Bioethanol Upgrading to Renewable Monomers Using Hierarchical Zeolites: Catalyst Preparation, Characterization, and Catalytic Studies
Abstract
:1. Introduction
2. Design and Preparation of Hierarchical Zeolite Catalysts
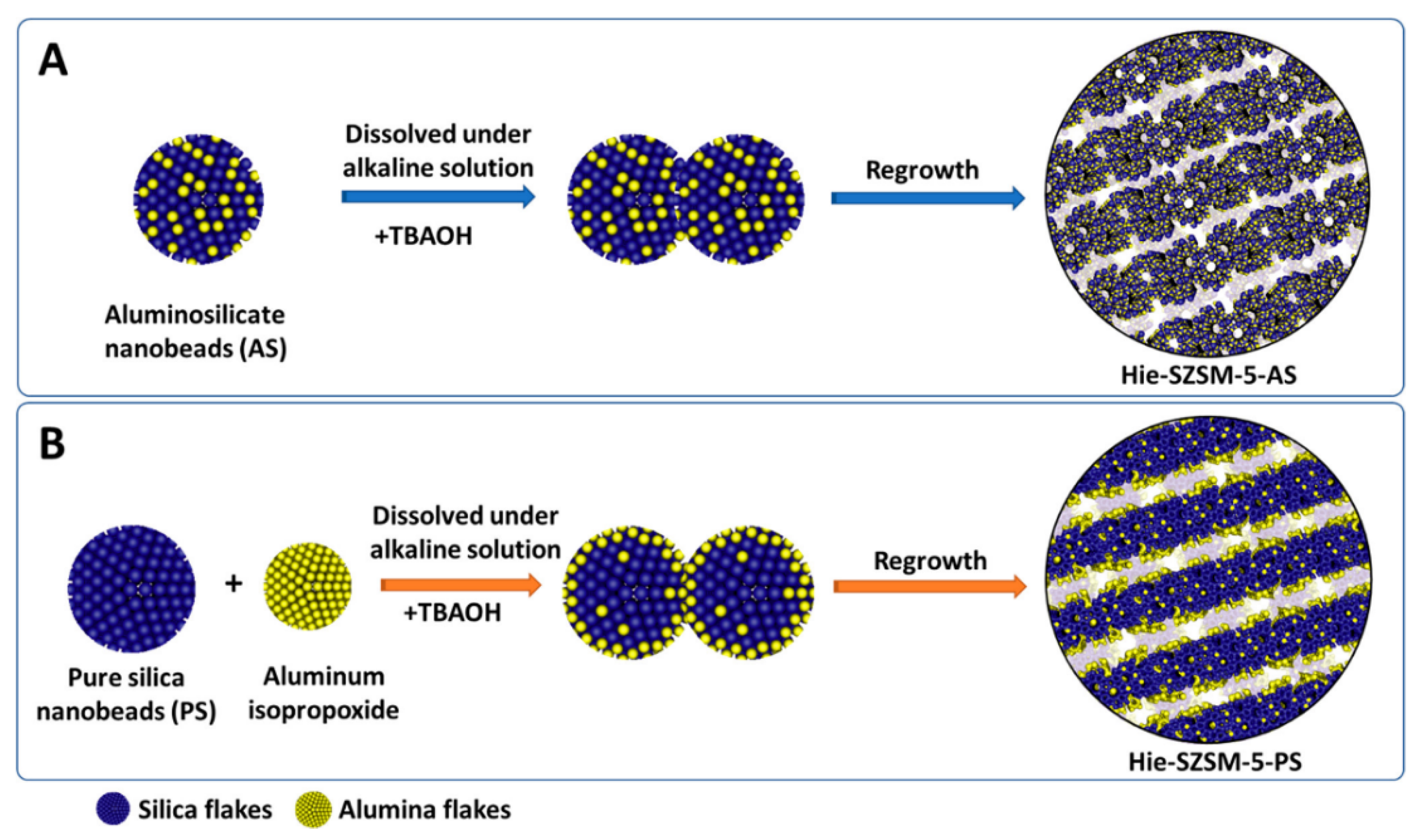
2.1. Bottom-Up Approach
2.2. Top-Down Approach
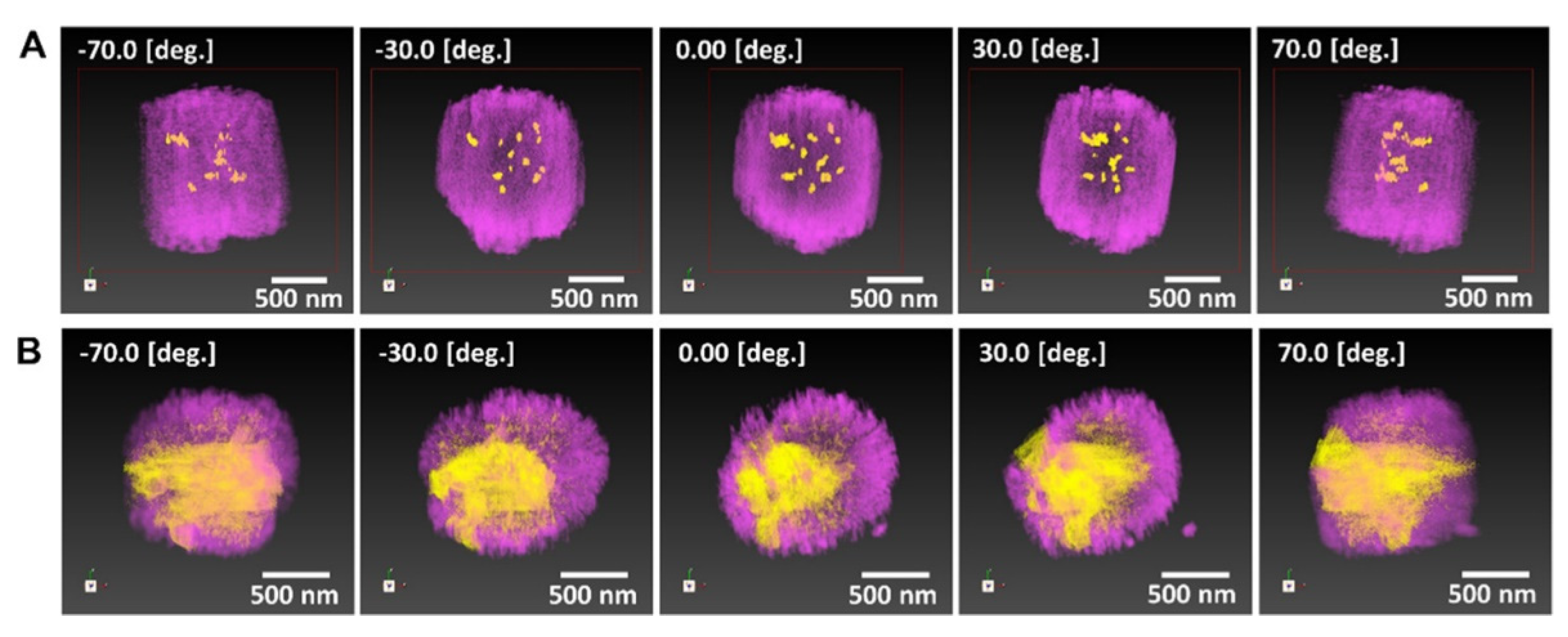
2.3. Incorporation of Metal Species into Zeolite Supports
3. Characterization of Hierarchical Zeolite Catalysts
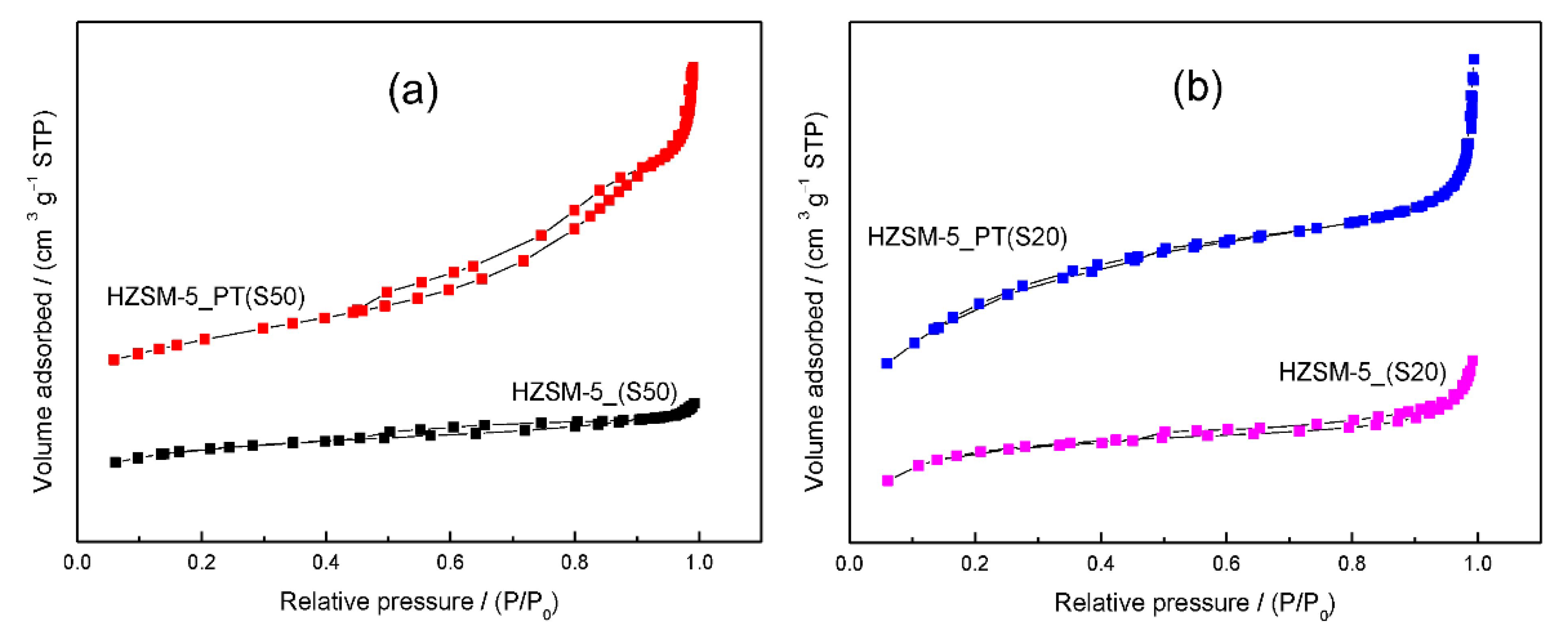
3.1. Textural Properties and Pore Architecture
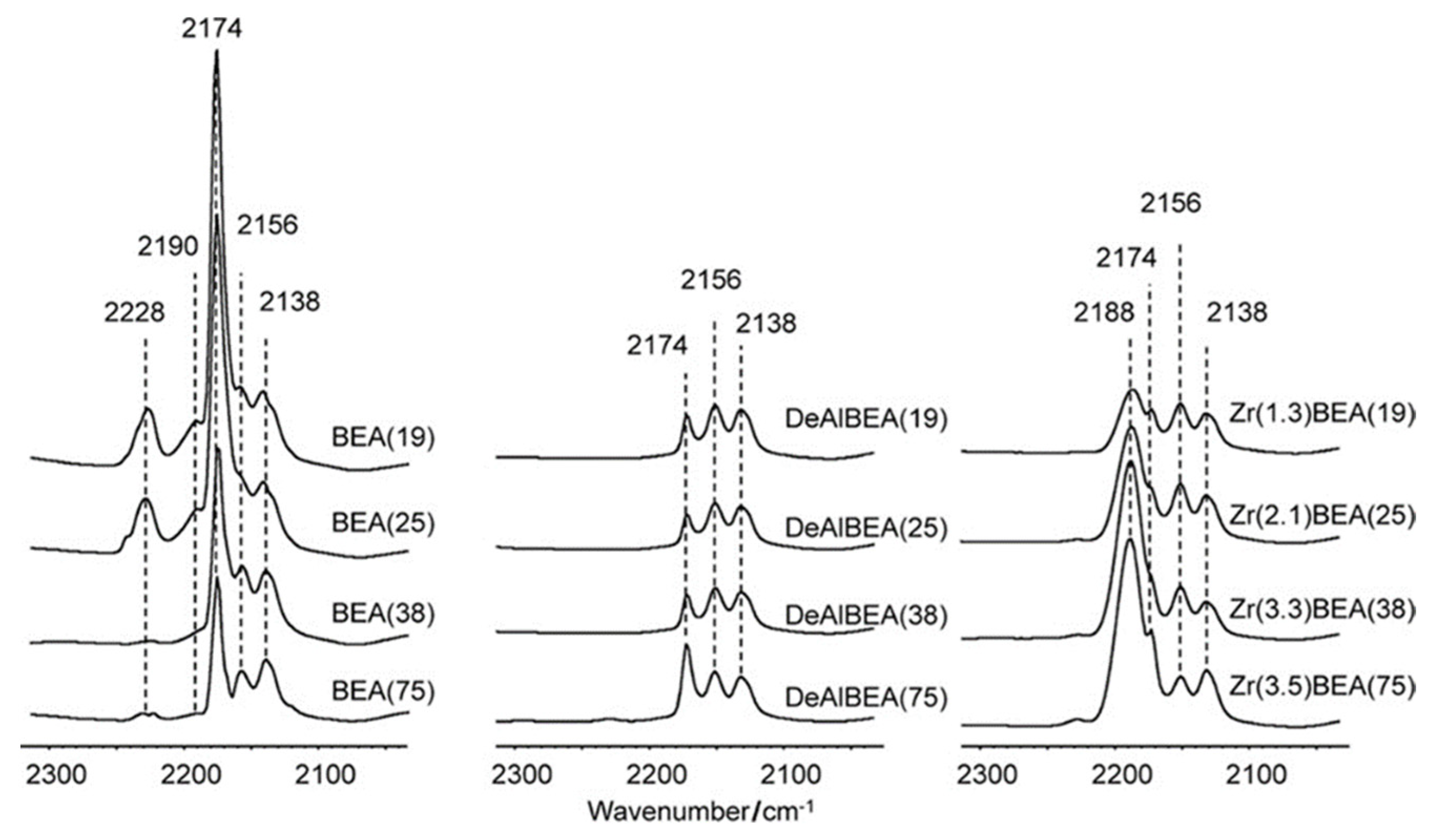
3.2. Acid-Base Properties and Active Species
4. Catalytic Behaviors of Hierarchical Zeolites in Bioethanol Conversion to Monomers
4.1. Catalytic Activities
4.1.1. Bioethanol Dehydration
4.1.2. Bioethanol to Hydrocarbons
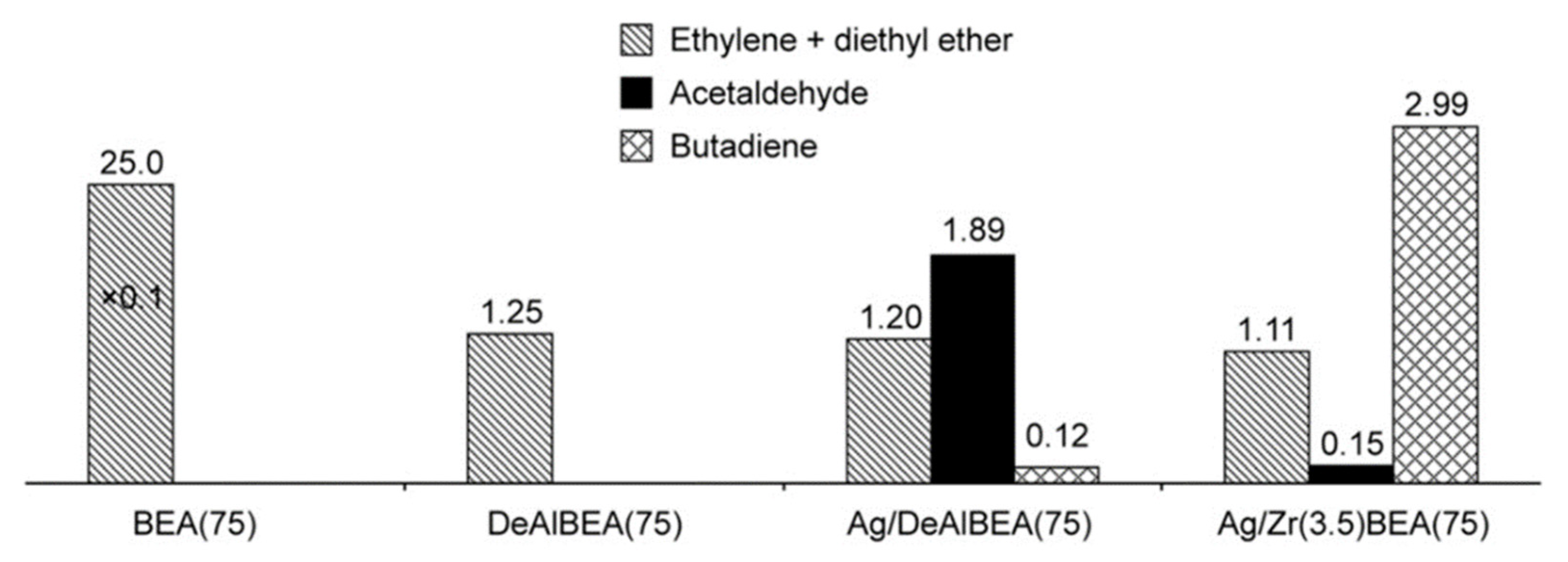
4.1.3. Bioethanol to Butadiene
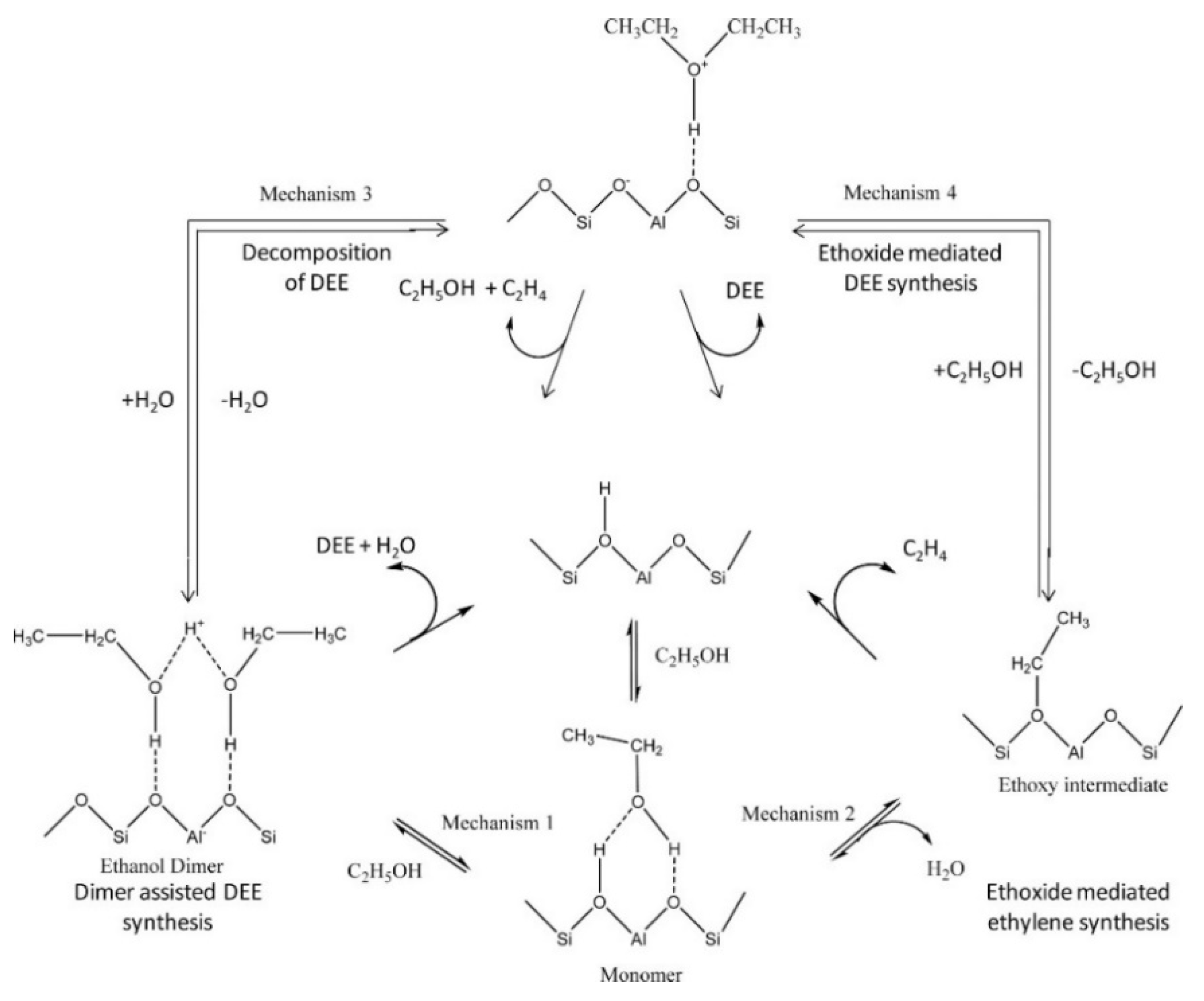
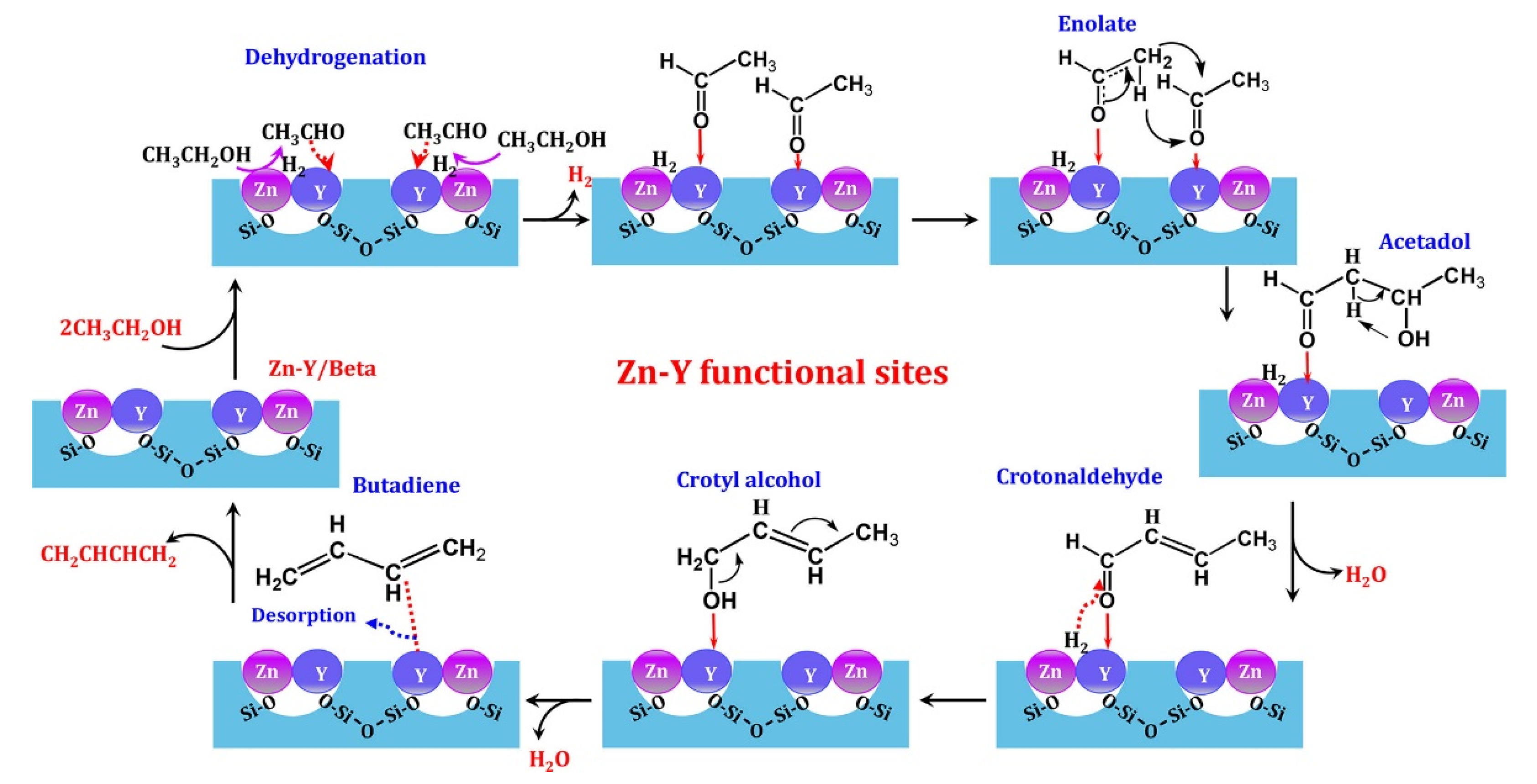
4.2. Mechanistic Studies
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Gucbilmez, Y.; Dogu, T.; Balci, S. Ethylene and acetaldehyde production by selective oxidation of ethanol using mesoporous V−MCM-41 catalysts. Ind. Eng. Chem. Res. 2006, 45, 3496–3502. [Google Scholar] [CrossRef]
- Capellán-Pérez, I.; Mediavilla, M.; de Castro, C.; Carpintero, Ó.; Miguel, L.J. Fossil fuel depletion and socio-economic scenarios: An integrated approach. Energy 2014, 77, 641–666. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Bridges, A.; Felder, F.A.; McKelvey, K.; Niyogi, I. Uncertainty in energy planning: Estimating the health impacts of air pollution from fossil fuel electricity generation. Energy Res. Soc. Sci. 2015, 6, 74–77. [Google Scholar] [CrossRef]
- Wang, L.; Templer, R.; Murphy, R.J. Environmental sustainability of bioethanol production from waste papers: Sensitivity to the system boundary. Energy Environ. Sci. 2012, 5, 8281–8293. [Google Scholar] [CrossRef]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Morone, P.; Strzałkowski, A.; Tani, A. Chapter 2—Biofuel transitions: An overview of regulations and standards for a more sustainable framework. In Biofuels for a More Sustainable Future; Ren, J., Scipioni, A., Manzardo, A., Liang, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–46. [Google Scholar]
- Mączyńska, J.; Krzywonos, M.; Kupczyk, A.; Tucki, K.; Sikora, M.; Pińkowska, H.; Bączyk, A.; Wielewska, I. Production and use of biofuels for transport in Poland and Brazil—The case of bioethanol. Fuel 2019, 241, 989–996. [Google Scholar] [CrossRef]
- Kempton, W.; Letendre, S.E. Electric vehicles as a new power source for electric utilities. Transp. Res. D Transp. Environ. 1997, 2, 157–175. [Google Scholar] [CrossRef]
- Hannan, M.A.; Azidin, F.A.; Mohamed, A. Hybrid electric vehicles and their challenges: A review. Renew. Sustain. Energy Rev. 2014, 29, 135–150. [Google Scholar] [CrossRef]
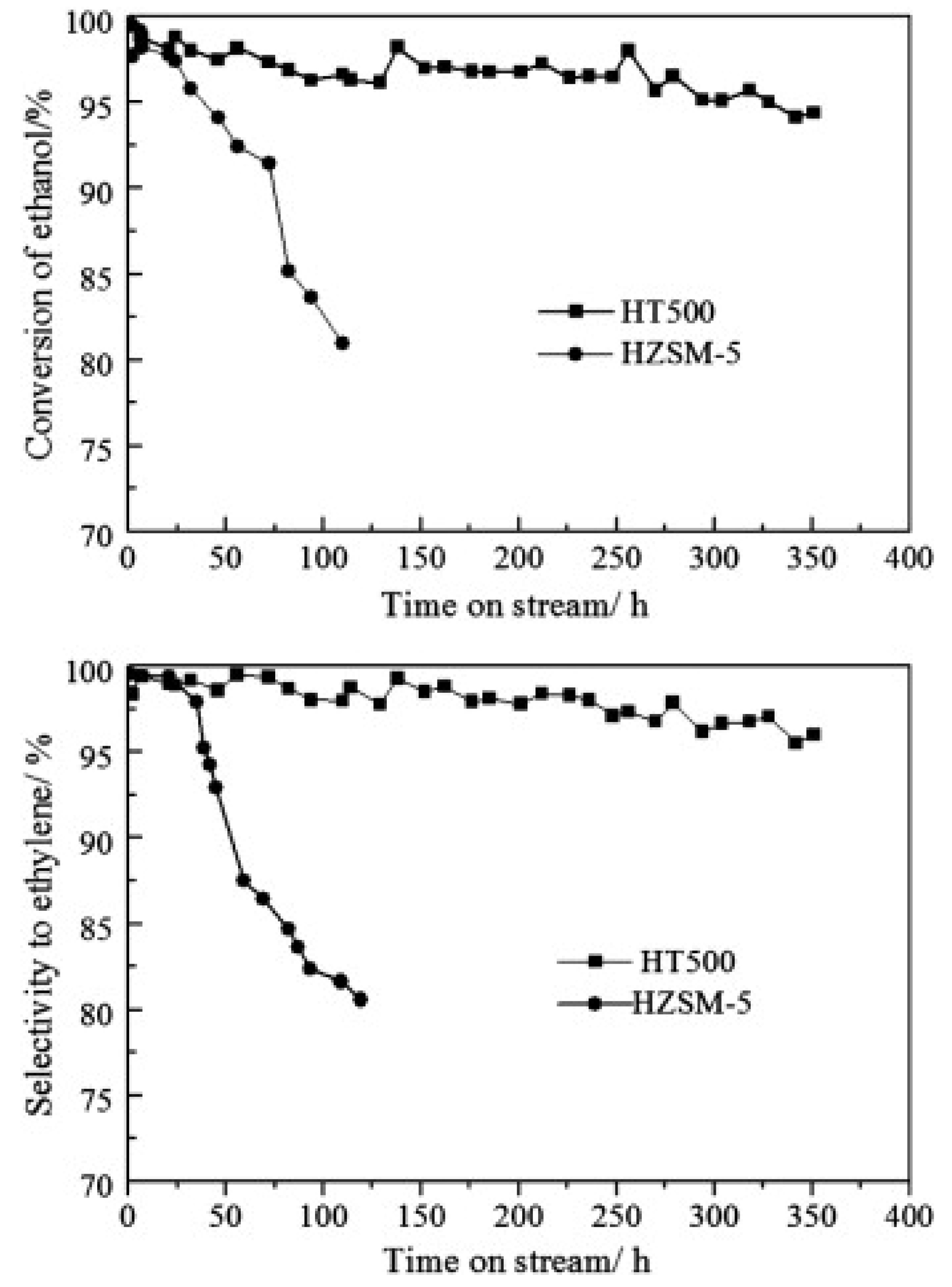
- Choopun, W.; Jitkarnka, S. Catalytic activity and stability of HZSM-5 zeolite and hierarchical uniform mesoporous MSU-SZSM-5 material during bio-ethanol dehydration. J. Clean. Prod. 2016, 135, 368–378. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J. Influence of HZSM-5-based catalyst deactivation on the performance of different reactor configurations for the conversion of bioethanol into hydrocarbons. Fuel 2021, 302, 121061. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Li, X.; Li, X.; Zhang, T. Catalytic conversion of ethanol into butadiene over high performance LiZnHf-MFI zeolite nanosheets. Green Chem. 2019, 21, 1006–1010. [Google Scholar] [CrossRef]
- Haro, P.; Ollero, P.; Trippe, F. Technoeconomic assessment of potential processes for bio-ethylene production. Fuel Process. Technol. 2013, 114, 35–48. [Google Scholar] [CrossRef]
- Yakovleva, I.S.; Banzaraktsaeva, S.P.; Ovchinnikova, E.V.; Chumachenko, V.A.; Isupova, L.A. Catalytic dehydration of bioethanol to ethylene. Catal. Ind. 2016, 8, 152–167. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y. Dehydration of ethanol to ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Kumar, R.V.; Cheetham, A.K. Carbon with hierarchical pores from carbonized metal-organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar] [CrossRef]
- Shetsiri, S.; Thivasasith, A.; Saenluang, K.; Wannapakdee, W.; Salakhum, S.; Wetchasat, P.; Nokbin, S.; Limtrakul, J.; Wattanakit, C. Sustainable production of ethylene from bioethanol over hierarchical ZSM-5 nanosheets. Sustain. Energy Fuels 2019, 3, 115–126. [Google Scholar] [CrossRef]
- Saenluang, K.; Imyen, T.; Wannapakdee, W.; Suttipat, D.; Dugkhuntod, P.; Ketkaew, M.; Thivasasith, A.; Wattanakit, C. Hierarchical nanospherical ZSM-5 nanosheets with uniform Al distribution for alkylation of benzene with ethanol. ACS Appl. Nano Mater. 2020, 3, 3252–3263. [Google Scholar] [CrossRef]
- Zhao, T.; Li, F.; Yu, H.; Ding, S.; Li, Z.; Huang, X.; Li, X.; Wei, X.; Wang, Z.; Lin, H. Synthesis of mesoporous ZSM-5 zeolites and catalytic cracking of ethanol and oleic acid into light olefins. Appl. Catal. A Gen. 2019, 575, 101–110. [Google Scholar] [CrossRef]
- Tarach, K.A.; Tekla, J.; Makowski, W.; Filek, U.; Mlekodaj, K.; Girman, V.; Choi, M.; Góra-Marek, K. Catalytic dehydration of ethanol over hierarchical ZSM-5 zeolites: Studies of their acidity and porosity properties. Catal. Sci. Technol. 2016, 6, 3568–3584. [Google Scholar] [CrossRef]
- Ramasamy, K.K.; Zhang, H.; Sun, J.; Wang, Y. Conversion of ethanol to hydrocarbons on hierarchical HZSM-5 zeolites. Catal. Today 2014, 238, 103–110. [Google Scholar] [CrossRef]
- Saenluang, K.; Thivasasith, A.; Dugkhuntod, P.; Pornsetmetakul, P.; Salakhum, S.; Namuangruk, S.; Wattanakit, C. In situ synthesis of Sn-beta zeolite nanocrystals for glucose to hydroxymethylfurfural (HMF). Catalysts 2020, 10, 1249. [Google Scholar] [CrossRef]
- Astafan, A.; Benghalem, M.A.; Pouilloux, Y.; Patarin, J.; Bats, N.; Bouchy, C.; Daou, T.J.; Pinard, L. Particular properties of the coke formed on nano-sponge *BEA zeolite during ethanol-to-hydrocarbons transformation. J. Catal. 2016, 336, 1–10. [Google Scholar] [CrossRef]
- Yang, G.; Wei, Y.; Xu, S.; Chen, J.; Li, J.; Liu, Z.; Yu, J.; Xu, R. Nanosize-enhanced lifetime of SAPO-34 catalysts in methanol-to-olefin reactions. J. Phys. Chem. C 2013, 117, 8214–8222. [Google Scholar] [CrossRef]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Yutthalekha, T.; Wattanakit, C.; Warakulwit, C.; Wannapakdee, W.; Rodponthukwaji, K.; Witoon, T.; Limtrakul, J. Hierarchical FAU-type zeolite nanosheets as green and sustainable catalysts for benzylation of toluene. J. Clean. Prod. 2017, 142, 1244–1251. [Google Scholar] [CrossRef]
- Takata, T.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Nanosized CHA zeolites with high thermal and hydrothermal stability derived from the hydrothermal conversion of FAU zeolite. Microporous Mesoporous Mater. 2016, 225, 524–533. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz Katie, A.; Agrawal Kumar, V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef] [Green Version]
- Souza de Carvalho Filho, J.F.; Maciel Pereira, M.; Gomes Aranda, D.A.; Monnerat Araujo Ribeiro de Almeida, J.; Falabella Sousa-Aguiar, E.; Nothaft Romano, P. Application of response surface methodology for ethanol conversion into hydrocarbons using ZSM-5 zeolites. Catalysts 2019, 9, 617. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Wu, H.S. Ethylene formation from ethanol dehydration using ZSM-5 catalyst. ACS Omega 2017, 2, 4287–4296. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, X.; Fang, Y.; Yi, X.; Hu, W.; Chu, Y.; Zhang, F.; Zheng, A.; Zhang, H.; Li, X. Catalytic dehydration of ethanol over post-treated ZSM-5 zeolites. J. Catal. 2014, 312, 204–215. [Google Scholar] [CrossRef]
- Tarach, K.A.; Tekla, J.; Filek, U.; Szymocha, A.; Tarach, I.; Góra-Marek, K. Alkaline-acid treated zeolite L as catalyst in ethanol dehydration process. Microporous Mesoporous Mater. 2017, 241, 132–144. [Google Scholar] [CrossRef]
- Sheng, Q.; Ling, K.; Li, Z.; Zhao, L. Effect of steam treatment on catalytic performance of HZSM-5 catalyst for ethanol dehydration to ethylene. Fuel Process. Technol. 2013, 110, 73–78. [Google Scholar] [CrossRef]
- Kuterasinski, L.; Filek, U.; Gackowski, M.; Zimowska, M.; Ruggiero-Mikolajczyk, M.; Jodlowski, P.J. Sonochemically prepared hierarchical MFI-type zeolites as active catalysts for catalytic ethanol dehydration. Ultrason. Sonochem. 2021, 74, 105581. [Google Scholar] [CrossRef]
- Klein, A.; Palkovits, R. Influence of structural parameters on the conversion of ethanol into 1,3-butadiene using mesoporous zeolites. Catal. Commun. 2017, 91, 72–75. [Google Scholar] [CrossRef]
- Yang, G.; Wang, L.; Jiang, H. Preparation of β zeolite with intracrystalline mesoporosity via surfactant -templating strategy and its application in ethanol-acetaldehyde to butadiene. Microporous Mesoporous Mater. 2021, 316, 110949. [Google Scholar] [CrossRef]
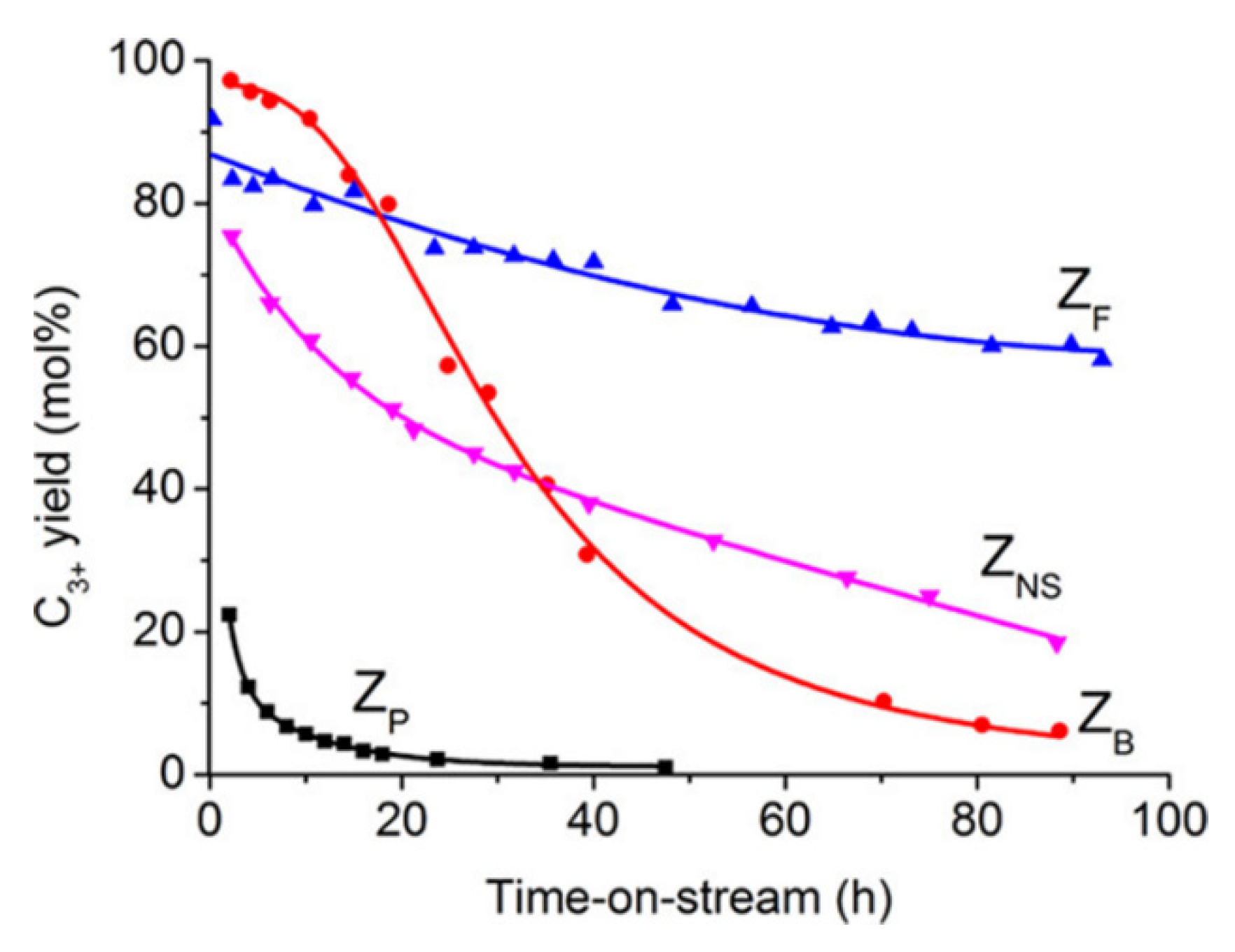
- Lakiss, L.; Ngoye, F.; Canaff, C.; Laforge, S.; Pouilloux, Y.; Qin, Z.; Tarighi, M.; Thomas, K.; Valtchev, V.; Vicente, A.; et al. On the remarkable resistance to coke formation of nanometer-sized and hierarchical MFI zeolites during ethanol to hydrocarbons transformation. J. Catal. 2015, 328, 165–172. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, D.; Zhai, X. Selective conversion of bio-ethanol to propene over nano-HZSM-5 zeolite: Remarkably enhanced catalytic performance by fluorine modification. Fuel Process. Technol. 2017, 167, 50–60. [Google Scholar] [CrossRef]
- Qin, Z.; Melinte, G.; Gilson, J.P.; Jaber, M.; Bozhilov, K.; Boullay, P.; Mintova, S.; Ersen, O.; Valtchev, V. The mosaic structure of zeolite crystals. Angew. Chem. Int. Ed. Engl. 2016, 55, 15049–15052. [Google Scholar] [CrossRef]
- Qin, Z.; Pinard, L.; Benghalem, M.A.; Daou, T.J.; Melinte, G.; Ersen, O.; Asahina, S.; Gilson, J.-P.; Valtchev, V. Preparation of single-crystal “house-of-cards”-like ZSM-5 and their performance in ethanol-to-hydrocarbon conversion. Chem. Mater. 2019, 31, 4639–4648. [Google Scholar] [CrossRef]
- Fang, Z.; Murayama, H.; Zhao, Q.; Liu, B.; Jiang, F.; Xu, Y.; Tokunaga, M.; Liu, X. Selective mild oxidation of methane to methanol or formic acid on Fe-MOR catalysts. Catal. Sci. Technol. 2019, 9, 6946–6956. [Google Scholar] [CrossRef]
- Yao, J.; Feng, X.; Fan, J.; He, Y.; Kosol, R.; Zeng, Y.; Liu, G.; Ma, Q.; Yang, G.; Tsubaki, N. Effects of mordenite zeolite catalyst synthesis conditions on dimethyl ether carbonylation. Microporous Mesoporous Mater. 2020, 306, 110431. [Google Scholar] [CrossRef]
- Gomes, G.J.; Costa, M.B.; Bittencourt, P.R.S.; Zalazar, M.F.; Arroyo, P.A. Catalytic improvement of biomass conversion: Effect of adding mesoporosity on MOR zeolite for esterification with oleic acid. Renew. Energy 2021, 178, 1–12. [Google Scholar] [CrossRef]
- Iadrat, P.; Horii, N.; Atithep, T.; Wattanakit, C. Effect of pore connectivity of pore-opened hierarchical MOR zeolites on catalytic behaviors and coke formation in ethanol dehydration. ACS Appl. Mater. Interfaces 2021, 13, 8294–8305. [Google Scholar] [CrossRef]
- Khatamian, M.; Saket Oskoui, M.; Darbandi, M. Synthesis and characterization of aluminium-free ZSM-5 type chromosilicates in different alkaline systems and investigation of their pore structures. Microporous Mesoporous Mater. 2013, 182, 50–61. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Effect of alkali metal cations modification on the acid/basic properties and catalytic activity of ZSM-5 in cracking of supercritical n-dodecane. Fuel 2019, 243, 155–161. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Bajec, D.; Djinović, P.; Likozar, B. One-step synthesis of glycidol from glycerol in a gas-phase packed-bed continuous flow reactor over HZSM-5 zeolite catalysts modified by CsNO3. Chem. Eng. J. 2020, 394, 124945. [Google Scholar] [CrossRef]
- Ketkaew, M.; Klinyod, S.; Saenluang, K.; Rodaum, C.; Thivasasith, A.; Kidkhunthod, P.; Wattanakit, C. Fine-tuning the chemical state and acidity of ceria incorporated in hierarchical zeolites for ethanol dehydration. Chem. Commun. 2020, 56, 11394–11397. [Google Scholar] [CrossRef] [PubMed]
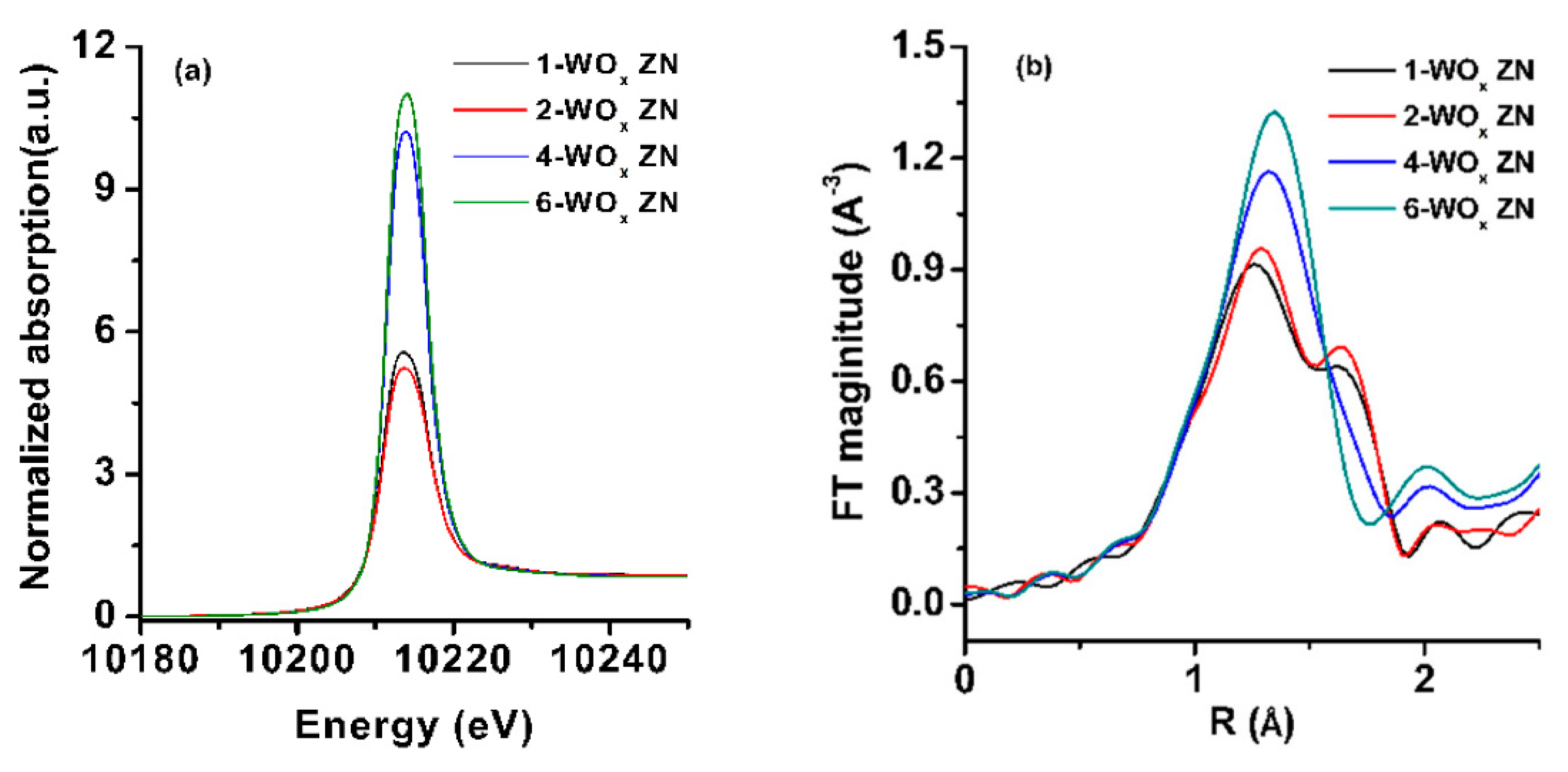
- Kim, H.; Numan, M.; Jo, C. Catalytic dehydration of ethanol over WOx nanoparticles supported on MFI (mobile five) zeolite nanosheets. Catalysts 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Vondrová, P.; Tišler, Z.; Kocík, J.; de Paz Carmona, H.; Murat, M. Comparison of doped ZSM-5 and ferrierite catalysts in the dehydration of bioethanol to ethylene in a flow reactor. React. Kinet. Mech. Catal. 2021, 132, 449–462. [Google Scholar] [CrossRef]
- Sarve, D.T.; Singh, S.K.; Ekhe, J.D. Kinetic and mechanistic study of ethanol dehydration to diethyl ether over Ni-ZSM-5 in a closed batch reactor. React. Kinet. Mech. Catal. 2020, 131, 261–281. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Struwe, K.; Lee, C.-H.; Chuang, H.-Y.; Sauer, J.; Yu, J.C.-C.; Nguyen, V.-H.; Huang, C.-W.; Wu, J.C.S. Ethanol conversion to selective high-value hydrocarbons over Ni/HZSM-5 zeolite catalyst. Catal. Commun. 2020, 144, 106067. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Tian, R.; Sun, C.; Dai, R.; Li, X.; Wu, X.; An, X.; Xie, X. Ni-hierarchical Beta zeolite catalysts were applied to ethanol steam reforming: Effect of sol gel method on loading Ni and the role of hierarchical structure. Mol. Catal. 2018, 453, 64–73. [Google Scholar] [CrossRef]
- Grzybek, G.; Greluk, M.; Tarach, K.; Pyra, K.; Słowik, G.; Rotko, M.; Góra-Marek, K. Bioethanol steam reforming over cobalt-containing USY and ZSM-5 commercial zeolite catalysts. Front. Mater. 2020, 7, 37–50. [Google Scholar] [CrossRef]
- da Costa-Serra, J.F.; Navarro, M.T.; Rey, F.; Chica, A. Sustainable production of hydrogen by steam reforming of ethanol using cobalt supported on nanoporous zeolitic material. Nanomaterials 2020, 10, 1934. [Google Scholar] [CrossRef]
- Narula, C.K.; Li, Z.; Casbeer, E.M.; Geiger, R.A.; Moses-Debusk, M.; Keller, M.; Buchanan, M.V.; Davison, B.H. Heterobimetallic zeolite, InV-ZSM-5, enables efficient conversion of biomass derived ethanol to renewable hydrocarbons. Sci. Rep. 2015, 5, 16039. [Google Scholar] [CrossRef] [Green Version]
- Said, S.; Aman, D.; Riad, M.; Mikhail, S. MoZn/AlPO4-5 zeolite: Preparation, structural characterization and catalytic dehydration of ethanol. J. Solid State Chem. 2020, 287, 121335. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I. Ag-promoted ZrBEA zeolites obtained by post-synthetic modification for conversion of ethanol to butadiene. ChemSusChem 2016, 9, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pang, J.; Wang, C.; Li, L.; Pan, X.; Zheng, M.; Zhang, T. Conversion of ethanol to 1,3-butadiene over high-performance Mg–ZrOx/MFI nanosheet catalysts via the two-step method. Green Chem. 2020, 22, 2852–2861. [Google Scholar] [CrossRef]
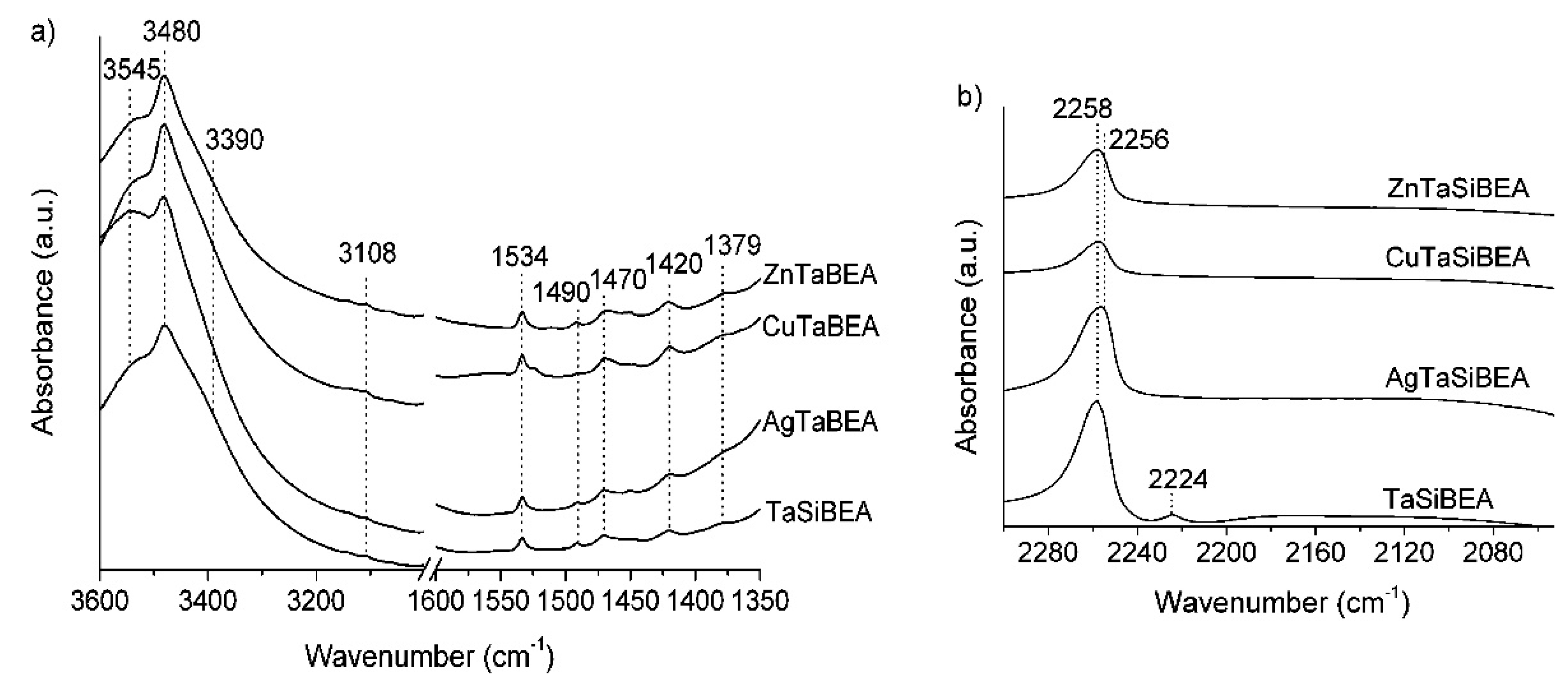
- Kyriienko, P.I.; Larina, O.V.; Soloviev, S.O.; Orlyk, S.M.; Calers, C.; Dzwigaj, S. Ethanol conversion into 1,3-butadiene by the Lebedev method over MTaSiBEA zeolites (M = Ag, Cu, Zn). ACS Sustain. Chem. Eng. 2017, 5, 2075–2083. [Google Scholar] [CrossRef] [Green Version]
- Kostyniuk, A.; Bajec, D.; Djinović, P.; Likozar, B. Allyl alcohol production by gas phase conversion reactions of glycerol over bifunctional hierarchical zeolite-supported bi- and tri-metallic catalysts. Chem. Eng. J. 2020, 397, 125430. [Google Scholar] [CrossRef]
- Karanwal, N.; Sibi, M.G.; Khan, M.K.; Myint, A.A.; Chan Ryu, B.; Kang, J.W.; Kim, J. Trimetallic Cu-Ni-Zn/H-ZSM-5 catalyst for the one-pot conversion of levulinic acid to high-yield 1,4-pentanediol under mild conditions in an aqueous medium. ACS Catal. 2021, 11, 2846–2864. [Google Scholar] [CrossRef]
- Li, J.; Xiao, G.; Guo, Z.; Lin, B.; Hu, Y.; Fu, M.; Ye, D. ZSM-5-supported V-Cu bimetallic oxide catalyst for remarkable catalytic oxidation of toluene in coal-fired flue gas. Chem. Eng. J. 2021, 419, 129675. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, J.; Su, Z.; Wang, P.; Tan, T.; Xiao, F.-S. Low-temperature dehydration of ethanol to ethylene over Cu–zeolite catalysts synthesized from Cu–tetraethylenepentamine. Ind. Eng. Chem. Res. 2020, 59, 17300–17306. [Google Scholar] [CrossRef]
- Dugkhuntod, P.; Maineawklang, N.; Saenluang, K.; Salakhum, S.; Rodaum, C.; Pornsetmetakul, P.; Wattanakit, C. Synthesis and characterization of Sn, Ge, and Zr isomorphous substituted MFI nanosheets for glucose isomerization to fructose. ChemPlusChem 2021, in press. [Google Scholar] [CrossRef]
- Makowski, W. Quasi-equilibrated temperature programmed desorption and adsorption: A new method for determination of the isosteric adsorption heat. Thermochim. Acta 2007, 454, 26–32. [Google Scholar] [CrossRef]
- Mlekodaj, K.; Tarach, K.; Datka, J.; Góra-Marek, K.; Makowski, W. Porosity and accessibility of acid sites in desilicated ZSM-5 zeolites studied using adsorption of probe molecules. Microporous Mesoporous Mater. 2014, 183, 54–61. [Google Scholar] [CrossRef]
- Soh, J.C.; Chong, S.L.; Hossain, S.S.; Cheng, C.K. Catalytic ethylene production from ethanol dehydration over non-modified and phosphoric acid modified Zeolite H-Y (80) catalysts. Fuel Process. Technol. 2017, 158, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.N.; Ruaux, V.; Massin, L.; Lorentz, C.; Afanasiev, P.; Maugé, F.; Bellière-Baca, V.; Rey, P.; Millet, J.M.M. Synthesis, characterization and study of lanthanum phosphates as light alcohols dehydration catalysts. Appl. Catal. B Environ. 2015, 166–167, 432–444. [Google Scholar] [CrossRef]
- Suttipat, D.; Saenluang, K.; Wannapakdee, W.; Dugkhuntod, P.; Ketkaew, M.; Pornsetmetakul, P.; Wattanakit, C. Fine-tuning the surface acidity of hierarchical zeolite composites for methanol-to-olefins (MTO) reaction. Fuel 2021, 286, 119306. [Google Scholar] [CrossRef]
- Damyanova, S.; Centeno, M.A.; Petrov, L.; Grange, P. Fourier transform infrared spectroscopic study of surface acidity by pyridine adsorption on Mo/ZrO2–SiO2(Al2O3) catalysts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 2495–2501. [Google Scholar] [CrossRef]
- Travert, A.; Vimont, A.; Sahibed-Dine, A.; Daturi, M.; Lavalley, J.-C. Use of pyridine CH(D) vibrations for the study of Lewis acidity of metal oxides. Appl. Catal. A Gen. 2006, 307, 98–107. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Tarach, K.; Choi, M. 2,6-Di-tert-butylpyridine sorption approach to quantify the external acidity in hierarchical zeolites. J. Phys. Chem. C 2014, 118, 12266–12274. [Google Scholar] [CrossRef]
- Sadowska, K.; Góra-Marek, K.; Datka, J. Accessibility of acid sites in hierarchical zeolites: Quantitative IR studies of pivalonitrile adsorption. J. Phys. Chem. C 2013, 117, 9237–9244. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Vimont, A.; Travert, A.; Ivanova, I.I. Spectroscopic evidence for open and closed Lewis acid sites in ZrBEA zeolites. J. Phys. Chem. C 2015, 119, 17633–17639. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2002; Volume 47, pp. 307–511. [Google Scholar]
- Huang, M.; Kaliaguine, S. Zeolite basicity characterized by pyrrole chemisorption: An infrared study. J. Chem. Soc. Faraday Trans. 1992, 88, 751–758. [Google Scholar] [CrossRef]
- Angelici, C.; Meirer, F.; van der Eerden, A.M.J.; Schaink, H.L.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J.M.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Ex situ and operando studies on the role of copper in Cu-promoted SiO2–MgO catalysts for the Lebedev ethanol-to-butadiene process. ACS Catal. 2015, 5, 6005–6015. [Google Scholar] [CrossRef]
- Reddy, G.R.; Balasubramanian, S.; Chennakesavulu, K. Zeolite encapsulated active metal composites and their photocatalytic studies for rhodamine-B, reactive red-198 and chloro-phenols. RSC Adv. 2015, 5, 81013–81023. [Google Scholar] [CrossRef]
- Hutson, N.D.; Reisner, B.A.; Yang, R.T.; Toby, B.H. Silver ion-exchanged zeolites Y, X, and low-silica X: Observations of thermally induced cation/cluster migration and the resulting effects on the equilibrium adsorption of nitrogen. Chem. Mater. 2000, 12, 3020–3031. [Google Scholar] [CrossRef]
- Ju, W.-S.; Matsuoka, M.; Iino, K.; Yamashita, H.; Anpo, M. The local structures of silver(I) ion catalysts anchored within zeolite cavities and their photocatalytic reactivities for the elimination of N2O into N2 and O2. J. Phys. Chem. B 2004, 108, 2128–2133. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Janas, J.; Mizera, J.; Gurgul, J.; Socha, R.P.; Che, M. Incorporation of copper in SiBEA zeolite as isolated lattice mononuclear Cu(II) species and its role in selective catalytic reduction of NO by ethanol. Catal. Lett. 2008, 126, 36–42. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.; Lu, J.; Feng, H. ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion. Phys. Chem. Chem. Phys. 2016, 18, 601–614. [Google Scholar] [CrossRef]
- Newalkar, B.L.; Olanrewaju, J.; Komarneni, S. Microwave-hydrothermal synthesis and characterization of zirconium substituted SBA-15 mesoporous silica. J. Phys. Chem. B 2001, 105, 8356–8360. [Google Scholar] [CrossRef]
- Tang, Y.; Zong, E.; Wan, H.; Xu, Z.; Zheng, S.; Zhu, D. Zirconia functionalized SBA-15 as effective adsorbent for phosphate removal. Microporous Mesoporous Mater. 2012, 155, 192–200. [Google Scholar] [CrossRef]
- Gao, X.; Fierro, J.L.G.; Wachs, I.E. Structural characteristics and catalytic properties of highly dispersed ZrO2/SiO2 and V2O5/ZrO2/SiO2 catalysts. Langmuir 1999, 15, 3169–3178. [Google Scholar] [CrossRef]
- Yamazoe, S.; Hitomi, Y.; Shishido, T.; Tanaka, T. XAFS study of tungsten L1- and L3-edges: Structural analysis of WO3 species loaded on TiO2 as a catalyst for photo-oxidation of NH3. J. Phys. Chem. C 2008, 112, 6869–6879. [Google Scholar] [CrossRef]
- Bi, J.; Guo, X.; Liu, M.; Wang, X. High effective dehydration of bio-ethanol into ethylene over nanoscale HZSM-5 zeolite catalysts. Catal. Today 2010, 149, 143–147. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, F.; Ludlow, D.K.; Rownaghi, A.A. Synthesis of SAPO-34@ZSM-5 and SAPO-34@silicalite-1 core–shell zeolite composites for ethanol dehydration. Ind. Eng. Chem. Res. 2018, 57, 1446–1453. [Google Scholar] [CrossRef]
- Ramasamy, K.K.; Wang, Y. Ethanol conversion to hydrocarbons on HZSM-5: Effect of reaction conditions and Si/Al ratio on the product distributions. Catal. Today 2014, 237, 89–99. [Google Scholar] [CrossRef]
- Pomalaza, G.; Arango Ponton, P.; Capron, M.; Dumeignil, F. Ethanol-to-butadiene: The reaction and its catalysts. Catal. Sci. Technol. 2020, 10, 4860–4911. [Google Scholar] [CrossRef]
- Kadam, S.A.; Shamzhy, M.V. IR Operando study of ethanol dehydration over MFI zeolite. Catal. Today 2018, 304, 51–57. [Google Scholar] [CrossRef]
- Kadam, S.A.; Shamzhy, M.V. IR Operando study of ethanol dehydration over MFI zeolites: Structure–activity relationships. J. Phys. Chem. C 2018, 122, 24055–24067. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Chu, Y.; Xu, J.; Wang, Q.; Qi, G.; Zhao, X.; Feng, N.; Deng, F. Observation of an oxonium ion intermediate in ethanol dehydration to ethene on zeolite. Nat. Commun. 2019, 10, 1961. [Google Scholar] [CrossRef]
- Van der Borght, K.; Galvita, V.V.; Marin, G.B. Ethanol to higher hydrocarbons over Ni, Ga, Fe-modified ZSM-5: Effect of metal content. Appl. Catal. A Gen. 2015, 492, 117–126. [Google Scholar] [CrossRef]
- Yan, T.; Dai, W.; Wu, G.; Lang, S.; Hunger, M.; Guan, N.; Li, L. Mechanistic insights into one-step catalytic conversion of ethanol to butadiene over bifunctional Zn-Y/beta zeolite. ACS Catal. 2018, 8, 2760–2773. [Google Scholar] [CrossRef]
- Maihom, T.; Khongpracha, P.; Sirijaraensre, J.; Limtrakul, J. Mechanistic studies on the transformation of ethanol into ethene over Fe-ZSM-5 zeolite. ChemPhysChem 2013, 14, 101–107. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iadrat, P.; Wattanakit, C. Bioethanol Upgrading to Renewable Monomers Using Hierarchical Zeolites: Catalyst Preparation, Characterization, and Catalytic Studies. Catalysts 2021, 11, 1162. https://doi.org/10.3390/catal11101162
Iadrat P, Wattanakit C. Bioethanol Upgrading to Renewable Monomers Using Hierarchical Zeolites: Catalyst Preparation, Characterization, and Catalytic Studies. Catalysts. 2021; 11(10):1162. https://doi.org/10.3390/catal11101162
Chicago/Turabian StyleIadrat, Ploychanok, and Chularat Wattanakit. 2021. "Bioethanol Upgrading to Renewable Monomers Using Hierarchical Zeolites: Catalyst Preparation, Characterization, and Catalytic Studies" Catalysts 11, no. 10: 1162. https://doi.org/10.3390/catal11101162
APA StyleIadrat, P., & Wattanakit, C. (2021). Bioethanol Upgrading to Renewable Monomers Using Hierarchical Zeolites: Catalyst Preparation, Characterization, and Catalytic Studies. Catalysts, 11(10), 1162. https://doi.org/10.3390/catal11101162





