Comment on Weber et al. Mayenite-Based Electride C12A7e−: A Reactivity and Stability Study. Catalysts 2021, 11, 334
Author Contributions
Funding
Conflicts of Interest
References
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.-W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef] [PubMed]
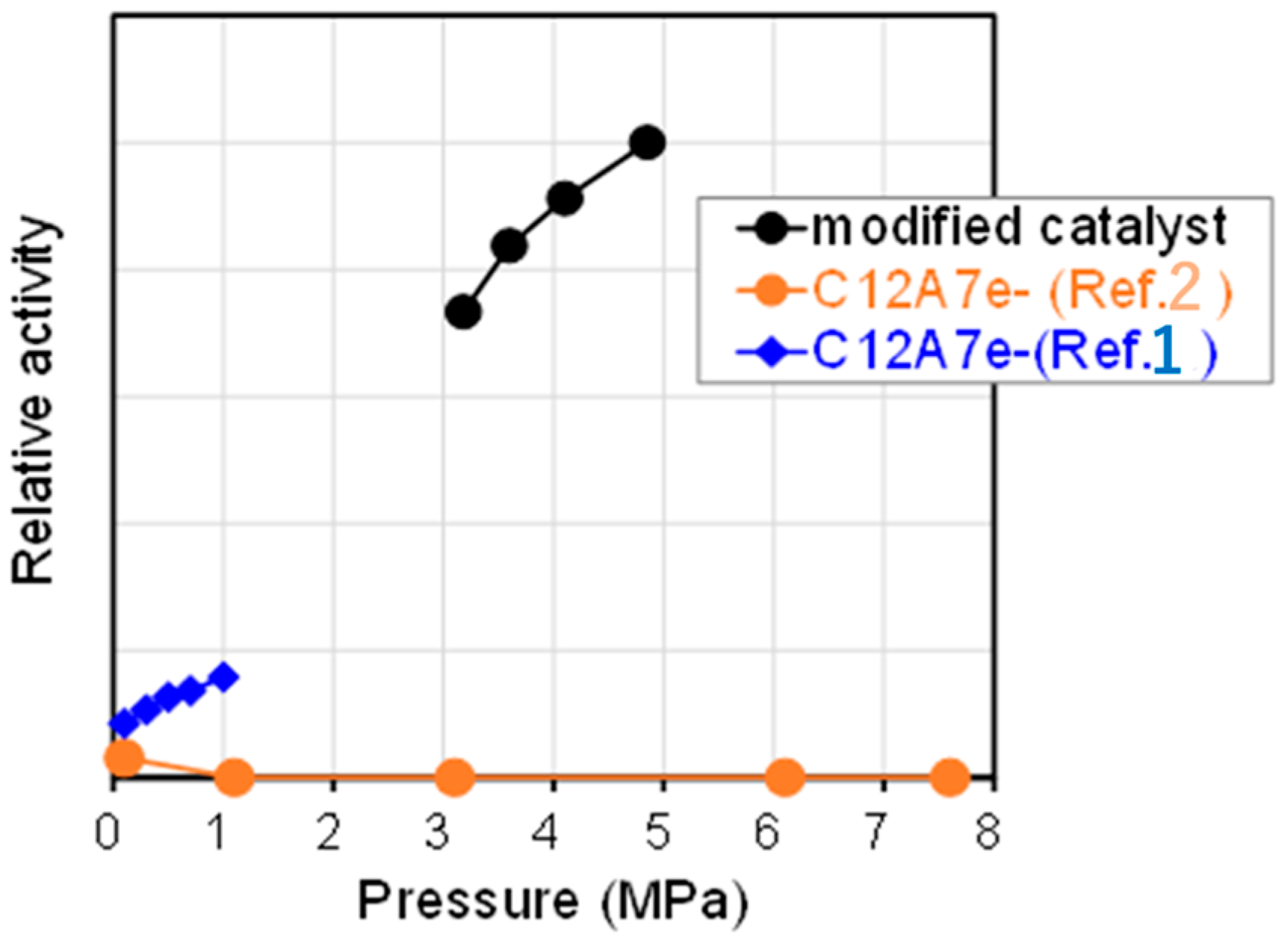
- Weber, S.; Schäfer, S.; Saccoccio, M.; Ortner, N.; Bertmer, M.; Seidel, K.; Berendts, S.; Lerch, M.; Gläser, R.; Kohlmann, H.; et al. Mayenite-Based Electride C12A7e−: A Reactivity and Stability Study. Catalysts 2021, 11, 334. [Google Scholar] [CrossRef]
- Kanbara, S.; Kitano, M.; Inoue, Y.; Yokoyama, T.; Hara, M.; Hosono, H.J. Mechanism Switching of Ammonia Synthesis Over Ru-Loaded Electride Catalyst at Metal–Insulator Transition. Am. Chem. Soc. 2015, 137, 14517–14524. [Google Scholar] [CrossRef] [PubMed]
- Kammert, J.; Moon, J.; Cheng, Y.; Daemen, L.L.; Irle, S.; Fung, V.; Liu, J.; Page, K.; Ma, X.; Phaneuf, V.; et al. Nature of Reactive Hydrogen for Ammonia Synthesis over a Ru/C12A7 Electride Catalyst. J. Am. Chem. Soc. 2020, 142, 7655–7667. [Google Scholar] [CrossRef] [PubMed]
- Matsuishi, S.; Nomura, T.; Hirano, M.; Kodama, K.; Shamoto, S.I.; Hosono, H. Drect Synthesis of Powdery Inorganic Electride [Ca24Al28O64]4+(e−)4 and Determination of Oxygen Stoichiometry. Chem. Mater. 2009, 21, 2589–2591. [Google Scholar] [CrossRef]
- Hosono, H. Electron Transfer from Support/Promotor to Metal Catalyst: Requirements for Effective Support. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Hosono, H.; Kitano, M. Advances in Materials and Applications of Inorganic Electrides. Chem. Rev. 2021, 121, 3121–3186. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Kitano, M.; Hosono, H. Comment on Weber et al. Mayenite-Based Electride C12A7e−: A Reactivity and Stability Study. Catalysts 2021, 11, 334. Catalysts 2021, 11, 1154. https://doi.org/10.3390/catal11101154
Inoue Y, Kitano M, Hosono H. Comment on Weber et al. Mayenite-Based Electride C12A7e−: A Reactivity and Stability Study. Catalysts 2021, 11, 334. Catalysts. 2021; 11(10):1154. https://doi.org/10.3390/catal11101154
Chicago/Turabian StyleInoue, Yasunori, Masaaki Kitano, and Hideo Hosono. 2021. "Comment on Weber et al. Mayenite-Based Electride C12A7e−: A Reactivity and Stability Study. Catalysts 2021, 11, 334" Catalysts 11, no. 10: 1154. https://doi.org/10.3390/catal11101154
APA StyleInoue, Y., Kitano, M., & Hosono, H. (2021). Comment on Weber et al. Mayenite-Based Electride C12A7e−: A Reactivity and Stability Study. Catalysts 2021, 11, 334. Catalysts, 11(10), 1154. https://doi.org/10.3390/catal11101154




