Insights into the Nature of the Active Sites of Pt-WOx/Al2O3 Catalysts for Glycerol Hydrogenolysis into 1,3-Propanediol
Abstract
:1. Introduction
2. Results
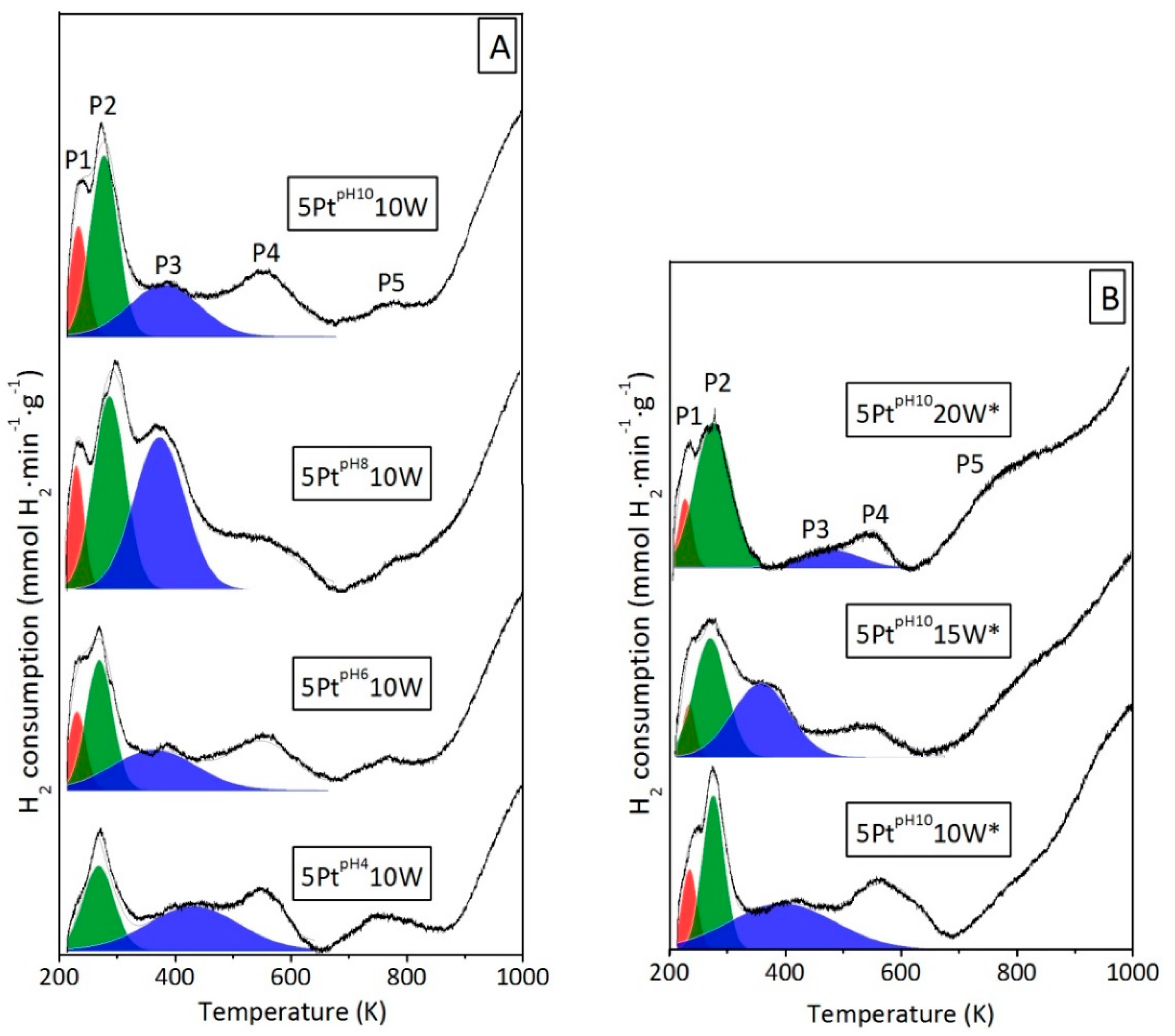
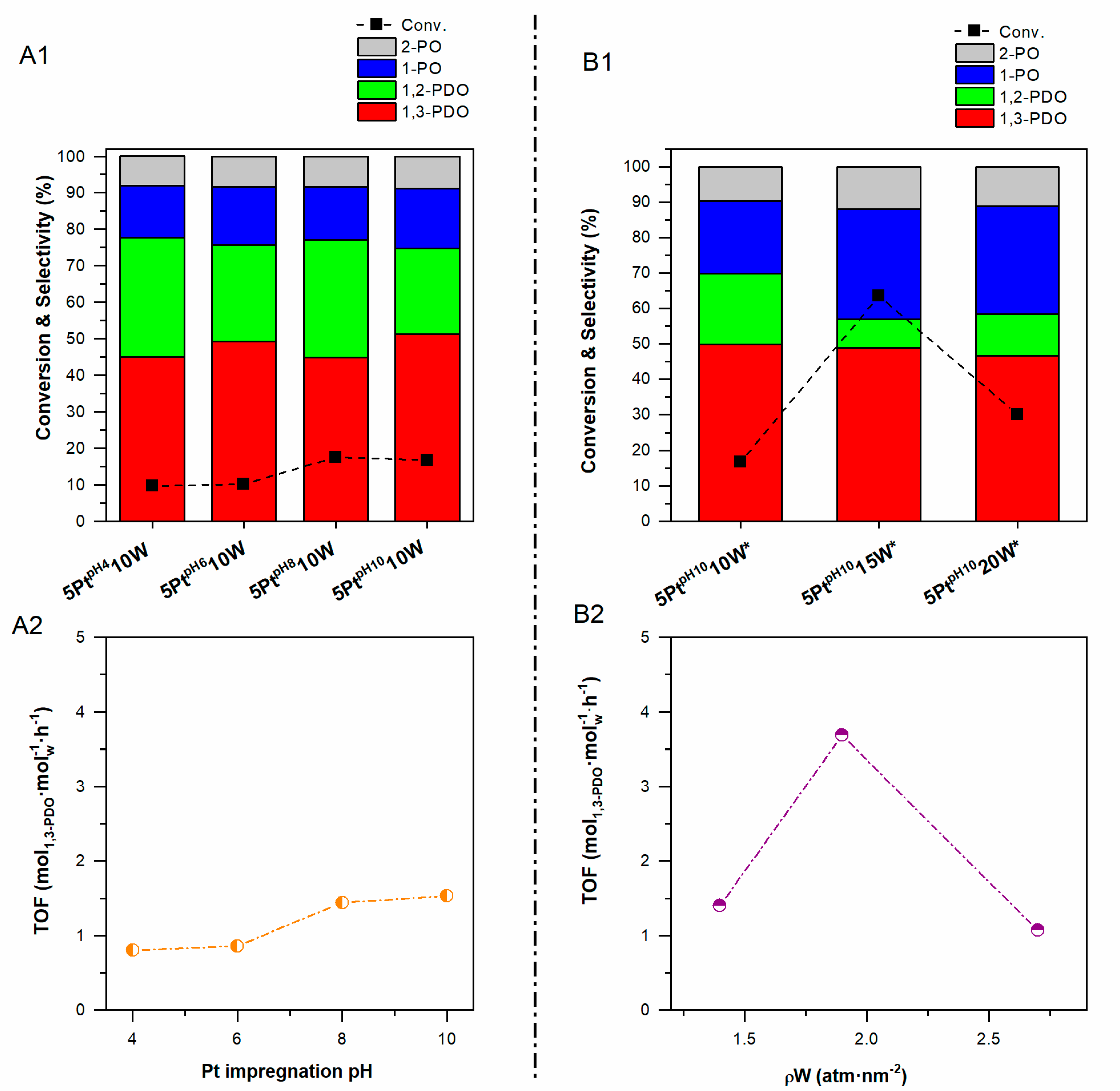
2.1. Effect of the pH Value of the Platinum Impregnation Solution
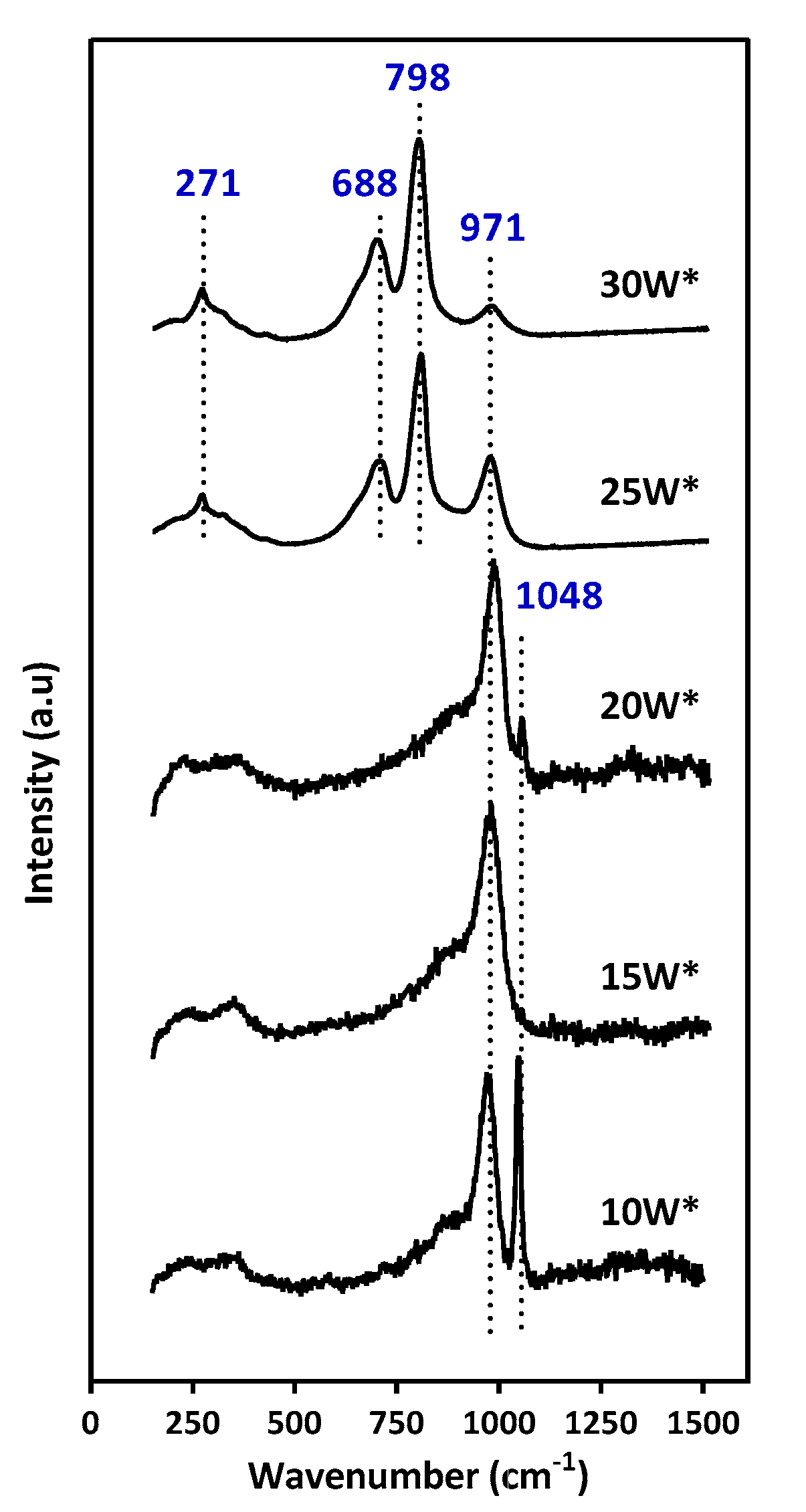
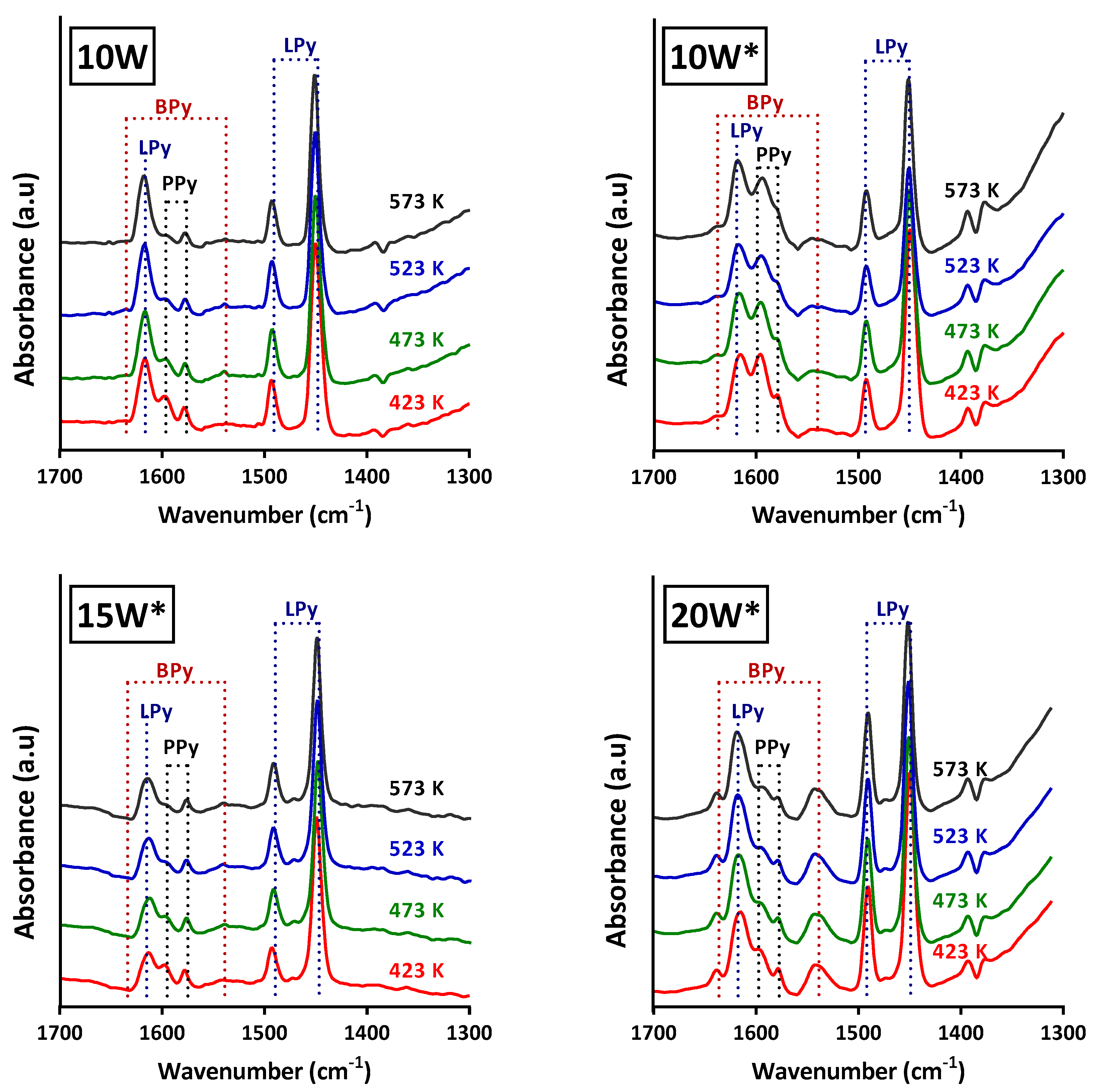
2.2. Effect of the pH Value of the Tungsten Impregnation Solution
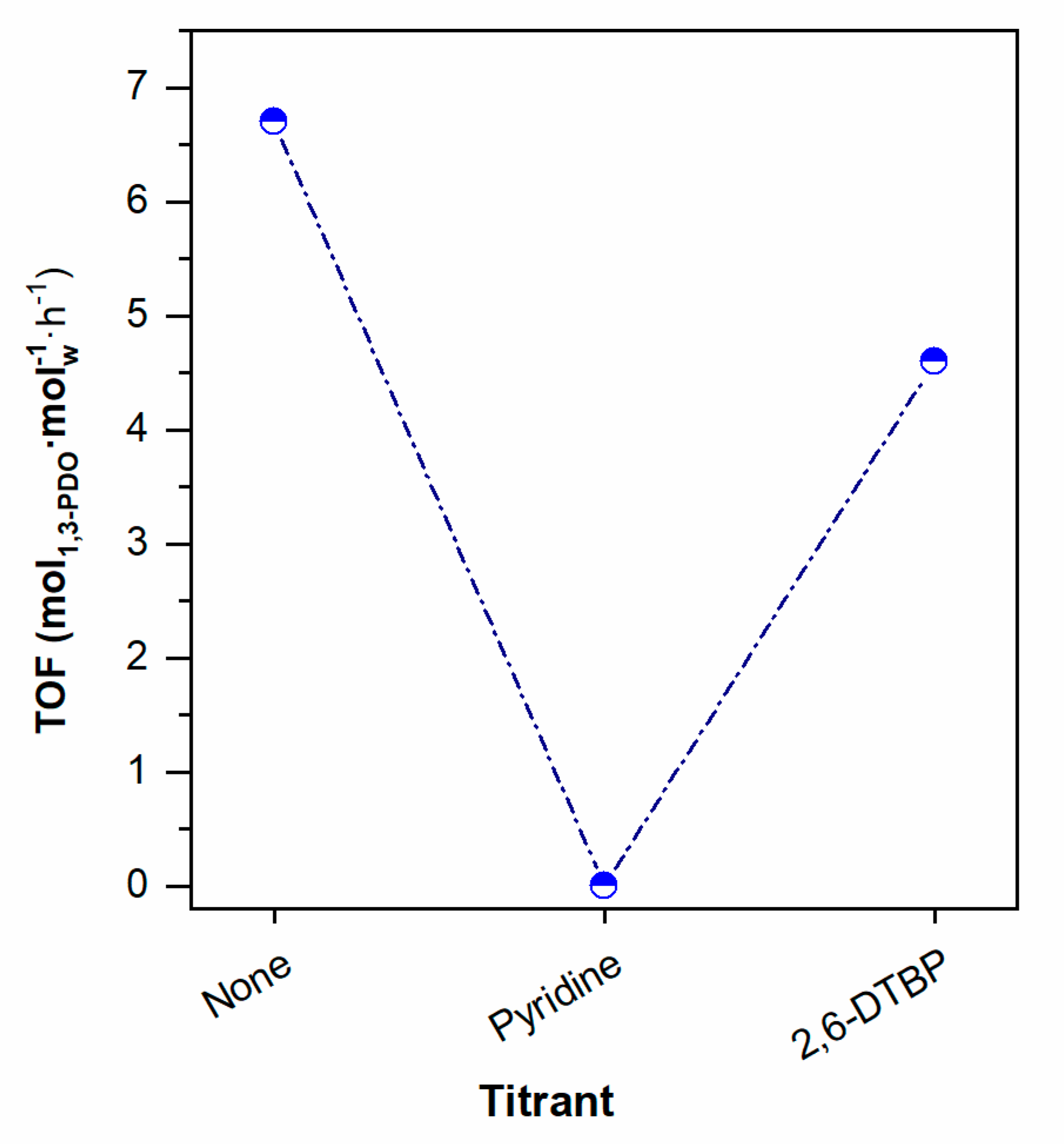
2.3. Effect of Pt Site Availability
2.4. Effect of the Alumina Surface Coverage
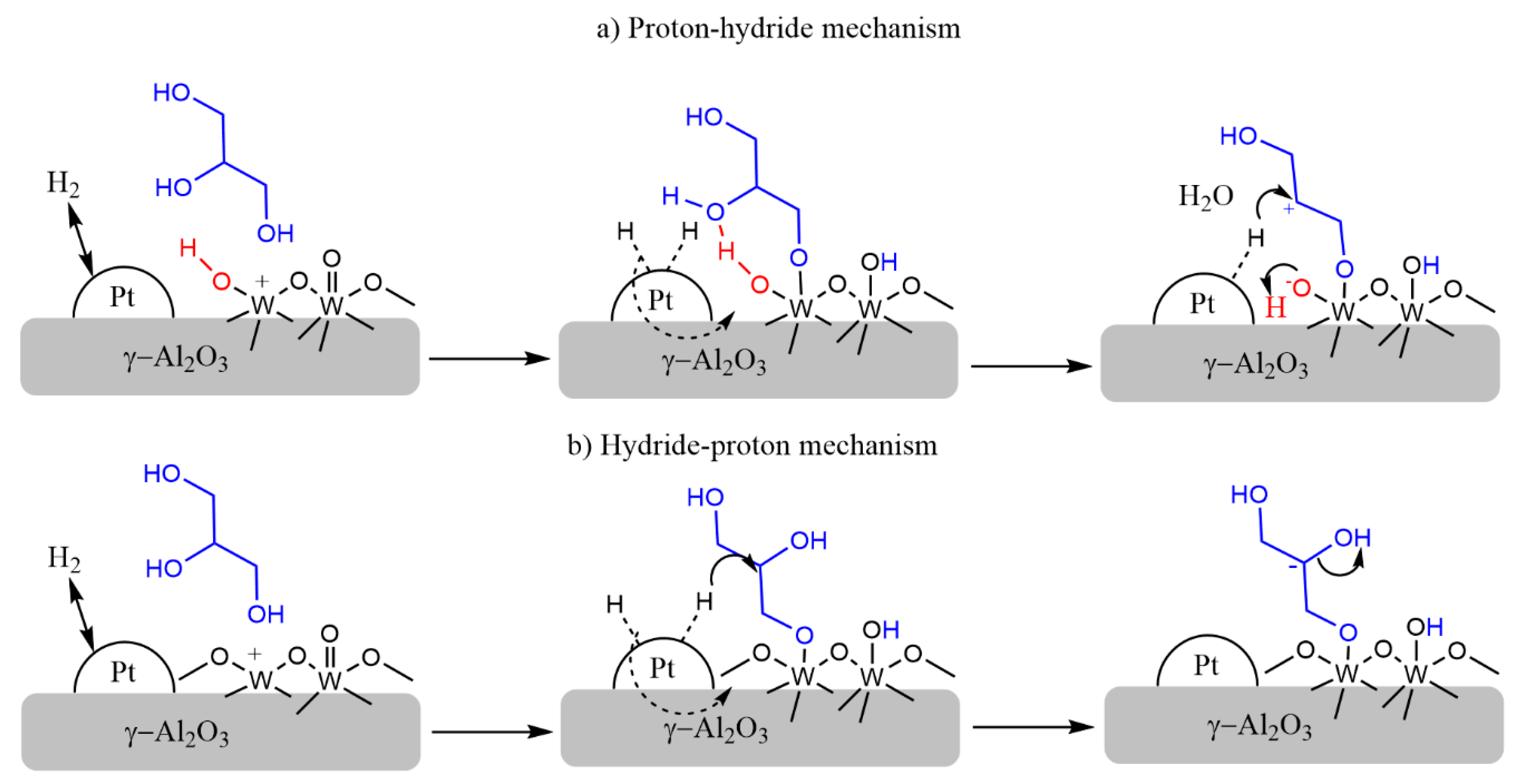
3. Discussion
4. Materials and Methods
4.1. Catalyst Synthesis
4.2. Catalyst Characterization
4.2.1. Chemical Analysis
4.2.2. N2 Physisorption
4.2.3. CO Chemisorption
4.2.4. Raman Spectroscopy
4.2.5. XPS
4.2.6. NH3-TPD
4.2.7. FTIR of Adsorbed Pyridine
4.3. Activity Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis goes bio: From carbohydrates and sugar alcohols to platform chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef]
- Chen, K.; Koso, S.; Kubota, T.; Nakagawa, Y.; Tomishige, K. Chemoselective Hydrogenolysis of Tetrahydropyran-2-methanol to 1,6-Hexanediol over Rhenium-Modified Carbon-Supported Rhodium Catalysts. ChemCatChem 2010, 2, 547–555. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, X.; Ren, J.; Wang, Y.; Lu, G. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst. Chem. Commun. 2011, 47, 3924–3926. [Google Scholar] [CrossRef]
- Soghrati, E.; Kok Poh, C.; Du, Y.; Gao, F.; Kawi, S.; Borgna, A. C–O Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol Over Bi-functional Nickel-Tungsten Catalysts. ChemCatChem 2018, 10, 4652–4664. [Google Scholar] [CrossRef]
- García-Fernández, S.; Gandarias, I.; Requies, J.; Güemez, M.B.; Bennici, S.; Auroux, A.; Arias, P.L. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1,3-propanediol. J. Catal. 2015, 323, 65–75. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Omar, L.; Perret, N.; Daniele, S. Self-Assembled Hybrid ZnO Nanostructures as Supports for Copper-Based Catalysts in the Hydrogenolysis of Glycerol. Catalysts 2021, 11, 516. [Google Scholar] [CrossRef]
- Gandarias, I.; Requies, J.; Arias, P.L.; Armbruster, U.; Martin, A. Liquid-phase glycerol hydrogenolysis by formic acid over Ni-Cu/Al2O3 catalysts. J. Catal. 2012, 290, 79–89. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Wang, Y.; Cheng, D. Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol. Catalysts 2021, 11, 232. [Google Scholar] [CrossRef]
- Chia, M.; Pagán-Torres, Y.J.; Hibbitts, D.; Tan, Q.; Pham, H.N.; Datye, A.K.; Neurock, M.; Davis, R.J.; Dumesic, J.A. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts. J. Am. Chem. Soc. 2011, 133, 12675–12689. [Google Scholar] [CrossRef]
- Arundhathi, R.; Mizugaki, T.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Highly selective hydrogenolysis of glycerol to 1,3-propanediol over a boehmite-supported platinum/tungsten catalyst. ChemSusChem 2013, 6, 1345–1347. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, Y.; Liu, L.; He, Q.; Dong, J.-X. Facilitating Pt–WOx Species Interaction for Efficient Glycerol Hydrogenolysis to 1,3-Propanediol. ChemCatChem 2021, 13, 3695–3705. [Google Scholar] [CrossRef]
- Xi, Z.; Hong, Z.; Huang, F.; Zhu, Z.; Jia, W.; Li, J. Hydrogenolysis of Glycerol on the ZrO2-TiO2 Supported Pt-WOx Catalyst. Catalysts 2020, 10, 312. [Google Scholar] [CrossRef] [Green Version]
- Aihara, T.; Kobayashi, H.; Feng, S.; Miura, H.; Shishido, T. Effect of WO3 loading on the activity of Pt/WO3/Al2O3 catalysts in selective hydrogenolysis of glycerol to 1,3-propanediol. Chem. Lett. 2017, 46, 1497–1500. [Google Scholar] [CrossRef]
- García-Fernández, S.; Gandarias, I.; Tejido-Núñez, Y.; Requies, J.; Arias, P.L. Influence of the Support of Bimetallic Platinum Tungstate Catalysts on 1,3-Propanediol Formation from Glycerol. ChemCatChem 2017, 9, 4508–4519. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Zhao, Y.; Wang, S.; Ma, X. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B Environ. 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Cui, J.; Zheng, H.; Li, Y. SiO2 promoted Pt/WOx/ZrO2 catalysts for the selective hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B Environ. 2014, 158–159, 391–399. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Li, Y. Chemical Promoting effect of WOx on selective hydrogenolysis of glycerol to 1,3-propanediol over bifunctional Pt–WOx/Al2O3 catalysts. J. Mol. Catal. A Chem. 2015, 398, 391–398. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, C. Hydrogenolysis of glycerol to 1,3-propanediol over Li2B4O7-modified tungsten–zirconium composite oxides supported platinum catalyst. React. Kinet. Mech. Catal. 2018, 124, 683–699. [Google Scholar] [CrossRef]
- Barton, D.; Soled, S.L.; Meitzner, G.D.; Fuentes, G.A.; Iglesia, E. Structural and Catalytic Characterization of Solid Acids Based on Zirconia Modified by Tungsten Oxide. J. Catal. 1999, 181, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z. Tuning Structural Properties of WO3 Thin Films for Photoelectrocatalytic Water Oxidation. Catalysts 2021, 11, 381. [Google Scholar] [CrossRef]
- Jelinska, A.; Bienkowski, K.; Jadwiszczak, M.; Pisarek, M.; Strawski, M.; Kurzydlowski, D.; Solarska, R.; Augustynski, J. Enhanced Photocatalytic Water Splitting on Very Thin WO3 Films Activated by High-Temperature Annealing. ACS Catal. 2018, 8, 10573–10580. [Google Scholar] [CrossRef]
- Brunelle, J.P. Preparation of Catalysts by Adsorption of Metal Complexes on Mineral Oxides. Pure Appl. Chem. 1979, 3, 211–232. [Google Scholar]
- Mulcahy, F.M.; Houalla, M.; Hercules, D.M. The effect of the isoelectric point on the adsorption of molybdates on fluoride-modified aluminas. J. Catal. 1987, 106, 210–215. [Google Scholar] [CrossRef]
- Kohler, S.D.; Ekerdt, J.G.; Kim, D.S.; Wachs, I.E. Relationship between structure and point of zero surface charge for molybdenum and tungsten oxides supported on alumina. Catal. Lett. 1992, 16, 231–239. [Google Scholar] [CrossRef]
- Hao, X.; Quach, L.; Korah, J.; Spieker, W.A.; Regalbuto, J.R. The control of platinum impregnation by PZC alteration of oxides and carbon. J. Mol. Catal. A Chem. 2004, 219, 97–107. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Otto, K. Identification of platinum phases on γ-alumina by XPS. Appl. Surf. Sci. 1988, 32, 246–252. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Weng, D.; Liu, S.; Si, Z.; Fan, J. Total oxidation of propane on Pt/WOx/Al2O3 catalysts by formation of metastable Pt+ species interacted with WOx clusters. J. Hazard. Mater. 2012, 225, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Vermaire, D.C.; van Berge, P.C. The preparation of WO3TiO2 and WO3Al2O3 and characterization by temperature-programmed reduction. J. Catal. 1989, 116, 309–317. [Google Scholar] [CrossRef]
- Cruz, J.; Avalos-Borja, M.; López Cordero, R.; Bañares, M.A.; Fierro, J.L.G.; Palacios, J.M.; López Agudo, A. Influence of pH of the impregnation solution on the phosphorus promotion in W/Al2O3 hydrotreating catalysts. Appl. Catal. A Gen. 2002, 224, 97–110. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Barton, D.G.; Shtein, M.; Wilson, R.D.; Soled, S.L.; Iglesia, E. Structure and Electronic Properties of Solid Acids Based on Tungsten Oxide Nanostructures. J. Phys. Chem. B 1999, 103, 630–640. [Google Scholar] [CrossRef]
- Wachs, I.E.; Kim, T.; Ross, E.I. Catalysis science of the solid acidity of model supported tungsten oxide catalysts. Catal. Today 2006, 116, 162–168. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.-W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on g-Al2O3. Science 2009, 325, 1670–1674. [Google Scholar] [CrossRef]
- Knözinger, H. Infrared Spectroscopy for the Characterization of Surface Acidity and Basicity. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Aihara, T.; Miura, H.; Shishido, T. Effect of perimeter interface length between 2D WO3 monolayer domain and γ-Al2O3 on selective hydrogenolysis of glycerol to 1,3-propanediol. Catal. Sci. Technol. 2019, 9, 5359–5367. [Google Scholar] [CrossRef]
- Amada, Y.; Shinmi, Y.; Koso, S.; Kubota, T.; Nakagawa, Y.; Tomishige, K. Reaction mechanism of the glycerol hydrogenolysis to 1,3-propanediol over Ir-ReOx/SiO2 catalyst. Appl. Catal. B Environ. 2011, 105, 117–127. [Google Scholar] [CrossRef]

| Entry | Catalyst/ γ-Al2O3 | W (wt%) 1 | Pt (wt%) 1 | Pt Dispersion (%) 2 | Pt Size (nm) 2 |
|---|---|---|---|---|---|
| 1 | 5PtpH410W | 9.0 | 3.5 | 30.8 | 3.0 |
| 2 | 5PtpH610W | 9.2 | 4.3 | 25.1 | 3.8 |
| 3 | 5PtpH810W | 9.1 | 4.3 | 25.9 | 3.6 |
| 4 | 5PtpH1010W | 9.3 | 4.3 | 30.5 | 3.1 |
| 5 | 5PtpH1010W * | 9.9 | 4.6 | 29.4 | 3.2 |
| 6 | 5PtpH1015W * | 14.6 | 4.9 | 21.2 | 4.4 |
| 7 | 5PtpH1020W * | 18.3 | 4.6 | 15.5 | 6.1 |
| 8 | 2.5PtpH1015W * | 14.2 | 2.5 | 27.9 | 3.4 |
| 9 | 10PtpH1015W * | 13.4 | 8.6 | 22.2 | 4.3 |
| Entry | Sample | Pt/Al | W/Al | BE (eV) | |||
|---|---|---|---|---|---|---|---|
| Bulk | Surface | Bulk | Surface | Pt 4d5/2 | W 4f7/2 | ||
| 1 | 5PtpH410W | 0.009 | 0.011 | 0.021 | 0.022 | 315.5 | 35.4 |
| 2 | 5PtpH610W | 0.010 | 0.012 | 0.022 | 0.023 | 315.5 | 35.5 |
| 3 | 5PtpH810W | 0.010 | 0.013 | 0.020 | 0.023 | 315.6 | 35.5 |
| 4 | 5PtpH1010W | 0.010 | 0.012 | 0.020 | 0.023 | 315.8 | 35.6 |
| 5 | 5PtpH1010W * | 0.010 | 0.010 | 0.024 | 0.029 | 315.6 | 35.4 |
| 6 | 5PtpH1015W * | 0.012 | 0.011 | 0.037 | 0.041 | 315.4 | 35.4 |
| 7 | 5PtpH1020W * | 0.011 | 0.011 | 0.049 | 0.063 | 315.5 | 35.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarauta-Córdoba, C.; Oregui Bengoechea, M.; Agirrezabal-Telleria, I.; Arias, P.-L.; Gandarias, I. Insights into the Nature of the Active Sites of Pt-WOx/Al2O3 Catalysts for Glycerol Hydrogenolysis into 1,3-Propanediol. Catalysts 2021, 11, 1171. https://doi.org/10.3390/catal11101171
Jarauta-Córdoba C, Oregui Bengoechea M, Agirrezabal-Telleria I, Arias P-L, Gandarias I. Insights into the Nature of the Active Sites of Pt-WOx/Al2O3 Catalysts for Glycerol Hydrogenolysis into 1,3-Propanediol. Catalysts. 2021; 11(10):1171. https://doi.org/10.3390/catal11101171
Chicago/Turabian StyleJarauta-Córdoba, Clara, Mikel Oregui Bengoechea, Iker Agirrezabal-Telleria, Pedro-Luis Arias, and Iñaki Gandarias. 2021. "Insights into the Nature of the Active Sites of Pt-WOx/Al2O3 Catalysts for Glycerol Hydrogenolysis into 1,3-Propanediol" Catalysts 11, no. 10: 1171. https://doi.org/10.3390/catal11101171
APA StyleJarauta-Córdoba, C., Oregui Bengoechea, M., Agirrezabal-Telleria, I., Arias, P.-L., & Gandarias, I. (2021). Insights into the Nature of the Active Sites of Pt-WOx/Al2O3 Catalysts for Glycerol Hydrogenolysis into 1,3-Propanediol. Catalysts, 11(10), 1171. https://doi.org/10.3390/catal11101171








