The Biocatalytic Production of 3-Hydroxypropionaldehyde and Evaluation of Its Stability
Abstract
:1. Introduction
2. Results and Discussion
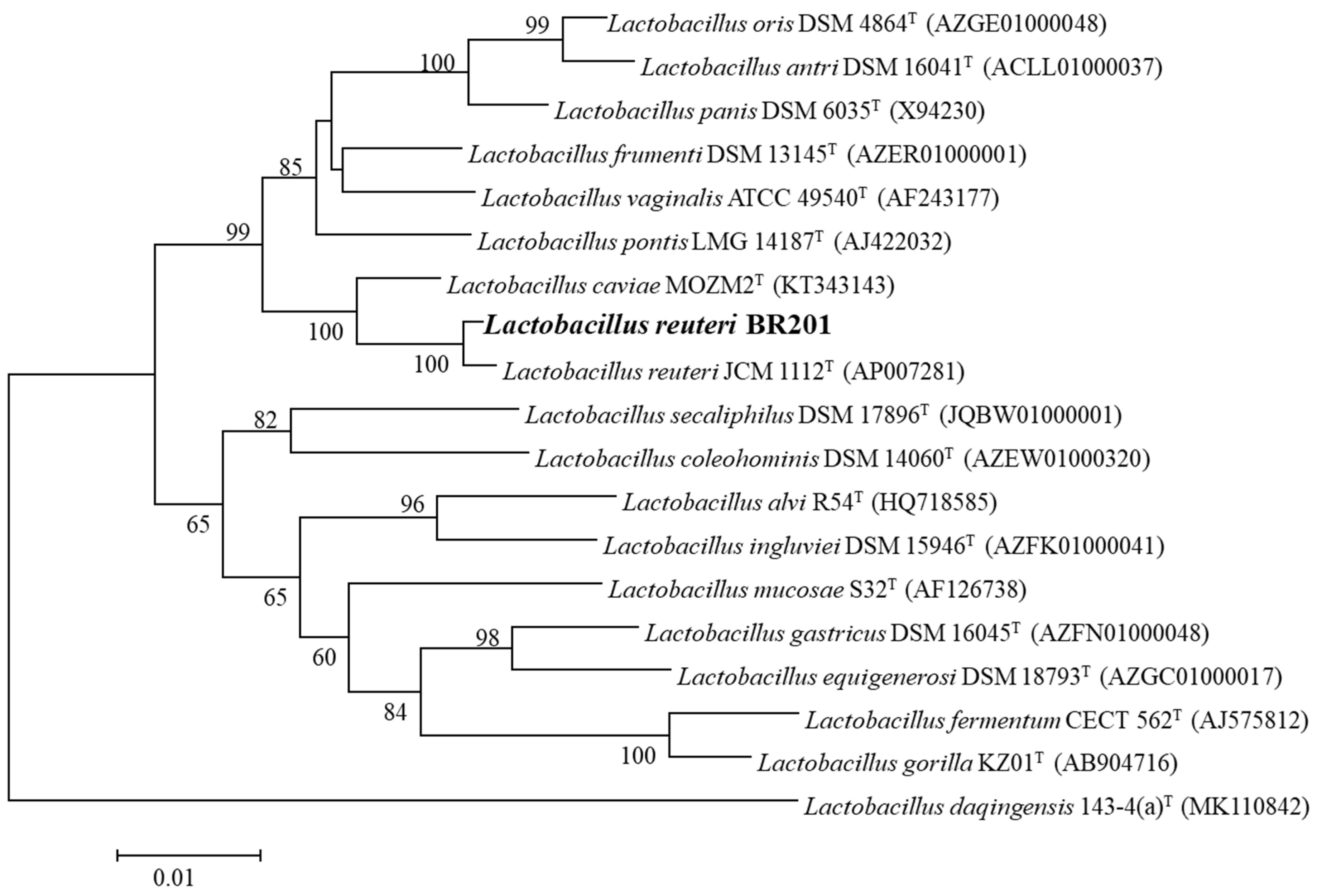
2.1. Isolation and Identification L. reuteri BR201
2.2. The Effect of Cell Growth for 3-HPA Bioconversion
2.3. The Effect of Cell Density
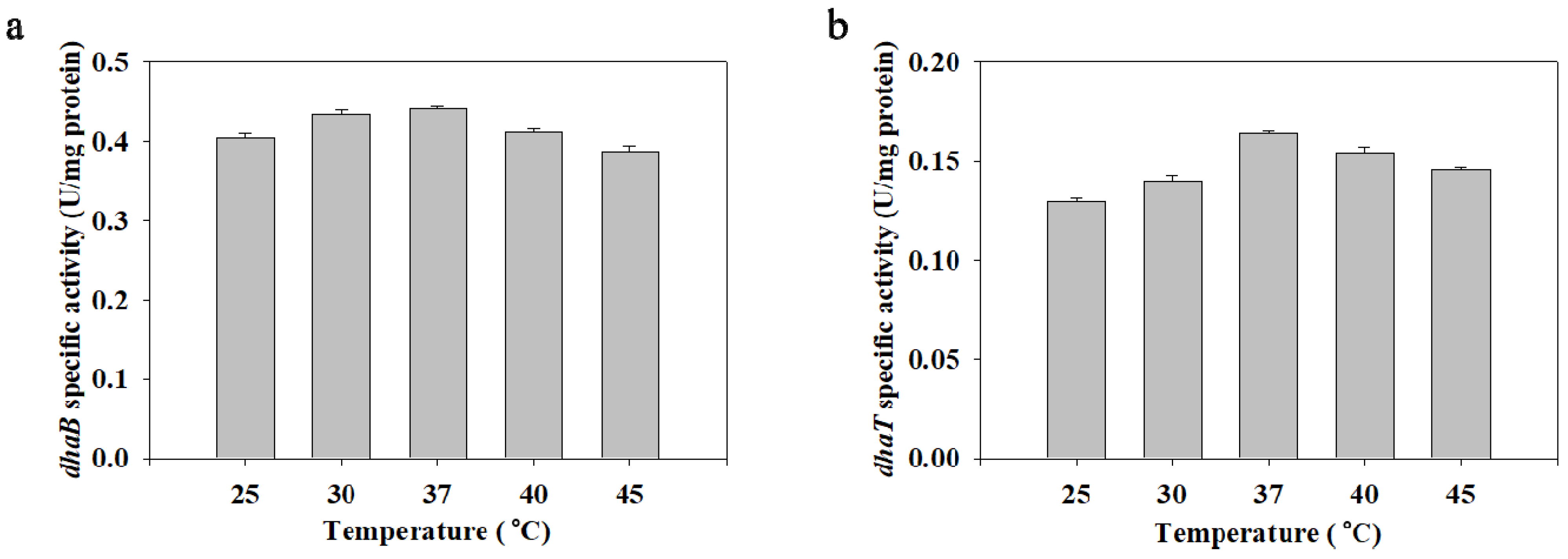
2.4. The Effect of Temperature
2.5. The Effect of Substrate Concentration
2.6. The Effect of Conversion Time
2.7. Antibacterial Activity and Stability of 3-HPA
3. Material and Methods
3.1. Materials and Growth Media
3.2. Isolation of L. reuteri Strain
3.3. Identification of L. reuteri BR201 by 16S rRNA Analysis
3.4. Cultivation of L. reuteri BR201
3.5. Cultivation Metabolite Analysis
3.6. 3-HPA Bioconversion
3.7. Measurement of Antimicrobial Activity
3.8. Acrolein Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vollenweider, S.; Lacroix, C. 3-Hydroxypropionaldehyde: Applications and Perspectives of Biotechnological Production. Appl. Microbiol. Biotechnol. 2004, 64, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; du Toit, M.; Kossmann, J. Influence of Environmental Parameters on Production of the Acrolein Precursor 3-Hydroxypropionaldehyde by Lactobacillus reuteri DSMZ 20016 and Its Accumulation by Wine Lactobacilli. Int. J. Food Microbiol. 2010, 137, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Lüthi-Peng, Q.; Schärer, S.; Puhan, Z. Production and Stability of 3-Hydroxypropionaldehyde in Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2002, 60, 73–80. [Google Scholar] [PubMed]
- Arqués, J.L.; Fernández, J.; Gaya, P.; Nuñez, M.; Rodríguez, E.; Medina, M. Antimicrobial Activity of Reuterin in Combination with Nisin Against Food-Borne Pathogens. Int. J. Food Microbiol. 2004, 95, 225–229. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Inactivation of Gram-Negative Pathogens in Refrigerated Milk by Reuterin in Combination with Nisin or the Lactoperoxidase System. Eur. Food Res. Technol. 2008, 227, 77–82. [Google Scholar] [CrossRef]
- Sobolov, M.; Smiley, K.L. Metabolism of Glycerol by an Acrolein-Forming Lactobacillus. J. Bacteriol. 1960, 79, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Talarico, T.L.; Casas, I.A.; Chung, T.C.; Dobrogosz, W.J. Production and Isolation of Reuterin, a Growth Inhibitor Produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1988, 32, 1854–1858. [Google Scholar] [CrossRef] [Green Version]
- Talarico, T.L.; Dobrogosz, W.J. Chemical Characterization of an Antimicrobial Substance Produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989, 33, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Hayek, S.A.; Ibrahim, S.A. Current Limitations and Challenges with Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2013, 4, 40133. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Ashok, S.; Park, S. Recent Advances in Biological Production of 3-Hydroxypropionic Acid. Biotechnol. Adv. 2013, 31, 945–961. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Determination of Lactobacillus reuteri Strain DSM 17938; FDA: Silver Sping, MD, USA, 2008.
- Lüthi-Peng, Q.; Dileme, F.; Puhan, Z. Effect of Glucose on Glycerol Bioconversion by Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2002, 59, 289–296. [Google Scholar] [PubMed]
- Vieira, P.B.; Kilikian, B.V.; Bastos, R.V.; Perpetuo, E.A.; Nascimento, C.A.O. Process Strategies for Enhanced Production of 1,3-Propanediol by Lactobacillus reuteri Using Glycerol as a Co-Substrate. Biochem. Eng. J. 2015, 94, 30–38. [Google Scholar] [CrossRef]
- Doleyres, Y.; Beck, P.; Vollenweider, S.; Lacroix, C. Production of 3-Hydroxypropionaldehyde Using a Two-Step Process with Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2005, 68, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M.J.A.; Vollenweider, S.; Mertes, P.; Lacroix, C. Bisulfite as Scavenger for Enhanced Biotechnological Production of 3-Hydroxypropionaldehyde by Lactobacillus reuteri. Biochem. Eng. J. 2013, 79, 239–245. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Dishisha, T.; Pyo, S.-H.; Hatti-Kaul, R. Semicarbazide-Functionalized Resin as a New Scavenger for In Situ Recovery of 3-Hydroxypropionaldehyde during Biotrans-Formation of Glycerolby Lactobacillus reuteri. J. Biotechnol. 2014, 192, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, C.; Schwab, C.; Zhang, J.; Stevens, M.J.A.; Bieri, C.; Ebert, M.-O.; McNeill, K.; Sturla, S.J.; Lacroix, C. Acrolein Contributes Strongly to Antimicrobial and Heterocyclic Amine Transformation Activities of Reuterin. Sci. Rep. 2016, 6, 36246. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.; Vollenweider, S.; Lacroix, C.; Zurich, E.T.H. 5-The Potential of Reuterin Produced by Lactobacillus reuteri as a Broad Spectrum Preservative in Food. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation; Lacroix, C., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 129–160. [Google Scholar]
- Abraham, K.; Andres, S.; Palavinskas, R.; Berg, K.; Appel, K.E.; Lampen, A. Toxicology and Risk Assessment of Acrolein in Food. Mol. Nutr. Food Res. 2011, 55, 1277–1290. [Google Scholar] [CrossRef]
- Ju, J.-H.; Heo, S.-Y.; Choi, S.-W.; Kim, Y.-M.; Kim, M.-S.; Kim, C.-H.; Oh, B.-R. Effective Bioconversion of 1,3-Propanediol from Biodiesel-Derived Crude Glycerol Using Organic Acid Resistance-Enhanced Lactobacillus reuteri JH83. Bioresour. Technol. 2021, 337, 125361. [Google Scholar] [CrossRef]
- Kang, T.S.; Korber, D.R.; Tanaka, T. Glycerol and Environmental Factors: Effects on 1,3-Propanediol Production and NAD+ Regenerationin Lactobacillus panis PM1. J. Appl. Microbiol. 2013, 115, 1003–1011. [Google Scholar] [CrossRef]
- Daniel, R.; Bobik, T.A.; Gottschalk, G. Biochemistry of Coenzyme B12-Dependent Glycerol and Diol Dehydratases and Organization of the Encoding Genes. FEMS Microbiol. Rev. 1998, 22, 553–566. [Google Scholar] [CrossRef]
- Mori, K.; Hosokawa, Y.; Yoshinaga, T.; Toraya, T. Diol Dehydratase-Reactivating Factor Is a Reactivase-Evidence for Multiple Turnovers and Subunit Swapping with Diol Dehy-Dratase. FEBS J. 2010, 227, 4931–4943. [Google Scholar] [CrossRef] [PubMed]
- Toraya, T.; Mori, K. A Reactivating Factor for Coenzyme B12-Dependent Diol Dehydratase. J. Biol. Chem. 1999, 274, 3372–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Yu, B. Efficient Production of Reuterin from Glycerol by Magnetically Immobilized Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2015, 99, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus reuteri CRL1098 Produces Cobalamin. J. Bacteriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Teusink, B.; Molenaar, D.; van Heck, M.; Wels, M.; Sieuwerts, S.; de Vos, W.M.; Hugenholtz, J. Effect of Amino Acid Availability on Vitamin B12 Production in Lactobacillus reuteri. Appl. Environ. Microbiol. 2009, 75, 3930. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.-H.; Wang, D.; Heo, S.-Y.; Kim, M.-S.; Seo, J.-W.; Kim, Y.-M.; Kim, D.-H.; Kang, S.-A.; Kim, C.-H.; Oh, B.-R. Enhancement of 1, 3-Propanediol Production from Industrial By-Productby Lactobacillus reuteri CH53. Microb. Cell Fact. 2020, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rasch, M. The Influence of Temperature, Salt and pH on the Inhibitory Effect of Reuterin on Escherichia coli. Int. J. Food Microbiol. 2002, 72, 225–231. [Google Scholar] [CrossRef]
- Yunmbam, M.K.; Roberts, J.F. The In Vitro Efficacy of Reuterin on the Culture and Bloodstream Forms of Trypanosoma brucei brucei. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 101, 235–238. [Google Scholar] [CrossRef]
- Vimont, A.; Fernandez, B.; Ahmed, G.; Fortin, H.-P.; Fliss, I. Quantitative Antifungal Activity of Reuterin Against Food Isolates of Yeasts and Moulds and Its Potential Application in Yogurt. Int. J. Food Microbiol. 2019, 289, 182–188. [Google Scholar] [CrossRef]
- El-Ziney, M.G.; van den Tempel, T.; Debevere, J.; Jakobsen, M. Application of Reuterin Produced by Lactobacillus reuteri 12002 for Meat Decontamination and Preservation. J. Food Prot. 1999, 62, 257–261. [Google Scholar] [CrossRef]
- Crouch, R.D.; Richardson, A.; Howard, J.L.; Harker, R.L.; Barker, K.H. The Aldol Addition and Condensation: The Effect of Conditions on Reaction Pathway. J. Chem. Educ. 2007, 84, 475. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C. Retro-Aldol Degradations of Unsaturated Aldehydes: Role in the Formation of c4-Heptenal from t2, c6-Nonadienal in Fish, Oyster and Other Flavors. J. Am. Oil Chem. Soc. 1987, 64, 132–138. [Google Scholar] [CrossRef]
- Buchan, B.W.; Riebe, K.M.; Ledeboer, N.A. Comparison of the MALDI Biotyper System Using Sepsityper Specimen Processing to Routine Microbiological Methods for Identification of Bacteria from Positive Blood Culture Bottles. J. Clin. Microbiol. 2012, 50, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.-S.; Cho, Y.-J.; Lee, K.; Yoon, S.-H.; Kim, M.; Na, H.; Park, S.-C.; Jeon, Y.S.; Lee, J.-H.; Yi, H.; et al. Introducing EzTaxon-E: A Prokaryotic 16S rRNA Gene Sequence Database with Phylotypes that Represent Uncultured Species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Ju, J.-H.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Lee, B.-S.; Kim, M.-S.; Kim, C.-H.; Seo, J.-W.; Oh, B.-R. Regulation of Lipid Accumulation Using Nitrogen for Microalgae Lipid Production in Schizochytrium sp. ABC101. Renew. Energy 2020, 153, 580–587. [Google Scholar] [CrossRef]
- Circle, S.J.; Stone, L.; Boruff, C.S. Acrolein Determination by Means of Tryptophane. A Colorimetric Micromethod. Ind. Eng. Chem. Anal. Ed. 1945, 17, 259–262. [Google Scholar] [CrossRef]
- Asare, P.T.; Zurfluh, K.; Greppi, A.; Lynch, D.; Schwab, C.; Stephan, R.; Lacroix, C. Reuterin Demonstrates Potent Antimicrobial Activity Against a Broad Panel of Human and Poultry Meat Campylobacter spp. Isolates. Microorganisms 2020, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Hanoune, B.; LeBris, T.; Allou, L.; Marchand, C.; Le Calve, S.J.A.E. Formaldehyde Measurements in Libraries: Comparison between Infrared Diode Laser Spectroscopy and a DNPH-Derivatization Method. Atmos. Environ. 2006, 40, 5768–5775. [Google Scholar] [CrossRef]
- Possanzini, M.; Di Palo, V.; Petricca, M.; Fratarcangeli, R.; Brocco, D.J.A.E. Measurements of Lower Carbonyls in Rome Ambient Air. Atmos. Environ. 1996, 30, 3757–3764. [Google Scholar] [CrossRef]
- Slemr, J.; Junkermann, W.; Volz-Thomas, A.J.A.E. Temporal Variations in Formaldehyde, Acetaldehyde and Acetone and Budget of Formaldehyde at a Rural Site in Southern Germany. Atmos. Environ. 1996, 30, 3667–3676. [Google Scholar] [CrossRef]


| Cultivation Time (h) | Regidual Glycerol (mM) | 3-HPA (mM) | 1,3-PDO (mM) |
|---|---|---|---|
| 4 | 137.5 ± 2.1 | 214.2 ± 2.6 | 37.8 ± 0.2 |
| 6 | 14.1 ± 0.1 | 324.1 ± 3.8 | 59.9 ± 0.6 |
| 8 | 11.4 ± 0.1 | 325.6 ± 4.3 | 61.8 ± 0.5 |
| 10 | 195.9 ± 1.2 | 168.5 ± 2.7 | 34.2 ± 0.4 |
| Pathogen | Response * at Different 3-HPA Concentration (mM) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | MIC (mM) | |
| E. coli ATCC 8739 | ++ | + | + | − | − | 3 |
| S. aureus ATCC 6538 | ++ | + | − | − | − | 2 |
| P. aeruginosa ATCC 9027 | ++ | + | − | − | − | 2 |
| C. albicans ATCC 10231 | ++ | + | + | + | − | 4 |
| A. niger ATCC 16404 | ++ | + | + | + | − | 4 |
| Temperature (°C) | Acrolein (mg/L) | ||||
|---|---|---|---|---|---|
| 0 Days | 7 Days | 14 Days | 21 Days | 28 Days | |
| 4 | 0.65 ± 0.02 | 0.67 ± 0.04 | 0.68 ± 0.05 | 0.69 ± 0.04 | 0.69 ± 0.03 |
| 25 | 1.04 ± 0.11 | 1.42 ± 0.08 | 1.54 ± 0.06 | 1.66 ± 0.12 | |
| 37 | 11.36 ± 0.37 | 14.43 ± 0.32 | 15.82 ± 0.14 | 15.96 ± 0.18 | |
| 50 | 12.77 ± 0.42 | 15.19 ± 0.28 | 16.54 ± 0.24 | 16.58 ± 0.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.-H.; Jeon, S.-G.; Lee, K.M.; Heo, S.-Y.; Kim, M.-S.; Kim, C.-H.; Oh, B.-R. The Biocatalytic Production of 3-Hydroxypropionaldehyde and Evaluation of Its Stability. Catalysts 2021, 11, 1139. https://doi.org/10.3390/catal11101139
Ju J-H, Jeon S-G, Lee KM, Heo S-Y, Kim M-S, Kim C-H, Oh B-R. The Biocatalytic Production of 3-Hydroxypropionaldehyde and Evaluation of Its Stability. Catalysts. 2021; 11(10):1139. https://doi.org/10.3390/catal11101139
Chicago/Turabian StyleJu, Jung-Hyun, Sang-Gyu Jeon, Kyung Min Lee, Sun-Yeon Heo, Min-Soo Kim, Chul-Ho Kim, and Baek-Rock Oh. 2021. "The Biocatalytic Production of 3-Hydroxypropionaldehyde and Evaluation of Its Stability" Catalysts 11, no. 10: 1139. https://doi.org/10.3390/catal11101139
APA StyleJu, J. -H., Jeon, S. -G., Lee, K. M., Heo, S. -Y., Kim, M. -S., Kim, C. -H., & Oh, B. -R. (2021). The Biocatalytic Production of 3-Hydroxypropionaldehyde and Evaluation of Its Stability. Catalysts, 11(10), 1139. https://doi.org/10.3390/catal11101139





