Sol-Gel Synthesis of Organically Modified Silica Particles as Efficient Palladium Catalyst Supports to Perform Hydrogenation Process
Abstract
:1. Introduction
2. Results and Discussion
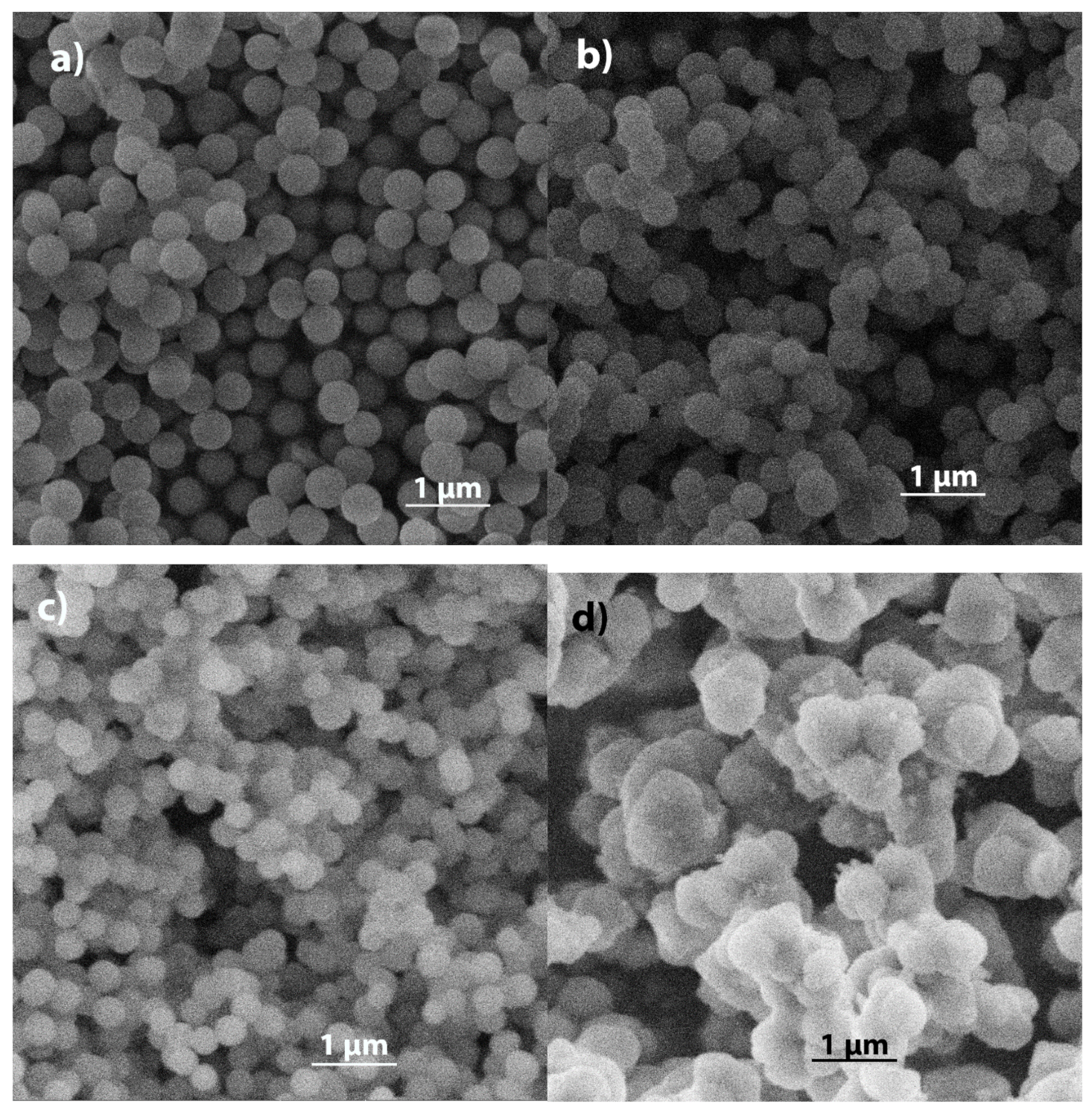
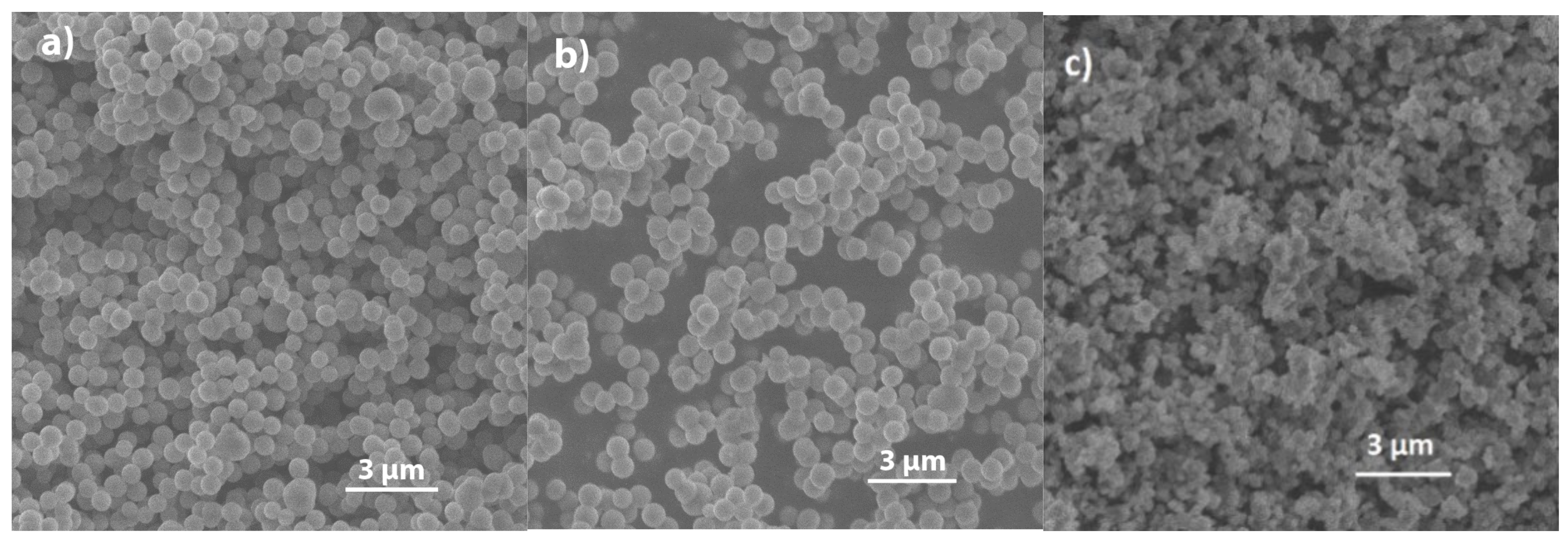
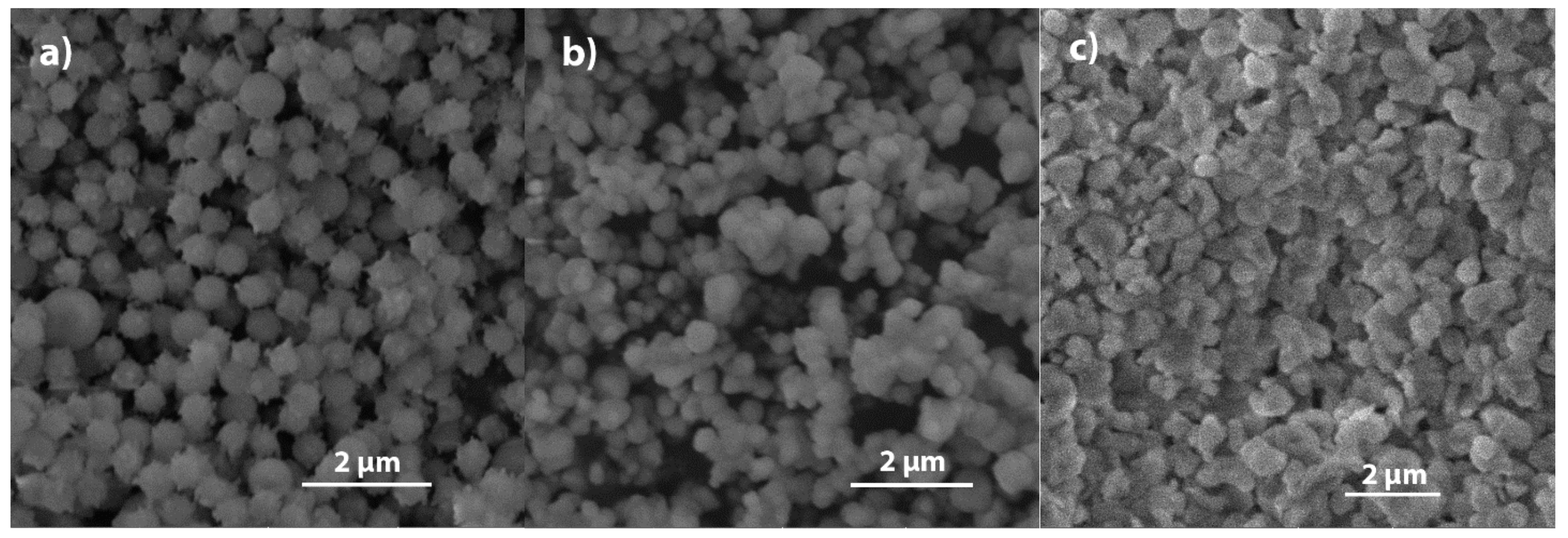
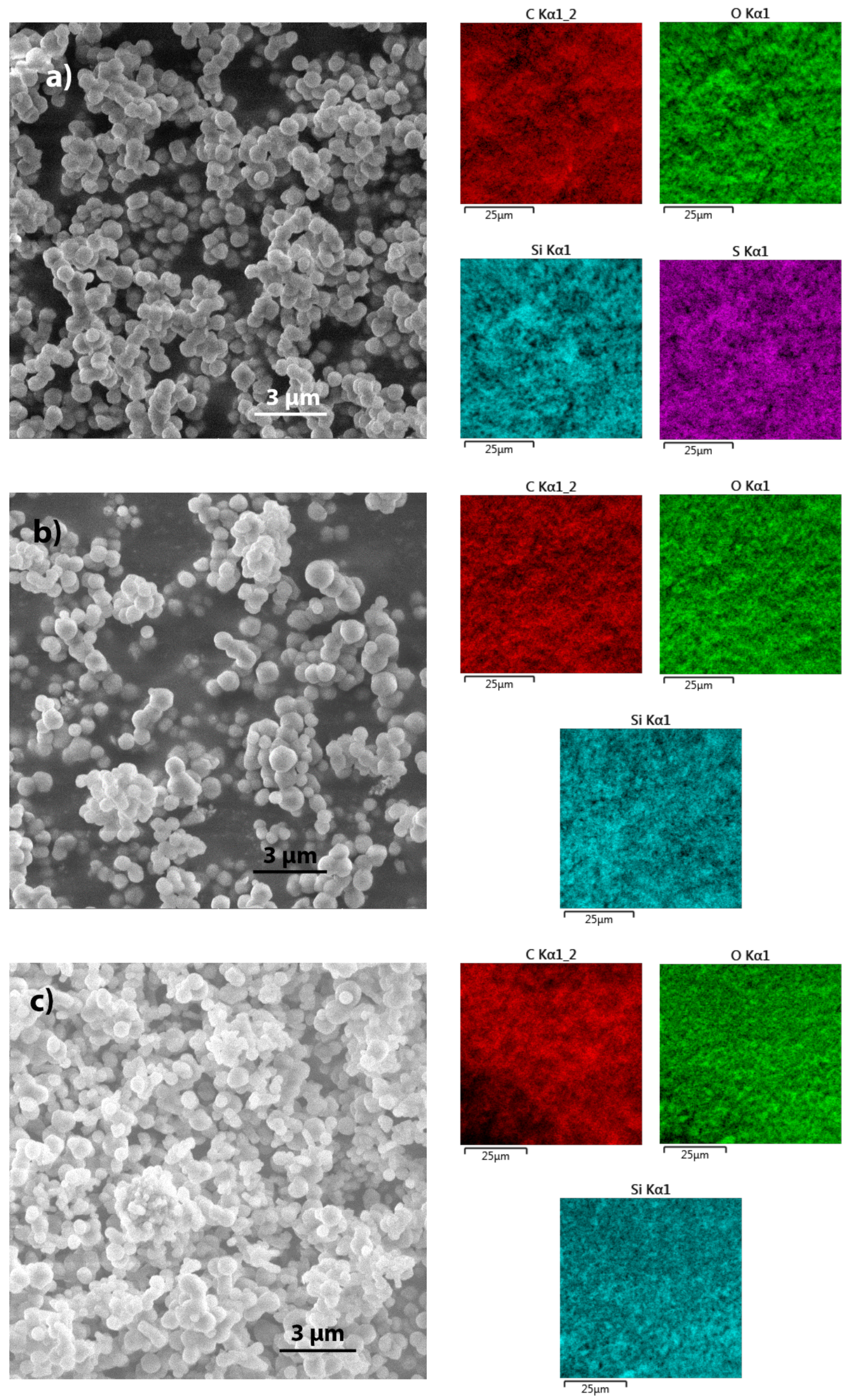
2.1. SEM
2.1.1. Amino-Modified Silica
2.1.2. Silica Modified with Methyl, Phenyl, and Mercapto Groups
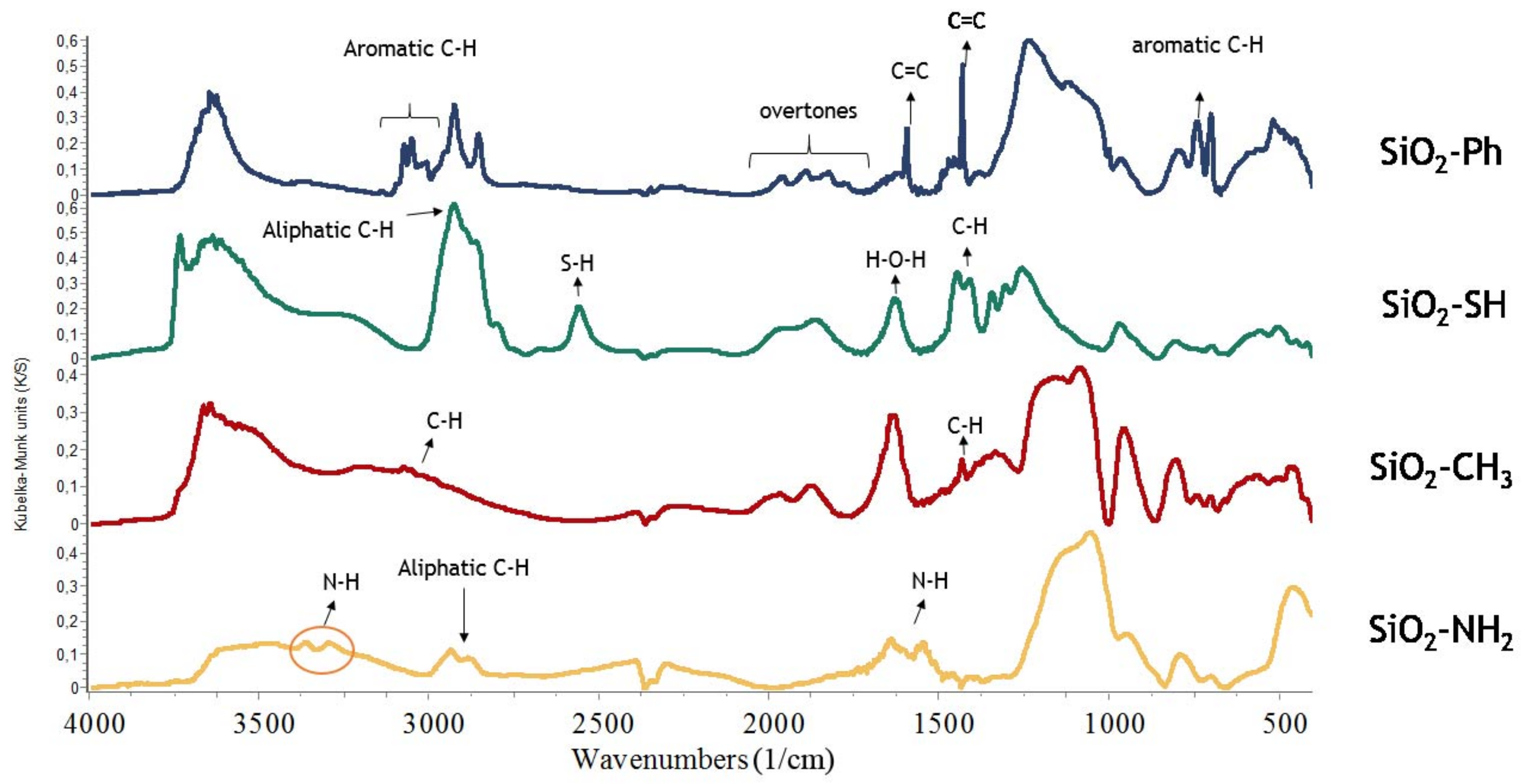
2.2. Infrared Spectroscopy
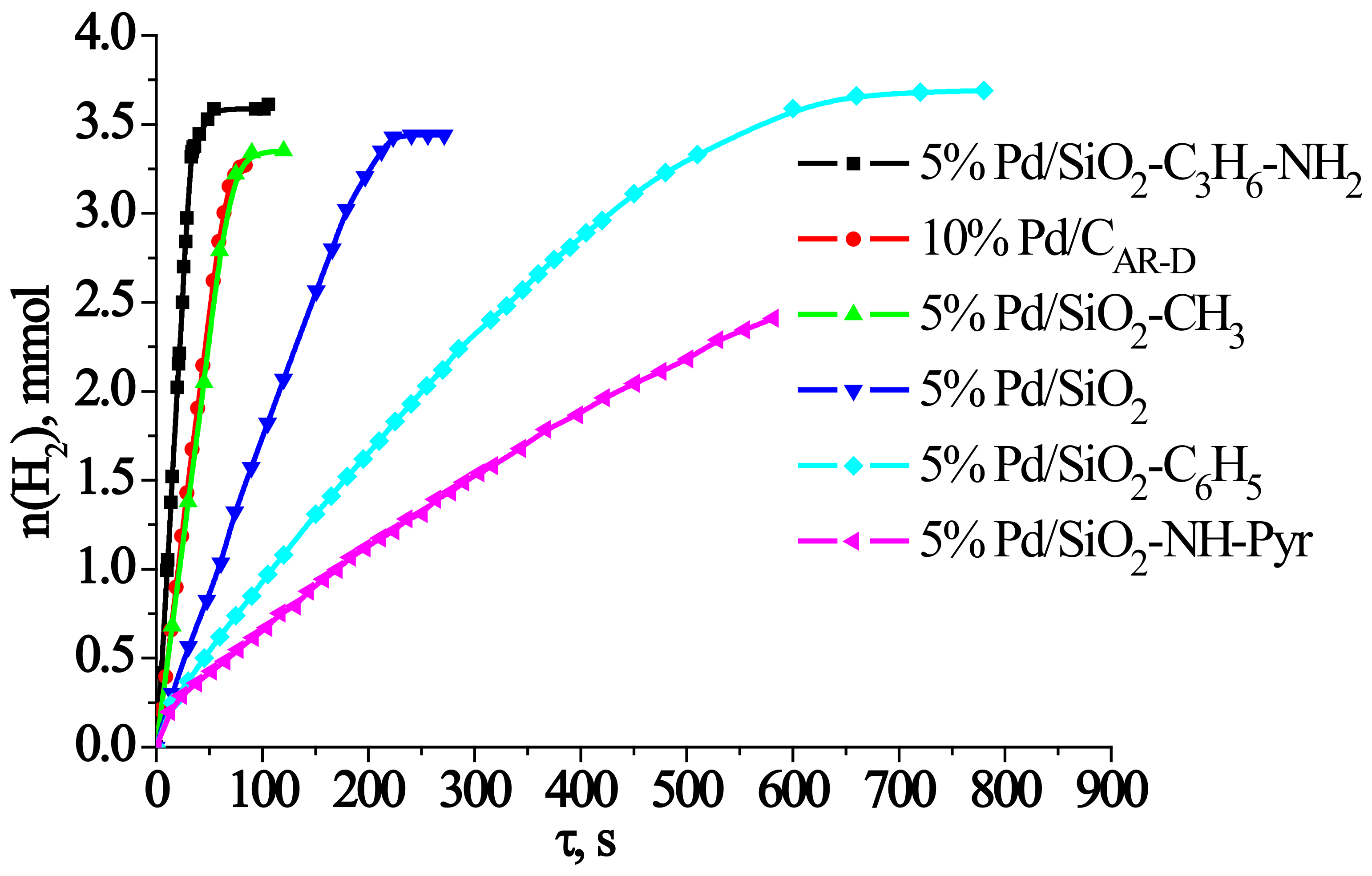
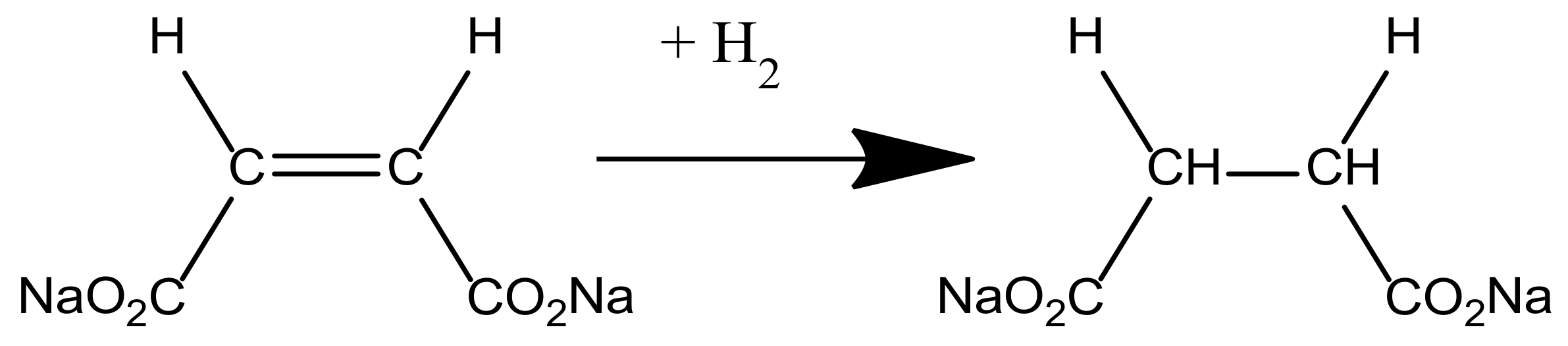
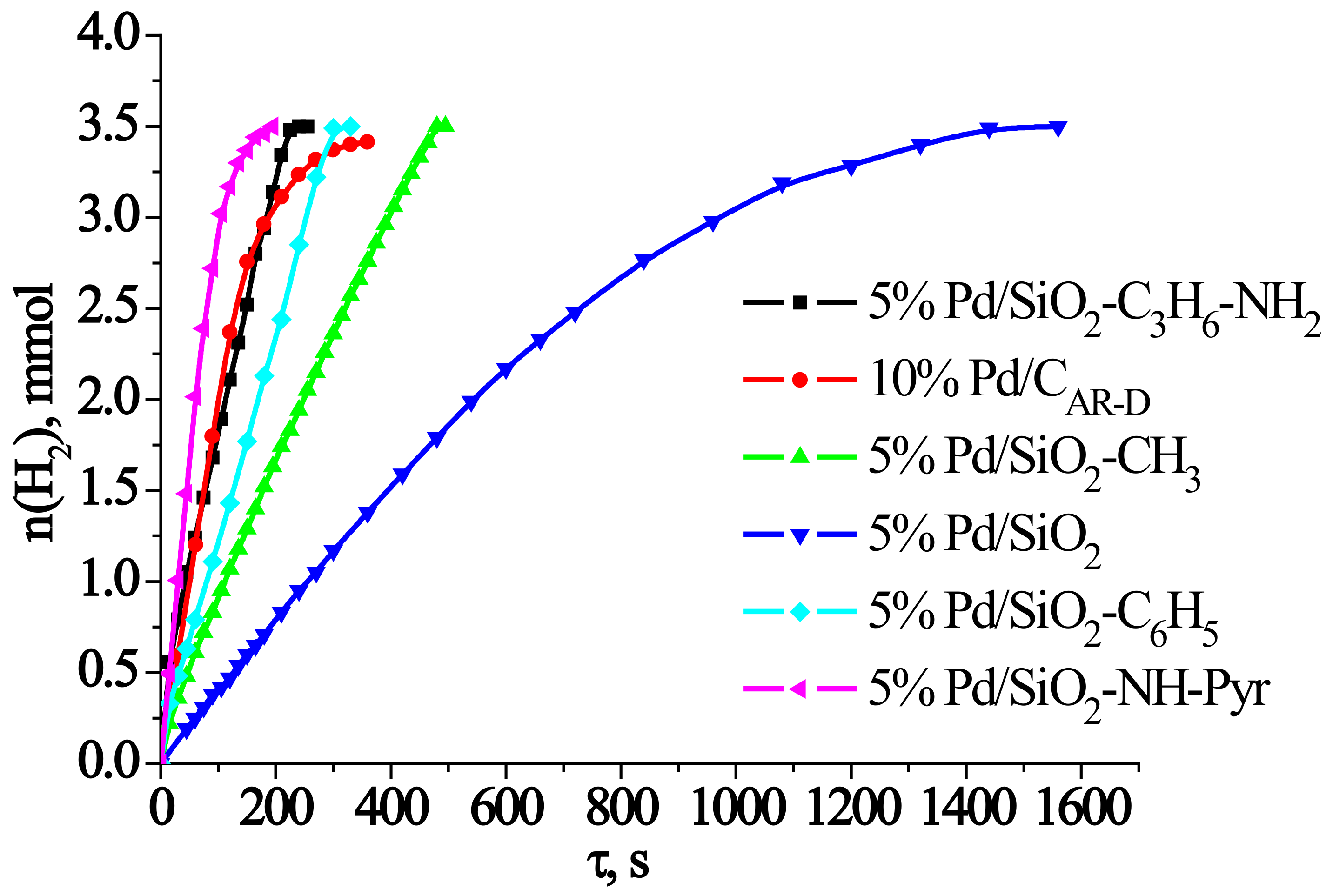
2.3. Analysis of the Catalytic Activity of the Developed Catalysts
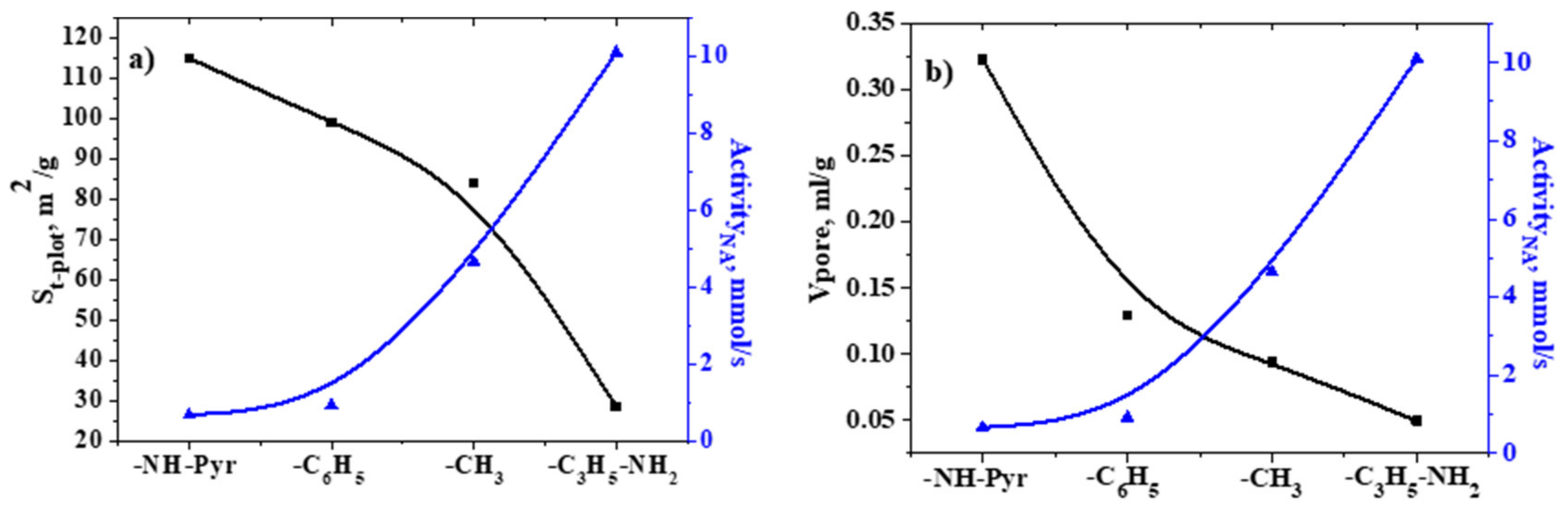
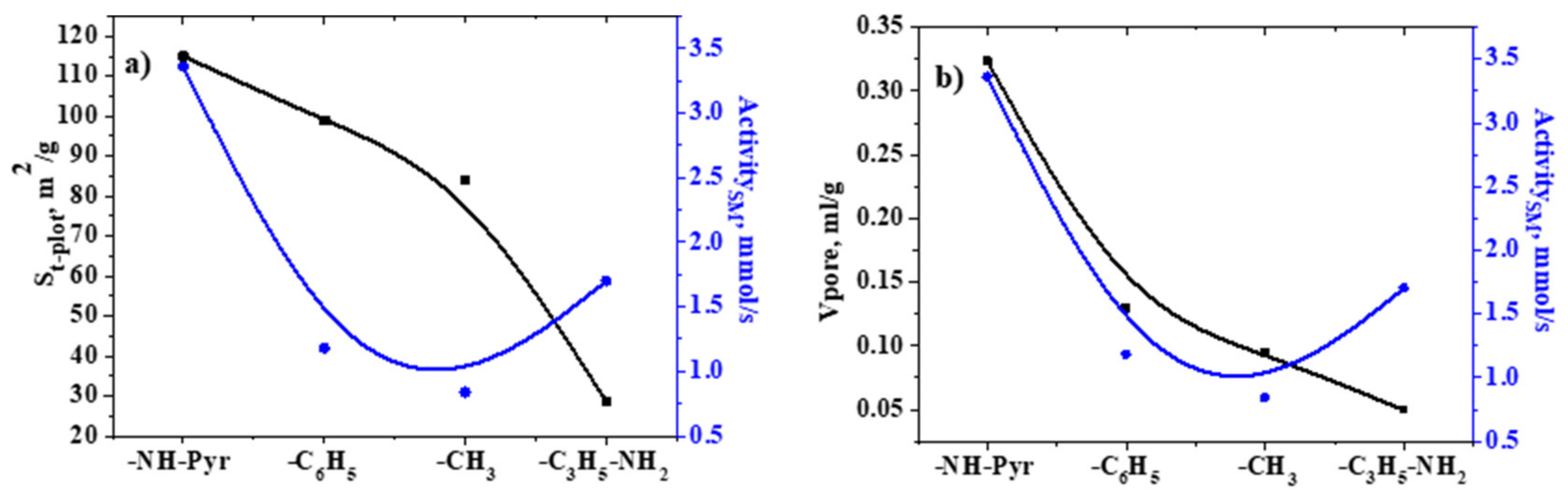
2.4. Analysis of Specific Surface Area and Porosity
2.5. X-ray Photoelectron Spectroscopy
3. Materials and Methods
3.1. Reagents
3.2. Synthesis Methodology
3.2.1. Aminomodified Silica Synthesis
3.2.2. Synthesis of Phenyl-, Methyl-, and Mercapto-Modified Silica Particles
3.2.3. Catalyst Synthesis Method
3.3. Research Methods
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Infrared Fourier Spectroscopy
3.3.3. Kinetics Investigation of Catalytic Process
3.3.4. Nitrogen Physisorption
3.3.5. X-ray Photoelectron Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Shah, Z.H.; Zhang, S.; Lu, R. Silica-based nanocomposites via reverse microemulsions: Classifications, preparations, and applications. Nanoscale 2014, 6, 4418–4437. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Mugica, L.C.; Rodríguez-Molina, B.; Ramos, S.; Kozina, A. Surface functionalization of silica particles for their efficient fluorescence and stereo selective modification. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 79–87. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface Modification of Silica Nanoparticles to Reduce Aggregation and Nonspecific Binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasir Baig, R.B.; Varma, R.S. Magnetic Silica-Supported Palladium Catalyst: Synthesis of Allyl Aryl Ethers in Water. Ind. Eng. Chem. Res. 2014, 53, 18625–18629. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. APTES-modified mesoporous silicas as the carriers for poorly water-soluble drug. Modeling of diflunisal adsorption and release. Appl. Surf. Sci. 2016, 368, 348–359. [Google Scholar] [CrossRef]
- Wang, A.; Yang, Y.; Qi, Y.; Qi, W.; Fei, J.; Ma, H.; Zhao, J.; Cui, W.; Li, J. Fabrication of Mesoporous Silica Nanoparticle with Well-Defined Multicompartment Structure as Efficient Drug Carrier for Cancer Therapy in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 8900–8907. [Google Scholar] [CrossRef]
- Rajanna, S.K.; Kumar, D.; Vinjamur, M.; Mukhopadhyay, M. Silica Aerogel Microparticles from Rice Husk Ash for Drug Delivery. Ind. Eng. Chem. Res. 2015, 54, 949–956. [Google Scholar] [CrossRef]
- Roy, I. Synthesis, Surface Modification, Characterization, and Biomedical In Vitro Applications of Organically Modified Silica (ORMOSIL) Nanoparticles. In Nanoparticles in Biology and Medicine: Methods and Protocols; Soloviev, M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 365–379. [Google Scholar]
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators. Acc. Chem. Res. 2020, 53, 1545–1556. [Google Scholar] [CrossRef]
- Wang, K.; Liu, P.; Ye, Y.; Li, J.; Zhao, W.; Huang, X. Fabrication of a novel laccase biosensor based on silica nanoparticles modified with phytic acid for sensitive detection of dopamine. Sens. Actuators B Chem. 2014, 197, 292–299. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, Y.; Zhu, Q.; Wang, K.; Liu, M.; Huang, X.; Shen, J. A novel glucose biosensor based on phosphonic acid-functionalized silica nanoparticles for sensitive detection of glucose in real samples. Electrochim. Acta 2013, 89, 278–283. [Google Scholar] [CrossRef]
- Putz, A.-M.; Mihaela, C.; Adina, N.; Grad, O.; Ianasi, C.; Ivankov, O.; Milanović, M.; Stijepović, I.; Almásy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef]
- Leśniewska, B.; Arciszewska, Ż.; Wawrzyńczak, A.; Jarmolińska, S.; Nowak, I.; Godlewska-Żyłkiewicz, B. Method development for determination of trace amounts of palladium in environmental water samples by ICP-MS/MS after pre-concentration on thiol-functionalized MCM-41 materials. Talanta 2020, 217, 121004. [Google Scholar] [CrossRef] [PubMed]
- Baccile, N.; Babonneau, F. Organo-modified mesoporous silicas for organic pollutant removal in water: Solid-state NMR study of the organic/silica interactions. Microporous Mesoporous Mater. 2008, 110, 534–5422. [Google Scholar] [CrossRef] [Green Version]
- Celik, G.; Ailawar, S.A.; Sohn, H.; Tang, Y.; Tao, F.F.; Miller, J.T.; Edmiston, P.L.; Ozkan, U.S. Swellable Organically Modified Silica (SOMS) as a Catalyst Scaffold for Catalytic Treatment of Water Contaminated with Trichloroethylene. ACS Catal. 2018, 8, 6796–6809. [Google Scholar] [CrossRef]
- Saptal, V.B.; Saptal, M.V.; Mane, R.S.; Sasaki, T.; Bhanage, B.M. Amine-Functionalized Graphene Oxide-Stabilized Pd Nanoparticles (Pd@APGO): A Novel and Efficient Catalyst for the Suzuki and Carbonylative Suzuki–Miyaura Coupling Reactions. ACS Omega 2019, 4, 643–649. [Google Scholar] [CrossRef]
- Hou, Z.; Theyssen, N.; Leitner, W. Palladium nanoparticles stabilised on PEG-modified silica as catalysts for the aerobic alcohol oxidation in supercritical carbon dioxide. Green Chem. 2007, 9, 127–132. [Google Scholar] [CrossRef]
- Corral, J.A.; López, M.I.; Esquivel, D.; Mora, M.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Preparation of Palladium-Supported Periodic Mesoporous Organosilicas and their Use as Catalysts in the Suzuki Cross-Coupling Reaction. Materials 2013, 6, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Ivashchenko, N.; Tertykh, V.; Yanishpolskii, V.; Khainakov, S.; Dikhtiarenko, A. Controlled reduction of palladium nanoparticles on surface of chemically modified silicas. Materialwissenschaft Werkstofftechnik 2011, 42, 64–69. [Google Scholar] [CrossRef]
- Lemay, M.; Pandarus, V.; Simard, M.; Marion, O.; Tremblay, L.; Béland, F. SiliaCat® S-Pd and SiliaCat DPP-Pd: Highly Reactive and Reusable Heterogeneous Silica-Based Palladium Catalysts. Top. Catal. 2010, 53, 1059–1062. [Google Scholar] [CrossRef]
- Carreño, G.A.; Schott, E.; Zarate, X.; Arratia-Perez, R.K.J.; Mardones, M.; Manriquez, J.M.I. Adsorption essays of palladium in modified silica gel with thiouronium groups: Experimental and theorical studies. J. Chil. Chem. Soc. 2010, 56, 692–696. [Google Scholar] [CrossRef]
- Veisi, H.; Abassi, P.; Mohammadi, P.; Tamoradi, T.; Karmakar, B. Gold nanoparticles decorated biguanidine modified mesoporous silica KIT-5 as recoverable heterogeneous catalyst for the reductive degradation of environmental contaminants. Sci. Rep. 2021, 11, 2734. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Abu-Reziq, R. Palladium Nanoparticles Supported on Magnetic Organic-Silica Hybrid Nanoparticles. J. Phys. Chem. C 2014, 118, 30045–30056. [Google Scholar] [CrossRef]
- Guin, D.; Baruwati, B.; Manorama, S.V. Pd on Amine-Terminated Ferrite Nanoparticles: A Complete Magnetically Recoverable Facile Catalyst for Hydrogenation Reactions. Org. Lett. 2007, 9, 1419–1421. [Google Scholar] [CrossRef]
- Lewis, L.N. Chemical catalysis by colloids and clusters. Chem. Rev. 1993, 93, 2693–2730. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, M.; Tai, C.-w.; Göthelid, E.; Oscarsson, S.; Bäckvall, J.-E. Small Pd Nanoparticles Supported in Large Pores of Mesocellular Foam: An Excellent Catalyst for Racemization of Amines. Chem. A Eur. J. 2011, 17, 13269–13273. [Google Scholar] [CrossRef]
- Verho, O.; Nagendiran, A.; Johnston, E.V.; Tai, C.-W.; Bäckvall, J.-E. Nanopalladium on Amino-Functionalized Mesocellular Foam: An Efficient Catalyst for Suzuki Reactions and Transfer Hydrogenations. ChemCatChem 2013, 5, 612–618. [Google Scholar] [CrossRef]
- Johnston, E.V.; Verho, O.; Kärkäs, M.D.; Shakeri, M.; Tai, C.-W.; Palmgren, P.; Eriksson, K.; Oscarsson, S.; Bäckvall, J.-E. Highly Dispersed Palladium Nanoparticles on Mesocellular Foam: An Efficient and Recyclable Heterogeneous Catalyst for Alcohol Oxidation. Chem. A Eur. J. 2012, 18, 12202–12206. [Google Scholar] [CrossRef]
- Verho, O.; Nagendiran, A.; Tai, C.-W.; Johnston, E.V.; Bäckvall, J.-E. Nanopalladium on Amino-Functionalized Mesocellular Foam as an Efficient and Recyclable Catalyst for the Selective Transfer Hydrogenation of Nitroarenes to Anilines. ChemCatChem 2014, 6, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Maddila, S.; Yalagala, K.; Jonnalagadda, S.B. Organo functionalized graphene with Pd nanoparticles and its excellent catalytic activity for Suzuki coupling reaction. Appl. Catal. A Gen. 2015, 505, 539–547. [Google Scholar] [CrossRef]
- Rana, S.; Maddila, S.; Jonnalagadda, S.B. Synthesis and characterization of Pd(ii) dispersed over diamine functionalized graphene oxide and its scope as a catalyst for selective oxidation. Catal. Sci. Technol. 2015, 5, 3235–3241. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Rana, S.; Parida, K. Pd(0) Nanoparticles Supported Organofunctionalized Clay Driving C–C Coupling Reactions under Benign Conditions through a Pd(0)/Pd(II) Redox Interplay. J. Phys. Chem. C 2014, 118, 1640–1651. [Google Scholar] [CrossRef]
- Hussain, Z.; Schwalm, C.; Rambo, R.; Dias, R.; Stieler, R.; Monteiro, A. Synthesis of Mono- and Bis-Pyrazoles Bearing Flexible p-Tolyl Ether and Rigid Xanthene Backbones, and Their Potential as Ligands in the Pd-Catalysed Suzuki–Miyaura Cross-Coupling Reaction. Catalysts 2019, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Karanjit, S.; Tamura, A.; Kashihara, M.; Ushiyama, K.; Shrestha, L.K.; Ariga, K.; Nakayama, A.; Namba, K. Hydrotalcite-Supported Ag/Pd Bimetallic Nanoclusters Catalyzed Oxidation and One-Pot Aldol Reaction in Water. Catalysts 2020, 10, 1120. [Google Scholar] [CrossRef]
- Keller, K.; Lott, P.; Stotz, H.; Maier, L.; Deutschmann, O. Microkinetic Modeling of the Oxidation of Methane Over PdO Catalysts—Towards a Better Understanding of the Water Inhibition Effect. Catalysts 2020, 10, 922. [Google Scholar] [CrossRef]
- Ramirez Côté, C.; Ciriminna, R.; Pandarus, V.; Béland, F.; Pagliaro, M. Comparing the Pyrophoricity of Palladium Catalysts for Heterogeneous Hydrogenation. Org. Process. Res. Dev. 2018, 22, 1852–1855. [Google Scholar] [CrossRef]
- Sauvage, J.-F.; Baker, R.H.; Hussey, A.S. The Hydrogenation of Cycloalkenes over Supported Palladium Catalysts. J. Am. Chem. Soc. 1961, 83, 3874–3877. [Google Scholar] [CrossRef]
- Suh, D.J.; Park, T.J.; Ihm, S.K. Characteristics of carbon-supported palladium catalysts for liquid-phase hydrogenation of nitroaromatics. Ind. Eng. Chem. Res. 1992, 31, 1849–1856. [Google Scholar] [CrossRef]
- Wei, Z.; Xie, Z.; Gao, L.; Wang, Y.; Sun, H.; Jian, Y.; Zhang, G.; Xu, L.; Yang, J.; Zhang, W.; et al. Highly Crystallized Pd/Cu Nanoparticles on Activated Carbon: An Efficient Heterogeneous Catalyst for Sonogashira Cross-Coupling Reaction. Catalysts 2020, 10, 192. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.; Bodenes, L.; Bennett, J.; Deplanche, K.; Macaskie, L.E. Hydrogenation of 2-Butyne-1,4-diol Using Novel Bio-Palladium Catalysts. Ind. Eng. Chem. Res. 2010, 49, 980–988. [Google Scholar] [CrossRef]
- Zhu, Y.; Bai, Z. Pd–Ce/ZIF-8 Nanocomposite for Catalytic Extraction of Sinomenine from Sinomenium acutum. Catalysts 2020, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Putz, A.-M.; Almásy, L.; Len, A.; Ianasi, C. Functionalized silica materials synthesized via co-condensation and post-grafting methods. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 323–332. [Google Scholar] [CrossRef]
- Ab Rahman, I.; Jafarzadeh, M.; Sipaut, C. Physical and optical properties of organo-modified silica nanoparticles prepared by sol–gel. J. Sol-Gel Sci. Technol. 2011, 59, 63–72. [Google Scholar] [CrossRef]
- Pirez, C.; Wilson, K.; Lee, A.F. An energy-efficient route to the rapid synthesis of organically-modified SBA-15 via ultrasonic template removal. Green Chem. 2014, 16, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Yoshitake, H. Highly-controlled synthesis of organic layers on mesoporous silica: Their structure and application to toxic ion adsorptions. J. Chem. 2005, 29, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Vojoudi, H.; Badiei, A.; Bahar, S.; Mohammadi Ziarani, G.; Faridbod, F.; Ganjali, M.R. Post-modification of nanoporous silica type SBA-15 by bis(3-triethoxysilylpropyl)tetrasulfide as an efficient adsorbent for arsenic removal. Powder Technol. 2017, 319, 271–278. [Google Scholar] [CrossRef]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef]
- Chen, H.; Fu, W.; Li, Z. Temperature and pH Responsive Janus Silica Nanoplates Prepared by the Sol–Gel Process and Postmodification. Langmuir 2020, 36, 273–278. [Google Scholar] [CrossRef]
- Costa, M.B.; Bizeto, M.A. Synthesis of a highly hydrophobic silica mesostructure by a modified co-condensation procedure and evaluation of its drug release capability. J. Porous Mater. 2018, 25, 1603–1609. [Google Scholar] [CrossRef]
- Xiao, J.; Jing, Y.; Yao, Y.; Wang, X.; Jia, Y. Synthesis of amidoxime-decorated 3D cubic mesoporous silica via self-assembly co-condensation as a superior uranium(VI) adsorbent. J. Mol. Liq. 2019, 277, 843–855. [Google Scholar] [CrossRef]
- Feinle, A.; Leichtfried, F.; Straßer, S.; Hüsing, N. Carboxylic acid-functionalized porous silica particles by a co-condensation approach. J. Sol-Gel Sci. Technol. 2017, 81, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Grün, M.; Lauer, I.; Unger, K.K. The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv. Mater. 1997, 9, 254–257. [Google Scholar] [CrossRef]
- Barrera, E.G.; Livotto, P.R.; Santos, J.H.Z.d. Hybrid silica bearing different organosilanes produced by the modified Stöber method. Powder Technol. 2016, 301, 486–492. [Google Scholar] [CrossRef]
- Kocherbitov, V.; Veryazov, V.; Söderman, O. Hydration of trimethylamine-N-oxide and of dimethyldodecylamine-N-oxide: An ab initio study. J. Mol. Struct. THEOCHEM 2007, 808, 111–118. [Google Scholar] [CrossRef]
- Ngo, D.; Baldelli, S. Adsorption of Dimethyldodecylamine Oxide and Its Mixtures with Triton X-100 at the Hydrophilic Silica/Water Interface Studied Using Total Internal Reflection Raman Spectroscopy. J. Phys. Chem. B 2016, 120, 12346–12357. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko, A.A.; Tarasyuk, I.A.; Marfin, Y.S.; Grzhegorzhevskii, K.V.; Muslimov, A.R.; Bondarenko, A.B.; Lebedev, M.D.; Kuz’min, I.A.; Vashurin, A.S.; Lepik, K.V.; et al. DDAO Controlled Synthesis of Organo-Modified Silica Nanoparticles with Encapsulated Fluorescent Boron Dipyrrins and Study of Their Uptake by Cancerous Cells. Molecules 2020, 25, 3802. [Google Scholar] [CrossRef] [PubMed]
- Northolt, M. The structure and properties of aramid fibres. In Developments in the Science and Technology of Composite Materials, Proceedings of the ECCM3 Third European Conference on Composite Materials, Bordeaux, France, 20–23 March 1989; Bunsell, A.R., Lamicq, P., Massiah, A., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 3–4. [Google Scholar]
- Amer, I.; Brandt, S. Synthesis and characterization of Poly(p-phenylenediamine) and its derivatives using aluminium triflate as a co-catalyst. Cogent Eng. 2018, 5, 1499701. [Google Scholar] [CrossRef]
- Qi, H.-R.; Shen, D.-X.; Jia, Y.-J.; An, Y.-C.; Wu, H.; Wei, X.-Y.; Zhang, Y.; Zhi, X.-X.; Liu, J.-G. Preparation and Properties of Electrospun Phenylethynyl—Terminated Polyimide Nano-Fibrous Membranes with Potential Applications as Solvent-Free and High-Temperature Resistant Adhesives for Harsh Environments. Nanomaterials 2021, 11, 1525. [Google Scholar] [CrossRef]
- Bahlol, H.S.; Foda, M.F.; Ma, J.; Han, H. Robust Synthesis of Size-Dispersal Triangular Silver Nanoprisms via Chemical Reduction Route and Their Cytotoxicity. Nanomaterials 2019, 9, 674. [Google Scholar] [CrossRef] [Green Version]
- El-Subbagh, H.I.; Al-Badr, A.A. Chapter 2—Cytarabine. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2009; Volume 34, pp. 37–113. [Google Scholar]
- Minigh, J. Hydrocortisone. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2008; pp. 1–9. [Google Scholar]
- Rigge, D.; Jones, M. Shelf lives of aseptically prepared medicines—Stability of hydrocortisone sodium succinate in PVC and non-PVC bags and in polypropylene syringes. J. Pharm. Biomed. Anal. 2005, 38, 332–336. [Google Scholar] [CrossRef]
- Rosa, A.M.; Sversut, R.A.; Silva, D.B.; Cardoso, T.F.M.; Amaral, M.S.; Carollo, A.R.H.; Kassab, N.M. Simultaneous Determination of Enrofloxacin, Silver Sulfadiazine, Hydrocortisone Acetate, Hydrocortisone Sodium Succinate, and Preservative Excipients in Pharmaceutical Preparations Using HPLC–DAD Method. Chromatographia 2017, 80, 1641–1649. [Google Scholar] [CrossRef]
- Doluda, V.; Sidorov, A.; Sulman, E.; Latypova, A.; Fillipov, D.; Lefedova, O. Synthesis, structure and catalytic properties of pd nanostructured materials in p-nitroaniline catalytic hydrogenation. Izvestiya Vysshikh Uchebnykh Zavedenii Khimiya Khimicheskaya Tekhnol. 2019, 62, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Krasnov, A.; Latypova, A.; Lefedova, O.; Sharonov, N.; Efremov, E.; Filippov, D. Kinetics of hydrogenation of nitrobenzene, 4-nitrotoluene, 4-nitroaniline and 2-chloro-4-nitroaniline on supported palladium catalyst. Izvestiya Vysshikh Uchebnykh Zavedenii Khimiya Khimicheskaya Tekhnol. 2016, 59, 68. [Google Scholar] [CrossRef]
- Burge, H.D.; Collins, D.J.; Davis, B.H. Intermediates in the Raney Nickel Catalyzed Hydrogenation of Nitrobenzene to Aniline. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 389–391. [Google Scholar] [CrossRef]
- Udayakumar, V.; Alexander, S.; Gayathri, V.; Shivakumaraiah; Viswanathan, B. Study on the influence of substituents upon the hydrogenation of nitrobenzene using a polymer-supported palladium-imidazole complex catalyst. React. Kinet. Mech. Catal. 2011, 103, 341–352. [Google Scholar] [CrossRef]
- Tallon, M.A. Reactions Involving Maleic Anhydride. In Handbook of Maleic Anhydride Based Materials: Syntheses, Properties and Applications; Musa, O.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–149. [Google Scholar]
- Korosteleva, P.O.; Ulitin, M.V.; Lukin, M.V.; Filippov, D.V. The kinetics of liquid-phase hydrogenation of sodium maleate by hydrogen adsorbed on the surface of Raney nickel. Russ. J. Phys. Chem. A 2009, 83, 1715–1719. [Google Scholar] [CrossRef]
- Hoffmann, F.; Fröba, M. Vitalising porous inorganic silica networks with organic functions—PMOs and related hybrid materials. Chem. Soc. Rev. 2011, 40, 608–620. [Google Scholar] [CrossRef]
- Smith, K.A. Polycondensation of methyltrimethoxysilane. Macromolecules 1987, 20, 2514–2520. [Google Scholar] [CrossRef]
- Brochier Salon, M.-C.; Bayle, P.-A.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N. Kinetics of hydrolysis and self condensation reactions of silanes by NMR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 83–91. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Latypova, A.R.; Lebedev, M.D.; Rumyantsev, E.V.; Filippov, D.V.; Lefedova, O.V.; Bykov, A.V.; Doluda, V.Y. Amino-Modified Silica as Effective Support of the Palladium Catalyst for 4-Nitroaniline Hydrogenation. Catalysts 2020, 10, 375. [Google Scholar] [CrossRef] [Green Version]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Belskaya, O.; Roman, M.; Gulyaeva, T.; Trenikhin, M.; Likholobov, V. Effect of the reduction conditions of the supported palladium precursor on the activity of Pd/C catalysts in hydrogenation of sodium 2,4,6-trinitrobenzoate. Russ. Chem. Bull. 2018, 67, 71–78. [Google Scholar] [CrossRef]



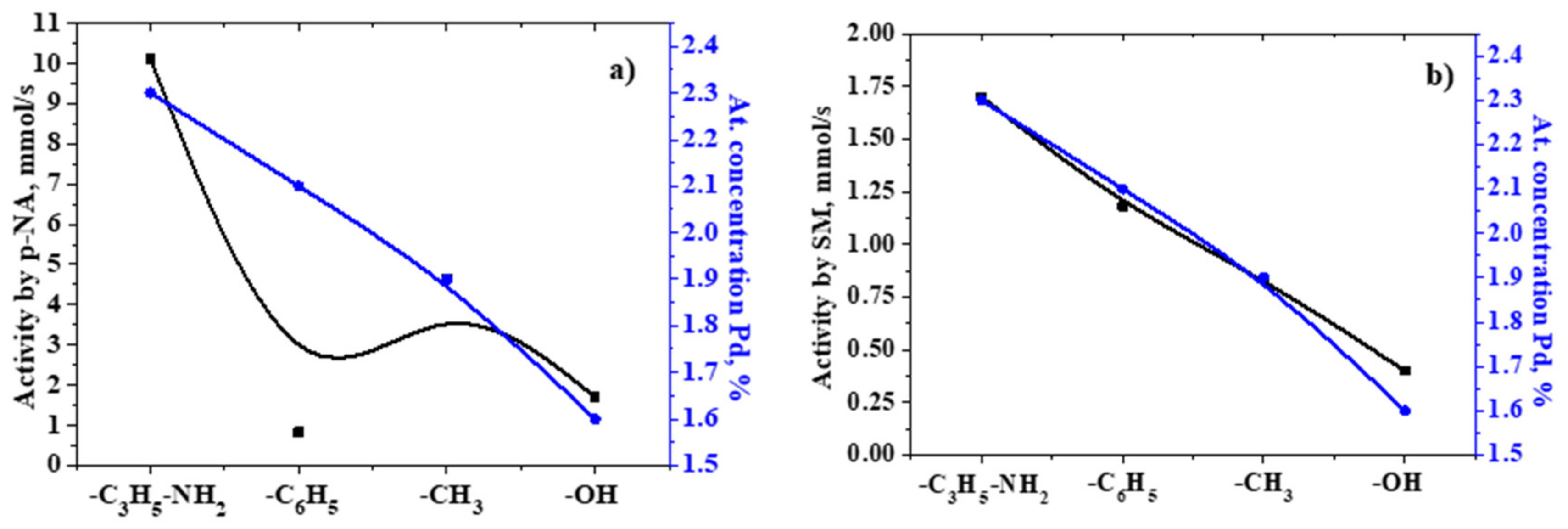
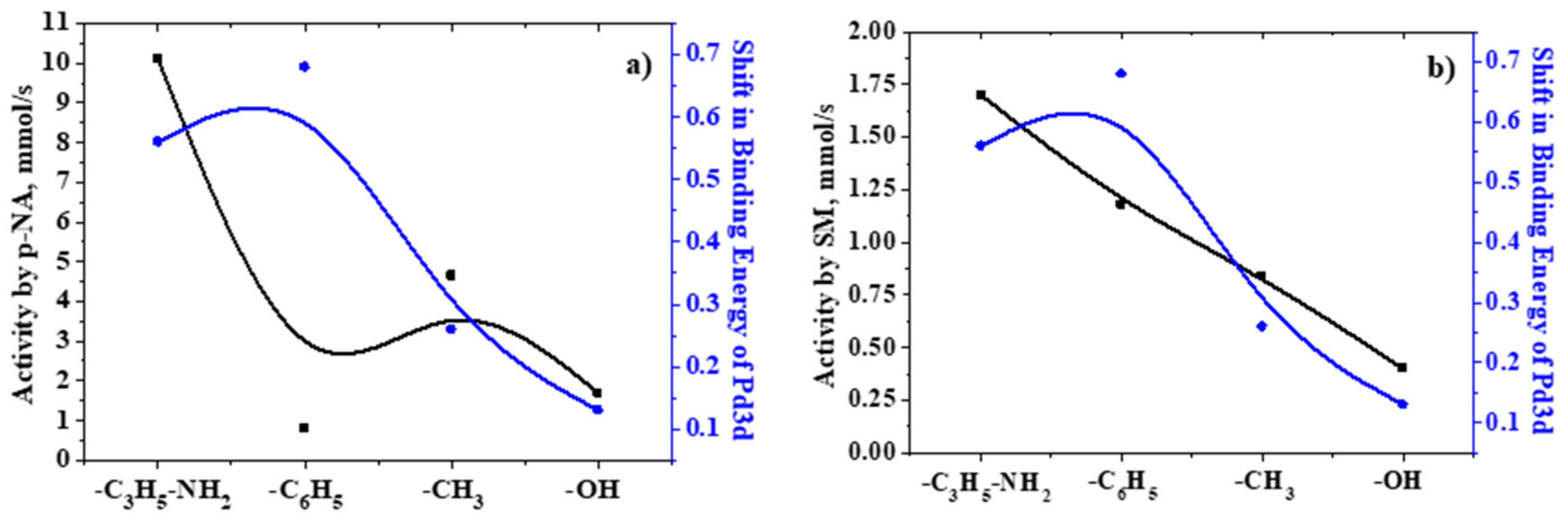
| Sample | r, mmol/s | ||||
|---|---|---|---|---|---|
| 1 Run | 2 Run | 3 Run | 4 Run | 5 Run | |
| 10% Pd/CAR-D | 4.88 | 4.20 | 3.90 | 3.60 | 3.30 |
| 5% Pd/SiO2 | 1.70 | 1.50 | 1.40 | 1.40 | 1.40 |
| 5% Pd/SiO2–CH3 | 4.65 | 3.50 | 2.70 | 2.53 | 2.47 |
| 5% Pd/SiO2–C3H6–NH2 [79] | 10.1 | 9.50 | 8.80 | 8.30 | 8.00 |
| 5% Pd/SiO2–C6H5 | 0.82 | 0.76 | 0.50 | 0.39 | 0.33 |
| Sample | Specific Surface Area, m2/g | Vpore, mL/g (BJH Method) | ||
|---|---|---|---|---|
| Langmuir | BET | t-Plot | ||
| SiO2–C3H6–NH2 | 8.07 (0.99219) | 14.01 (0.99409) | 28.51 (0.99660) | 0.05 |
| SiO2–C6H5 | 55.19 (0.99370) | 70.23 (0.99971) | 99.00 (0.99994) | 0.32 |
| SiO2–NH–Pyr | 68.08 (0.9965) | 81.37 (0.99954) | 114.95 (0.99989) | 0.13 |
| SiO2–CH3 | 81.89 (0.99890) | 84.40 (0.99998) | 84.03 (0.99995) | 0.09 |
| Sample | TEOS, mL | TEOS, M | APTMOS, mL | APTMOS, M | W, % (APTMOS) |
|---|---|---|---|---|---|
| NH2-0.5-0.2 | 0.50 | 0.070 | 0.20 | 0.032 | 29 |
| NH2-0.75-0.3 | 0.75 | 0.100 | 0.30 | 0.052 | 29 |
| NH2-1-0.4 | 1.00 | 0.140 | 0.40 | 0.069 | 29 |
| NH2-0.4-0.1 | 0.40 | 0.056 | 0.10 | 0.017 | 20 |
| NH2-0.3-0.2 | 0.30 | 0.042 | 0.20 | 0.034 | 40 |
| NH2-0.25-0.25 | 0.25 | 0.035 | 0.25 | 0.043 | 50 |
| NH2-0.2-0.3 | 0.20 | 0.028 | 0.30 | 0.052 | 60 |
| Sample | TMOS, mL | TMOS, M | R-TMOS, mL | R-TMOS, M | W, % (R-TMOS) |
|---|---|---|---|---|---|
| SH-0.33-0.2 | 0.33 | 0.07 | 0.20 | 0.032 | 41 |
| SH-0.33-0.13 | 0.33 | 0.07 | 0.13 | 0.021 | 31 |
| Ph-0.33-0.2 | 0.33 | 0.07 | 0.20 | 0.032 | 42 |
| Ph-0.33-0.13 | 0.33 | 0.07 | 0.13 | 0.021 | 32 |
| Me-0.33-0.14 | 0.33 | 0.07 | 0.14 | 0.032 | 29 |
| Me-0.33-0.09 | 0.33 | 0.07 | 0.09 | 0.021 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latypova, A.R.; Lebedev, M.D.; Tarasyuk, I.A.; Sidorov, A.I.; Rumyantsev, E.V.; Vashurin, A.S.; Marfin, Y.S. Sol-Gel Synthesis of Organically Modified Silica Particles as Efficient Palladium Catalyst Supports to Perform Hydrogenation Process. Catalysts 2021, 11, 1175. https://doi.org/10.3390/catal11101175
Latypova AR, Lebedev MD, Tarasyuk IA, Sidorov AI, Rumyantsev EV, Vashurin AS, Marfin YS. Sol-Gel Synthesis of Organically Modified Silica Particles as Efficient Palladium Catalyst Supports to Perform Hydrogenation Process. Catalysts. 2021; 11(10):1175. https://doi.org/10.3390/catal11101175
Chicago/Turabian StyleLatypova, Adele R., Maxim D. Lebedev, Ilya A. Tarasyuk, Alexander I. Sidorov, Evgeniy V. Rumyantsev, Artur S. Vashurin, and Yuriy S. Marfin. 2021. "Sol-Gel Synthesis of Organically Modified Silica Particles as Efficient Palladium Catalyst Supports to Perform Hydrogenation Process" Catalysts 11, no. 10: 1175. https://doi.org/10.3390/catal11101175
APA StyleLatypova, A. R., Lebedev, M. D., Tarasyuk, I. A., Sidorov, A. I., Rumyantsev, E. V., Vashurin, A. S., & Marfin, Y. S. (2021). Sol-Gel Synthesis of Organically Modified Silica Particles as Efficient Palladium Catalyst Supports to Perform Hydrogenation Process. Catalysts, 11(10), 1175. https://doi.org/10.3390/catal11101175







