Will It Be Possible to Put into Practice the Mitigation of Ventilation Air Methane Emissions? Review on the State-of-the-Art and Emerging Materials and Technologies
Abstract
:1. Introduction
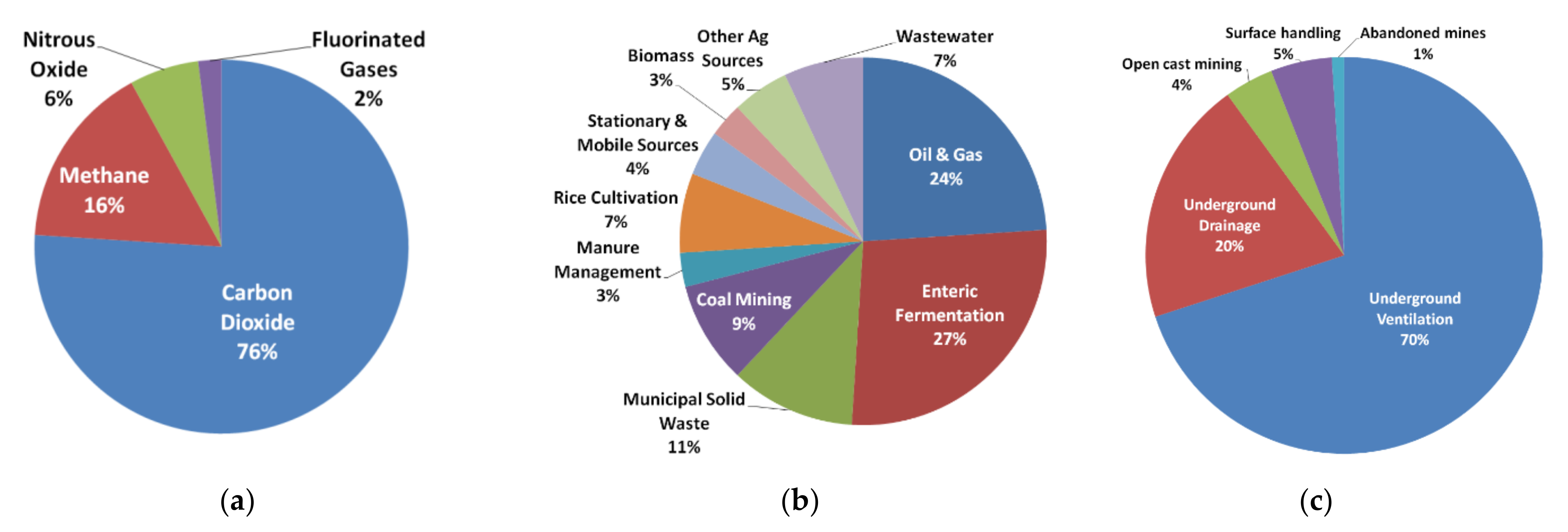
2. Characteristics of the VAM
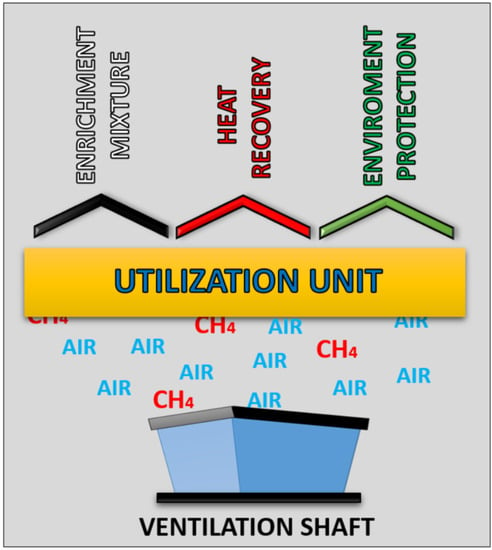
3. A Contemporary Approach to VAM Utilization
3.1. Enrichment Methods
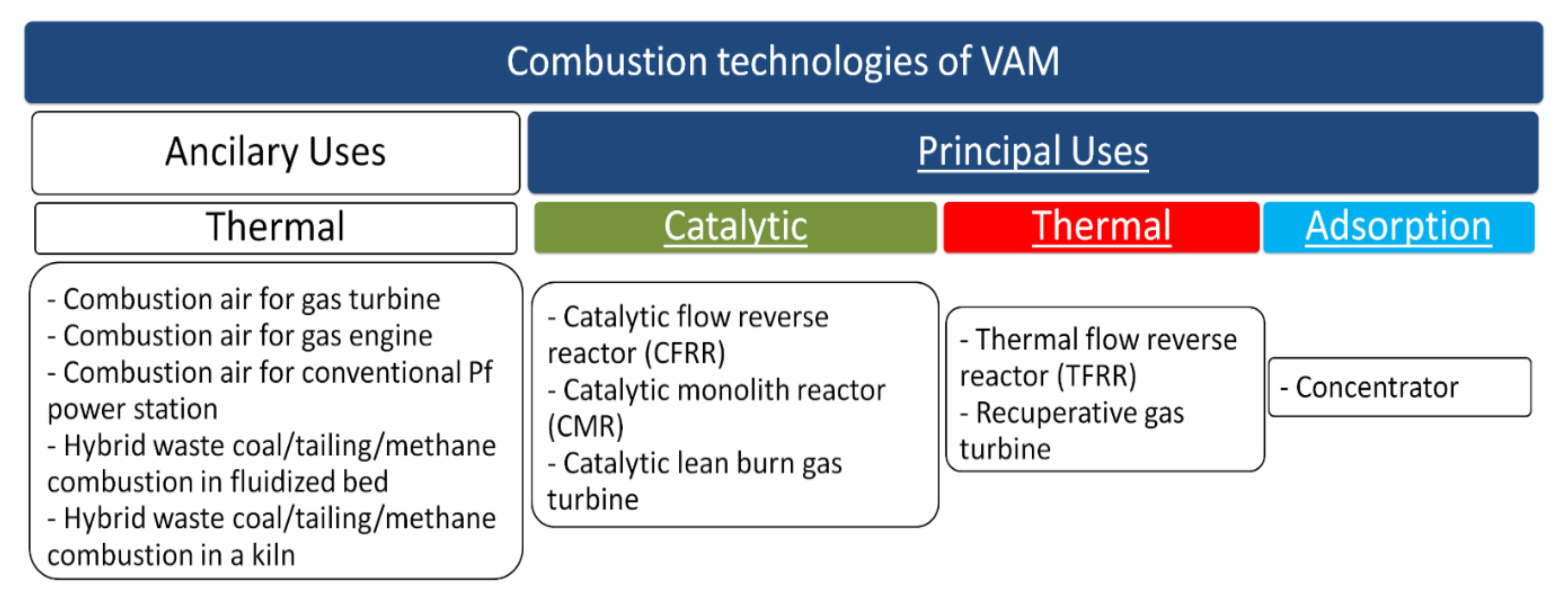
3.2. Oxidation Methods
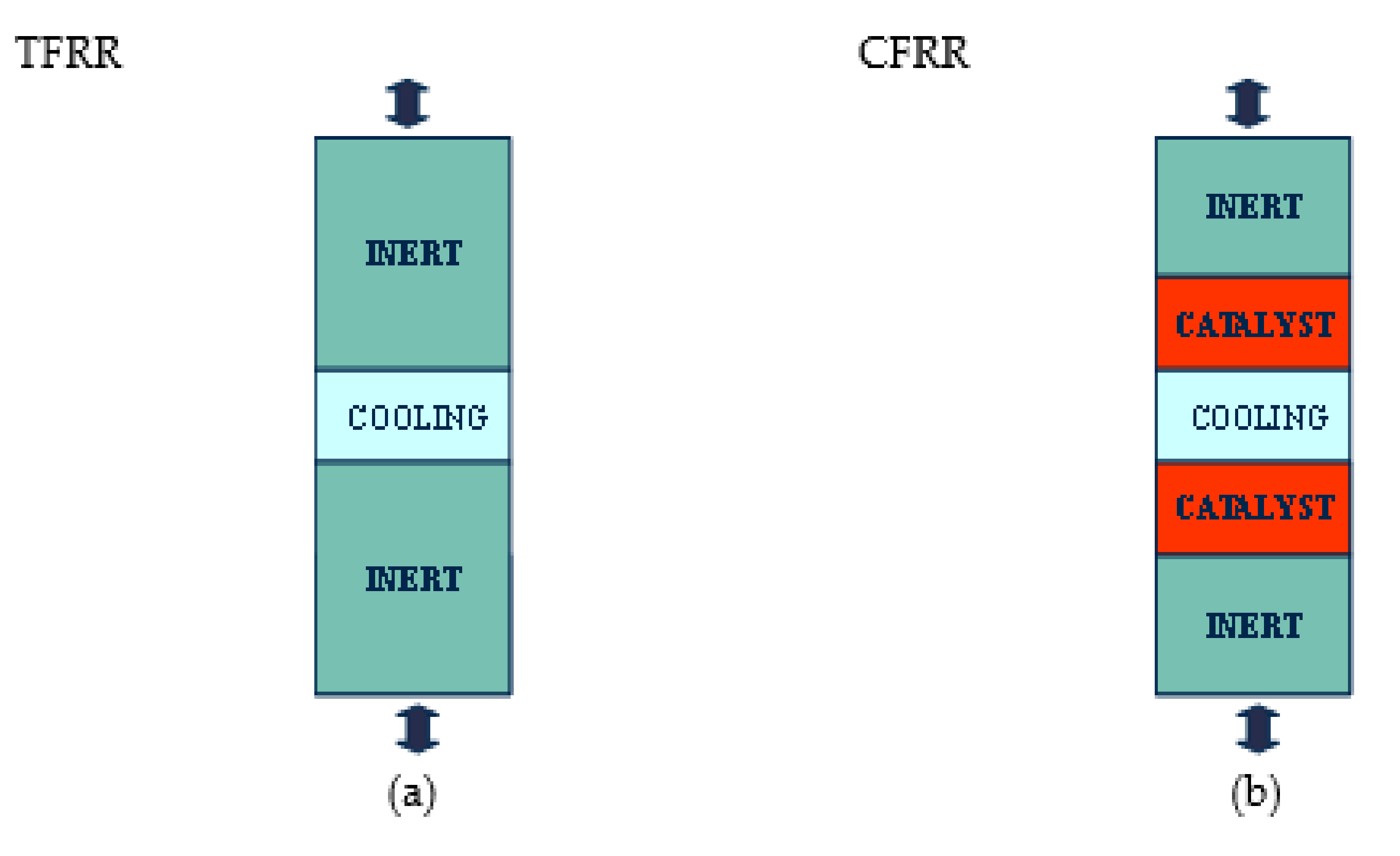
3.2.1. Implemented Technology
3.2.2. Development of Catalytic Technologies
Role of Support
Poison Resistance and Stability Tests
Catalysts Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, Y.; Liu, S.B.; Zhang, Q.; Tao, M.X.; Zhao, M.J.; Hong, F. Coalbed methane genesis, occurrence and accumulation in China. Pet. Sci. 2012, 9, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Cashdollar, K.L.; Zlochower, I.A.; Green, G.M.; Thomas, R.A.; Hertzberg, M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process. Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Su, S.; Chen, H.W.; Teakle, P.; Xue, S. Characteristics of coal mine ventilation air flows. J. Environ. Manag. 2008, 86, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Gosiewski, K.; Pawlaczyk, A.; Jaschik, M. Energy recovery from ventilation air methane via reverse-flow reactors. Energy 2015, 92, 13–23. [Google Scholar] [CrossRef]
- Singh, H.; Mallick, J. Utilization of Ventilation Air Methane in Indian Coal Mines: Prospects and Challenges. Glob. Chall. Policy Framew. Sustain. Dev. Min. Mineral. Foss. Energy Resour. (Gcpf2015) 2015, 11, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, R.O.; Noor, Z.Z.; Abba, A.H.; Abu Hassan, M.A.; Din, M.F.M. Methane emission by sectors: A comprehensive review of emission sources and mitigation methods. Renew. Sustain. Energy Rev. 2012, 16, 5059–5070. [Google Scholar] [CrossRef]
- Kholod, N.; Evans, M.; Pilcher, R.C.; Roshchanka, V.; Ruiz, F.; Cote, M.; Collings, R. Global methane emissions from coal mining to continue growing even with declining coal production. J. Clean. Prod. 2020, 256, 120489. [Google Scholar] [CrossRef]
- Derwent, R.G. Global Warming Potential (GWP) for Methane: Monte Carlo Analysis of the Uncertainties in Global Tropospheric Model Predictions. Atmosphere 2020, 11, 486. [Google Scholar] [CrossRef]
- Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 15 June 2021).
- Wang, W.; Ren, J.D.; Li, X.J.; Li, H.B.; Li, D.Y.; Li, H.M.; Song, Y. Enrichment experiment of ventilation air methane (0.5%) by the mechanical tower. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Su, S.; Agnew, J. Catalytic combustion of coal mine ventilation air methane. Fuel 2006, 85, 1201–1210. [Google Scholar] [CrossRef]
- Holmes, R.I. Mitigating Ventilation Air Methane Cost-Effectively from a Colliery in Australia. J. Appl. Eng. Sci. 2016, 6, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Kumar, J. Fugitive methane emissions from Indian coal mining and handling activities: Estimates, mitigation and opportunities for its utilization to generate clean energy. In Proceedings of the 5th International Conference on Advances in Energy Research (ICAER), IIT Bombay, Mumbai, India, 15–17 December 2015; pp. 336–348. [Google Scholar]
- Baris, K. Assessing ventilation air methane (VAM) mitigation and utilization opportunities: A case study at Kozlu Mine, Turkey. Energy Sustain. Dev. 2013, 17, 13–23. [Google Scholar] [CrossRef]
- Dreger, M.; Kedzior, S. Methane emissions and demethanation of coal mines in the Upper Silesian Coal Basin between 1997 and 2016. Environ. Socio-Econ. Stud. 2019, 7, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Hinde, P.; Mitchell, I.; Riddell, M. COMET (TM)—A New Ventilation Air Methane (VAM) Abatement Technology. Johns. Matthey Technol. Rev. 2016, 60, 211–221. [Google Scholar] [CrossRef]
- Setiawan, A.; Friggieri, J.; Kennedy, E.M.; Dlugogorski, B.Z.; Stockenhuber, M. Catalytic combustion of ventilation air methane (VAM)—Long term catalyst stability in the presence of water vapour and mine dust. Catal. Sci. Technol. 2014, 4, 1793–1802. [Google Scholar] [CrossRef]
- Fernandez, J.; Marin, P.; Diez, F.V.; Ordonez, S. Experimental demonstration and modeling of an adsorption-enhanced reverse flow reactor for the catalytic combustion of coal mine ventilation air methane. Chem. Eng. J. 2015, 279, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.S.; Su, S.; Yu, X.X.; Yin, J.J.; Villella, A.; Jara, M.; Loney, M. Site Trials of Ventilation Air Methane Enrichment with Two-Stage Vacuum, Temperature, and Vacuum Swing Adsorption. Ind. Eng. Chem. Res. 2020, 59, 15732–15741. [Google Scholar] [CrossRef]
- Tremain, P.; Maddocks, A.; Moghtaderi, B. Stone Dust Looping for Ventilation Air Methane Abatement: A 1 m(3)/s Pilot-Scale Study. Energy Fuels 2019, 33, 12568–12577. [Google Scholar] [CrossRef]
- Bae, J.S.; Jin, Y.G.; Huynh, C.; Su, S. Biomass-derived carbon composites for enrichment of dilute methane from underground coal mines. J. Environ. Manag. 2018, 217, 373–380. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Gao, D.; Sun, T.; Zhang, C.; Wang, S. Influence of metal oxides on the performance of Pd/Al2O3 catalysts for methane combustion under lean-fuel conditions. Fuel Process. Technol. 2013, 111, 55–61. [Google Scholar] [CrossRef]
- Setiawan, A.; Kennedy, E.M.; Dlugogorski, B.Z.; Adesina, A.A.; Stockenhuber, M. The stability of Co3O4, Fe2O3, Au/Co3O4 and Au/Fe2O3 catalysts in the catalytic combustion of lean methane mixtures in the presence of water. Catal. Today 2015, 258, 276–283. [Google Scholar] [CrossRef]
- Haojie, G.; Zhongqing, Y.; Jingyu, R.; Li, Z.; Yunfei, Y.; Mingnv, G. Low-concentration methane combustion over a Cu/g-Al2O3 catalyst: Effects of water. RSC Adv. 2015, 5, 18915–18921. [Google Scholar]
- UNFCCC. Text of the Kyoto Protocol. Available online: https://unfccc.int/process-and-meetings/the-kyoto-protocol/what-is-the-kyoto-protocol/kyoto-protocol-targets-for-the-first-commitment-period (accessed on 15 June 2021).
- Su, S.; Beath, A.; Guo, H.; Mallett, C. An assessment of mine methane mitigation and utilization technologies. Prog. Energy Combust. Sci. 2005, 31, 123–170. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Wu, S.; Che, D. Utilization of combustible waste gas as a supplementary fuel in coal-fired boilers. Int. J. Energy Res. 2018, 42, 1677–1692. [Google Scholar] [CrossRef]
- Warmuziński, K.; Jaschik, M.; Olejnik, S.; Tańczyk, M.; Jaschik, J. Analiza kosztów utylizacji kopalnianego powietrza wentylacyjnego w produkcji ciepła i energii elektrycznej. Chem. Inżynieria Ekol. 2004, 11, 242–250. [Google Scholar]
- SU, S.; YU, X. Progress in developing an innovative lean burn catalytic turbine technology for fugitive methane mitigation and utilization. Front. Energy 2011, 5(2), 229–235. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.S.; Li, Z.Y.; Zhang, C.Z.; Xing, Y. Vacuum Exhaust Process in Pilot-Scale Vacuum Pressure Swing Adsorption for Coal Mine Ventilation Air Methane Enrichment. Energies 2018, 11, 1030. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Yu, X.X. A 25 kWe low concentration methane catalytic combustion gas turbine prototype unit. Energy 2015, 79, 428–438. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.J.; Liu, H.Q.; Ribeiro, A.M.; Li, P.; Yu, J.G.; Rodrigues, A.E. Enrichment of ventilation air methane by adsorption with displacement chromatography technology: Experiment and numerical simulation. Chem. Eng. Sci. 2016, 149, 215–228. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Yuan, D.S.; Chen, G.M. Demonstration and its validation for ventilation air methane (VAM) thermal oxidation and energy recovery project. Appl. Therm. Eng. 2015, 90, 75–85. [Google Scholar] [CrossRef]
- He, X.Z.; Lei, L.F. Optimizing methane recovery: Techno-economic feasibility analysis of N-2-selective membranes for the enrichment of ventilation air methane. Sep. Purif. Technol. 2021, 259, 118180. [Google Scholar] [CrossRef]
- Du, J.W.; Li, H.J.; Wang, L.G. Thermodynamic stability conditions, methane enrichment, and gas uptake of ionic clathrate hydrates of mine ventilation air. Chem. Eng. J. 2015, 273, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Bałys, M.; Szczurowski, J.; Czepirski, L. Adsorption technology for ventilation air methane enrichment; Agencja Wydawniczo-Poligraficzna ART-TEKST: Krakow, Poland, 2016; pp. 253–257. [Google Scholar]
- Bae, J.S.; Su, S.; Yu, X.X. Enrichment of Ventilation Air Methane (VAM) with Carbon Fiber Composites. Environ. Sci. Technol. 2014, 48, 6043–6049. [Google Scholar] [CrossRef]
- Ursueguia, D.; Diaz, E.; Ordonez, S. Densification-Induced Structure Changes in Basolite MOFs: Effect on Low-Pressure CH(4)Adsorption. Nanomaterials 2020, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.P.; Zhao, Q.H.; Tao, L.F.; Xiao, P.; Webley, P.A.; Li, K.G. Enrichment of low grade CH4 from N-2/CH4 mixtures using vacuum swing adsorption with activated carbon. Chem. Eng. Sci. 2021, 229, 116152. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, R.N.; Zhang, X.; Han, Y.W.; Bian, W.J.; Ma, Y.; Xue, M. Enrichment and Separation of Methane Gas by Vacuum Pressure Swing Adsorption. Adsorpt. Sci. Technol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Liu, B.; Smit, B. Molecular Simulation Studies of Separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C 2010, 114, 8515–8522. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of Methane and Nitrogen by Adsorption on Carbon Molecular Sieve. Sep. Sci. Technol. 2005, 40, 2721–2743. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, S.; Li, L.; Wang, P.; Li, X.; Che, Y.; Li, X. Preparation of Carbon Molecular Sieves Used for CH4/N2 Separation. J. Chem. Eng. Data 2018, 63, 1737–1744. [Google Scholar] [CrossRef]
- Majumdar, B.; Bhadra, S.J.; Marathe, R.P.; Farooq, S. Adsorption and Diffusion of Methane and Nitrogen in Barium Exchanged ETS-4. Ind. Eng. Chem. Res. 2011, 50, 3021–3034. [Google Scholar] [CrossRef]
- Ouyang, S.; Xu, S.; Song, N.; Jiao, S. Coconut shell-based carbon adsorbents for ventilation air methane enrichment. Fuel 2013, 113, 420–425. [Google Scholar] [CrossRef]
- Bae, J.S.; Su, S.; Yu, X.X. Two-Stage Enrichment of Ventilation Air Methane with Vacuum, Temperature, and Vacuum Swing Adsorption (VTVSA) Processes. Ind. Eng. Chem. Res. 2019, 58, 21700–21707. [Google Scholar] [CrossRef]
- Matros, Y.S.; Bunimovich, G.A. Reverse-Flow Operation in Fixed Bed Catalytic Reactors. Catal. Rev.-Sci. Eng. 1996, 38, 1–68. [Google Scholar] [CrossRef]
- Zagoruiko, A.N.; Bobrova, L.; Vernikovskaya, N.; Zazhigalov, S. Unsteady-state operation of reactors with fixed catalyst beds. Rev. Chem. Eng. 2021, 37, 193–225. [Google Scholar] [CrossRef]
- Ventilation Air Methane (VAM) Utilization Technologies. Available online: https://www.epa.gov/sites/production/files/2017-01/documents/vam_technologies-1-2017.pdf.pdf (accessed on 27 November 2020).
- Stolaroff, J.K.; Bhattacharyya, S.; Smith, C.A.; Bourcier, W.L.; Cameron-Smith, P.J.; Aines, R.D. Review of Methane Mitigation Technologies with Application to Rapid Release of Methane from the Arctic. Environ. Sci. Technol. 2012, 46, 6455–6469. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A. Catalytic or thermal reversed flow combustion of coal mine ventilation air methane: What is better choice and when? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
- Mao, M.M.; Shi, J.R.; Liu, Y.Q.; Gao, M.; Chen, Q. Experimental investigation on control of temperature asymmetry and nonuniformity in a pilot scale thermal flow reversal reactor. Appl. Therm. Eng. 2020, 175, 115375. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Y.Q.; Sun, P.; Meng, J.; Liu, R.X. Dehydrogenation characteristics of lean methane in a thermal reverse-flow reactor. Int. J. Hydrog. Energy 2019, 44, 5137–5142. [Google Scholar] [CrossRef]
- Lan, B.; Li, Y.R.; Zhao, X.S.; Kang, J.D. Industrial-Scale Experimental Study on the Thermal Oxidation of Ventilation Air Methane and the Heat Recovery in a Multibed Thermal Flow-Reversal Reactor. Energies 2018, 11, 1578. [Google Scholar] [CrossRef] [Green Version]
- Gosiewski, K.; Pawlaczyk, A.; Jaschik, M. Thermal combustion of lean methane—Air mixtures: Flow reversal research and demonstration reactor model and its validation. Chem. Eng. J. 2012, 207-208, 76–84. [Google Scholar] [CrossRef]
- Setiawan, A.; Kennedy, E.M.; Stockenhuber, M. Development of Combustion Technology for Methane Emitted from Coal-Mine Ventilation Air Systems. Energy Technol. 2017, 5, 521–538. [Google Scholar] [CrossRef]
- Yin, J.J.; Su, S.; Yu, X.X.; Bae, J.S.; Jin, Y.G.; Villella, A.; Jara, M.; Ashby, M.; Cunnington, M.; Loney, M. Site Trials and Demonstration of a Novel Pilot Ventilation Air Methane Mitigator. Energy Fuels 2020, 34, 9885–9893. [Google Scholar] [CrossRef]
- Gao, P.F.; Gou, X.L. Experimental Research on the Thermal Oxidation of Ventilation Air Methane in a Thermal Reverse Flow Reactor. ACS Omega 2019, 4, 14886–14894. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.; Vega, A.; Diez, F.V.; Ordonez, S. Control of regenerative catalytic oxidizers used in coal mine ventilation air methane exploitation. Process. Saf. Environ. Protect. 2020, 134, 333–342. [Google Scholar] [CrossRef]
- Salomons, S.; Hayes, R.E.; Poirier, M.; Sapoundjiev, H. Flow reversal reactor for the catalytic combustion of lean methane mixtures. Catal. Today 2003, 83, 59–69. [Google Scholar] [CrossRef]
- Wang, Y.; Man, C.; Che, D. Catalytic Combustion of Ventilation Air Methane in a Reverse-Flow Reactor. Energy Fuels 2010, 24, 4841–4848. [Google Scholar] [CrossRef]
- Somers, J.M.; Schultz, H.L. Thermal oxidation of coal mine ventilation air methane. In Proceedings of the 12th U.S./North American Mine Ventilation Symposium, Reno, NV, USA, 9–11 June 2008; pp. 301–305. [Google Scholar]
- Shanxi LuAn Group Gaohe Mine VAM Destruction and Utilization Project. Available online: https://cdm.unfccc.int/Projects/DB/TUEV-RHEIN1352801900.72/view (accessed on 15 June 2021).
- MEGTEC Systems Inc. Leaflet: Ventilation Air Methane (VAM) Processing. MEGTEC Solutions for VAM Abatement, Energy Recovery & Utilization 2014. [Google Scholar]
- U.S. EPA. U.S. Underground Coal Mine Ventilation Air Methane Exhaust Characterization; U.S. EPA: Washington, DC, USA, 2010; pp. 1–16. [Google Scholar]
- Shen, X.; Zhang, B.; Zhang, X.; Wu, S. Explosion behaviors of mixtures of methane and air with saturated water vapor. Fuel 2016, 177, 15–18. [Google Scholar] [CrossRef]
- Chen, J.; Wen, G.; Yan, S.; Lan, X.; Xiao, L. Oxidation and Characterization of Low-Concentration Gas in a High-Temperature Reactor. Processes 2020, 8, 481. [Google Scholar] [CrossRef] [Green Version]
- Best Practice Guidance for Effective Methane Drainage and Use in Coal Mines. Available online: https://unece.org/DAM/energy/se/pdfs/cmm/pub/BestPractGuide_MethDrain_es31.pdf (accessed on 30 June 2021).
- Zheng, B.; Liu, Y.Q.; Liu, R.X.; Meng, J. Catalytic oxidation of coal mine ventilation air methane in a preheat catalytic reaction reactor. Int. J. Hydrog. Energy 2015, 40, 3381–3387. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Y.Q.; Sun, P.; Sun, J.J.; Meng, J. Oxidation of lean methane in a two-chamber preheat catalytic reactor. Int. J. Hydrog. Energy 2017, 42, 18643–18648. [Google Scholar] [CrossRef]
- Sekizawa, K.; Widjaja, H.; Maeda, S.; Ozawa, Y.; Eguchi, K. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catal. Today 2000, 59, 69–74. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Tylus, W.; Kępiński, L. Pd-based monolithic catalysts on metal supports for catalytic combustion of methane. Appl. Catal. B Environ. 2004, 49, 27–37. [Google Scholar] [CrossRef]
- Xiao, L.-h.; Sun, K.-p.; Xu, X.-l.; Li, X.-n. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition–precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M.; Baranowska, K. Methane combustion over bimetallic Ru-Re/γ-Al2O3 catalysts: Effect of Re and pretreatments. Appl. Catal. B Environ. 2016, 194, 22–31. [Google Scholar] [CrossRef]
- Lin, W.; Lin, L.; Zhu, Y.X.; Xie, Y.C.; Scheurell, K.; Kemnitz, E. Novel Pd/SnxZr1-xO2 catalysts for methane total oxidation at low temperature and their O-18-isotope exchange behavior. Appl. Catal. B-Environ. 2005, 57, 175–181. [Google Scholar] [CrossRef]
- Guo, T.Y.; Du, J.P.; Wu, J.T.; Wang, S.; Li, J.P. Structure and kinetic investigations of surface-stepped CeO2-supported Pd catalysts for low-concentration methane oxidation. Chem. Eng. J. 2016, 306, 745–753. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Specchia, S. Catalytic Performance of Pd/Co3O4 on SiC and ZrO2 Open Cell Foams for Process Intensification of Methane Combustion in Lean Conditions. Ind. Eng. Chem. Res. 2017, 56, 6625–6636. [Google Scholar] [CrossRef]
- Xiong, J.X.; Wu, K.; Yang, J.; Liu, P.; Song, L.H.; Zhang, J.; Fu, M.L.; Chen, L.M.; Huang, H.M.; Wu, J.L.; et al. The effect of existence states of PdOx supported by Co3O4 nanoplatelets on catalytic oxidation of methane. Appl. Surf. Sci. 2021, 539, 14. [Google Scholar] [CrossRef]
- Lamarino, M.; Chirone, R.; Lisi, L.; Pirone, R.; Salatino, P.; Russo, G. Cu/γ-Al2O3 catalyst for the combustion of methane in a fluidized bed reactor. Catal. Today 2002, 75, 317–324. [Google Scholar] [CrossRef]
- Marín, P.; Ordóñez, S.; Díez, F.V. Performance of silicon-carbide foams as supports for Pd-based methane combustion catalysts. J. Chem. Technol. Biotechnol. 2012, 87, 360–367. [Google Scholar] [CrossRef]
- Hosseiniamoli, H.; Bryant, G.; Kennedy, E.M.; Mathisen, K.; Nicholson, D.; Sankar, G.; Setiawan, A.; Stockenhuber, M. Understanding Structure-Function Relationships in Zeolite-Supported Pd Catalysts for Oxidation of Ventilation Air Methane. Acs Catal. 2018, 8, 5852–5863. [Google Scholar] [CrossRef]
- Guo, T.Y.; Nie, X.R.; Du, J.P.; Li, J.P. Enhanced properties of Pd/CeO2-nanorods modified with alkaline-earth metals for catalytic oxidation of low-concentration methane. Rsc Adv. 2018, 8, 38641–38647. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.F.; Li, W.Z.; Lin, Q.Z.; Zheng, X.S.; Pan, H.B.; Pi, D.; Shao, C.Y.; Hu, C.; Zhang, H.T. Catalytic performance of Pd-NiCo2O4/SiO2 in lean methane combustion at low temperature. J. Energy Inst. 2018, 91, 733–742. [Google Scholar] [CrossRef]
- Li, Y.; Armor, J.N. Catalytic combustion of methane over palladium exchanged zeolites. Appl. Catal. B Environ. 1994, 3, 275–282. [Google Scholar] [CrossRef]
- Miniajluk, N.; Trawczyński, J.; Zawadzki, M.; Tylus, W. LaMnO3 (La0.8Sr0.2MnO3) Perovskites for Lean Methane Combustion: Effect of Synthesis Method. Adv. Mater. Phys. Chem. 2018, 08, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, V.R.; Patil, V.P.; Jana, P.; Uphade, B.S. Nano-gold supported on Fe2O3: A highly active catalyst for low temperature oxidative destruction of methane green house gas from exhaust/waste gases. Appl. Catal. A Gen. 2008, 350, 186–190. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, H.; Dong, B.; Ni, Y.; Kong, A.; Shan, Y. High Efficient Mesoporous Co3O4Nanocatalysts For Methane Combustion at Low Temperature. Chem. Sel. 2016, 1, 979–983. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, W.; Hu, R.; Chang, G.; Li, C.; Wang, L. Catalytic activity of spinel oxides MgCr2O4 and CoCr2O4 for methane combustion. Mater. Res. Bull. 2014, 57, 268–273. [Google Scholar] [CrossRef]
- Kruemek, P.; Mattathankul, S.; Triamnak, N.; Chotigkrai, N. Iop. A facile synthesis of CuCe and CuCeFe mixed-oxide catalysts by solution combustion method for catalytic methane combustion. In Proceedings of the 26th Regional Symposium on Chemical Engineering (RSCE), Kuala Lumpur, Malaysia, 30–31 October 2019. [Google Scholar]
- Hosseiniamoli, H.; Setiawan, A.; Adesina, A.A.; Kennedy, E.M.; Stockenhuber, M. The stability of Pd/TS-1 and Pd/silicalite-1 for catalytic oxidation of methane—Understanding the role of titanium. Catal. Sci. Technol. 2020, 10, 1193–1204. [Google Scholar] [CrossRef]
- Zedan, A.F.; AlJaber, A.S. Combustion Synthesis of Non-Precious CuO-CeO2 Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation. Materials 2019, 12, 878. [Google Scholar] [CrossRef] [Green Version]
- Kucharczyk, B. Activity of monolithic Pd/Al2O3 catalysts in the combustion of mine ventilation air methane. Pjct 2011, 13, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Narui, K.; Yata, H.; Furuta, K.; Nishida, A.; Kohtoku, Y.; Matsuzaki, T. Effects of addition of Pt to PdO/Al2O3 catalyst on catalytic activity for methane combustion and TEM observations of supported particles. Appl. Catal. A Gen. 1999, 179, 165–173. [Google Scholar] [CrossRef]
- Ishihara, T.; Shigematsu, H.; Abe, Y.; Takita, Y. Effects of Additives on the Activity of Palladium Catalysts for Methane Combustion. Chem. Lett. 1993, 22, 407–410. [Google Scholar] [CrossRef]
- Xiong, J.; Mo, S.; Song, L.; Fu, M.; Chen, P.; Wu, J.; Chen, L.; Ye, D. Outstanding stability and highly efficient methane oxidation performance of palladium-embedded ultrathin mesoporous Co2MnO4 spinel catalyst. Appl. Catal. A Gen. 2020, 598, 117571. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.T.; Al-Qaradawi, S.Y. Exploring halloysite nanotubes as catalyst support for methane combustion: Influence of support pretreatment. Appl. Clay Sci. 2021, 201, 9. [Google Scholar] [CrossRef]
- Geng, H.J.; Yang, Z.Q.; Zhang, L.; Ran, J.Y.; Chen, Y.R. Experimental and kinetic study of methane combustion with water over copper catalyst at low-temperature. Energy Convers. Manag. 2015, 103, 244–250. [Google Scholar] [CrossRef]
- Gao, X.J.; Jin, Z.H.; Hu, R.S.; Hu, J.N.; Bai, Y.Q.; Wang, P.; Zhang, J.; Zhao, C.X. Double perovskite anti-supported rare earth oxide catalyst CeO2/La2CoFeO6 for efficient ventilation air methane combustion. J. Rare Earths 2021, 39, 398–408. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Specchia, S.; Finocchio, E.; Busca, G.; Palmisano, P.; Specchia, V. Surface chemistry and reactivity of ceria–zirconia-supported palladium oxide catalysts for natural gas combustion. J. Catal. 2009, 263, 134–145. [Google Scholar] [CrossRef]
- Zanoletti, M.; Godard, F.; Perrier, M. Effect of support on the apparent activity of palladium oxide in catalytic methane combustion. Can. J. Chem. Eng. 2020, 98, 2205–2213. [Google Scholar] [CrossRef]
- Wang, S.; Gao, D.N.; Wang, S.D. Steady and Transient Characteristics of Catalytic Flow Reverse Reactor Integrated with Central Heat Exchanger. Ind. Eng. Chem. Res. 2014, 53, 12644–12654. [Google Scholar] [CrossRef]
- Coney, C.; Stere, C.; Millington, P.; Raj, A.; Wilkinson, S.; Caracotsios, M.; McCullough, G.; Hardacre, C.; Morgan, K.; Thompsett, D.; et al. Spatially-resolved investigation of the water inhibition of methane oxidation over palladium. Catal. Sci. Technol. 2020, 10, 1858–1874. [Google Scholar] [CrossRef]
- Stasinska, B.; Machocki, A.; Antoniak, K.; Rotko, M.; Figueiredo, J.L.; Gonçalves, F. Importance of palladium dispersion in Pd/Al2O3 catalysts for complete oxidation of humid low-methane–air mixtures. Catal. Today 2008, 137, 329–334. [Google Scholar] [CrossRef]
- Ordonez, S.; Hurtado, P.; Sastre, H.; Diez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulphur dioxide: Development of a deactivation model. Appl. Catal. A-Gen. 2004, 259, 41–48. [Google Scholar] [CrossRef]
- Nawrat, S.; Gatnar, K. Ocena stanu i możliwości utylizacji metanu z powietrza wentylacyjnego podziemnych kopalń węgla kamiennego. Polityka Energetyczna 2008, 11, 69–84. [Google Scholar]
- Su, S.; Ren, T.; Balusu, R.; Beath, A.; Guo, H.; Mallett, C. Development of Two Case Studies on Mine Methane Capture and Utilisation in China. CSIRO - Report P2006/17; CSIRO: Kenmore, Australia, 2006. [Google Scholar]
- Carothers, P.; Deo, M. Technical and Economic Assessment: Mitigation of Methane Emissions from Coal Mine Ventilation Air. Coalbed Methane Outreach Program; U.S. EPA: Washington, DC, USA, 2000; p. 96. [Google Scholar]
- Tsyrulnikov, P.G.; Sal’nikov, V.S.; Drozdov, V.A.; Noskov, A.S.; Chumakova, N.A.; Ermolaev, V.K.; Malakhova, I.V. Deep Oxidation of Methane on Alumina–Manganese and Pt-Containing Catalysts. J. Catal. 2001, 198, 164–171. [Google Scholar] [CrossRef]
- Gogin, L.L.; Matros, L.L.; Ivanov, A.G. Ekologiia i Kataliz [Ecology and Catalysis]; (in Russian). Izd. Nauka: Novosibirsk, Russia, 1990; p. 107. [Google Scholar]
- Stasińska, B. Ograniczenie emisji metanu z kopalń węglowych poprzez katalityczne oczyszczanie powietrza wentylacyjnego. Polityka Energetyczna 2009, 12, 123–132. [Google Scholar]
- Mallett, C.; Su, S. Progress in Developing Ventilation Air Methane Mitigation and Utilization Technologies. In Proceedings of the Third International Methane and Nitrous Oxide Mitigation Conference, Beijing, China, 17–21 November 2003; 2003. [Google Scholar]
- Sapoundjiev, H.; Trottier, R.; Aube, F. Heat recovery from lean industrial emissions. Enviromental and economic benefits of CFRR technology. Greenh. Gas. Control. Technol. 1999, 805–810. [Google Scholar]
- Marin, P.; Hevia, M.A.G.; Ordonez, S.; Diez, F.V. Combustion of methane lean mixtures in reverse flow reactors: Comparison between packed and structured catalyst beds. Catal. Today 2005, 105, 701–708. [Google Scholar] [CrossRef]
- Urbani, C.; Marin, P.; Diez, F.V.; Ordonez, S. Catalytic combustion of sulphur-containing methane lean emissions in a reverse-flow reactor with integrated adsorption. Chem. Eng. J. 2016, 285, 39–48. [Google Scholar] [CrossRef]
- Fernandez, J.; Marin, P.; Diez, F.V.; Ordonez, S. Coal mine ventilation air methane combustion in a catalytic reverse flow reactor: Influence of emission humidity. Fuel Process. Technol. 2015, 133, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Universitaet Stuttgart; European Union. European Union Project (Contract, No. ICA2-CT-2000-10035): Recovery of methane from vent gases of coal mines and its efficient utilization as a high temperature heat source – Final Report; Universitaet Stuttgart: Stuttgart, Germany, 2003. [Google Scholar]
- Gosiewski, K. Efficiency of heat recovery versus maximum catalyst temperature in the reverse-flow combustion of methane. Chem. Eng. J. 2005, 107, 19–25. [Google Scholar] [CrossRef]
- Sapoundjiev, H.; Aubé, F.; Trottier, R. Report: Elimination of dilute methane emissions from underground mine and oil and natural gas production sectors. CANMET, Natural Resources Canada; Canadian Environment Industry Association: Ottawa, ON, Canada, 1999. [Google Scholar]
- Slepterev, A.A.; Salnikov, V.S.; Tsyrulnikov, P.G.; Noskov, A.S.; Tomilov, V.N.; Chumakova, N.A.; Zagoruiko, A.N. Homogeneous high-temperature oxidation of methane. React. Kinet. Catal. Lett. 2007, 91, 273–282. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A.; Warmuzinski, K.; Jaschik, M. A study on thermal combustion of lean methane—Air mixtures: Simplified reaction mechanism and kinetic equations. Chem. Eng. J. 2009, 154, 9–16. [Google Scholar] [CrossRef]
- Tsyrulnikov, P.G.; Tsybulya, S.V.; Kryukova, G.N.; Boronin, A.I.; Koscheev, S.V.; Starostina, T.G.; Bubnov, A.V.; Kudrya, E.N. Phase transformations in the thermoactivated MnOx–Al2O3 catalytic system. J. Mol. Catal. A Chem. 2002, 170, 213–220. [Google Scholar] [CrossRef]
- Gosiewski, K.; Machej, T.; Janas, J.; Sadowska, H.; Warmuziński, K. Kinetyka katalitycznego spalania metanu w małym stężeniu. Inżynieria Chem. I Proces. 2001, 22, 599–612. [Google Scholar]
- Gosiewski, K.; Warmuziński, K.; Tańczyk, M.; Moszczyński, M.; Jaschik, J.; Zielińska, I.; Giełzak, K. Projekt badawczy KBN nr 3 TO9C 042 18: Katalityczne usuwanie metanu z górniczych gazów wentylacyjnych w reaktorach niestacjonarnych ze wstępnym wzbogacaniem mieszaniny gazowej metodą adsorpcji zmiennociśnieniowej; Institute of Chemical Engineering PAS: Gliwice, Poland, 2001–2003. [Google Scholar]
- Bos, A.N.R.; Lange, J.P.; Kabra, G. A novel reverse flow reactor with integrated separation. Chem. Eng. Sci. 2007, 62, 5661–5662. [Google Scholar] [CrossRef]
- Fernandez, J.; Marin, P.; Diez, F.V.; Ordonez, S. Combustion of coal mine ventilation air methane in a regenerative combustor with integrated adsorption: Reactor design and optimization. Appl. Therm. Eng. 2016, 102, 167–175. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Stasińska, B.; Nawrat, S. Studies on work of a prototype installation with two types of catalytic bed in the reactor for oxidation of methane from mine ventilation air. Fuel Process. Technol. 2017, 166, 8–16. [Google Scholar] [CrossRef]
- Salamon, E.; Cornejo, I.; Mmbaga, J.P.; Kolodziej, A.; Lojewska, J.; Hayes, R.E. Investigations of a three channel autogenous reactor for lean methane combustion. Chem. Eng. Process.-Process. Intensif. 2020, 153, 9. [Google Scholar] [CrossRef]
- Yonggang, J.; Shi, S. Proof-of-Concept Photocatalytic Destruction of Methane for Coal Mining Fugitive Emissions Abatement. Available online: https://www.acarp.com.au/abstracts.aspx?repId=C24061 (accessed on 20 June 2021).

| Parameter 1 | Australia | Turky | |||
|---|---|---|---|---|---|
| Mine A [3] | Mine B [3] | Mine C [3] | Mine D [3] | Kozlu Mine—Turky [14] | |
| Flow rate [m3/s] | 230–260 | 120–195 | 370–390 | 200–230 | 85–97 |
| Relative humidity [%] | 100 | 85–100 | 74.5–83.5 | 73–99.8 | No data |
| Dust loading [mg/m3] | 0.13–4.47 | 0.25–3.87 | 0.67–3.82 | 0.21–3.19 | No data |
| Methane conc. [vol.%] | 0.08–0.92 | 0.16–1.4 | 0.04–0.19 | 0.32–1.68 | 0.6–0.9 |
| Kind of SA | Ref. | Kind of Adsorbent | BET [m2/g] | Gas Concentration [vol.%] | Scale of Installation | Process | |
|---|---|---|---|---|---|---|---|
| Feed | Result | ||||||
| PSA | [36] | Coconut shell activated carbon | 1085 | 0.3 | 1 | φ 20 × 1500 mm Bed: 0.16 kg | Laboratory |
| VSA | [39] | Commercial activated carbon | 700 | 4.7 8.05 11.7 17 | 14.3 21.8 29.3 42.2 | φ 20 × 900 mm | Laboratory |
| TVSA | [37] | Honeycomb monolithic carbon fiber composite | 458–577 | 0.27 0.32 0.53 0.93 | 2.5 5 7 12 | 30 × 100 × 200 mm | Laboratory 20 L/min |
| VPSA | [30] | Coconut shell activated carbon | 1155 | 0.2 | 1.2 | Two columns: φ 1000 × 1840 mm φ 550 × 1440 mm | Pilot plant Real VAM + coal mine methane 8300 L/min |
| [40] | Activated carbon | 1124.3 | 0.1 0.3 0.5 0.7 | 0.21 0.59 0.89 1.2 | φ 60 × 500 mm | Simulation and experimental (with similar increase ratio) | |
| [32] | Activated carbon | 1077.49 | 1 | 53.5 | φ 27 × 617 mm | Laboratory; displacement of CH4 by use CO2 | |
| [45] | Coconut shell activated carbon | 329 | 0.42 | 1.09 | φ 17 × 500 mm | Laboratory 0.65 L/min | |
| VTVSA | [46] | Honeycomb monolithic carbon fiber composite | 604 | 0.3 0.6 0.98 | 19.28 24.24 36.92 | Two columns: 134 × 184 × 2000 mm 123 × 168 × 1000 mm Bed: 9.97 kg | Simulation 30 L/min |
| [19] | 0.58 0.73 | 28.37 32.04 | Prototype Real VAM 22–60 L/min | ||||
| Ref. | Company | Location | CH4 Conc. [vol.%] | Flow Rate [m3/h] | Number of Units |
|---|---|---|---|---|---|
| [62] | MEGTEC | West Cliff Colliery in New South Wales in Australia | 0.9–1 | 250,000 | 4 |
| [49] | Dürr System | Marshal County Mine, West Virginia, USA | 1.2 | 270,000 | 3 |
| [49,63] | Dürr System | Gaohe Mine of the LuAn Mining Group in Shanxi Province in China | 1.2 | 1,080,000 | 12 |
| [64] | MEGTEC | Da Tong Mine, ChonQing Province in China | 0.5 | 375,000 | 6 |
| [33] | MEGTEC SHENGDONG Cor. | Dafosi Coal Mine, Binchang Shanxi Province in China | 0.9–1.1 | 300,000 | 5 |
| [65] | Biothermica, JWR | JWR No. 7 w Brookwood, Alabama in USA | 0.9 | 51,000 | 1 |
| Ref. | Catalyst | Range of | Tign [°C] | Tx [°C] x- Conversion [%] | Reactor | Experimental Conditions | |
|---|---|---|---|---|---|---|---|
| Temp. [°C] | CH4 [vol.%] in Air | ||||||
| [69] | Pd 0.7 g/L | 530–560 | 0.8–1.2 | 350 | No data | PCRR | 1000 Nm3/h, SV = 2600–11,500 1/h; MB |
| [70] | Pd 0.7 g/L | 420–540 | 0.6–0.8 | No data | T94 = ~450 | 2CPCRR | 2000 Nm3/h, SV = 3800–8100 1/h; CMB |
| [71] | Pd/ZrO2–MOx Pd/SnO2–MOx | 800 | 1 | ~300 ~300 | T90 = ~500 T90 = ~440 | FR | SV = 48,000 1/h |
| [22] | Pd/Al2O3–MOx | 600 | 0.4 | 300 | T90 = 410 | FBTR | GHSV = 80,000 1/h; 4% water GHSV = 160,000 1/h; 8% water; pellets |
| [72] | Pd/Al2O3–ZrSiO4 Pd/Al2O3–SiO2 | 250–650 | 1 | 250 270 | T90 = 415 T90 = 447 | FR | GHSV = 5800 1/h; MC |
| [73] | Pd/CeO2 | 200–560 | 1 | 200 | T100 = 300 | FR | GHSV = 50,000 1/h; 10% water |
| [74] | Ru-Re/Al2O3 | 200–600 | 0.8 | 200 | T95 = 525 | FBR | GHSV = 60,000 1/h |
| [23] | Au/Co3O4 Au/Fe2O3 | 250–650 | 0.6 | 290 375 | T90 = 463 T90 = 600 | TMR | GHSV = 100,000 1/h; 3% water; nanoparticles |
| [75] | Pd/SnxZr1-xO2 | 250–500 | 1 | 250 | T90 = 445 | U-MR | GHSV = 48,000 1/h |
| [76] | Pd/LOC-Zr | 200–500 | 1 | 200 | T90 = 380 | FBMR | GHSV = 16,000 mL/(m h) |
| [77] | Pd/Co3O4 | 250–650 | 0.5–1 | 200 | T90 = 325 | FR | WHSV = 30–90 Nl/(gcat h); OCF |
| [78] | Pd/Co3O4 | 200–380 | 1 | 200 | T90 = 375 | FBR | WHSV = 30,000 mL/(g h); 5% water; 3 types of Pd supports |
| [79] | Cu/γ-Al2O3 | 350–750 | 0.15–0.3 | 400 | T50 = 525–540 | FBR | Additional test in fluidized bed reactor; spheres |
| [80] | Pd/SiC | 300–550 | 0.08–0.17 | 250 | T100 = 550 | MTR | 0.5 l/min; foam |
| [81] | Pd/HZSM-5 | 400 | 0.7 | 350 | T90 = 420 | FBMR | GHSV = 100,000 1/h; 30,000 ppm water; zeolite |
| [82] | PdCa/CeO2-ND | 200–450 | 1 | 200 | T90 = 380–450 | FBMR | GHSV = 16,000 mL/(g h) |
| [83] | Pd-NiCo2O4/SiO2 | 250–550 | 1 | 250 | T90 = 355 | TR | WHSV = 30,000 mL/(g h) |
| [84] | Pd/HZSM-5 PdO/Al2O3 | 175–375 | 1 | 200 250 | T100 = 280 T100 = 370 | FR | GHSV = 30,000 1/h; zeolite |
| [85] | LaMnO3 La0.8Sr0.2MnO3 | 200–550 | 0.6 | ~200 | T90 = 375 | TR | GHSV = 40,000 1/h |
| [86] | Au/Fe2O3 | 300–600 | 1 | 300 | T95 = 488 | FBR | GHSV = 51,000 1/h |
| [87] | Co3O4 | 200–450 | 1 | 200 | T90 = 360 | No data | GHSV = 168,000 mL/(gcat h) |
| [88] | MgCr2O4 CoCr2O4 | 350–800 | 1 | 350 380 | T90 = 684 T90 = 736.7 | FBR | GHSV = 48,000 mL/(g h) |
| [89] | CuCe CuCeFe | 300–500 | 1 | No data | T20 = 413 T25 = 440 | FR | T57 = 500, Tin = 300 |
| [90] | Pd/TS-1 Pd/Silicate-1 | 300–550 | 0.7 | 303 305 | T90 = 385 T90 = 410 | FBMR | GHSV = 100,000 1/h; 30,000–40,000 ppm of water (80–100% RH); zeolite |
| [91] | CuO-CeO2 | 300–600 | 0.1 | 300 | T90 = 575 | FR | WHSV = 78,000 mL/(g h) |
| [92] | (0.5–2%)Pd/Al2O3 | 250–550 | 0.5–1 | 255 | T90 = 390 | FR | GHSV = 4500–7000 1/h; Metal monolith |
| [93] | PdO-Pt/α- Al2O3 PdO/α- Al2O3 | 350 | 0.5 | No data | T97 = 350 T87 = 350 | FBR | GHSV = 18,000 1/h |
| [94] | Pd/NiO | 330–730 | 1 | ~280 | T90 = 748 | FR | GHSV = 100,000 1/h |
| [17] | 1 wt% Pd Al2O3 | 250–500 | 0.7 | ~250 | T90 = 45 | FR | GHSV = 100,000 1/h; test with water and dust mine |
| [95] | Co2MnO4 Pd/Co2MnO4 | 200–475 | 1 | ~250 ~225 | T90 = 373 T90 = 324 | FBR | WHSV = 15,000–120,000 mL/(g h) |
| [96] | Pd/HNT | 200–450 | 1 | ~250 | T100 = 375 | FBMR | GHSV = 72,000 mL/(g h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk-Kurek, A.; Suwak, M. Will It Be Possible to Put into Practice the Mitigation of Ventilation Air Methane Emissions? Review on the State-of-the-Art and Emerging Materials and Technologies. Catalysts 2021, 11, 1141. https://doi.org/10.3390/catal11101141
Pawlaczyk-Kurek A, Suwak M. Will It Be Possible to Put into Practice the Mitigation of Ventilation Air Methane Emissions? Review on the State-of-the-Art and Emerging Materials and Technologies. Catalysts. 2021; 11(10):1141. https://doi.org/10.3390/catal11101141
Chicago/Turabian StylePawlaczyk-Kurek, Anna, and Mikołaj Suwak. 2021. "Will It Be Possible to Put into Practice the Mitigation of Ventilation Air Methane Emissions? Review on the State-of-the-Art and Emerging Materials and Technologies" Catalysts 11, no. 10: 1141. https://doi.org/10.3390/catal11101141
APA StylePawlaczyk-Kurek, A., & Suwak, M. (2021). Will It Be Possible to Put into Practice the Mitigation of Ventilation Air Methane Emissions? Review on the State-of-the-Art and Emerging Materials and Technologies. Catalysts, 11(10), 1141. https://doi.org/10.3390/catal11101141