Recent Manganese Oxide Octahedral Molecular Sieves (OMS–2) with Isomorphically Substituted Cationic Dopants and Their Catalytic Applications
Abstract
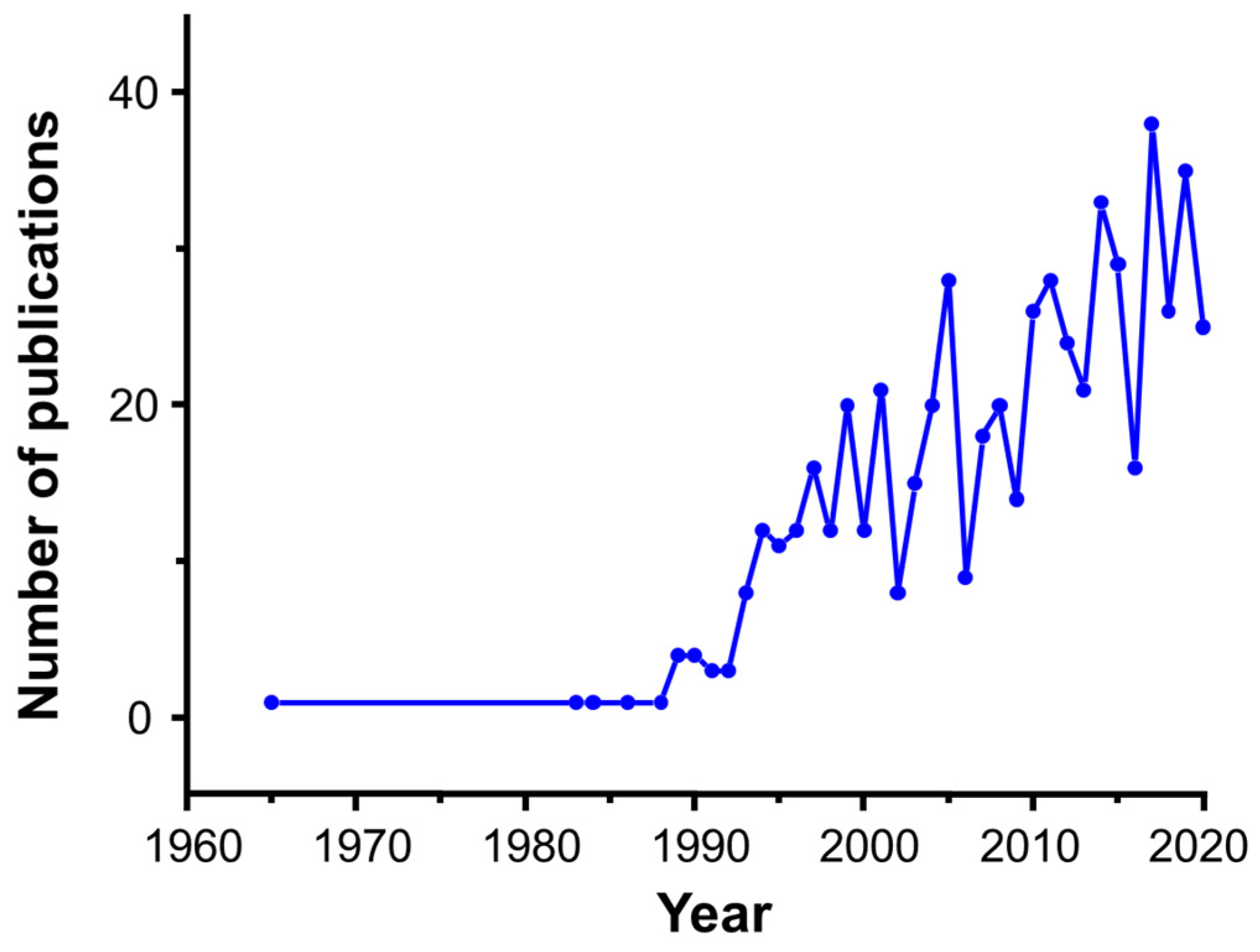
1. Introduction
2. Octahedral Molecular Sieves (OMS)
2.1. General Aspects
2.2. Manganese Octahedral Molecular Sieves
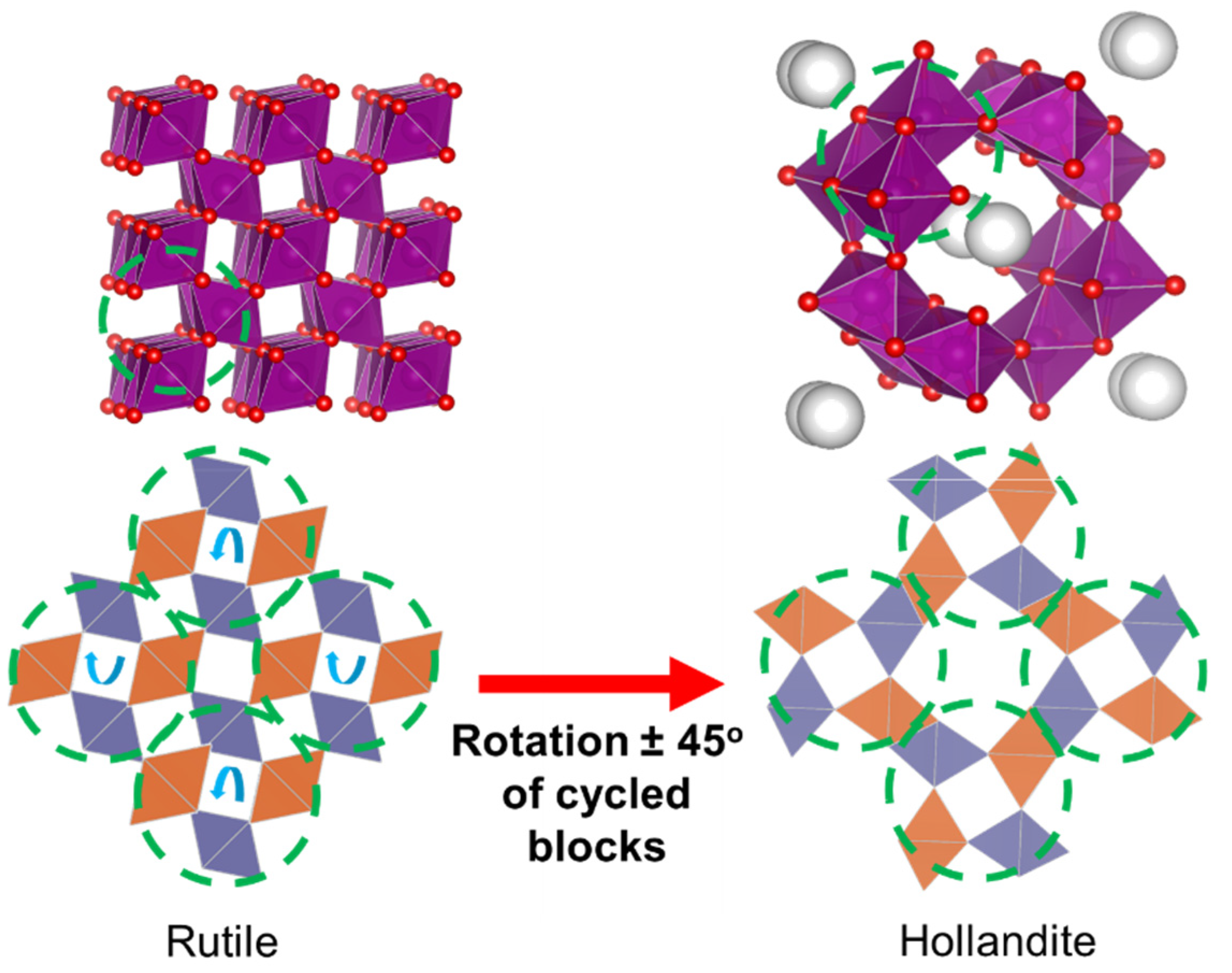
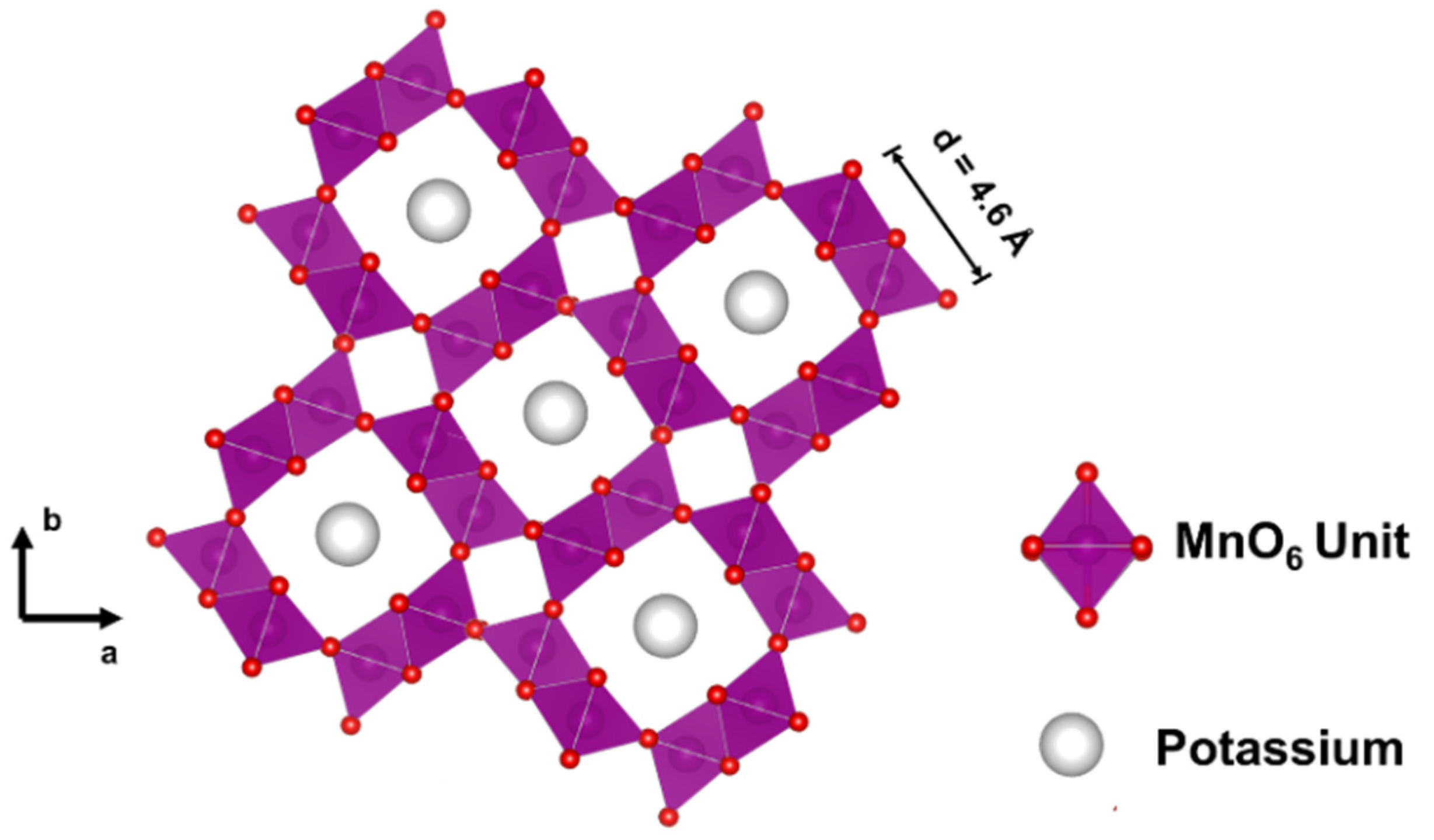
2.3. Cryptomelane (OMS–2)
2.3.1. General Aspects
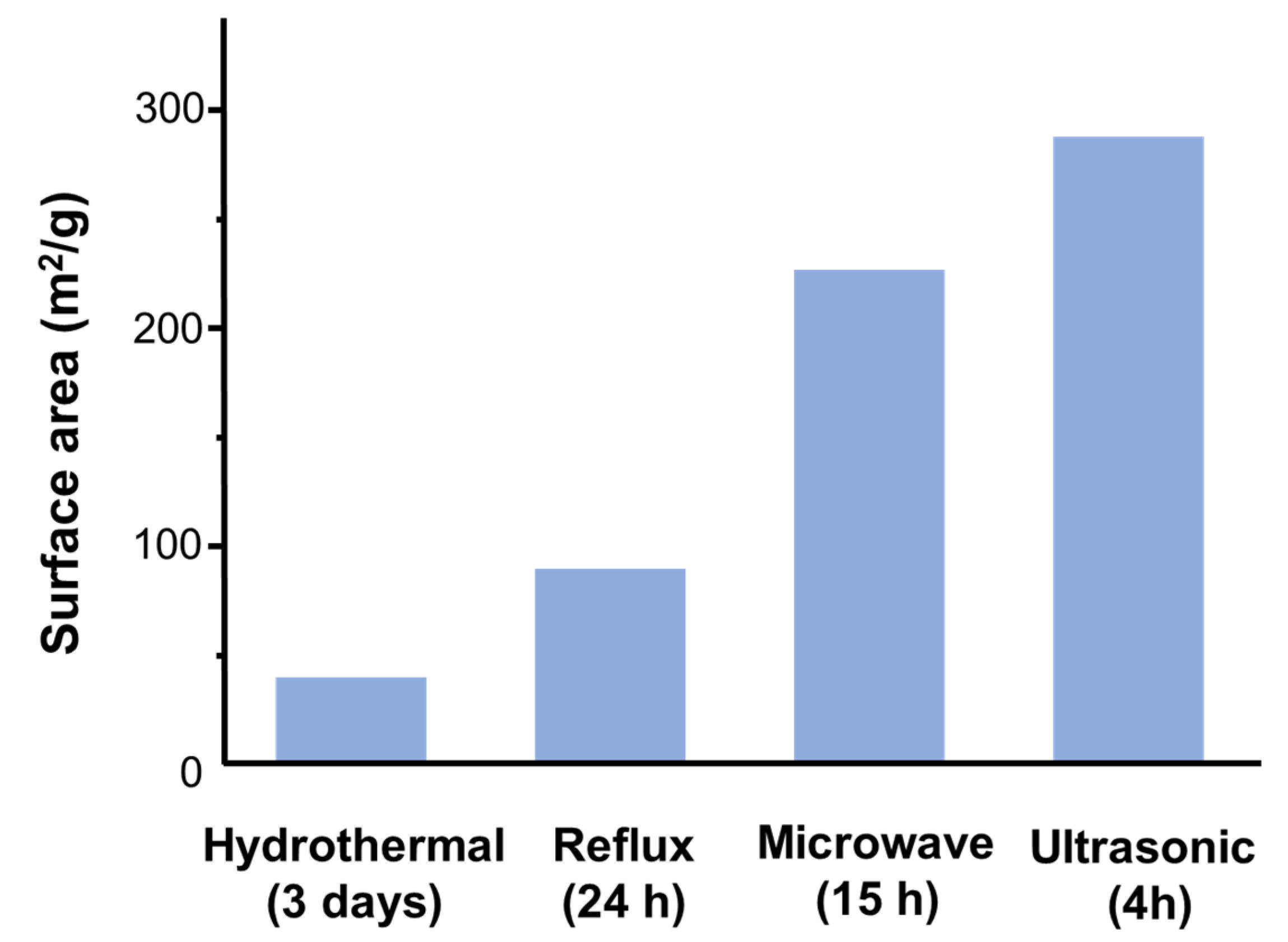
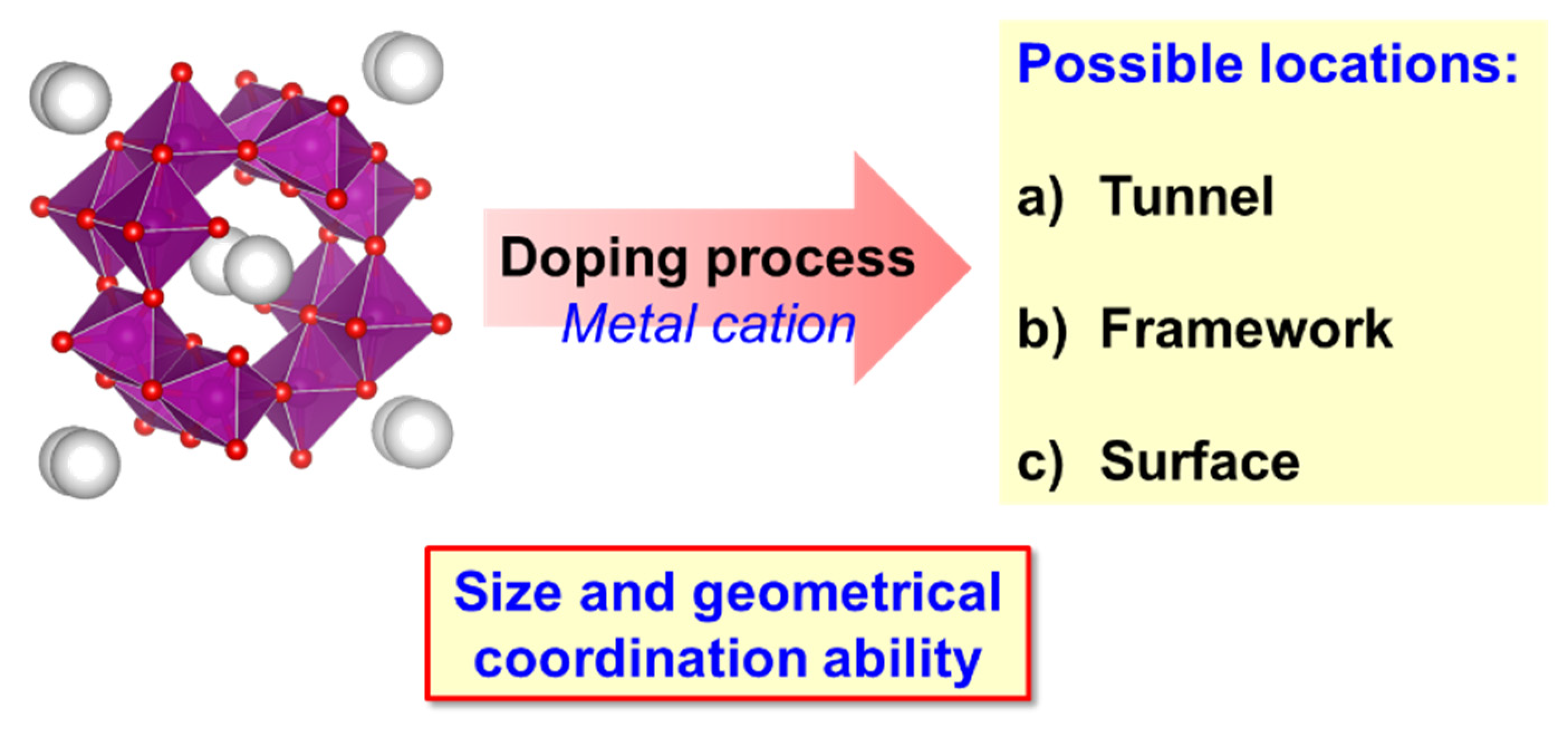
2.3.2. Assessment of Doping Processes
3. [M]–K–OMS–2 Materials
3.1. Characterization Data
3.1.1. [Ag]–K–OMS–2
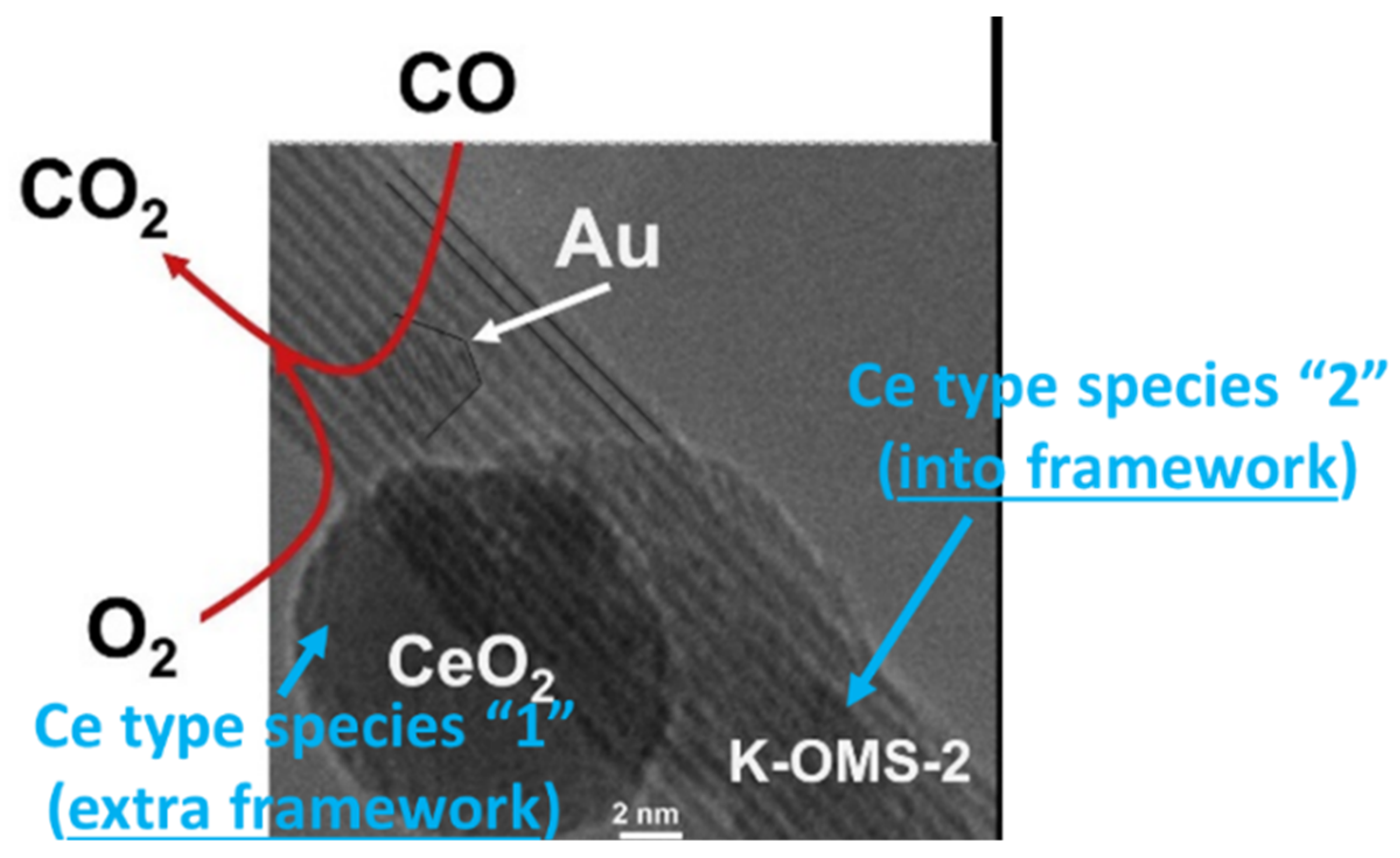
3.1.2. [Ce]–K–OMS–2
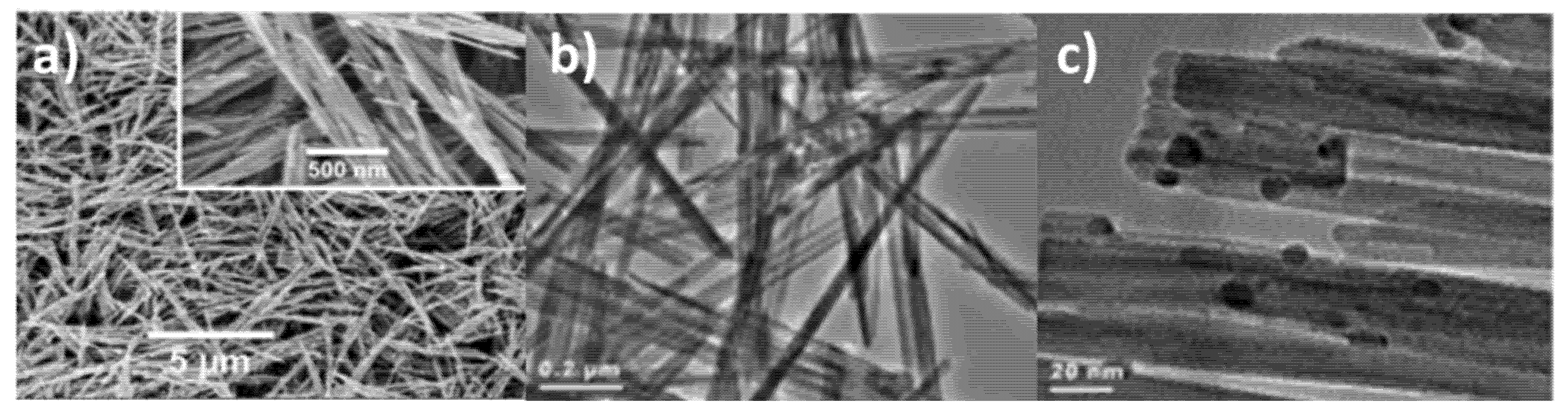
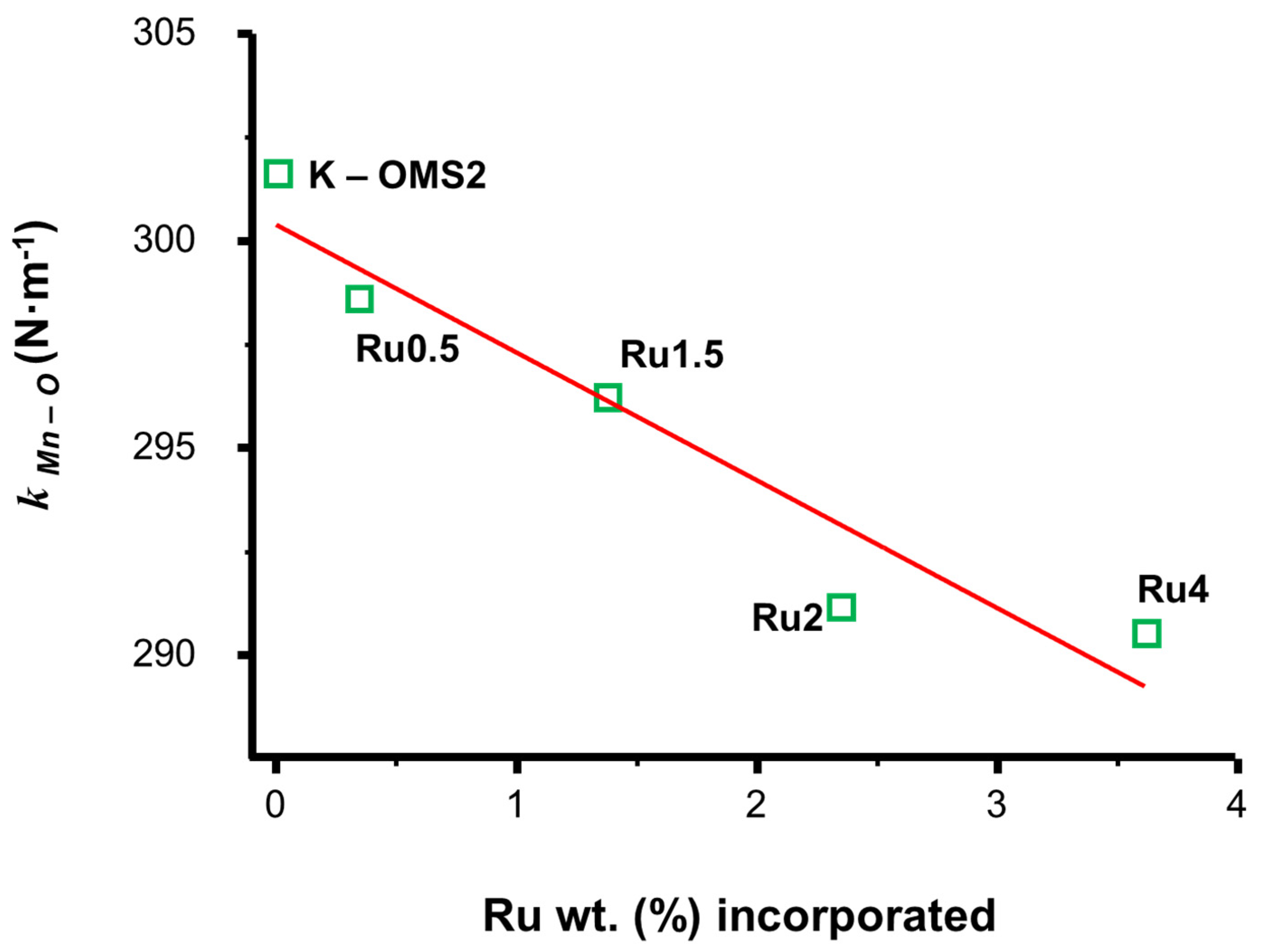
3.1.3. [Ru]–K–OMS–2
3.1.4. [Ti]–K–OMS–2
3.1.5. Doping with High-Valence Cations: [Mo]–K–OMS–2, [W]–K–OMS–2 and [V]–K–OMS–2
3.1.6. [Nb]–K–OMS–2
3.1.7. [In]–K–OMS–2
3.1.8. [Zn]–K–OMS–2 and [Zr]–K–OMS–2
3.2. Catalytic Applications
3.2.1. [Ce]–K–OMS–2 as a Catalyst for General Pollutant Control Processes
- An O3 molecule is adsorbed on the surface of the catalyst, and then dissociates into an oxygen molecule and an atomic oxygen species.
- The remaining atomic oxygen species react with another ozone molecule to form an adsorbed peroxide species (O22−) or superoxide (O2−) and an oxygen molecule.
- Adsorbed O22− or O2− decompose into oxygen molecules and desorb from the active site of catalysts
3.2.2. [Ru]–K–OMS–2 as a Catalyst for Fine Chemicals
3.2.3. [Ag]–, [Nb]–, [Mo]–, [V]–, [Cu]– and [Zn]–K–OMS–2 as Catalysts for CO Oxidation
3.2.4. Other High-Impact Applications
4. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neculita, C.M.; Rosa, E. A review of the implications and challenges of manganese removal from mine drainage. Chemosphere 2019, 214, 491–510. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, B.-S.; Chon, C.-M. Characterization of iron and manganese minerals and their associated microbiota in different mine sites to reveal the potential interactions of microbiota with mineral formation. Chemosphere 2018, 191, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Costas, M. Z=25, manganeso, Mn. El metal del centro generador de O2 en la fotosíntesis. Anales de Química de la RSEQ 2019, 115, 87. [Google Scholar]
- Bertini, I.; Gray, H.; Stiefel, E.; Valentine, J. Biological Inorganic Chemistry; University Science Books: Sausalito, CA, USA, 2007. [Google Scholar]
- Luo, C.; Tian, Z.; Yang, B.; Zhang, L.; Yan, S. Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal. Chem. Eng. J. 2013, 234, 256–265. [Google Scholar] [CrossRef]
- Wan, S.; Ding, W.; Wang, Y.; Wu, J.; Gu, Y.; He, F. Manganese oxide nanoparticles impregnated graphene oxide aggregates for cadmium and copper remediation. Chem. Eng. J. 2018, 350, 1135–1143. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Shi, L.; Yin, Y.; Zhang, L.-C.; Wu, Z.; Duan, X.; Wang, S.; Sun, H. Manganese oxide integrated catalytic ceramic membrane for degradation of organic pollutants using sulfate radicals. Water Res. 2019, 167, 115110. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-Temperature Selective Catalytic Reduction (SCR) of NO with NH3 by Using Mn, Cr, and Cu Oxides Supported on Hombikat TiO2. Angew. Chem. Int. Ed. 2001, 40, 2479–2482. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Brimblecombe, R.; Felton, G.A.N.; Pryadun, R.S.; Sheats, J.E.; Spiccia, L.; Swiegers, G.F. Development of Bioinspired Mn4O4−Cubane Water Oxidation Catalysts: Lessons from Photosynthesis. Acc. Chem. Res. 2009, 42, 1935–1943. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Gorlin, Y.; Jaramillo, T.F. A Bifunctional Nonprecious Metal Catalyst for Oxygen Reduction and Water Oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, Y.; Wang, H.; Key, J.; Brett, D.; Ji, S.; Yin, S.; Shen, P.K. A cost effective, highly porous, manganese oxide/carbon supercapacitor material with high rate capability. J. Mater. Chem. A 2016, 4, 5390–5394. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Zhao, Y.; Jia, B. A Review on the Synthesis of Manganese Oxide Nanomaterials and Their Applications on Lithium-Ion Batteries. J. Nanomater. 2013, 2013, 736375. [Google Scholar] [CrossRef]
- Margreth, M.; Schlink, R.; Steinbach, A. Water Determination by Karl Fischer Titration. In Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Pizarro, P.; Coronado, J.M. Assessing Cr incorporation in Mn2O3/Mn3O4 redox materials for thermochemical heat storage applications. J. Energy Storage 2021, 33, 102028. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef]
- Wu, M.; Hou, P.; Dong, L.; Cai, L.; Chen, Z.; Zhao, M.; Li, J. Manganese dioxide nanosheets: From preparation to biomedical applications. Int. J. Nanomed. 2019, 14, 4781–4800. [Google Scholar] [CrossRef]
- Kim, T.; Momin, E.; Choi, J.; Yuan, K.; Zaidi, H.; Kim, J.; Park, M.; Lee, N.; McMahon, M.T.; Quinones-Hinojosa, A.; et al. Mesoporous Silica-Coated Hollow Manganese Oxide Nanoparticles as Positive T1 Contrast Agents for Labeling and MRI Tracking of Adipose-Derived Mesenchymal Stem Cells. J. Am. Chem. Soc. 2011, 133, 2955–2961. [Google Scholar] [CrossRef]
- Birgisson, S.; Saha, D.; Iversen, B.B. Formation Mechanisms of Nanocrystalline MnO2 Polymorphs under Hydrothermal Conditions. Cryst. Growth Des. 2018, 18, 827–838. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, S.; Dai, Y.; Liu, C.-C.; Zhang, H. Effect of MnO2 Phase Structure on the Oxidative Reactivity toward Bisphenol A Degradation. Environ. Sci. Technol. 2018, 52, 11309–11318. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fan, Y.; Ye, R.; Tang, Y.; Cao, X.; Yin, Z.; Zeng, Z. MnO2-Based Materials for Environmental Applications. Adv. Mater. 2021, 33, 2004862. [Google Scholar] [CrossRef]
- Chen, B.-R.; Sun, W.; Kitchaev, D.A.; Mangum, J.S.; Thampy, V.; Garten, L.M.; Ginley, D.S.; Gorman, B.P.; Stone, K.H.; Ceder, G.; et al. Understanding crystallization pathways leading to manganese oxide polymorph formation. Nat. Commun. 2018, 9, 2553. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.L.; Tian, S.; Li, L.; Yue, Y.B.; Wu, Y.P.; Guan, S.Y.; Zhu, K. Nano-LiCoO2 as cathode material of large capacity and high rate capability for aqueous rechargeable lithium batteries. Electrochem. Commun. 2010, 12, 1524–1526. [Google Scholar] [CrossRef]
- Biswal, A.; Chandra Tripathy, B.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic manganese dioxide (EMD): A perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Dose, W.M.; Donne, S.W. Heat treated electrolytic manganese dioxide for primary Li/MnO2 batteries: Effect of manganese dioxide properties on electrochemical performance. Electrochim. Acta 2013, 105, 305–313. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Structure–Property Relationship of Bifunctional MnO2 Nanostructures: Highly Efficient, Ultra-Stable Electrochemical Water Oxidation and Oxygen Reduction Reaction Catalysts Identified in Alkaline Media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Kitchaev, D.A.; Dacek, S.T.; Sun, W.; Ceder, G. Thermodynamics of Phase Selection in MnO2 Framework Structures through Alkali Intercalation and Hydration. J. Am. Chem. Soc. 2017, 139, 2672–2681. [Google Scholar] [CrossRef]
- Suib, S.L. Porous Manganese Oxide Octahedral Molecular Sieves and Octahedral Layered Materials. Acc. Chem. Res. 2008, 41, 479–487. [Google Scholar] [CrossRef]
- Ghosh, S.K. Diversity in the Family of Manganese Oxides at the Nanoscale: From Fundamentals to Applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef]
- Pistoia, G.; Antonini, A.; Zane, D.; Pasquali, M. Synthesis of Mn spinels from different polymorphs of MnO2. J. Power Sources 1995, 56, 37–43. [Google Scholar] [CrossRef]
- Chukhrov, F.V.; Gorshkov, A.I.; Sivtsov, A.V.; Berezovskaya, V.V.; Dikov, Y.P.; Dubinina, G.A.; Varinov, N.N. Akhtenskite—The natural analog oF ε-MnO2. Int. Geol. Rev. 1989, 31, 1068–1072. [Google Scholar] [CrossRef]
- Hunter, J.C. Preparation of a new crystal form of manganese dioxide: λ-MnO2. J. Solid State Chem. 1981, 39, 142–147. [Google Scholar] [CrossRef]
- Hendriks, R.; Cunha, D.M.; Singh, D.P.; Huijben, M. Enhanced Lithium Transport by Control of Crystal Orientation in Spinel LiMn2O4 Thin Film Cathodes. ACS Appl. Energy Mater. 2018, 1, 7046–7051. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, J.K.; Yaylian, R.; Huang, A.; Meng, Y.S. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. Int. Mater. Rev. 2020, 65, 356–387. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Song, J.; Zhu, T.; Xu, W. Structure-Activity Relationship of Manganese Oxide Catalysts for the Catalytic Oxidation of (chloro)-VOCs. Catalysts 2019, 9, 726. [Google Scholar] [CrossRef]
- Dey, S.; Praveen Kumar, V. V The performance of highly active manganese oxide catalysts for ambient conditions carbon monoxide oxidation. Curr. Res. Green Sustain. Chem. 2020, 3, 100012. [Google Scholar] [CrossRef]
- Shen, X.-F.; Ding, Y.-S.; Liu, J.; Han, Z.-H.; Budnick, J.I.; Hines, W.A.; Suib, S.L. A Magnetic Route to Measure the Average Oxidation State of Mixed-Valent Manganese in Manganese Oxide Octahedral Molecular Sieves (OMS). J. Am. Chem. Soc. 2005, 127, 6166–6167. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Gu, Q.; Sheng, A.; Zhang, B. Tunable Mn Oxidation State and Redox Potential of Birnessite Coexisting with Aqueous Mn(II) in Mildly Acidic Environments. Minerals 2020, 10, 690. [Google Scholar] [CrossRef]
- Bernardini, S.; Bellatreccia, F.; Della Ventura, G.; Sodo, A. A Reliable Method for Determining the Oxidation State of Manganese at the Microscale in Mn Oxides via Raman Spectroscopy. Geostand. Geoanal. Res. 2021, 45, 223–244. [Google Scholar] [CrossRef]
- Pan, G.-H.; Song, R.-J.; Li, J.-H. Radical-mediated synthesis of γ-lactones by copper-catalyzed intermolecular carboesterification of alkenes with α-carbonyl alkyl bromides and H2O. Org. Chem. Front. 2018, 5, 179–183. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Liao, J.; Li, J.; Wu, W.; Jiang, H. MnO2-promoted carboesterification of alkenes with anhydrides: A facile approach to γ-lactones. Chem. Commun. 2016, 52, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Ndolomingo, M.J.; Meijboom, R. Noble and Base-Metal Nanoparticles Supported on Mesoporous Metal Oxides: Efficient Catalysts for the Selective Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Lett. 2019, 149, 2807–2822. [Google Scholar] [CrossRef]
- Nie, J.; Liu, H. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on manganese oxide catalysts. J. Catal. 2014, 316, 57–66. [Google Scholar] [CrossRef]
- Kamimura, A.; Nozaki, Y.; Ishikawa, S.; Inoue, R.; Nakayama, M. K-birnessite MnO2: A new selective oxidant for benzylic and allylic alcohols. Tetrahedron Lett. 2011, 52, 538–540. [Google Scholar] [CrossRef]
- Fu, X.; Feng, J.; Wang, H.; Ng, K.M. Manganese oxide hollow structures with different phases: Synthesis, characterization and catalytic application. Catal. Commun. 2009, 10, 1844–1848. [Google Scholar] [CrossRef]
- Maji, B.; Yamamoto, H. Proline-Tetrazole-Catalyzed Enantioselective N-Nitroso Aldol Reaction of Aldehydes with In Situ Generated Nitrosocarbonyl Compounds. Angew. Chem. Int. Ed. 2014, 53, 8714–8717. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Ruan, L.; Lv, X.; Lv, Y.; Su, J.; Wen, Y. TG–FTIR analysis of pyrolusite reduction by major biomass components. Chin. J. Chem. Eng. 2015, 23, 1691–1697. [Google Scholar] [CrossRef]
- Sarmah, B.; Srivastava, R.; Manjunathan, P.; Shanbhag, G.V. Green and Sustainable Tandem Catalytic Approach for Fine-Chemicals Synthesis Using Octahedral MnO2 Molecular Sieve: Catalytic Activity versus Method of Catalyst Synthesis. ACS Sustain. Chem. Eng. 2015, 3, 2933–2943. [Google Scholar] [CrossRef]
- Yang, Y.; Su, X.; Zhang, L.; Kerns, P.; Achola, L.; Hayes, V.; Quardokus, R.; Suib, S.L.; He, J. Intercalating MnO2 Nanosheets With Transition Metal Cations to Enhance Oxygen Evolution. ChemCatChem 2019, 11, 1689–1700. [Google Scholar] [CrossRef]
- Lu, F.; Huang, J.; Wu, Q.; Zhang, Y. Mixture of α-Fe2O3 and MnO2 powders for direct conversion of syngas to light olefins. Appl. Catal. A Gen. 2021, 621, 118213. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, K.; Dong, F.; Lin, M.; Sun, Y.; Tang, Z. Textual properties of Cu–Mn mixed oxides and application for methyl formate synthesis from syngas. J. Ind. Eng. Chem. 2017, 54, 117–125. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Yang, Y.; Miao, S.; Shen, F. Effect of the Mechanism of H2S on Elemental Mercury Removal Using the MnO2 Sorbent during Coal Gasification. Energy Fuels 2018, 32, 4453–4460. [Google Scholar] [CrossRef]
- Sherman, J.D. Synthetic zeolites and other microporous oxide molecular sieves. Proc. Natl. Acad. Sci. USA 1999, 96, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Auroux, A. Molecular Sieves—Science and Technology: Acidity and Basicity; Karge, H., Weitkamp, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 6. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. A Neutral Templating Route to Mesoporous Molecular Sieves. Science 1995, 267, 865–867. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Nieto, J.M.L. The selective oxidative activation of light alkanes. From supported vanadia to multicomponent bulk V-containing catalysts. Top. Catal. 2006, 41, 3–15. [Google Scholar] [CrossRef]
- Sadakane, M.; Kodato, K.; Kuranishi, T.; Nodasaka, Y.; Sugawara, K.; Sakaguchi, N.; Nagai, T.; Matsui, Y.; Ueda, W. Molybdenum–Vanadium-Based Molecular Sieves with Microchannels of Seven-Membered Rings of Corner-Sharing Metal Oxide Octahedra. Angew. Chem. Int. Ed. 2008, 47, 2493–2496. [Google Scholar] [CrossRef]
- Zhu, Q.; Yin, S.; Zhou, M.; Wang, J.; Chen, C.; Hu, P.; Jiang, X.; Zhang, Z.; Li, Y.; Ueda, W. Aerobic Alcohol Oxidation by a Zeolitic Octahedral Metal Oxide based on Iron Vanadomolybdates Under Mild Conditions. ChemCatChem 2021, 13, 1763–1771. [Google Scholar] [CrossRef]
- Corma, A.; Corresa, E.; Mathieu, Y.; Sauvanaud, L.; Al-Bogami, S.; Al-Ghrami, M.S.; Bourane, A. Crude oil to chemicals: Light olefins from crude oil. Catal. Sci. Technol. 2017, 7, 12–46. [Google Scholar] [CrossRef]
- Besnardiere, J.; Ma, B.; Torres-Pardo, A.; Wallez, G.; Kabbour, H.; González-Calbet, J.M.; Von Bardeleben, H.J.; Fleury, B.; Buissette, V.; Sanchez, C.; et al. Structure and electrochromism of two-dimensional octahedral molecular sieve h’-WO3. Nat. Commun. 2019, 10, 327. [Google Scholar] [CrossRef]
- Corma, A.; Navarro, M.T. From micro to mesoporous molecular sieves: Adapting composition and structure for catalysis. In Impact of Zeolites and Other Porous Materials on the New Technologies at the Beginning of the New Millennium; Aiello, R., Giordano, G., Testa, Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 142, pp. 487–501. ISBN 0167-2991. [Google Scholar]
- Martínez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef]
- Suib, S.L. (Ed.) Introduction. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. ix–x. ISBN 978-0-444-53874-1. [Google Scholar]
- Brock, S.L.; Duan, N.; Tian, Z.R.; Giraldo, O.; Zhou, H.; Suib, S.L. A Review of Porous Manganese Oxide Materials. Chem. Mater. 1998, 10, 2619–2628. [Google Scholar] [CrossRef]
- DeGuzman, R.N.; Shen, Y.-F.; Neth, E.J.; Suib, S.L.; O’Young, C.-L.; Levine, S.; Newsam, J.M. Synthesis and Characterization of Octahedral Molecular Sieves (OMS-2) Having the Hollandite Structure. Chem. Mater. 1994, 6, 815–821. [Google Scholar] [CrossRef]
- Suib, S.L.; Iton, L.E. Magnetic Studies of Manganese Oxide Octahedral Molecular Sieves: A New Class of Spin Glasses. Chem. Mater. 1994, 6, 429–433. [Google Scholar] [CrossRef]
- Suib, S.L. Microporous manganese oxides. Curr. Opin. Solid State Mater. Sci. 1998, 3, 63–70. [Google Scholar] [CrossRef]
- Tian, Z.-R.; Tong, W.; Wang, J.-Y.; Duan, N.-G.; Krishnan, V.V.; Suib, S.L. Manganese Oxide Mesoporous Structures: Mixed-Valent Semiconducting Catalysts. Science 1997, 276, 926–930. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zerger, R.P.; DeGuzman, R.N.; Suib, S.L.; McCurdy, L.; Potter, D.I.; O’Young, C.L. Manganese Oxide Octahedral Molecular Sieves: Preparation, Characterization, and Applications. Science 1993, 260, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, S.R.; Carrado, K.A.; Yuchs, S.E.; Shen, Y.F.; Cao, H.; Suib, S.L. The structure of new synthetic manganese oxide octahedral molecular sieves. Phys. B Condens. Matter 1995, 208–209, 674–676. [Google Scholar] [CrossRef]
- Shen, X.-F.; Ding, Y.-S.; Liu, J.; Cai, J.; Laubernds, K.; Zerger, R.P.; Vasiliev, A.; Aindow, M.; Suib, S.L. Control of Nanometer-Scale Tunnel Sizes of Porous Manganese Oxide Octahedral Molecular Sieve Nanomaterials. Adv. Mater. 2005, 17, 805–809. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Férey, G. Crystal Chemistry. From Basic to Tools for Materials Creation; Word Scientific Publishing Company: Singapore, 2017. [Google Scholar]
- Plug, C.M. On the relationship between the structure of CaFe2O4 and hollandite. J. Solid State Chem. 1982, 41, 23–26. [Google Scholar] [CrossRef]
- Bursill, L.A. Structural relationships between [beta]-gallia, rutile, hollandite, psilomelane, ramsdellite and gallium titanate type structures. Acta Crystallogr. Sect. B 1979, 35, 530. [Google Scholar] [CrossRef]
- De Guzman, R.N.; Awaluddin, A.; Shen, Y.-F.; Tian, Z.R.; Suib, S.L.; Ching, S.; O’Young, C.-L. Electrical Resistivity Measurements on Manganese Oxides with Layer and Tunnel Structures: Birnessites, Todorokites, and Cryptomelanes. Chem. Mater. 1995, 7, 1286–1292. [Google Scholar] [CrossRef]
- Ching, S.; Krukowska, K.S.; Suib, S.L. A new synthetic route to todorokite-type manganese oxides. Inorg. Chim. Acta 1999, 294, 123–132. [Google Scholar] [CrossRef]
- Yin, Y.-G.; Xu, W.-Q.; Shen, Y.-F.; Suib, S.L.; O’Young, C.L. Studies of Oxygen Species in Synthetic Todorokite-like Manganese Oxide Octahedral Molecular Sieves. Chem. Mater. 1994, 6, 1803–1808. [Google Scholar] [CrossRef]
- Cerdá-Moreno, C.; Chica, A.; Keller, S.; Rautenberg, C.; Bentrup, U. Ni-sepiolite and Ni-todorokite as efficient CO2 methanation catalysts: Mechanistic insight by operando DRIFTS. Appl. Catal. B Environ. 2020, 264, 118546. [Google Scholar] [CrossRef]
- Fuertes, A.; Da Costa-Serra, J.F.; Chica, A. New Catalysts based on Ni-Birnessite and Ni-Todorokite for the Efficient Production of Hydrogen by Bioethanol Steam Reforming. Energy Procedia 2012, 29, 181–191. [Google Scholar] [CrossRef][Green Version]
- Bletsa, E.; Zaccone, C.; Miano, T.; Terzano, R.; Deligiannakis, Y. Natural Mn-todorokite as an efficient and green azo dye–degradation catalyst. Environ. Sci. Pollut. Res. 2020, 27, 9835–9842. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Komaba, S.; Abe, K.; Yashiro, H. Synthesis of metal-doped todorokite-type MnO2 and its cathode characteristics for rechargeable lithium batteries. J. Power Sources 2005, 146, 310–314. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, D.; Bai, X.; Xie, H.; Liu, X.; Jiang, X.; Lin, H.; He, H. High-Cycle-Performance Aqueous Magnesium Ions Battery Capacitor Based on a Mg-OMS-1/Graphene as Cathode and a Carbon Molecular Sieves as Anode. ACS Sustain. Chem. Eng. 2019, 7, 6113–6121. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, K.; Cang, R.; Zhu, K.; Yan, J.; Cheng, K.; Wang, G.; Cao, D. The synthesis of 1×1 magnesium octahedral molecular sieve with controllable size and shape for aqueous magnesium ion battery cathode material. J. Electroanal. Chem. 2017, 807, 37–44. [Google Scholar] [CrossRef]
- Jakubek, T.; Hudy, C.; Indyka, P.; Nowicka, E.; Golunski, S.; Kotarba, A. Effect of noble metal addition to alkali-exchanged cryptomelane on the simultaneous soot and VOC combustion activity. Catal. Commun. 2019, 132, 105807. [Google Scholar] [CrossRef]
- Malz, R., Jr.; Kumar, R.; Garces, L.J.; Suib, S.L. Process for Preparing Ortho Substituted Phenylamines. WO2004072028A2, 26 August 2004. [Google Scholar]
- Kumar, R.; Garces, L.J.; Son, Y.-C.; Suib, S.L.; Malz, R.E. Manganese oxide octahedral molecular sieve catalysts for synthesis of 2-aminodiphenylamine. J. Catal. 2005, 236, 387–391. [Google Scholar] [CrossRef]
- Yang, L.; Ma, J.; Li, X.; He, G.; Zhang, C.; He, H. Improving the catalytic performance of ozone decomposition over Pd-Ce-OMS-2 catalysts under harsh conditions. Catal. Sci. Technol. 2020, 10, 7671–7680. [Google Scholar] [CrossRef]
- Hou, J.; Li, Y.; Mao, M.; Zhao, X.; Yue, Y. The effect of Ce ion substituted OMS-2 nanostructure in catalytic activity for benzene oxidation. Nanoscale 2014, 6, 15048–15058. [Google Scholar] [CrossRef]
- Sultana, S.; Ye, Z.; Veerapandian, S.K.P.; Löfberg, A.; De Geyter, N.; Morent, R.; Giraudon, J.-M.; Lamonier, J.-F. Synthesis and catalytic performances of K-OMS-2, Fe/K-OMS-2 and Fe-K-OMS-2 in post plasma-catalysis for dilute TCE abatement. Catal. Today 2018, 307, 20–28. [Google Scholar] [CrossRef]
- Lin, Y.; Minghua, H.; Jvcheng, G.; Ziqin, X. Catalytic oxidation of toluene over Co-modified manganese oxide octahedral molecular sieves (OMS-2) synthesized by different methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 32017. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Zhang, S.; Wang, S.; Deng, S.; Wang, B.; Yu, G. Catalytic removal of gaseous HCBz on Cu doped OMS: Effect of Cu location on catalytic performance. Appl. Catal. B Environ. 2014, 150–151, 167–178. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J. Ce ion substitution position effect on catalytic activity of OMS-2 for benzene oxidation. Mater. Res. Bull. 2019, 118, 110497. [Google Scholar] [CrossRef]
- Pahalagedara, L.R.; Dharmarathna, S.; King’ondu, C.K.; Pahalagedara, M.N.; Meng, Y.T.; Kuo, C.H.; Suib, S.L. Microwave-Assisted Hydrothermal Synthesis of α-MnO2: Lattice Expansion via Rapid Temperature Ramping and Framework Substitution. J. Phys. Chem. C 2014, 118, 20363–20373. [Google Scholar] [CrossRef]
- Shen, X.; Morey, A.M.; Liu, J.; Ding, Y.; Cai, J.; Durand, J.; Wang, Q.; Wen, W.; Hines, W.A.; Hanson, J.C.; et al. Characterization of the Fe-Doped Mixed-Valent Tunnel Structure Manganese Oxide KOMS-2. J. Phys. Chem. C 2011, 115, 21610–21619. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; He, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B Environ. 2017, 201, 503–510. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Feng, X.; Qiu, G.; Tan, W.; Liu, F. Large-scale size-controlled synthesis of cryptomelane-type manganese oxide OMS-2 in lateral and longitudinal directions. J. Mater. Chem. 2011, 21, 5223–5225. [Google Scholar] [CrossRef]
- Sabaté, F.; Jordá, J.L.; Sabater, M.J.; Corma, A. Synthesis of isomorphically substituted Ru manganese molecular sieves and their catalytic properties for selective alcohol oxidation. J. Mater. Chem. A 2020, 8, 3771–3784. [Google Scholar] [CrossRef]
- Fan, C.; Lu, A.; Li, Y.; Wang, C. Synthesis, characterization, and catalytic activity of cryptomelane nanomaterials produced with industrial manganese sulfate. J. Colloid Interface Sci. 2008, 327, 393–402. [Google Scholar] [CrossRef]
- Su, Y.; Wang, L.-C.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Microwave-accelerated solvent-free aerobic oxidation of benzyl alcohol over efficient and reusable manganese oxides. Catal. Commun. 2007, 8, 2181–2185. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, W.; Suib, S.L.; Qiu, G.; Liu, F. Dissolution and phase transformation processes of hausmannite in acidic aqueous systems under anoxic conditions. Chem. Geol. 2018, 487, 54–62. [Google Scholar] [CrossRef]
- Dharmarathna, S.; King’ondu, C.K.; Pahalagedara, L.; Kuo, C.-H.; Zhang, Y.; Suib, S.L. Manganese octahedral molecular sieve (OMS-2) catalysts for selective aerobic oxidation of thiols to disulfides. Appl. Catal. B Environ. 2014, 147, 124–131. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Qiu, G.; Liu, F.; Feng, X. Size-controlled synthesis and formation mechanism of manganese oxide OMS-2 nanowires under reflux conditions with KMnO4 and inorganic acids. Solid State Sci. 2016, 55, 152–158. [Google Scholar] [CrossRef]
- Dharmarathna, S.; King’ondu, C.K.; Pedrick, W.; Pahalagedara, L.; Suib, S.L. Direct Sonochemical Synthesis of Manganese Octahedral Molecular Sieve (OMS-2) Nanomaterials Using Cosolvent Systems, Their Characterization, and Catalytic Applications. Chem. Mater. 2012, 24, 705–712. [Google Scholar] [CrossRef]
- Tian, H.; He, J.; Zhang, X.; Zhou, L.; Wang, D. Facile synthesis of porous manganese oxide K-OMS-2 materials and their catalytic activity for formaldehyde oxidation. Microporous Mesoporous Mater. 2011, 138, 118–122. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kobayashi, H.; Wang, Y.; Oishi, T.; Ogasawara, Y.; Mizuno, N. Green oxidative synthesis of primary amides from primary alcohols or aldehydes catalyzed by a cryptomelane-type manganese oxide-based octahedral molecular sieve, OMS-2. Catal. Sci. Technol. 2013, 3, 318–327. [Google Scholar] [CrossRef]
- Bi, X.; Huang, Y.; Liu, X.; Yao, N.; Zhao, P.; Meng, X.; Astruc, D. Oxidative degradation of aqueous organic contaminants over shape-tunable MnO2 nanomaterials via peroxymonosulfate activation. Sep. Purif. Technol. 2021, 275, 119141. [Google Scholar] [CrossRef]
- Dinh, M.T.N.; Nguyen, C.C.; Phan, M.D.; Duong, M.K.; Nguyen, P.H.D.; Lancelot, C.; Nguyen, D.L. Novel cryptomelane nanosheets for the superior catalytic combustion of oxygenated volatile organic compounds. J. Hazard. Mater. 2021, 417, 126111. [Google Scholar] [CrossRef]
- Jin, L.; Reutenauer, J.; Opembe, N.; Lai, M.; Martenak, D.J.; Han, S.; Suib, S.L. Studies on Dehydrogenation of Ethane in the Presence of CO2 over Octahedral Molecular Sieve (OMS-2) Catalysts. ChemCatChem 2009, 1, 441–444. [Google Scholar] [CrossRef]
- Sriskandakumar, T.; Opembe, N.; Chen, C.-H.; Morey, A.; King’ondu, C.; Suib, S.L. Green Decomposition of Organic Dyes Using Octahedral Molecular Sieve Manganese Oxide Catalysts. J. Phys. Chem. A 2009, 113, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wu, X.; Yang, S.; Li, C.; Tang, F.; Chen, J.; Chen, Y.; Xiang, Y.; Wu, X.; He, Z. Cryptomelane-Type KMn8O16 as Potential Cathode Material—For Aqueous Zinc Ion Battery. Front. Chem. 2018, 6, 352. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.H.; Lee, S.C.; Kim, J.; Lee, D.; Woo, H.C. Properties of a manganese oxide octahedral molecular sieve (OMS-2) for adsorptive desulfurization of fuel gas for fuel cell applications. Fuel Process. Technol. 2015, 131, 238–246. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Huang, J.; Pelliccione, C.J.; Tong, X.; Cheng, S.; Wu, L.; Zhu, Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Synthesis of cryptomelane type [small alpha]-MnO2 (KxMn8O16) cathode materials with tunable K+ content: The role of tunnel cation concentration on electrochemistry. J. Mater. Chem. A 2017, 5, 16914–16928. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Huang, J.; Wu, L.; Bock, D.C.; Zhu, Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Potassium-Based α-Manganese Dioxide Nanofiber Binder-Free Self-Supporting Electrodes: A Design Strategy for High Energy Density Batteries. Energy Technol. 2016, 4, 1358–1368. [Google Scholar] [CrossRef]
- Rasul, S.; Suzuki, S.; Yamaguchi, S.; Miyayama, M. Manganese oxide octahedral molecular sieves as insertion electrodes for rechargeable Mg batteries. Electrochim. Acta 2013, 110, 247–252. [Google Scholar] [CrossRef]
- Liu, T.; Li, Q.; Xin, Y.; Zhang, Z.; Tang, X.; Zheng, L.; Gao, P.-X. Quasi free K cations confined in hollandite-type tunnels for catalytic solid (catalyst)-solid (reactant) oxidation reactions. Appl. Catal. B Environ. 2018, 232, 108–116. [Google Scholar] [CrossRef]
- Kona, J.R.; King’ondu, C.K.; Howell, A.R.; Suib, S.L. OMS-2 for Aerobic, Catalytic, One-pot Alcohol Oxidation-Wittig Reactions: Efficient Access to α,β-Unsaturated Esters. ChemCatChem 2014, 6, 749–752. [Google Scholar] [CrossRef]
- Ferlin, F.; Marini, A.; Ascani, N.; Ackermann, L.; Lanari, D.; Vaccaro, L. Heterogeneous Manganese-Catalyzed Oxidase C−H/C−O Cyclization to Access Pharmaceutically Active Compounds. ChemCatChem 2020, 12, 449–454. [Google Scholar] [CrossRef]
- Opembe, N.N.; Guild, C.; King’ondu, C.; Nelson, N.C.; Slowing, I.I.; Suib, S.L. Vapor-Phase Oxidation of Benzyl Alcohol Using Manganese Oxide Octahedral Molecular Sieves (OMS-2). Ind. Eng. Chem. Res. 2014, 53, 19044–19051. [Google Scholar] [CrossRef]
- Makwana, V.D.; Garces, L.J.; Liu, J.; Cai, J.; Son, Y.-C.; Suib, S.L. Selective oxidation of alcohols using octahedral molecular sieves: Influence of synthesis method and property–activity relations. Catal. Today 2003, 85, 225–233. [Google Scholar] [CrossRef]
- Schurz, F.; Bauchert, J.M.; Merker, T.; Schleid, T.; Hasse, H.; Gläser, R. Octahedral molecular sieves of the type K-OMS-2 with different particle sizes and morphologies: Impact on the catalytic properties in the aerobic partial oxidation of benzyl alcohol. Appl. Catal. A Gen. 2009, 355, 42–49. [Google Scholar] [CrossRef]
- Sabaté, F.; Navas, J.; Sabater, M.J.; Corma, A. Synthesis of γ-lactones from easily and accessible reactants catalyzed by Cu–MnOx catalysts. C. R. Chim. 2018, 21, 164–173. [Google Scholar] [CrossRef]
- Sabaté, F.; Jordà, J.L.; Sabater, M.J. Ruthenium isomorphic substitution into Manganese Oxide Octahedral Molecular Sieve OMS-2: Comparative physic-chemical and catalytic studies of Ru versus Abundant Metal Cationic Dopants. Catal. Today 2021. [Google Scholar] [CrossRef]
- Pan, F.; Liu, W.; Yu, Y.; Yin, X.; Wang, Q.; Zheng, Z.; Wu, M.; Zhao, D.; Zhang, Q.; Lei, X.; et al. The effects of manganese oxide octahedral molecular sieve chitosan microspheres on sludge bacterial community structures during sewage biological treatment. Sci. Rep. 2016, 6, 37518. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Wei, Y.; Liu, J. Ordered micro/macro porous K-OMS-2/SiO2 nanocatalysts: Facile synthesis, low cost and high catalytic activity for diesel soot combustion. Sci. Rep. 2017, 7, 43894. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Centeno, M.A.; Ivanova, S.; Eloy, P.; Gaigneaux, E.M.; Odriozola, J.A. Cu-modified cryptomelane oxide as active catalyst for CO oxidation reactions. Appl. Catal. B Environ. 2012, 123–124, 27–35. [Google Scholar] [CrossRef]
- Davó-Quiñonero, A.; Navlani-García, M.; Lozano-Castelló, D.; Bueno-López, A. CuO/cryptomelane catalyst for preferential oxidation of CO in the presence of H2: Deactivation and regeneration. Catal. Sci. Technol. 2016, 6, 5684–5692. [Google Scholar] [CrossRef]
- Ousmane, M.; Perrussel, G.; Yan, Z.; Clacens, J.M.; De Campo, F.; Pera-Titus, M. Highly selective direct amination of primary alcohols over a Pd/K-OMS-2 catalyst. J. Catal. 2014, 309, 439–452. [Google Scholar] [CrossRef]
- Hou, J.; Li, Y.; Liu, L.; Ren, L.; Zhao, X. Effect of giant oxygen vacancy defects on the catalytic oxidation of OMS-2 nanorods. J. Mater. Chem. A 2013, 1, 6736–6741. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Sabaté, F.; Sabater, M.J. Electrochemical Analysis of Catalytic and Oxygen Interfacial Transfer Effects on MnO2 Deposited on Gold Electrodes. J. Phys. Chem. C 2018, 122, 10939–10947. [Google Scholar] [CrossRef]
- Davó-Quiñonero, A.; Such-Basáñez, I.; Juan-Juan, J.; Lozano-Castelló, D.; Stelmachowski, P.; Grzybek, G.; Kotarba, A.; Bueno-López, A. New insights into the role of active copper species in CuO/Cryptomelane catalysts for the CO-PROX reaction. Appl. Catal. B Environ. 2019, 118372. [Google Scholar] [CrossRef]
- Bellussi, G.; Fattore, V. Isomorphous Substitution in Zeolites: A Route for the Preparation of Novel Catalysts. In Zeolite Chemistry and Catalysis; Jacobs, P.A., Jaeger, N.I., Kubelková, L., Wichterlov, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 69, pp. 79–92. ISBN 0167-2991. [Google Scholar]
- Delgado, D.; Concepción, P.; Trunschke, A.; López Nieto, J.M. Tungsten–niobium oxide bronzes: A bulk and surface structural study. Dalton Trans. 2020, 49, 13282–13293. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Recio, I.; Azor-Lafarga, A.; Ruiz-González, M.L.; Hernando, M.; Parras, M.; Calvino, J.J.; Fernández-Díaz, M.T.; Portehault, D.; Sanchez, C.; González-Calbet, J.M. Unambiguous localization of titanium and iron cations in doped manganese hollandite nanowires. Chem. Commun. 2020, 56, 4812–4815. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, J.; Willis, W.S.; Suib, S.L. Framework Doping of Iron in Tunnel Structure Cryptomelane. Chem. Mater. 2001, 13, 2413–2422. [Google Scholar] [CrossRef]
- King’ondu, C.K.; Opembe, N.; Chen, C.; Ngala, K.; Huang, H.; Iyer, A.; Garcés, H.F.; Suib, S.L. Manganese Oxide Octahedral Molecular Sieves (OMS-2) Multiple Framework Substitutions: A New Route to OMS-2 Particle Size and Morphology Control. Adv. Funct. Mater. 2011, 21, 312–323. [Google Scholar] [CrossRef]
- El-Sawy, A.M.; King’ondu, C.K.; Kuo, C.-H.; Kriz, D.A.; Guild, C.J.; Meng, Y.; Frueh, S.J.; Dharmarathna, S.; Ehrlich, S.N.; Suib, S.L. X-ray Absorption Spectroscopic Study of a Highly Thermally Stable Manganese Oxide Octahedral Molecular Sieve (OMS-2) with High Oxygen Reduction Reaction Activity. Chem. Mater. 2014, 26, 5752–5760. [Google Scholar] [CrossRef]
- Li, W.; Cui, X.; Zeng, R.; Du, G.; Sun, Z.; Zheng, R.; Ringer, S.P.; Dou, S.X. Performance modulation of α-MnO2 nanowires by crystal facet engineering. Sci. Rep. 2015, 5, 8987. [Google Scholar] [CrossRef]
- Shah, S.I.; Khan, T.; Khan, R.; Khan, S.A.; Khattak, S.A.; Khan, G. Study of structural, optical and dielectric properties of α-MnO2 nanotubes (NTS). J. Mater. Sci. Mater. Electron. 2019, 30, 19199–19205. [Google Scholar] [CrossRef]
- Feng, Q.; Kanoh, H.; Miyai, Y.; Ooi, K. Alkali Metal Ions Insertion/Extraction Reactions with Hollandite-Type Manganese Oxide in the Aqueous Phase. Chem. Mater. 1995, 7, 148–153. [Google Scholar] [CrossRef]
- Calvert, C.; Joesten, R.; Ngala, K.; Villegas, J.; Morey, A.; Shen, X.; Suib, S.L. Synthesis, Characterization, and Rietveld Refinement of Tungsten-Framework-Doped Porous Manganese Oxide (K-OMS-2) Material. Chem. Mater. 2008, 20, 6382–6388. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.-F.; Suib, S.L.; O’Young, C.L. Characterization of Manganese Oxide Octahedral Molecular Sieve (M−OMS-2) Materials with Different Metal Cation Dopants. Chem. Mater. 2002, 14, 940–948. [Google Scholar] [CrossRef]
- Hu, R.; Cheng, Y.; Xie, L.; Wang, D. Effect of Doped Ag on Performance of Manganese Oxide Octahedral Molecular Sieve for CO Oxidation. Chin. J. Catal. 2007, 28, 463–468. [Google Scholar] [CrossRef]
- Hu, R.; Yan, C.; Xie, L.; Cheng, Y.; Wang, D. Selective oxidation of CO in rich hydrogen stream over Ag/OMS-2 catalyst. Int. J. Hydrog. Energy 2011, 36, 64–71. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Z.; Shi, J.; Liu, Z.; Zhou, J.; Shangguan, W. Modified manganese oxide octahedral molecular sieves M′-OMS-2 (M′=Co,Ce,Cu) as catalysts in post plasma-catalysis for acetaldehyde degradation. Catal. Today 2015, 256, 178–185. [Google Scholar] [CrossRef]
- Yue, L.; Hu, M.; Tian, M.; Liao, X.; Xu, Z.; Shi, L.; He, C. Insight Into the Role of Ceria on OMS-2 and OL Materials for Catalytic Degradation of Toluene. Front. Environ. Chem. 2020, 1, 12. [Google Scholar] [CrossRef]
- Nur, H.; Hayati, F.; Hamdan, H. On the location of different titanium sites in Ti–OMS-2 and their catalytic role in oxidation of styrene. Catal. Commun. 2007, 8, 2007–2011. [Google Scholar] [CrossRef]
- Chen, C.-H.; Njagi, E.C.; Chen, S.-Y.; Horvath, D.T.; Xu, L.; Morey, A.; Mackin, C.; Joesten, R.; Suib, S.L. Structural Distortion of Molybdenum-Doped Manganese Oxide Octahedral Molecular Sieves for Enhanced Catalytic Performance. Inorg. Chem. 2015, 54, 10163–10171. [Google Scholar] [CrossRef]
- Genuino, H.C.; Meng, Y.; Horvath, D.T.; Kuo, C.H.; Seraji, M.S.; Morey, A.M.; Joesten, R.L.; Suib, S.L. Enhancement of Catalytic Activities of Octahedral Molecular Sieve Manganese Oxide for Total and Preferential CO Oxidation through Vanadium Ion Framework Substitution. ChemCatChem 2013, 5, 2306–2317. [Google Scholar] [CrossRef]
- Polverejan, M.; Villegas, J.C.; Suib, S.L. Higher Valency Ion Substitution into the Manganese Oxide Framework. J. Am. Chem. Soc. 2004, 126, 7774–7775. [Google Scholar] [CrossRef]
- Legutko, P.; Gryboś, J.; Fedyna, M.; Janas, J.; Wach, A.; Szlachetko, J.; Adamski, A.; Yu, X.; Zhao, Z.; Kotarba, A.; et al. Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance. Catalysts 2020, 10, 1390. [Google Scholar] [CrossRef]
- Wasalathanthri, N.D.; Guild, C.; Nizami, Q.A.; Dissanayake, S.L.; He, J.; Kerns, P.; Fee, J.; Achola, L.; Rathnayake, D.; Weerakkody, C.; et al. Niobium-substituted octahedral molecular sieve (OMS-2) materials in selective oxidation of methanol to dimethoxymethane. RSC Adv. 2019, 9, 32665–32673. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Garcia-Martinez, J.; Suib, S.L. Adsorptive and Acidic Properties, Reversible Lattice Oxygen Evolution, and Catalytic Mechanism of Cryptomelane-Type Manganese Oxides as Oxidation Catalysts. J. Am. Chem. Soc. 2008, 130, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Özacar, M.; Poyraz, A.S.; Genuino, H.C.; Kuo, C.-H.; Meng, Y.; Suib, S.L. Influence of silver on the catalytic properties of the cryptomelane and Ag-hollandite types manganese oxides OMS-2 in the low-temperature CO oxidation. Appl. Catal. A Gen. 2013, 462–463, 64–74. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Sobolev, V.I.; Vodyankina, O.V. Silica-supported silver-containing OMS-2 catalysts for ethanol oxidative dehydrogenation. Catal. Today 2016, 278, 164–173. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Li, H.; Huang, X.; Shen, W. Facile synthesis of Ag–OMS-2 nanorods and their catalytic applications in CO oxidation. Microporous Mesoporous Mater. 2008, 116, 586–592. [Google Scholar] [CrossRef]
- Qu, Z.; Bu, Y.; Qin, Y.; Wang, Y.; Fu, Q. The improved reactivity of manganese catalysts by Ag in catalytic oxidation of toluene. Appl. Catal. B Environ. 2013, 132–133, 353–362. [Google Scholar] [CrossRef]
- O’Donnell, R.; Ralphs, K.; Grolleau, M.; Manyar, H.; Artioli, N. Doping Manganese Oxides with Ceria and Ceria Zirconia Using a One-Pot Sol–Gel Method for Low Temperature Diesel Oxidation Catalysts. Top. Catal. 2020, 63, 351–362. [Google Scholar] [CrossRef]
- Wang, R.; Li, J. OMS-2 Catalysts for Formaldehyde Oxidation: Effects of Ce and Pt on Structure and Performance of the Catalysts. Catal. Lett. 2009, 131, 500–505. [Google Scholar] [CrossRef]
- Xie, J.; Chen, L.; Zhou, W.-F.; Au, C.-T.; Yin, S.-F. Selective oxidation of p-chlorotoluene to p-chlorobenzaldehyde over metal-modified OMS-2 molecular sieves. J. Mol. Catal. A Chem. 2016, 425, 110–115. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Zhai, S.; Sun, P.; Wu, Z. The positive effect of Ca2+ on cryptomelane-type octahedral molecular sieve (K-OMS-2) catalysts for chlorobenzene combustion. J. Colloid Interface Sci. 2020, 576, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Diao, G.; Ye, F.; Sun, M.; Zhou, J.; Li, Y.; Liu, Y. Promoting Effect of Ce in Ce/OMS-2 Catalyst for Catalytic Combustion of Dimethyl Ether. Catal. Lett. 2011, 141, 111–119. [Google Scholar] [CrossRef]
- Santos, V.P.; Soares, O.S.G.P.; Bakker, J.J.W.; Pereira, M.F.R.; Órfão, J.J.M.; Gascon, J.; Kapteijn, F.; Figueiredo, J.L. Structural and chemical disorder of cryptomelane promoted by alkali doping: Influence on catalytic properties. J. Catal. 2012, 293, 165–174. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Bakker, J.J.W.; Soares, O.S.G.P.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Gascon, J.; Kapteijn, F. Stabilized gold on cerium-modified cryptomelane: Highly active in low-temperature CO oxidation. J. Catal. 2014, 309, 58–65. [Google Scholar] [CrossRef]
- Adjimi, S.; García-Vargas, J.M.; Díaz, J.A.; Retailleau, L.; Gil, S.; Pera-Titus, M.; Guo, Y.; Giroir-Fendler, A. Highly efficient and stable Ru/K-OMS-2 catalyst for NO oxidation. Appl. Catal. B Environ. 2017, 219, 459–466. [Google Scholar] [CrossRef]
- Molleti, J.; Tiwari, M.S.; Yadav, G.D. Novel synthesis of Ru/OMS catalyst by solvent-free method: Selective hydrogenation of levulinic acid to γ-valerolactone in aqueous medium and kinetic modelling. Chem. Eng. J. 2018, 334, 2488–2499. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2020, 10, 1152–1160. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Chen, Y.; Xu, G.-L.; Zhang, X.-R.; Chen, Z.; Li, J.-T.; Huang, L.; Amine, K.; Sun, S.-G. RuO2 nanoparticles supported on MnO2 nanorods as high efficient bifunctional electrocatalyst of lithium-oxygen battery. Nano Energy 2016, 28, 63–70. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Demethoxylation of guaiacol and methoxybenzenes over carbon-supported Ru–Mn catalyst. Appl. Catal. B Environ. 2016, 182, 193–203. [Google Scholar] [CrossRef]
- Yang, L.; Ma, J.; Li, X.; Zhang, C.; He, H. Enhancing Oxygen Vacancies of Ce-OMS-2 via Optimized Hydrothermal Conditions to Improve Catalytic Ozone Decomposition. Ind. Eng. Chem. Res. 2020, 59, 118–128. [Google Scholar] [CrossRef]
- Ahrens, L.H. The use of ionization potentials Part 1. Ionic radii of the elements. Geochim. Cosmochim. Acta 1952, 2, 155–169. [Google Scholar] [CrossRef]
- Hayati, F.; Chandren, S.; Hamdan, H.; Nur, H. The Role of Ti and Lewis Acidity in Manganese Oxide Octahedral Molecular Sieves Impregnated with Titanium in Oxidation Reactions. Bull. Chem. React. Eng. Catal. 2014, 9, 28–38. [Google Scholar] [CrossRef]
- Gao, T.; Glerup, M.; Krumeich, F.; Nesper, R.; Fjellvåg, H.; Norby, P. Microstructures and Spectroscopic Properties of Cryptomelane-type Manganese Dioxide Nanofibers. J. Phys. Chem. C 2008, 112, 13134–13140. [Google Scholar] [CrossRef]
- Tabassum, L.; Tasnim, H.; Meguerdichian, A.G.; Willis, W.S.; Macharia, J.; Price, C.; Dang, Y.; Suib, S.L. Enhanced Catalytic Activity of a Vanadium-Doped Mesoporous Octahedral Molecular Sieve-2 (K-OMS-2) toward Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 12185–12193. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging Transparent Electrodes Based on Thin Films of Carbon Nanotubes, Graphene, and Metallic Nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Minami, T. Chapter Five—Transparent Conductive Oxides for Transparent Electrode Applications. In Oxide Semiconductors; Svensson, B.G., Pearton, S.J., Jagadish, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, Y.; Chen, C.-H.; Zhao, L.; Suib, S.L. Framework Doping of Indium in Manganese Oxide Materials: Synthesis, Characterization, and Electrocatalytic Reduction of Oxygen. Chem. Mater. 2008, 20, 2069–2071. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Chen, Z.; Huang, Z.; Jing, G. Low-valence or tetravalent cation doping of manganese oxide octahedral molecular sieve (K-OMS-2) materials for nitrogen oxide emission abatement. Catal. Sci. Technol. 2019, 9, 4108–4117. [Google Scholar] [CrossRef]
- Nguyen Dinh, M.T.; Giraudon, J.M.; Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Lamonier, J.F. Manganese oxide octahedral molecular sieve K-OMS-2 as catalyst in post plasma-catalysis for trichloroethylene degradation in humid air. J. Hazard. Mater. 2016, 314, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, J.; Liu, F.; He, H.; Zhang, R. The Effects of Mn2+ Precursors on the Structure and Ozone Decomposition Activity of Cryptomelane-Type Manganese Oxide (OMS-2) Catalysts. J. Phys. Chem. C 2015, 119, 23119–23126. [Google Scholar] [CrossRef]
- Tseng, L.-T.; Lu, Y.; Fan, H.M.; Wang, Y.; Luo, X.; Liu, T.; Munroe, P.; Li, S.; Yi, J. Magnetic properties in α-MnO2 doped with alkaline elements. Sci. Rep. 2015, 5, 9094. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; He, H. Recent advances in catalytic decomposition of ozone. J. Environ. Sci. 2020, 94, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, S.S.; Verkhov, V.A.; Svetlichnyi, V.A.; Liotta, L.F.; La Parola, V.; Izaak, T.I.; Vodyankina, O. V Oxidative dehydrogenation of ethanol on modified OMS-2 catalysts. Catal. Today 2020, 357, 503–510. [Google Scholar] [CrossRef]
- Makwana, V.D.; Son, Y.-C.; Howell, A.R.; Suib, S.L. The Role of Lattice Oxygen in Selective Benzyl Alcohol Oxidation Using OMS-2 Catalyst: A Kinetic and Isotope-Labeling Study. J. Catal. 2002, 210, 46–52. [Google Scholar] [CrossRef]
- Gu, Y.; Min, Y.; Li, L.; Lian, Y.; Sun, H.; Wang, D.; Rummeli, M.H.; Guo, J.; Zhong, J.; Xu, L.; et al. Crystal Splintering of β-MnO2 Induced by Interstitial Ru Doping Toward Reversible Oxygen Conversion. Chem. Mater. 2021, 33, 4135–4145. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, K.-G.; Kim, H.K.; Hwang, S.-J. MnO2-based nanostructured materials for various energy applications. Mater. Chem. Front. 2021, 5, 3549–3575. [Google Scholar] [CrossRef]
- Garcia, C.; Truttmann, V.; Lopez, I.; Haunold, T.; Marini, C.; Rameshan, C.; Pittenauer, E.; Kregsamer, P.; Dobrezberger, K.; Stöger-Pollach, M.; et al. Dynamics of Pd Dopant Atoms inside Au Nanoclusters during Catalytic CO Oxidation. J. Phys. Chem. C 2020, 124, 23626–23636. [Google Scholar] [CrossRef]
- López-Hernández, I.; García, C.; Truttmann, V.; Pollitt, S.; Barrabés, N.; Rupprechter, G.; Rey, F.; Palomares, A.E. Evaluation of the silver species nature in Ag-ITQ2 zeolites by the CO oxidation reaction. Catal. Today 2020, 345, 22–26. [Google Scholar] [CrossRef]
- Sarma, B.B.; Plessow, P.N.; Agostini, G.; Concepción, P.; Pfänder, N.; Kang, L.; Wang, F.R.; Studt, F.; Prieto, G. Metal-Specific Reactivity in Single-Atom Catalysts: CO Oxidation on 4d and 5d Transition Metals Atomically Dispersed on MgO. J. Am. Chem. Soc. 2020, 142, 14890–14902. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, M.; Ye, Q.; Dong, N.; Dai, H. Enhanced Performance of the OMS-2-Supported CuOx Catalysts for Carbon Monoxide, Ethyl Acetate, and Toluene Oxidation. Catalysts 2021, 11, 713. [Google Scholar] [CrossRef]
- Chen, J.; Tang, X.; Liu, J.; Zhan, E.; Li, J.; Huang, X.; Shen, W. Synthesis and Characterization of Ag−Hollandite Nanofibers and Its Catalytic Application in Ethanol Oxidation. Chem. Mater. 2007, 19, 4292–4299. [Google Scholar] [CrossRef]
- Li, L.; King, D.L. Synthesis and Characterization of Silver Hollandite and Its Application in Emission Control. Chem. Mater. 2005, 17, 4335–4343. [Google Scholar] [CrossRef]
- Genuino, H.C.; Seraji, M.S.; Meng, Y.; Valencia, D.; Suib, S.L. Combined experimental and computational study of CO oxidation promoted by Nb in manganese oxide octahedral molecular sieves. Appl. Catal. B Environ. 2015, 163, 361–369. [Google Scholar] [CrossRef]
- Genuino, H.C.; Valencia, D.; Suib, S.L. Insights into the structure–property–activity relationship in molybdenum-doped octahedral molecular sieve manganese oxides for catalytic oxidation. Catal. Sci. Technol. 2018, 8, 6493–6502. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, Y.; Li, Y.; Liu, H. Highly effective UV–Vis-IR and IR photothermocatalytic CO abatement on Zn doped OMS-2 nanorods. Appl. Surf. Sci. 2019, 483, 827–834. [Google Scholar] [CrossRef]
- Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Unexpected stability of CuO/Cryptomelane catalyst under Preferential Oxidation of CO reaction conditions in the presence of CO2 and H2O. Appl. Catal. B Environ. 2017, 217, 459–465. [Google Scholar] [CrossRef]
- He, J.; Chen, S.-Y.; Tang, W.; Dang, Y.; Kerns, P.; Miao, R.; Dutta, B.; Gao, P.-X.; Suib, S.L. Microwave-assisted integration of transition metal oxide nanocoatings on manganese oxide nanoarray monoliths for low temperature CO oxidation. Appl. Catal. B Environ. 2019, 255, 117766. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Monteverde Videla, A.H.A.; Jakubek, T.; Kotarba, A.; Specchia, S. The Effect of Fe, Co, and Ni Structural Promotion of Cryptomelane (KMn8O16) on the Catalytic Activity in Oxygen Evolution Reaction. Electrocatalysis 2018, 9, 762–769. [Google Scholar] [CrossRef]




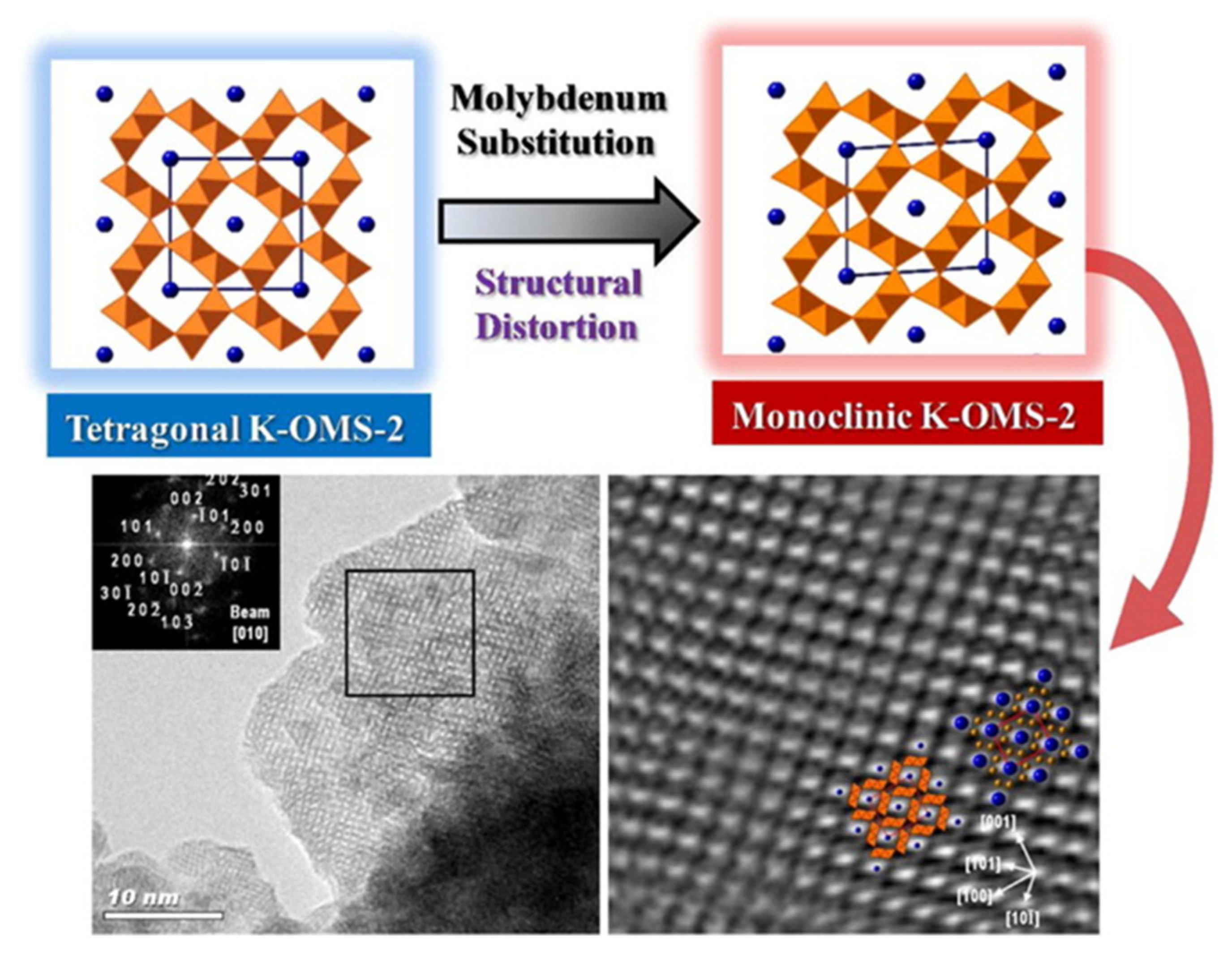
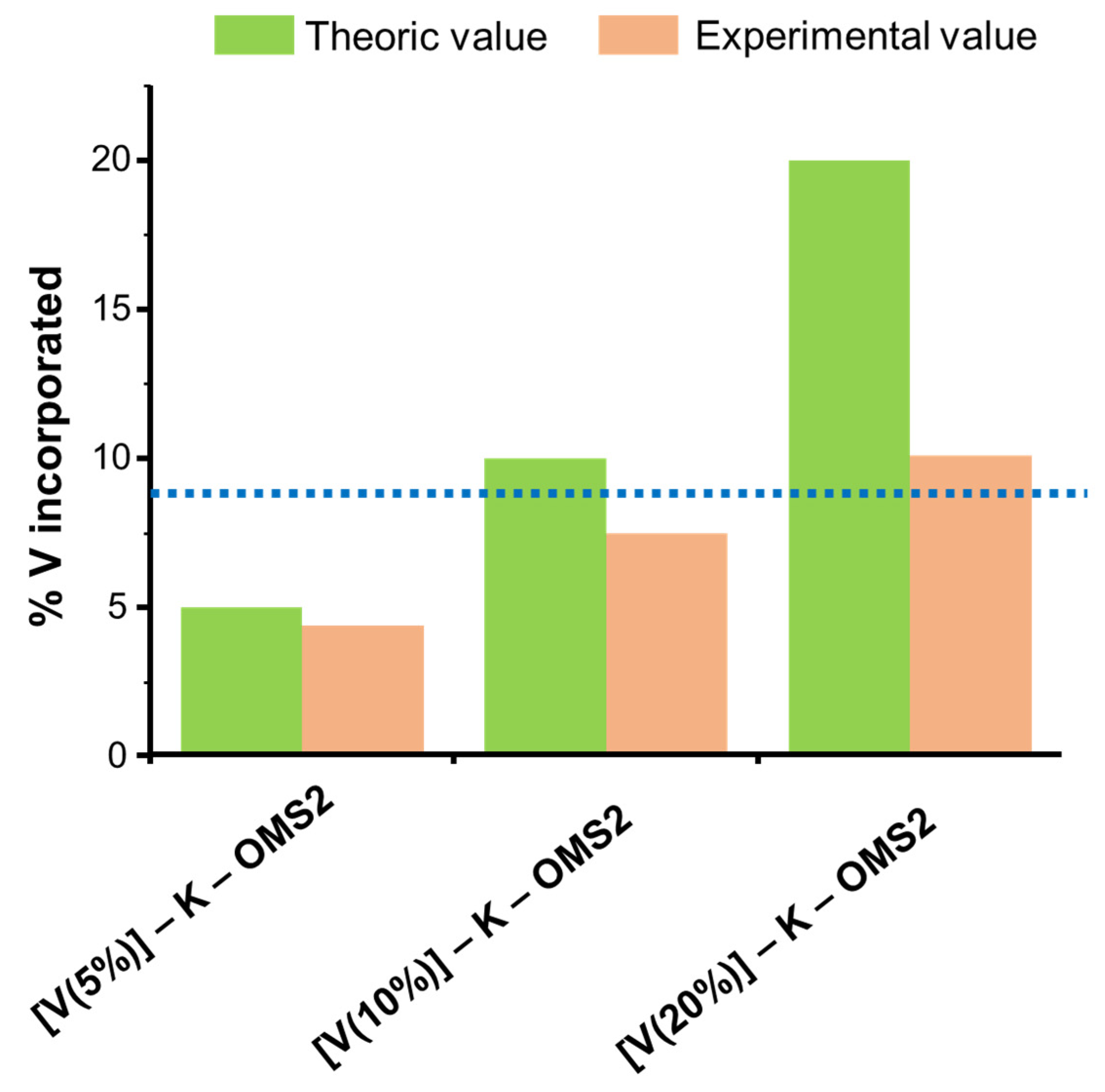
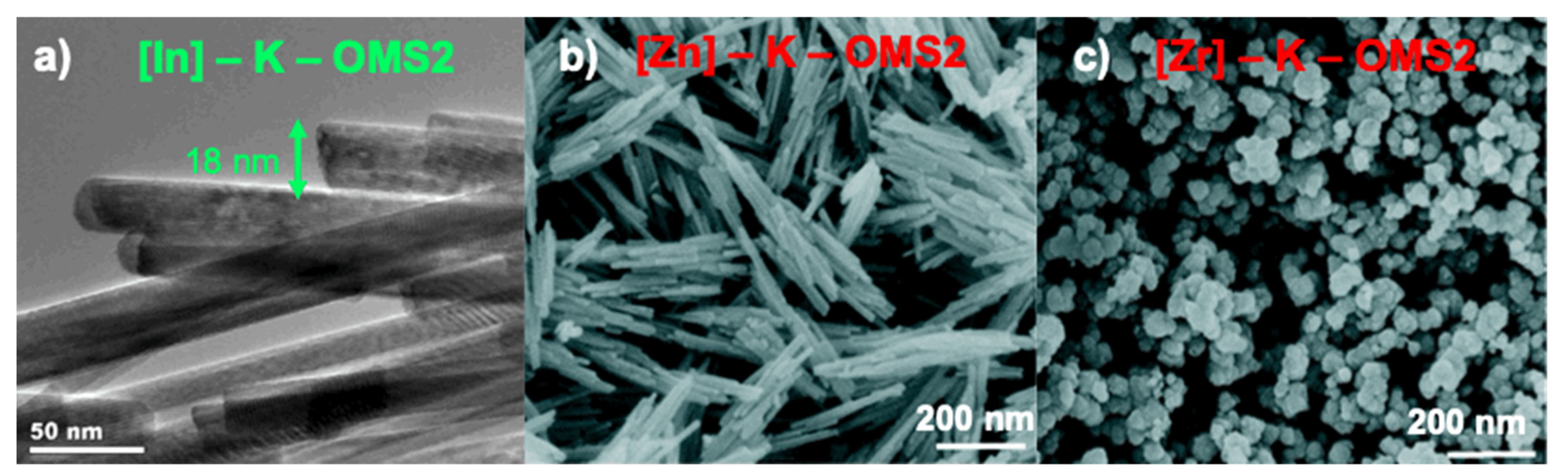

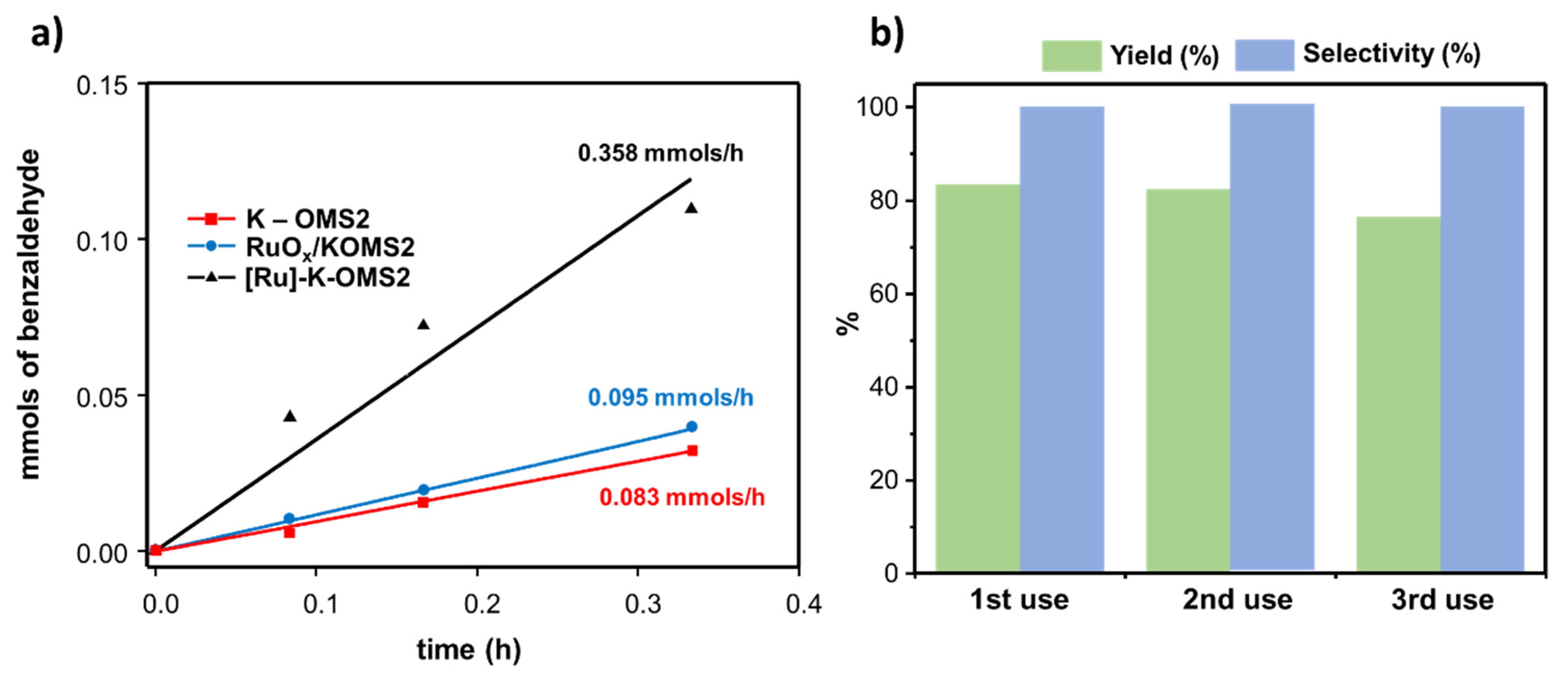

| Form | Name | Formula | Space Group | Structure (m × n, Dimension Tunnel Structure) | Cell [a] | Oxidation States of Mn |
|---|---|---|---|---|---|---|
| α–MnO2 | Hollandite | Ba(Mn4+6Mn3+2)O16 | Tetragonal/monoclinic I4/m | (2 × 2) tunnel | hcp | +4, +3 |
| β–MnO2 | Pyrolusite | Mn4+O2 | Tetragonal, P42/mnm | (1 × 1) tunnel | +4 | |
| γ–MnO2 | Nsutite | (Mn4+,Mn2+)(O,OH)2 | Hexagonal/Orthorombic, (n.d.) | (1 × 1)/(1 × 2) | +4, +2 | |
| R–MnO2 | Ramsdellite | Mn4+O2 | Orthorombic, Pnma | (1 × 2) tunnel | +4 | |
| ε–MnO2 | Akhtenskite | Mn4+O2 | Hexagonal, P63/mmc | dense | +4 | |
| δ–MnO2 | Birnessite | Na0.5(Mn4+Mn3+)O4·1.5H2O | Monoclinic, P63/mmc | (1 × ∞) layer | hcp/fcc | +3, +4 |
| λ–MnO2 | Spinel | (Li)Mn2O4 | Spinel, Fd3m | (1 × 1) tunnel | fcc | +3, +4 |
| Metal Incorporated into the Framework | Synthesis | Composition Range (% wt.) | Surface Area Range, N2, 77K (m2/g) | Morphology | Reaction Examples | References |
|---|---|---|---|---|---|---|
| Ag | Hydrothermal, reflux, microwave assisted, solid state | 0–2 | 80–160 | Nanorod (50 nm–1 μm) | CO oxidation | [153,154] |
| Ce | Reflux | 0–8 | 72–200 | Nanorod (500 nm) | VOC and ozone degradation | [106,155,156] |
| Ru | Reflux | 0–4 | 84–131 [a] | Nanorod (150–500 nm) | Oxidation of alcohols | [108,133] |
| Ti | Reflux | 0–2 | 152–155 | Nanorod (n.a.) | Oxidation of styrene | [157] |
| Mo | Reflux | 0–10 | 100–210 | Nanorod (50–200) | CO oxidation | [150,158] |
| W | Reflux | 0–10 | 110–190 | Nanorod (50–200) | CO oxidation | [151] |
| V | Reflux | 0–10 | 120–190 | Nanorod (50–200) | CO oxidation | [159,160] |
| Nb | Reflux | 0–30 | 147–220 | Nanorod (51–184) | Oxidation of methanol | [161,162] |
| In | See Section 3.1.6, Section 3.1.7 and Section 3.1.8 | |||||
| Zn | ||||||
| Zr | ||||||
| Sample | Incorporated Ag Content (% wt.) | BET Surface Value (m2/g) |
|---|---|---|
| 1 [a] | 0 | 76.9 |
| 2 | 0.1 | 79.1 |
| 3 | 0.5 | 79.7 |
| 4 | 1.5 | 81.2 |
| 5 | 2.0 | 81.8 |
| Sample | Desorption Temperature (°C) | Peak Area (a.u.) | |||||
|---|---|---|---|---|---|---|---|
| Peak I | Peak II | Peak III | Peak I | Peak II | Peak III | Total | |
| K–OMS–2 | 108 | 492 | 581 | 302 | 422 | 448 | 1172 |
| [Ce]–K–OMS–2 | 132 | 340 | 650 | 305 | 903 | 723 | 1931 |
| K–OMS–2 | [W(1.33%)]–K–OMS–2 | [W(2%)]–K–OMS–2 | |
|---|---|---|---|
| a (Å), b (Å) | 9.815 | 9.804 | 9.816 |
| c (Å) | 2.847 | 2.852 | 2.855 |
| cell volume (Å3) | 274.3 | 274.1 | 275.1 |
| Catalysts | K/Mn + Metal + K (%) [a] | Mn/Mn + Metal + K (%) [a] | Metallic Dopant Species (% abundance) [b] | Surface Area (m2/g) [c] |
|---|---|---|---|---|
| K–OMS–2 | 6.37 | 93.6 | - | 137 |
| [Ce]–K–OMS–2 | 4.82 | 86.7 | Ce3+ (30%), Ce4+ (70%) | 200 |
| [Co]–K–OMS–2 | 6.40 | 85.1 | Co3+ (95%) [d], Co2+(5%) [e] | 115 |
| [Fe]–K–OMS–2 | 6.31 | 83.6 | Fe2+ (36%) [d], Fe3+(64%) [e], | 52 |
| Dopant [M] | Material | Location of M [a] | Reference |
|---|---|---|---|
| Ag | [Ag]–K–OMS–2 | F, T, S | [164,204,205] |
| Nb | [Nb]–K–OMS–2 | F | [206] |
| Mo | [Mo]–K–OMS–2 | F | [207] |
| V | [V]–K–OMS–2 | F | [159] |
| Cu | [Cu]–K–OMS–2 | F, S | [136,141] |
| Zn | [Zn]–K–OMS–2 | F | [208] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabaté, F.; Sabater, M.J. Recent Manganese Oxide Octahedral Molecular Sieves (OMS–2) with Isomorphically Substituted Cationic Dopants and Their Catalytic Applications. Catalysts 2021, 11, 1147. https://doi.org/10.3390/catal11101147
Sabaté F, Sabater MJ. Recent Manganese Oxide Octahedral Molecular Sieves (OMS–2) with Isomorphically Substituted Cationic Dopants and Their Catalytic Applications. Catalysts. 2021; 11(10):1147. https://doi.org/10.3390/catal11101147
Chicago/Turabian StyleSabaté, Ferran, and María J. Sabater. 2021. "Recent Manganese Oxide Octahedral Molecular Sieves (OMS–2) with Isomorphically Substituted Cationic Dopants and Their Catalytic Applications" Catalysts 11, no. 10: 1147. https://doi.org/10.3390/catal11101147
APA StyleSabaté, F., & Sabater, M. J. (2021). Recent Manganese Oxide Octahedral Molecular Sieves (OMS–2) with Isomorphically Substituted Cationic Dopants and Their Catalytic Applications. Catalysts, 11(10), 1147. https://doi.org/10.3390/catal11101147







