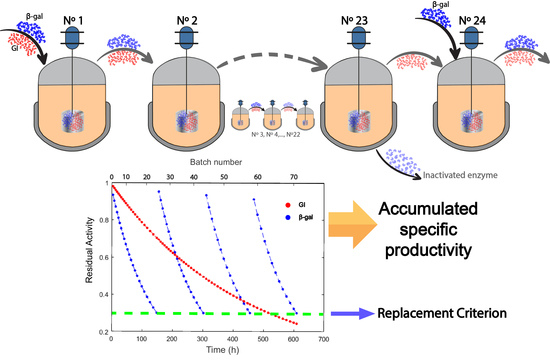
Catalyst Replacement Policy on Multienzymatic Systems: Theoretical Study in the One-Pot Sequential Batch Production of Lactofructose Syrup
Abstract
:1. Introduction
2. Results and Discussions
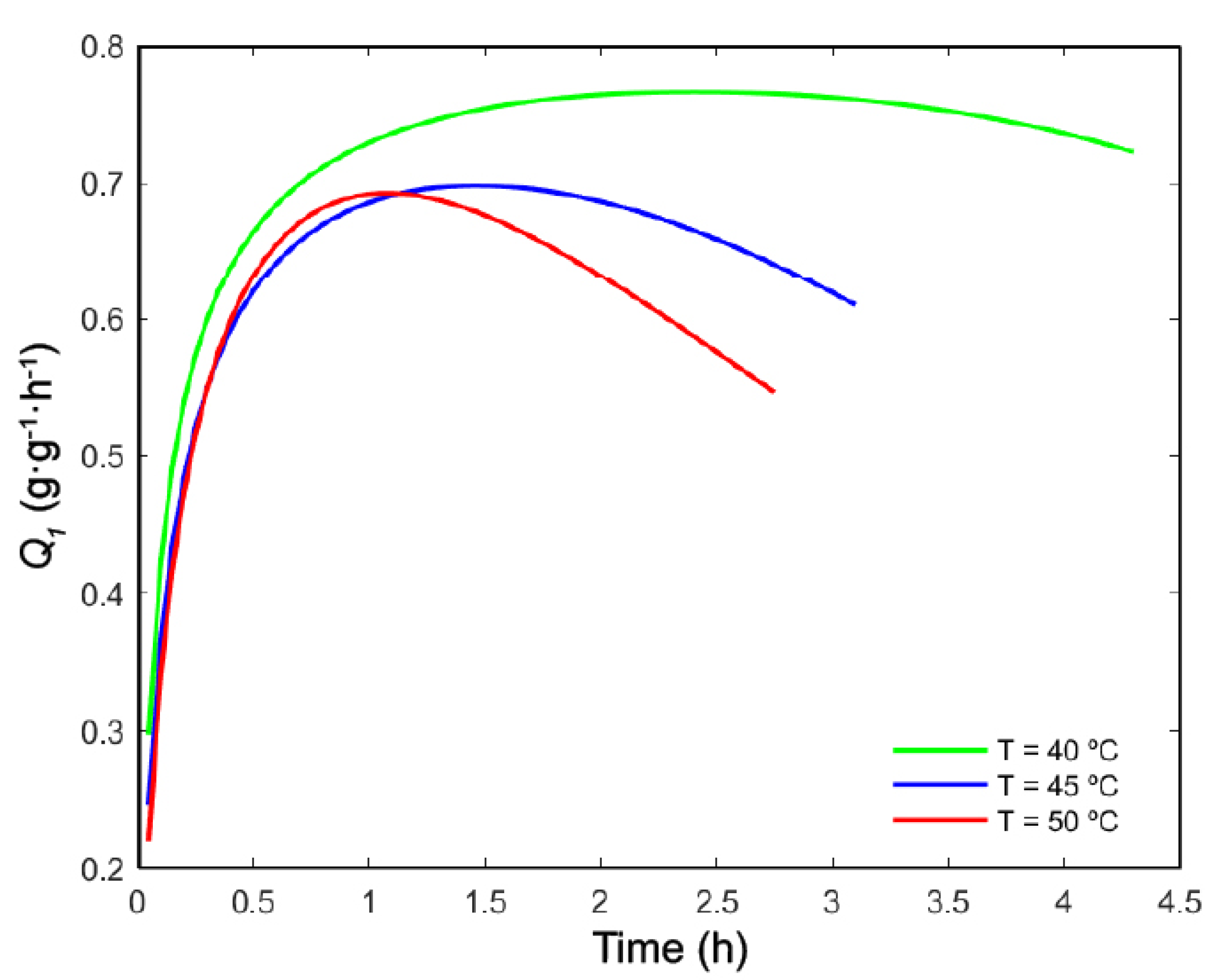
2.1. Selection of Operating Conditions
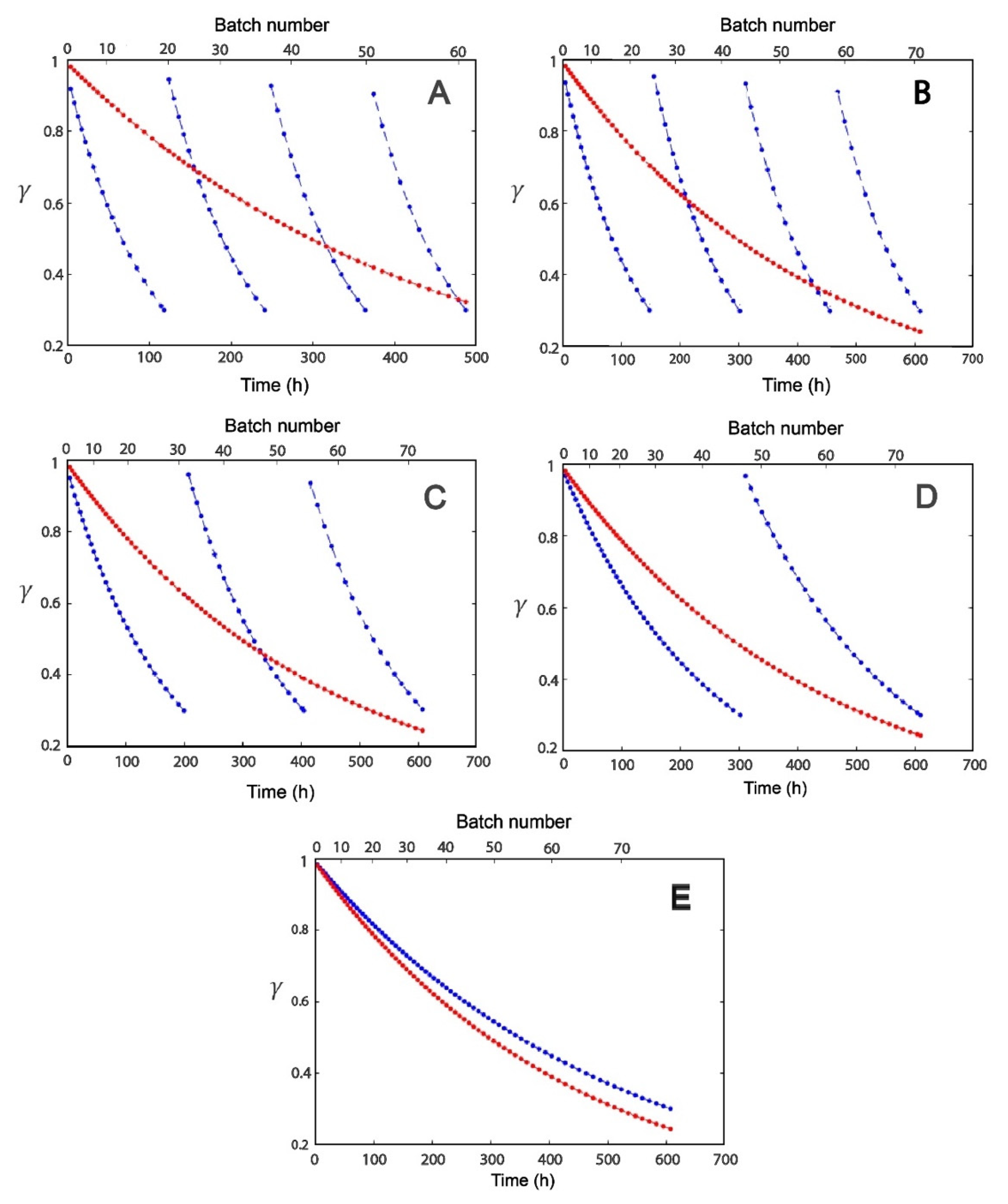
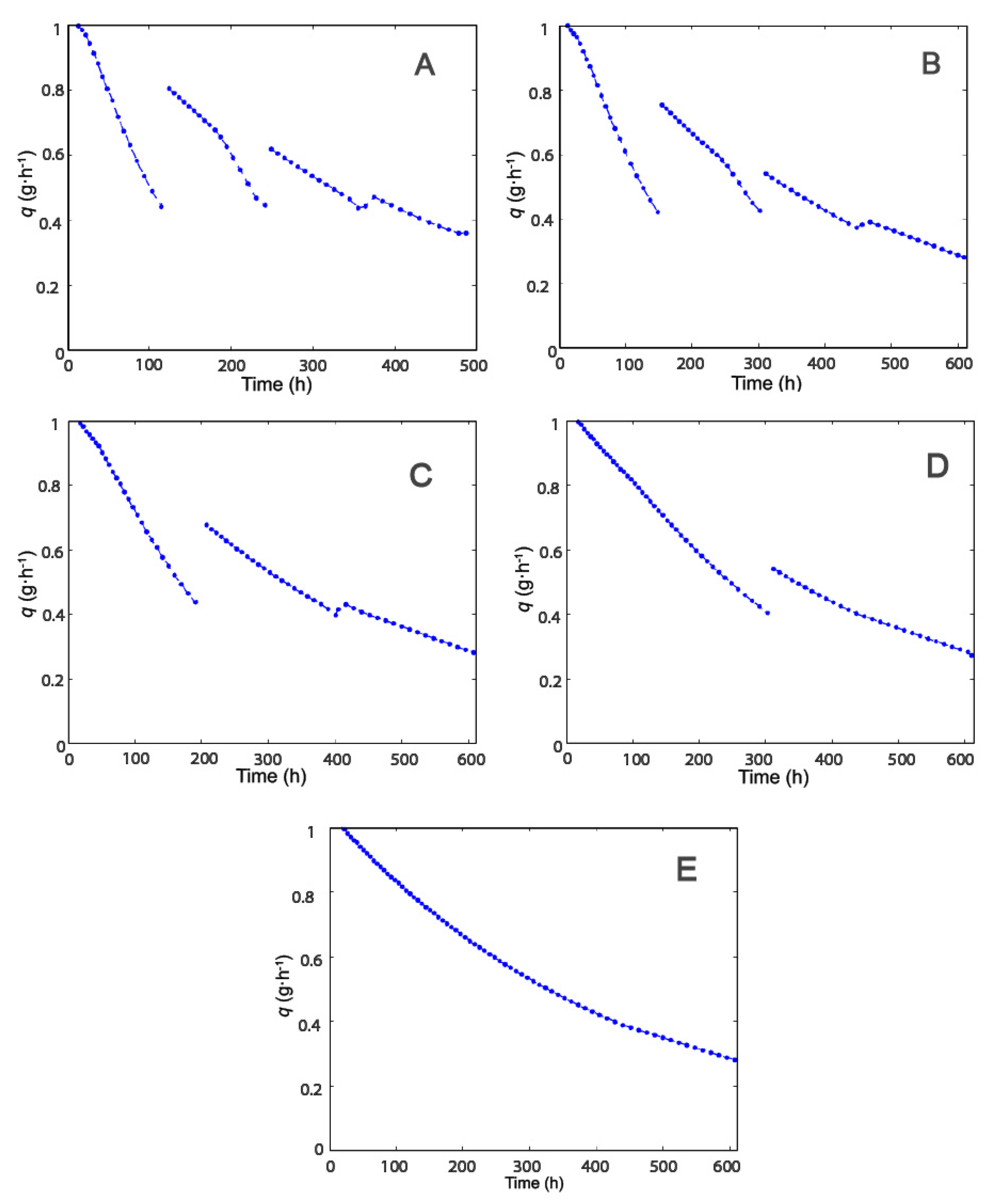
2.2. Effect of Modulation of β-gal Stability on the Production of Lactofructose Syrup in One-Pot Sequential Batch Operation
3. Materials and Methods
3.1. Materials
3.2. Modeling
3.2.1. Enzyme Kinetics
3.2.2. Enzyme Inactivation
3.2.3. Mathematical Model for the One-Pot Production of Lactofructose Syrup in Sequential Batch Operation
3.2.4. Reactor Operation Conditions
3.3. Metrics Used in Reactor Operations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| β-gal: | β-galactosidase |
| GI: | glucose isomerase |
| a1: | specific activity of β-gal (IU/g) |
| a2: | specific activity of GI (IU/g) |
| e1: | enzyme concentration of β-gal (IU/L) |
| e2: | enzyme concentration of GI (IU/L) |
| Ea: | activation energy (Kcal/mol) |
| Fru: | fructose concentration (M) |
| Frue: | equilibrium concentration of fructose (M) |
| Gal: | galactose concentration (M) |
| Glu: | glucose concentration (M) |
| Glu0: | initial concentration of glucose (M) |
| Glue: | equilibrium concentration of glucose (M) |
| ΔH°: | standard enthalpy change of dissociation of β-gal-lactose into β-gal and lactose (Kcal/mol) |
| ΔH°I: | standard enthalpy change of dissociation of β-gal-galactose into β-gal and galactose (Kcal/mol) |
| kcat1: | catalytic rate constant of β-gal (mol/(h·IU)) |
| kD1: | thermal decay constant of β-gal (h−1) |
| kD2: | thermal decay constant of glucose isomerase (h−1) |
| kD’: | thermal decay constant under operational conditions (h−1) |
| Ke: | equilibrium constant of GI |
| KI: | inhibition constant of β-gal by galactose (M) |
| Km1: | Michaelis-Menten constant for β-gal (M) |
| Km2: | apparent Michaelis-Menten constant of GI (M) |
| Kmf: | Michaelis-Menten constant for fructose (mol) |
| Kmg: | Michaelis-Menten constant for glucose (mol) |
| Lac: | lactose concentration (M) |
| Lac0: | initial concentration of lactose (M) |
| q: | productivity (g/h) |
| qa: | accumulated productivity of fructose (g/h) |
| Qj: | accumulated specific productivity of j catalyst (j =1: β-gal, j =2: GI) (g·g−1·h−1) |
| R: | universal gas constant (J/(mol·K)) |
| T: | temperature (K or °C) |
| t: | time (h) |
| η: | modulation factor |
| m1: | batch numbers in a use cycle of β-gal (−) |
| m2: | batch numbers in a use cycle of GI (−) |
| M1: | catalyst mass of β-gal (g) |
| M2: | catalyst mass of GI (g) |
| v1: | reaction rate of β-gal (mol/(g·h)) |
| v2: | reaction rate of GI (mol/(g·h)) |
| Vr: | reaction volume (mL) |
| Vm2: | maximum apparent reaction rate of glucose isomerization (mol/(g·h)) |
| Vmf: | maximum reaction rate for fructose isomerization into glucose (mol/(g·h)) |
| Vmg: | maximum reaction rate for glucose isomerization into fructose (mol/(g·h)) |
| xLac: | lactose conversion |
| γ1: | residual activity of β-gal |
| γ2: | residual activity of GI |
| MWF: | fructose molecular weight (g/mol) |
References
- Katsimpouras, C.; Stephanopoulos, G. Enzymes in biotechnology: Critical platform technologies for bioprocess development. Curr. Opin. Biotechnol. 2021, 69, 91–102. [Google Scholar] [CrossRef]
- Torres-León, C.; Chávez-González, M.L.; Hernández-Almanza, A.; Martínez-Medina, G.A.; Ramírez-Guzmán, N.; Londoño-Hernández, L.; Aguilar, C.N. Recent advances on the microbiological and enzymatic processing for conversion of food wastes to valuable bioproducts. Curr. Opin. Food Sci. 2021, 38, 40–45. [Google Scholar] [CrossRef]
- Suresh, A.; Shravan Ramgopal, D.; Panchamoorthy Gopinath, K.; Arun, J.; SundarRajan, P.; Bhatnagar, A. Recent advancements in the synthesis of novel thermostable biocatalysts and their applications in commercially important chemoenzymatic conversion processes. Bioresour. Technol. 2021, 323, 124558. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, B.; Tan, J.; Zhu, L.; Li, L. Immobilized multienzymatic systems for catalysis of cascade reactions. Process Biochem. 2016, 51, 1193–1203. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Streamlining design, engineering, and applications of enzymes for sustainable biocatalysis. ACS Sustain. Chem. Eng. 2021, 9, 8032–8052. [Google Scholar] [CrossRef]
- Santacoloma, P.A.; Sin, G.; Gernaey, K.V.; Woodley, J.M. Multienzyme-catalyzed processes: Next-generation biocatalysis. Org. Process Res. Dev. 2011, 15, 203–212. [Google Scholar] [CrossRef]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F. Wiring step-wise reactions with immobilized multi-enzyme systems. Biocatal. Biotransform. 2018, 36, 184–194. [Google Scholar] [CrossRef]
- Torres, P.; Batista-Viera, F. Immobilized trienzymatic system with enhanced stabilization for the biotransformation of lactose. Molecules 2017, 22, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Dubey, N.C.; Tripathi, B.P. Nature inspired multienzyme immobilization: Strategies and concepts. ACS Appl. Bio Mater. 2021, 4, 1077–1114. [Google Scholar] [CrossRef]
- Riva, S.; Fessner, W.D. Cascade Biocatalysis: Integrating Stereoselective and Environmentally Friendly Reactions; John Wiley & Sons: Weinheim, Germany, 2014; pp. 1–465. ISBN 9783527335220. [Google Scholar]
- Xue, R.; Woodley, J.M. Process technology for multi-enzymatic reaction systems. Bioresour. Technol. 2012, 115, 183–195. [Google Scholar] [CrossRef]
- Wang, Z.; Sundara Sekar, B.; Li, Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals. Bioresour. Technol. 2021, 323, 124551. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Corberan, V.C.; Fernandez-Lafuente, R. Multi-combilipases: Co-immobilizing lipases with very different stabilities combining immobilization via interfacial activation and ion exchange. The reuse of the most stable co-immobilized enzymes after inactivation of the least stable ones. Catalysts 2020, 10, 1207. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Ottone, C.; Romero, O. Co-immobilized carrier-free enzymes for lactose upgrading. Curr. Opin. Green Sustain. Chem. 2021, in press. [Google Scholar]
- Illanes, A. Enzyme Biocatalysis: Principles and Applications; Springer: Dordrecht, The Netherlands, 2008; pp. 1–391. [Google Scholar] [CrossRef]
- Illanes, A.; Wilson, L.; Raiman, L. Design of immobilized enzyme reactors for the continuous production of fructose syrup from whey permeate. Bioprocess Eng. 1999, 21, 509–515. [Google Scholar] [CrossRef]
- Velasco-Lozano, S. Immobilization of enzymes as cross-linked enzyme aggregates: General strategy to obtain robust biocatalysts. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2100, pp. 345–361. [Google Scholar]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2021, 51, 107584. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; López-Gallego, F. Characterization and evaluation of immobilized enzymes for applications in flow reactors. Curr. Opin. Green Sustain. Chem. 2020, 25, 100349. [Google Scholar] [CrossRef]
- Adloor, S.D.; Pons, T.; Vassiliadis, V.S. An optimal control approach to scheduling and production in a process using decaying catalysts. Comput. Chem. Eng. 2020, 135, 106743. [Google Scholar] [CrossRef]
- Shafi, A.; Ahmed, F.; Husain, Q. β-Galactosidase mediated synthesized nanosupport for the immobilization of same enzyme: Its stability and application in the hydrolysis of lactose. Int. J. Biol. Macromol. 2021, 184, 57–67. [Google Scholar] [CrossRef]
- Neifar, S.; Cervantes, F.V.; Bouanane-Darenfed, A.; BenHlima, H.; Ballesteros, A.O.; Plou, F.J.; Bejar, S. Immobilization of the glucose isomerase from Caldicoprobacter algeriensis on Sepabeads EC-HA and its efficient application in continuous High Fructose Syrup production using packed bed reactor. Food Chem. 2020, 309, 125710. [Google Scholar] [CrossRef]
- Guerrero, C.; Súarez, S.; Aburto, C.; Ubilla, C.; Ramírez, N.; Vera, C.; Illanes, A. Comparison of batch and repeated batch operation of lactulose synthesis with cross-linked aggregates of Bacillus circulans β-galactosidase. Process Biochem. 2020, 94, 224–234. [Google Scholar] [CrossRef]
- Jia, D.X.; Zhou, L.; Zheng, Y.G. Properties of a novel thermostable glucose isomerase mined from Thermus oshimai and its application to preparation of high fructose corn syrup. Enzym. Microb. Technol. 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Guerrero, C.; Vera, C.; Illanes, A. Optimisation of synthesis of oligosaccharides derived from lactulose (fructosyl-galacto-oligosaccharides) with β-galactosidases of different origin. Food Chem. 2013, 138, 2225–2232. [Google Scholar] [CrossRef]
- Vera, C.; Guerrero, C.; Illanes, A.; Conejeros, R. A pseudo steady-state model for galacto-oligosaccharides synthesis with β-galactosidase from Aspergillus oryzae. Biotechnol. Bioeng. 2011, 108, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Guerrero, C.; Illanes, A. Determination of the transgalactosylation activity of Aspergillus oryzae β-galactosidase: Effect of pH, temperature, and galactose and glucose concentrations. Carbohydr. Res. 2011, 346, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hartley, B.S.; Hanlon, N.; Jackson, R.J.; Rangarajan, M. Glucose isomerase: Insights into protein engineering for increased thermostability. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1543, 294–335. [Google Scholar] [CrossRef]
- Converti, A.; Del Borghi, M. Simultaneous effects of immobilization and substrate protection on the thermodynamics of glucose isomerase activity and inactivation. Enzym. Microb. Technol. 1997, 21, 511–517. [Google Scholar] [CrossRef]
- Amaral-Fonseca, M.; Morellon-Sterling, R.; Fernández-Lafuente, R.; Tardioli, P.W. Optimization of simultaneous saccharification and isomerization of dextrin to high fructose syrup using a mixture of immobilized amyloglucosidase and glucose isomerase. Catal. Today 2021, 362, 175–183. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Breiter, J.; Clawin-Rädecker, I.; Dau, A. A novel bi-enzymatic system for lactose conversion. Int. J. Food Sci. Technol. 2013, 48, 1396–1403. [Google Scholar] [CrossRef]
- Motiei, M.; Mirahmadi-Zare, S.Z.; Nasr-Esfahani, M.H. Chemical stabilization of γ-polyglutamate by chitosan and the effect of co-solvents on the stability. Biophys. Chem. 2021, 275, 106605. [Google Scholar] [CrossRef] [PubMed]
- Piszkiewicz, S.; Pielak, G.J. Protecting enzymes from stress-induced inactivation. Biochemistry 2019, 58, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Gupta, N.; Datta, S. Using the b-glucosidase catalyzed reaction product glucose to improve the ionic liquid tolerance of Β-glucosidases. Biotechnol. Biofuels 2016, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A.; Wilson, L.; Tomasello, G. Temperature optimization for reactor operation with chitin-immobilized lactase under modulated inactivation. Enzym. Microb. Technol. 2000, 27, 270–278. [Google Scholar] [CrossRef]
- Prestrelski, S.J.; Tedeschi, N.; Arakawa, T.; Carpenter, J.F. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys. J. 1993, 65, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.Q.; Shi, Y.; Wu, X.B.; Zhan, X.L.; Zhou, H.T.; Chen, Q.X. Heat inactivation kinetics of Hypocrea orientalis β-glucosidase with enhanced thermal stability by glucose. Int. J. Biol. Macromol. 2015, 81, 1012–1018. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A.; Wilson, L.; Tomasello, G. Effect of modulation of enzyme inactivation on temperature optimization for reactor operation with chitin-immobilized lactase. J. Mol. Catal.-B Enzym. 2001, 11, 531–540. [Google Scholar] [CrossRef]
- Chen, K.C.; Wu, J.Y. Substrate protection of immobilized glucose isomerase. Biotechnol. Bioeng. 1987, 30, 817–824. [Google Scholar] [CrossRef]
- Villaume, I.; Thomas, D.; Legoy, M.D. Catalysis may increase the stability of an enzyme: The example of horse liver alcohol dehydrogenase. Enzym. Microb. Technol. 1990, 12, 506–509. [Google Scholar] [CrossRef]
- Illanes, A.; Altamirano, C.; Zuñiga, M.E. Thermal inactivation of immobilized penicillin acylase in the presence of substrate and products. Biotechnol. Bioeng. 1996, 50, 609–616. [Google Scholar] [CrossRef]
- Faqir, N.M.; Abu-Reesh, I.M. Optimum temperature operation mode for glucose isomerase reactor operating at constant glucose conversion. Bioprocess Eng. 1998, 19, 11–17. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.B.; Ryu, D.D.Y. Optimization of operating temperature for continuous glucose isomerase reactor system. Biotechnol. Bioeng. 1981, 23, 1237–1254. [Google Scholar] [CrossRef]
- Mateo, C.; Bolivar, J.M.; Godoy, C.A.; Rocha-Martin, J.; Pessela, B.C.; Curiel, J.A.; Muñoz, R.; Guisan, J.M.; Fernández-Lorente, G. Improvement of enzyme properties with a two-step immobilizaton process on novel heterofunctional supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, C.; Vera, C.; Serna, N.; Illanes, A. Immobilization of Aspergillus oryzae β-galactosidase in an agarose matrix functionalized by four different methods and application to the synthesis of lactulose. Bioresour. Technol. 2017, 232, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.M.; Tehrany, M.S.; Safari, I. Kinetics of glucose isomerization to fructose by immobilized glucose isomerase (Sweetzyme IT). Ind. Eng. Chem. Res. 2009, 48, 3271–3278. [Google Scholar] [CrossRef]
- Ospina, S.S.; Lopez-Munguia, A.; Gonzalez, R.L.; Quintero, R. Characterization and use of a penicillin acylase biocatalyst. J. Chem. Technol. Biotechnol. 1992, 53, 205–213. [Google Scholar] [CrossRef] [PubMed]



| Enzyme | Parameter | Value | Unit | Reference |
|---|---|---|---|---|
| β-gal | 6.74 | mol/(h·IU) | [29,30] | |
| 7.02 | kcal/mol | |||
| 1.01 × 107 | M | |||
| 7.21 | kcal/mol | |||
| 1.32 × 102 | M | |||
| 5.52 | kcal/mol | |||
| GI | 1.46 × 1015 | mol/(h·IU) | [49] | |
| 29.5 | kcal/mol | |||
| 1.35 × 1021 | M | |||
| 32.9 | kcal/mol | |||
| 5.27 × 1013 | M | |||
| 21.2 | kcal/mol | |||
| 4.10 × 104 | - | |||
| 7.03 | kcal/mol |
| Parameters | β-gal | GI |
|---|---|---|
| (Kcal/mol) | 73.46 | 34.83 |
| (h−1) | 1.64 × 1049 | 5.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.; Arancibia, V.; Cid, D.; Romero, O.; Illanes, A.; Wilson, L. Catalyst Replacement Policy on Multienzymatic Systems: Theoretical Study in the One-Pot Sequential Batch Production of Lactofructose Syrup. Catalysts 2021, 11, 1167. https://doi.org/10.3390/catal11101167
Silva P, Arancibia V, Cid D, Romero O, Illanes A, Wilson L. Catalyst Replacement Policy on Multienzymatic Systems: Theoretical Study in the One-Pot Sequential Batch Production of Lactofructose Syrup. Catalysts. 2021; 11(10):1167. https://doi.org/10.3390/catal11101167
Chicago/Turabian StyleSilva, Pablo, Vanessa Arancibia, Daniela Cid, Oscar Romero, Andrés Illanes, and Lorena Wilson. 2021. "Catalyst Replacement Policy on Multienzymatic Systems: Theoretical Study in the One-Pot Sequential Batch Production of Lactofructose Syrup" Catalysts 11, no. 10: 1167. https://doi.org/10.3390/catal11101167
APA StyleSilva, P., Arancibia, V., Cid, D., Romero, O., Illanes, A., & Wilson, L. (2021). Catalyst Replacement Policy on Multienzymatic Systems: Theoretical Study in the One-Pot Sequential Batch Production of Lactofructose Syrup. Catalysts, 11(10), 1167. https://doi.org/10.3390/catal11101167