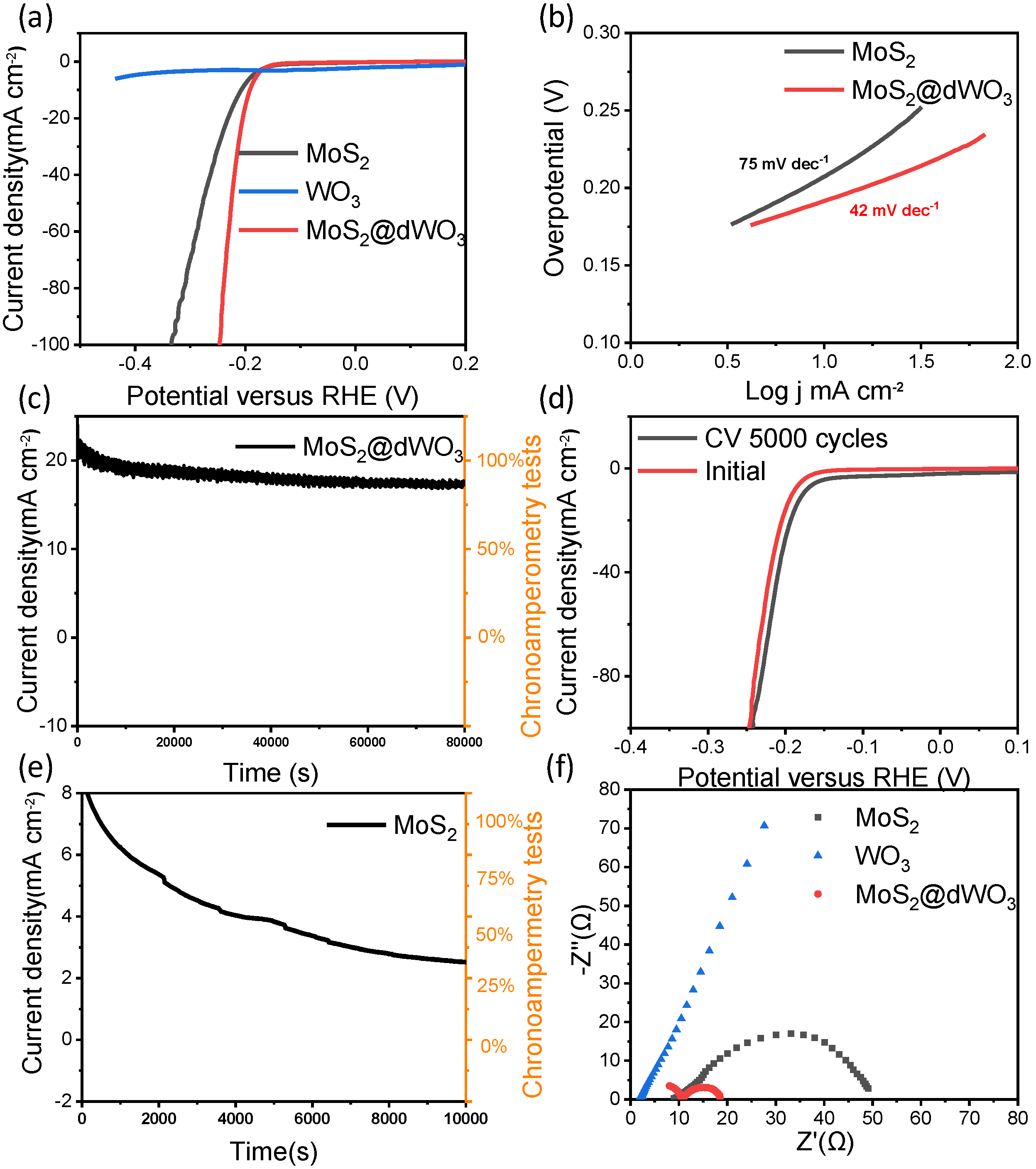
In order to assess HER performance of MoS2@dWO3 heterostructure catalyst, the electrocatalytic measurements were carried out using a standard three-electrode cell in N2-saturated 0.5-M H2SO4 electrolyte. Linear-sweep voltammetry (LSV) curves of MoS2@dWO3 heterostructure catalyst, control samples of pristine WO3 and pure MoS2 are shown in Figure 3a. Pristine WO3 demonstrates the most limited HER activity reflected by the low current density, attributing to the lack of active sites and its poor conductivity. The pure MoS2 exhibited significantly better activity than the pure WO3, as exhibiting an overpotential of 210 mV at 10 mA cm−2. The improved activity of MoS2 originated from the active-edge sites of 2D MoS2 nanosheets. The MoS2@dWO3 heterostructure catalyst was shown the most active catalyst, reaching the highest activity with an overpotential of 191 mV at 10 mA cm−2 and the lowest Tafel slope of 42 mV dec−1, as shown in Figure 3b. The much improved activity could be attributed to the in situ grown ultrasmall MoS2 nanosheets, more specifically, the oxygen-incorporated MoS2 active edge sites, plus the synergetic effects brought by the intimate and large interfacial contact between defective WO3 and oxygen-incorporated MoS2.
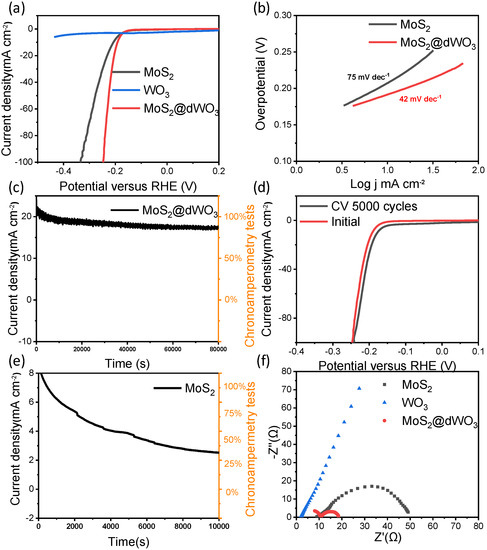
Figure 3.
Electrocatalytic hydrogen evolution reaction performance. (a) Polarization curves of MoS2, WO3 and MoS2@dWO3 (b) corresponding Tafel plots of MoS2 and MoS2@dWO3; (c) degree of decline of MoS2@dWO3 through the 80,000 s continuous operation (d) polarization curves of MoS2@dWO3 initially and after 5000 cyclic voltammetry (CV) sweeps; (e) degree of decline of MoS2 through the 10,000 s continuous operation; (f) electrochemical impedance spectroscopy of MoS2, WO3 and MoS2@dWO3.
In the environment of strong acidity and high current density, the long-term stability and durability of heterostructure catalyst are very important for the practical application of electrochemical hydrogen production. Therefore, the chronoamperometry is used to further perform the process at a constant potential of −0.21 V versus RHE. As shown in Figure 3c, we can observe that the hetero-catalyst runs continuously and stably for 80,000 s at a current density of ~20 mA cm−2 which implying its good durability of heterojunction under HER working condition. Meanwhile, a long-term cycling test of MoS2@dWO3 heterostructure catalyst was also carried out, as shown in Figure 3d. After 5000 cycles at a scan rate of 100 mV s−1 between −0.45 and 0.3 V vs. RHE, the polarization curve before and after the cycle remain similar, and a negligible activity increase is observed, which means that superior stability of prepared heterostructure. Compared to the latest reported MoS2 based and other nonprecious metal based HER electrocatalysts, ours heterostructure catalyst demonstrate comparable or even more efficient performance, which is more significant for practical application for electrodes in harsh working conditions (Table 1).

Table 1.
Comparison of hydrogen evolution reaction (HER) catalytic performance of the different electrocatalysts.
As control samples, the pure WO3 nanosheets were measured and exhibited poor HER activity. The pure MoS2 nanosheets prepared without WO3 templates showed a poor long-term stability and durability, in Figure 3e. These experimental results shown that the formation of heterostructure nanosheets is crucial for the enhanced HER performance. Intuitively, by comparing the TEM image of MoS2 prepared without WO3, the effect of WO3 on the catalyst preparation is expected to be significant for the confined growth of MoS2 nanosheets. Indeed, the theoretical simulations indicate that the catalytic activity of the edge sites of layered MoS2 could be effectively impacted by underlying substrates [49]. These results demonstrated that this in situ wet etching strategy is a unique method for constructing heterostructures, which can improve electronic modulation and increase the active edge sites by oxygen incorporation, leading to an enhanced HER performance of MoS2-based catalysts. The excellent electrocatalytic activity and electrical conductivity of heterostructure catalysts was further examined by electrochemical impedance spectroscopy (EIS). As shown as in Figure 3f, the charge transfer resistance (Rct) of MoS2@dWO3 nanosheets is significantly lower than original MoS2 and WO3 in the EIS, which means that the heterogeneous interface makes the barrier minimize of the charge transfer barrier and improves the electronic conductivity as well. At the same time, it also shown that WO3 is an excellent matrix material, which can promote the transmission of protons at the interface [50].
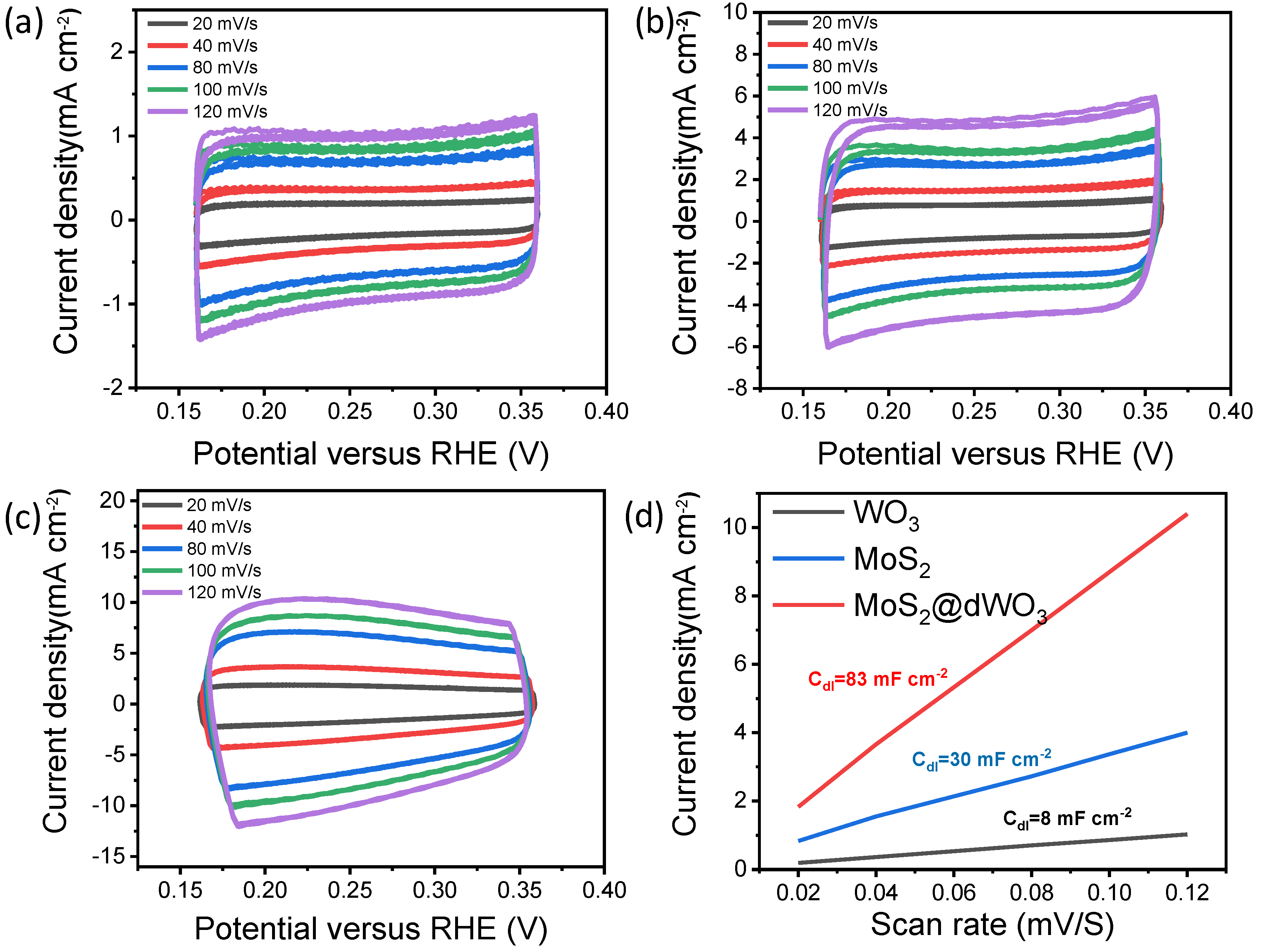
In order to further evaluate the effective electrochemical active surface area (ECSA) of MoS2, WO3 and MoS2@dWO3, the electrochemical double-layer capacity (Cdl) was further examined as shown in Figure 4. The double layer capacitance (Cdl) of MoS2@dWO3 is 83 mF cm−2, which is higher than that of pristine WO3 (8 mF cm−2) and pure MoS2 (30 mF cm−2). The plots in Figure 4d show that the MoS2@dWO3 have a large active surface area, which can be attributed to the heterostructure with rich active edge sites. Our experiment measurement indicates a strong dependence in the catalytic activity of the MoS2 film on the WO3 substrate.
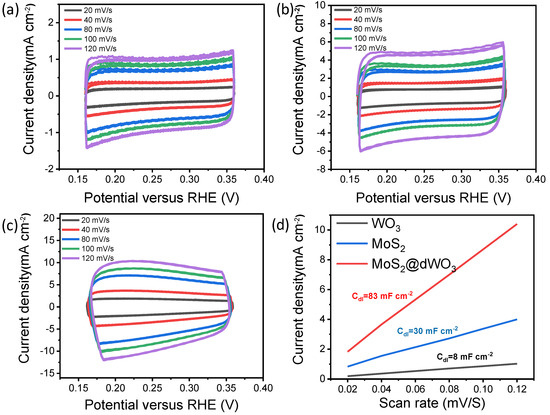
Figure 4.
Effective electrochemical active surface area tests (ECSA) of (a) WO3, (b) MoS2 and (c) MoS2@dWO3; (d) electrochemical double-layer capacity (Cdl) of MoS2, WO3 and MoS2@dWO3.
The high HER activity and good stability of the optimized catalyst constructed with heterostructure and designed electronic modulations can be mainly attributed to the following aspects: (i) the heterostructure increases the number of active edges as active sites for hydrogen evolution catalysis; (ii) the WO3 nanoframes with rich oxygen vacancies serve as an anisotropic substrate to promote electron transport; (iii) the oxygen incorporation in MoS2 can enhance the overall intrinsic conductivity and help improve electron structure; (iiii) the synergistic effect between MoS2 and WO3 is beneficial to simultaneously improve the catalytic activity and stability of the material. All in all, in order to achieve high-efficiency electrocatalytic hydrogen evolution, the integrated MoS2@dWO3 heterostructure catalyst were successfully prepared by constructing heterostructural and electronic modulations, paving a new way for improving the activity of various multielement electrocatalysts.
3. Materials and Methods
3.1. Chemicals and Reagents
Nafion (5% Alfa Aesar, Haverhill, MA, USA), (NH4)2MoS4(99.95%; Alfa Aesar, Haverhill, MA, USA), Na2WO4·2H2O (99.5%; Alfa Aesar, Haverhill, MA, USA) were obtained from Alfa Aesar (Alfa Aesar, Haverhill, MA, USA). Ethanol, methanol, isopropanol, dimethylformamide, citric acid, hydrochloric, glucose, H2SO4 (98%) were purchased from Beijing Chemical Regent Company (Beijing Chemical Regent Co., Ltd.; Beijing, China).
3.2. Synthesis of WO3 Nanosheets
We synthesized WO3 nanosheets through a facile hydrothermal method. First, 0.5 mmol of Na2WO4·2H2O were added to 20 mL of deionized water to form a clear solution, and then 0.75-mmol citric acid and 5-mmol glucose was slowly injected into it. After ultrasonic stirring for 20 min, we slowly dropped 10 mL of HCl (3 M) into the above mixed solution. After magnetic stirring for 60 min, the mixed solution was transferred into a 50-mL Teflon autoclave and heated at 120 ℃ for 6 h. The precipitates were centrifuged and washed with ethanol and water several times and then dried in vacuum at 60 ℃ for 4 h.
3.3. Synthesis of MoS2@dWO3 Heterostructure Nanosheets
First, we weighed 5 mg of the WO3 nanosheets prepared above and added it into 25 mL DMF under ultrasonication for 10 min at room temperature. Then, under ultrasonic stirring, the solution was added into 5 mL of DMF containing 1 mg of (NH4)2MoS4 for 20 min. The mixed solution was then transferred into a 30-mL Teflon autoclave and heated at 180 ℃ for 10 h. The precipitates were centrifuged and washed with ethanol and water 5 times and then dried in vacuum at 60 ℃ for 4 h.
3.4. Characterizations
We observed the size and morphology of these prepared nanocomposites through a JEM-1200EX (JEOL; Tokyo, Japan) transmission electron microscope (TEM) operating at 100 kV and JEM-2100 F (JEOL; Tokyo, Japan)transmission electron microscope operating at 200 kV. At the same time, a Bruker AXS D8 Advance X-ray diffractometer (Bruker Daltonics Inc., Karlsruhe, Germany) was used to test the X-ray diffraction (XRD) patterns of these obtained samples with Cu Kα radiation (λ = 1.5418 Å). The operation current and voltage were 40 mA and 40 kV (2θ ranging from 5° to 80°), respectively. Finally, in order to explore the combined state of the surface, we used the X-ray photoelectron spectrum (XPS) to measure it on AXIS ULTRA DLD (AXIS; Manchester, UK). We compared the standard binding-energy table and the XPS total spectrum to determine the energy axis of each element. All electrochemical measurements in this article were performed on an electrochemical workstation (CHI 660E, CH Instrument, Inc., Shanghai, China).
3.5. Fabrication of Electrodes
First, we weighed 1.0 mg of carbon black (XC-72) and 2.2 g of MoS2@dWO3 hybrid nanocrystals, added into 3.0 mL ethanol and then sonicated the mixture for 60-min before transferring the catalyst to the surface of the carbon black. Finally, we took 1 mL of the mixed solution, added isopropanol (750 µL), ethanol (250 µL) and nafion (5%, 20 µL) to form a homogeneous catalyst ink.
Then, we weighed 1.0 mg of carbon black (XC-72) and 2.2 g of MoS2 hybrid nanocrystals. To this was added into 3.0 mL ethanol. Then, we sonicated the mixture for 60 min. Finally, we took 1 mL of the mixed solution, added isopropanol (750 µL), ethanol (250 µL) and nafion (5%, 20 µL) to form a homogeneous catalyst ink. The ink of pure MoS2 and WO3 nanocatalyst was prepared the same way.
Finally, the nanocatalyst ink (8.7 μL) was dropped onto the glass carbon (GC) electrode (3-mm in diameter) and then dried for 2 h before electrocatalytic tests, yielding a catalyst loading of 0.28 mg cm−2 approximately.
3.6. Catalytic Measurements
All electrochemical measurements in this article were performed on the electrochemical station (CHI 660E, CH Instrument, Inc., Shanghai, China). The nanocatalyst inks were dropped onto a glassy carbon electrode (GCE) as the working electrode. At the same time, a carbon rod was used as the counter electrode, and a saturated calomel electrode (SCE) was used as reference electrode, respectively. The electrolyte solution was a 0.5-M H2SO4 solution; N2 was passed through for 30 min before the test. The HER performance was tested by linear-sweep voltammetry with sweeping the potential from 0.20 to −0.60 V vs. RHE at a scan rate of 2 mV s−1. The cyclic voltammetry was tested under the potential of 0.20 and −0.20 V vs. RHE at the scan rate of 5 mV s−1. Electrochemical impedance spectroscopy measurement was carried out from 10,000 Hz to 0.01 Hz. The electrochemical stability was tested by chronoamperometry (j–t) at a constant potential of −0.21 V vs. RHE. To estimate the electrochemical active surface area of the sample, cyclic voltammetry was tested under the potential window of 0.16 and 0.36 V with various scan rate of 20, 40, 60, 80, 100, 120 mV s−1.
The saturated calomel electrode (SCE) was used as the reference electrode in the all electrocatalytic measurements. It be calibrated in regard to reversible hydrogen electrode in the 0.5-M H2SO4 at H2-saturated environment. The Pt wires were used as the working electrode and counter electrode. The cyclic voltammetry (CV) was test at a scan rate of 1.0 mV/s, and the average value of potential at which the current crossed zero was the thermodynamic potential for the hydrogen electrode reaction. The CV curve is shown in Figure S3. Therefore, in this work, E(RHE) = E(SCE) + 0.242 V.
4. Conclusions
In conclusion, we successfully synthesized the MoS2@dWO3 heterostructure catalyst with enlarged MoS2 interlayer spacing and defective WO3 substrate using in situ etching method. Importantly, the in situ etching process achieved oxygen-incorporated MoS2 via construct hetero-interface at the surface of WO3 nanoframes. The MoS2@dWO3 heterostructure catalyst with rich edge active sites and high overall conductivity could deliver substantially enhanced HER activity and long-term durability in strong acid environment. The resulting nanocomposite exhibits highly activity with a low overpotential of 191 mV at 10 mA cm−2, with a Tafel slope of 42 mV dec−1 and long-term stability toward hydrogen evolution reaction. The results indicate that the MoS2@dWO3 heterostructure catalyst has large potential in water-splitting devices, and our study highlights a promising approach to design and synthesis of other nanostructure catalyst in miscellaneous promising applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/9/977/s1, Figure S1: (a) TEM image and (b) XRD patterns of the pure WO3. Figure S2: TEM image of the pure MoS2. Figure S3: the cyclic voltammetry of RHE calibration.
Author Contributions
X.L. performed data analysis and wrote the study. C.W. contributed to the study design and scientific discussion of the results. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial supports from the Youth Innovation Promotion Association CAS (2020180), CAS STS project (KFJ-STS-ZDTP-068), Shanxi Major Project (20181102026) and the Innovation Fund of Institute of Coal Chemistry (SCJJ-2020-12).
Acknowledgments
This work was supported by Junzhong Wang and Leyu Wang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Voiry, D.; Yang, J.; Chhowalla, M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Han, J.; Wang, X.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W.; Liu, S.; Gao, T.; Zhang, Z.; et al. 2D transition metal dichalcogenides: Design, modulation, and challenges in electrocatalysis. Adv. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Guo, J.; Huo, F.; Cheng, Y.; Xiang, Z. PAF-1 as oxygen tank to in -situ synthesize edge -exposed O-MoS2 for highly efficient hydrogen evolution. Catal. Today 2020, 347, 56–62. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Xiong, S.; Wu, X.; Shan, Y.; Chu, P.K. Synergistic WO3.2H2O nanoplates/WS2 hybrid catalysts for high-efficiency hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 13966–13972. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Liu, Y.; Long, R.; Li, M.; Chen, S.; Qi, Z.; Wang, C.; Song, L.; Jiang, J.; et al. Defective tungsten oxide hydrate nanosheets for boosting aerobic coupling of amines: Synergistic catalysis by oxygen vacancies and bronsted acid sites. Small 2017, 13, 1701354. [Google Scholar] [CrossRef]
- Jain, A.; Sadan, M.B.; Ramasubramaniam, A. Promoting active sites for hydrogen evolution in MoSe2 via transition-metal dopin. J. Phys. Chem. C 2020, 124, 12324–12336. [Google Scholar] [CrossRef]
- Luo, J.; Xu, P.; Zhang, D.; Wei, L.; Zhou, D.; Xu, W.; Li, J.; Yuan, D. Synthesis of 3D-MoO2 microsphere supported MoSe2 as an efficient electrocatalyst for hydrogen evolution reaction. Nanotechnology 2017, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, E.; He, F.; Shi, C.; He, C.; Li, J.; Zhao, N. 2D sandwich-like carbon-coated ultrathin TiO2@defect-rich MoS2 hybrid nanosheets: Synergistic-effect-promoted electrochemical performance for lithium ion batteries. Nano Energy 2016, 26, 541–549. [Google Scholar] [CrossRef]
- Fu, W.; He, H.; Zhang, Z.; Wu, C.; Wang, X.; Wang, H.; Zeng, Q.; Sun, L.; Wang, X.; Zhou, J.; et al. Strong interfacial coupling of MoS2/g-C3N4 van de Waals solids for highly active water reduction. Nano Energy 2016, 27, 44–50. [Google Scholar] [CrossRef]
- Sun, Y.; Alimohammadi, F.; Zhang, D.; Guo, G. Enabling colloidal synthesis of edge-oriented MoS2 with expanded interlayer spacing for enhanced HER catalysis. Nano Lett. 2017, 17, 1963–1969. [Google Scholar] [CrossRef]
- Ye, W.; Ren, C.; Liu, D.; Wang, C.; Zhang, N.; Yan, W.; Song, L.; Xiong, Y. Maneuvering charge polarization and transport in 2H-MoS2 for enhanced electrocatalytic hydrogen evolution reaction. Nano Res. 2016, 9, 2662–2671. [Google Scholar] [CrossRef]
- Xu, J.; Cui, J.; Guo, C.; Zhao, Z.; Jiang, R.; Xu, S.; Zhuang, Z.; Huang, Y.; Wang, L.; Li, Y. Ultrasmall Cu7S4 @MoS2 hetero-nanoframes with abundant active edge sites for ultrahigh-performance hydrogen evolution. Angew. Chem. Int. Ed. Engl. 2016, 55, 6502–6505. [Google Scholar] [CrossRef]
- Jiang, R.; He, C.; Guo, C.; Chen, W.; Luo, J.; Chen, Y.; Wang, L. Edge-contact geometry and anion-deficit construction for activating ultrathin MoS2 on W17O47 in the hydrogen evolution reaction. Inorg. Chem. 2019, 58, 11241–11247. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Late, D.J.; Kumar, A.; Patel, S.; Singh, J. 2D layered transition metal dichalcogenides (MoS2): Synthesis, applications and theoretical aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Mo, Q.; Peng, L.; Cao, S.; Wang, J.; Wu, C.; Li, C.; Guo, J.; Liu, B.; et al. Epitaxial MoS2 nanosheets on nitrogen doped graphite foam as a 3D electrode for highly efficient electrochemical hydrogen evolution. Electrochim. Acta 2018, 292, 407–418. [Google Scholar] [CrossRef]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 nanosheets with widened interlayer spacing for high-efficiency removal of mercury in aquatic systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Wang, Z.; Chen, J.G.; Liu, C.-j. Porous MS2/MO2 (M = W, Mo) nanorods as efficient hydrogen evolution reaction catalysts. Acs. Catal. 2016, 6, 6585–6590. [Google Scholar] [CrossRef]
- Geng, S.; Yang, W.; Yu, Y.S. Building MoS2/S-doped g-C3N4 layered heterojunction electrocatalysts for efficient hydrogen evolution reaction. J. Catal. 2019, 375, 441–447. [Google Scholar] [CrossRef]
- Sivasankaran, R.P.; Rockstroh, N.; Kreyenschulte, C.R.; Bartling, S.; Lund, H.; Acharjya, A.; Junge, H.; Thomas, A.; Brueckner, A. Influence of MoS2 on activity and stability of carbon nitride in photocatalytic hydrogen production. Catalysts 2019, 9, 695. [Google Scholar] [CrossRef]
- Kusmierek, E. Evaluating the effect of WO3 on electrochemical and corrosion properties of TiO2-RuO2-coated titanium anodes with low content of RuO2. Electrocatalysis 2020, 11, 555–566. [Google Scholar] [CrossRef]
- Lu, H.; Chen, X.; Dai, W.; Zhang, K.; Liu, C.; Dong, H. Prickly pear-like three-dimensional porous MoS2: Synthesis, characterization and advanced hydrogen evolution reaction. Catalysts 2018, 8, 580. [Google Scholar] [CrossRef]
- Yang, S.-Z.; Gong, Y.; Manchanda, P.; Zhang, Y.-Y.; Ye, G.; Chen, S.; Song, L.; Pantelides, S.T.; Ajayan, P.M.; Chisholm, M.F.; et al. Rhenium-doped and stabilized MoS2 atomic layers with basal-plane catalytic activity. Adv. Mater. 2018, 30, 1803477. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xing, Z.; Zhao, T.; Qiu, Y.; Tao, B.; Li, Z.; Zhou, W. Hollow flower-like polyhedral alpha-Fe2O3/defective MoS2/Ag Z-scheme heterojunctions with enhanced photocatalytic-Fenton performance via surface plasmon resonance and photothermal effects. Appl. Catal. B-Environ. 2020, 272, 118978. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, J.; Peng, X.; Zhong, L.; Sun, R. Porous carbon coupled with an interlaced MoP-MoS2 heterojunction hybrid for efficient hydrogen evolution reaction. J. Energy Chem. 2020, 45, 45–51. [Google Scholar] [CrossRef]
- Chen, R.; Song, Y.; Wang, Z.; Gao, Y.; Sheng, Y.; Shu, Z.; Zhang, J.; Li, X.a. Porous nickel disulfide/reduced graphene oxide nanohybrids with improved electrocatalytic performance for hydrogen evolution. Catal. Commun. 2016, 85, 26–29. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Wang, Y.; Wang, X.-L.; Li, S.-L.; Huang, W.; Dong, L.-Z.; Liu, C.-H.; Li, Y.-F.; Lan, Y.-Q. Molybdenum disulfide/nitrogen-doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600116. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, L.; Zou, G.; Sun, C.; Hu, J.; Xiao, X.; Gao, L. Optimizing MoS2 edges by alloying isovalent W for robust hydrogen evolution activity. Acs. Catal. 2018, 8, 9529–9536. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-processed two-dimensional MoS2 nanosheets: Preparation, hybridization, and applications. Angew. Chem. Int. Ed. 2016, 55, 8816–8838. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, J.; Xi, P.; Tao, K.; Gao, D. P dopants triggered new basal plane active sites and enlarged interlayer spacing in MoS2 nanosheets toward electrocatalytic hydrogen evolution. ACS Energy Lett. 2017, 2, 745–752. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Yang, L.; Liu, P.; Li, J.; Xiang, B. Two-dimensional material molybdenum misulfides as electrocatalysts for hydrogen evolution. Catalysts 2017, 7, 285. [Google Scholar] [CrossRef]
- Latorre-Sánchez, M.; Esteve-Adell, I.; Primo, A.; García, H. Innovative preparation of MoS2–graphene heterostructures based on alginate containing (NH4)2MoS4 and their photocatalytic activity for H2 generation. Carbon 2015, 81, 587–596. [Google Scholar] [CrossRef]
- Joe, J.; Bae, C.; Kim, E.; Ho, T.A.; Yang, H.; Park, J.H.; Shin, H. Mixed-phase (2H and 1T) MoS2 catalyst for a highly efficient and stable Si photocathode. Catalysts 2018, 8, 580. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; Song, H.; Wu, H.; Yan, S.; Xu, X.; Shi, Y. Fabrication of C/Co-FeS2/CoS2 with highly efficient hydrogen evolution reaction. Catalysts 2019, 9, 556. [Google Scholar] [CrossRef]
- Hong, S.; Rhee, C.K.; Sohn, Y. Photoelectrochemical hydrogen evolution and CO2 reduction over MoS2/Si and MoSe2/Si nanostructures by combined photoelectrochemical deposition and rapid-thermal annealing process. Catalysts 2019, 9, 494. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, G.; Pan, A.; Liang, S. Oxygen-incorporated MoS2 nanosheets with expanded interlayers for hydrogen evolution reaction and pseudocapacitor applications. Acs. Appl. Mater. Interfaces 2016, 8, 33681–33689. [Google Scholar] [CrossRef]
- Reddy, B.M.; Sreekanth, P.M.; Yamada, Y.; Xu, Q.; Kobayashi, T. Surface characterization of sulfate, molybdate and tungstate promoted TiO2-ZrO2 solid acid catalysts by XPS and other techniques. Appl. Catal. A. Gen. 2002, 228, 269–278. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, N.; Dai, C.; Xu, R.; Wu, B.; Yu, G.; Chen, B.; Du, Y. H-2-directing strategy on in situ synthesis of Co-MoS2 with highly expanded interlayer for elegant HER activity and its mechanism. Adv. Energy Mater. 2020, 10, 2000291. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Zhou, C.; Du, S.; Zhen, D.; Chen, B.; Li, J.; Wu, Q.; Iru, Y.; Chen, D. A modulated electronic state strategy designed to integrate active HER and OER components as hybrid heterostructures for efficient overall water splitting. Appl. Catal. B. Environ. 2020, 260, 118197. [Google Scholar] [CrossRef]
- Gao, M.R.; Liang, J.X.; Zheng, Y.R.; Xu, Y.F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.H. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Xu, S.; Kong, D.; Cha, J.J.; Zheng, G.; Hsu, P.; Yan, K.; Brandshaw, D.; Prinz, F.B.; et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Hao, X.; Zhou, J.; Song, D.; Wang, D.; Hou, L.; Gao, F. Fluorine- and nitrogen-codoped MoS2 with a catalytically active basal plane. Acs. Appl. Mater. Interfaces 2017, 9, 27715–27719. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Kozarenko, O.A.; Dyadyun, V.S.; Koshechko, V.G.; Pokhodenko, V.D. Efficient mechanochemical preparation of graphene-like molybdenum disulfide and graphene-based composite electrocatalysts for hydrogen evolution reaction. Electrocatalysis 2019, 10, 477–488. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Anupama, V.R.; Arun, P.S.; Jineesh, P.; Suji, L. Synthesis and development of nano WO3 catalyst incorporated Ni–P coating for electrocatalytic hydrogen evolution reaction. Int. J. Hydrog. Energy 2016, 41, 10090–10102. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).