A Facile Synthesis of Bi2O3/CoFe2O4 Nanocomposite with Improved Synergistic Photocatalytic Potential for Dye Degradation
Abstract
:1. Introduction
2. Results
2.1. Phase Analysis
2.2. Elemental Analysis
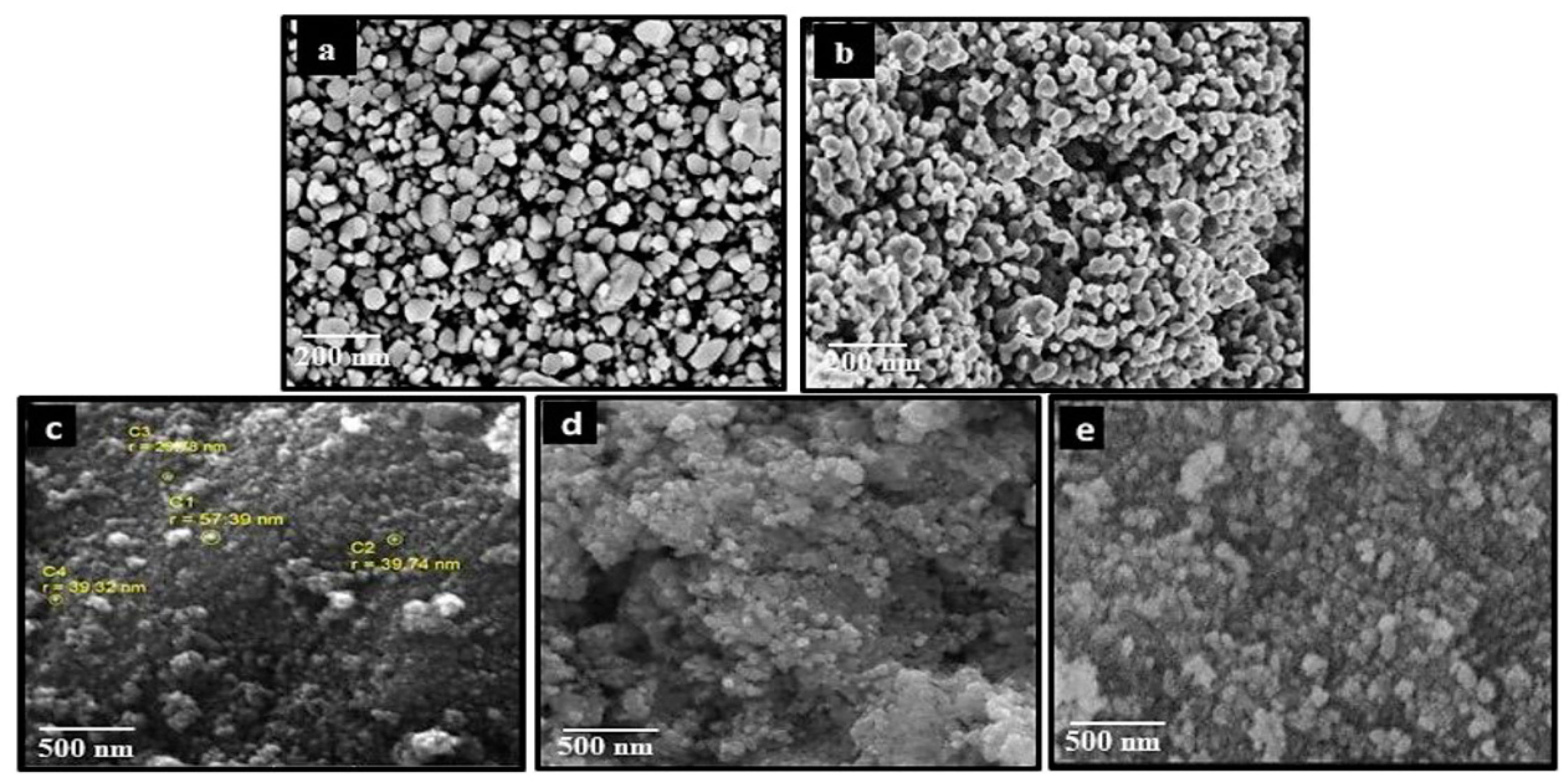
2.3. Morphological Analysis
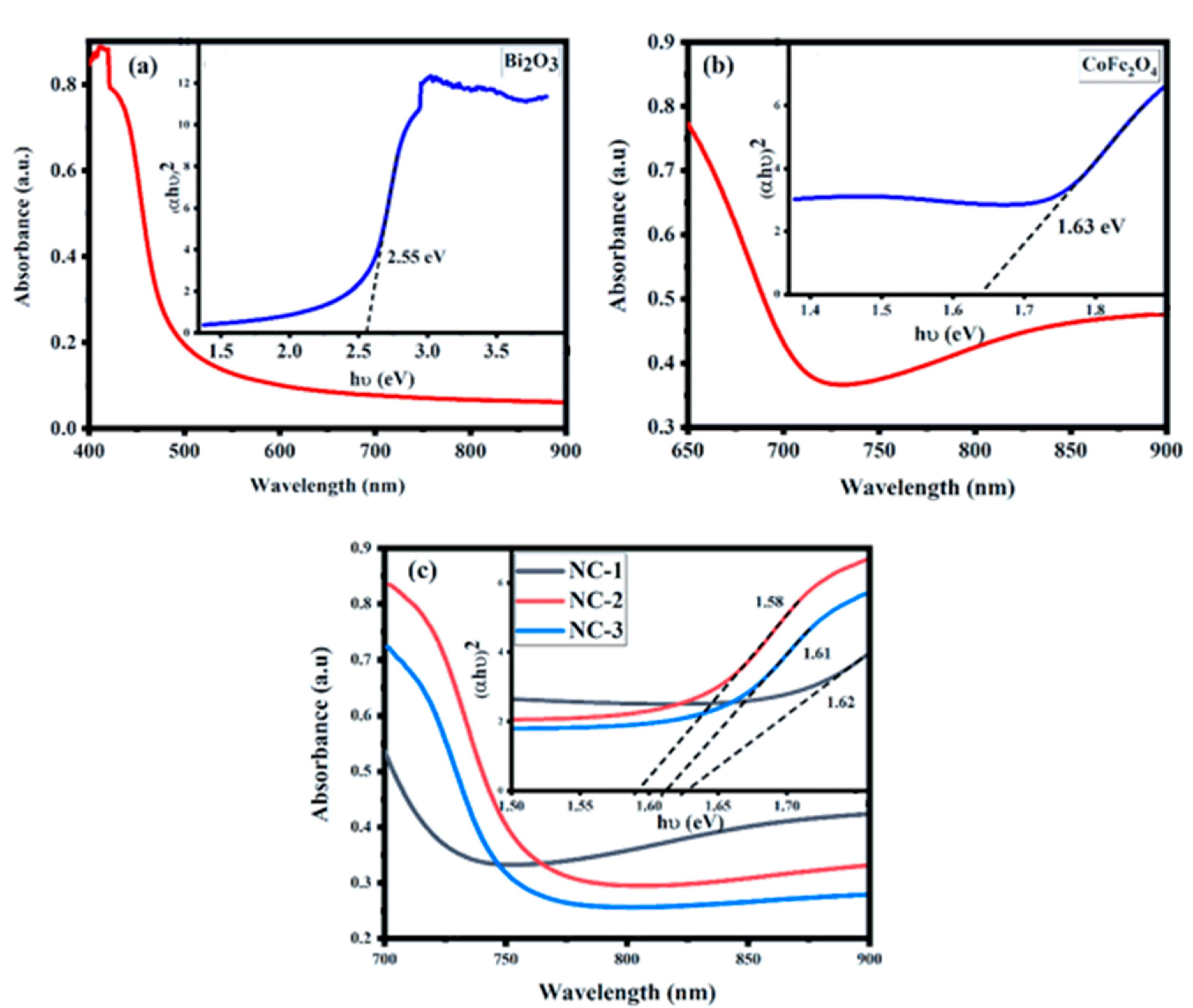
2.4. Diffuse Reflectance Spectroscopy and Tauc Plots
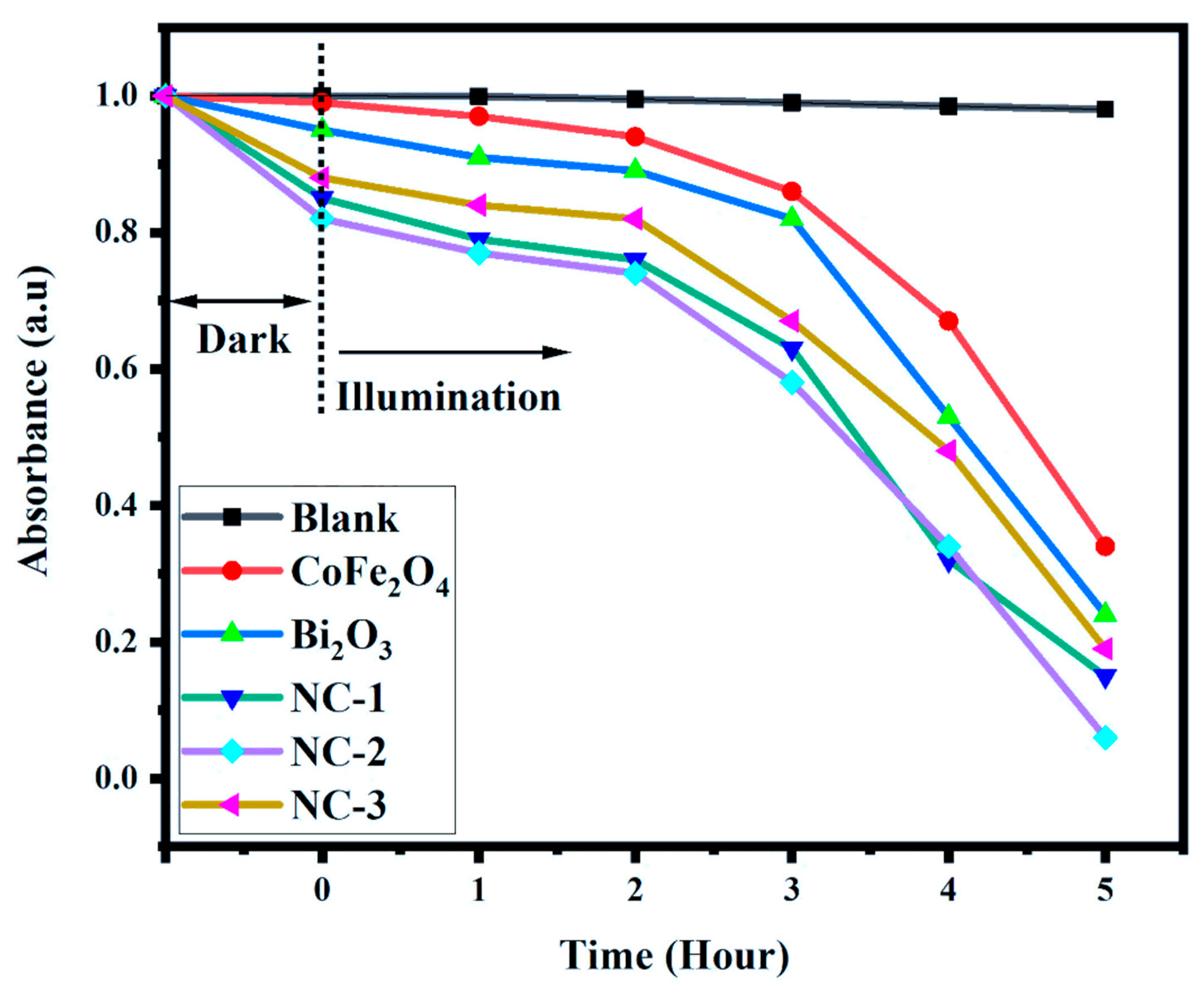
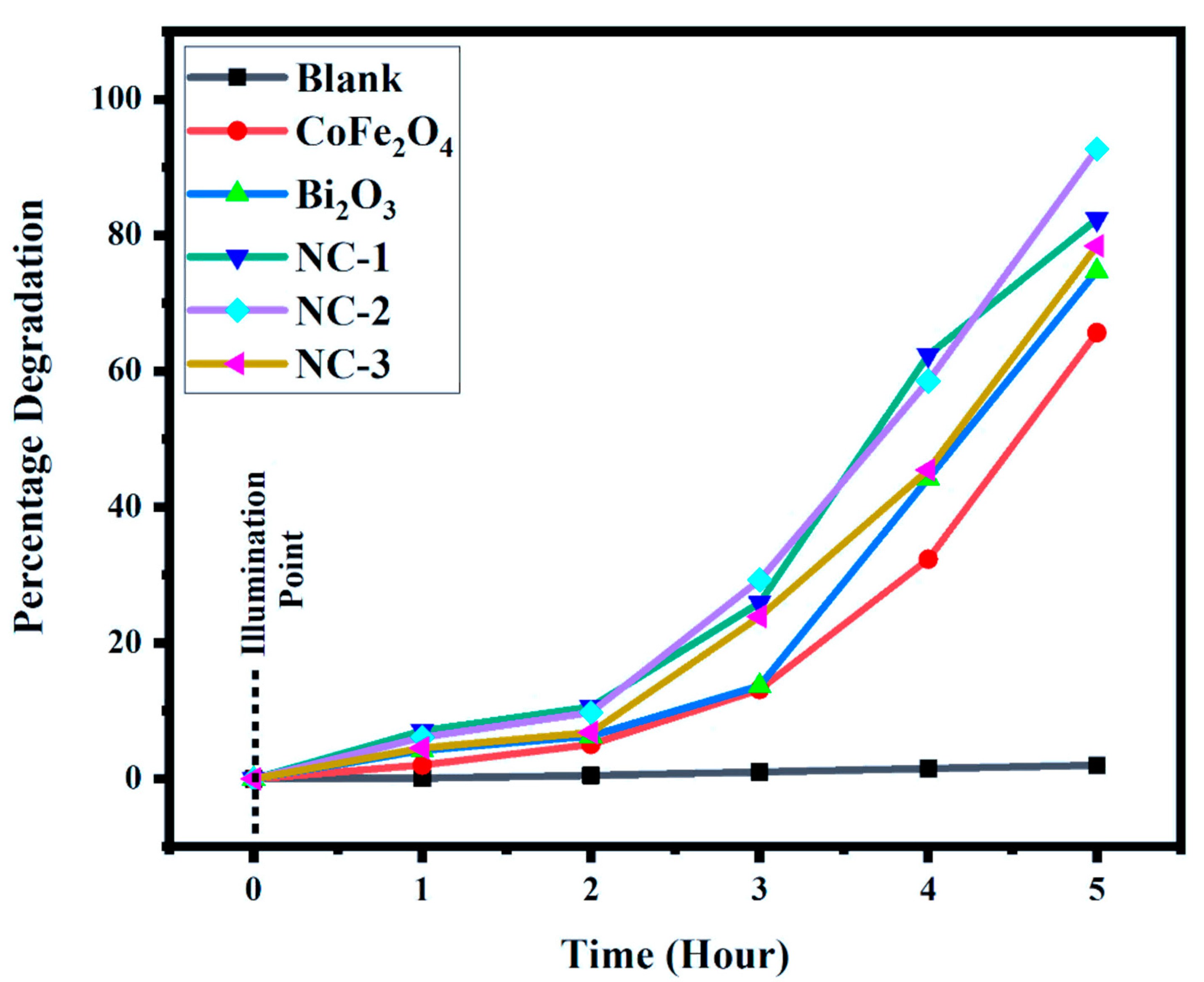
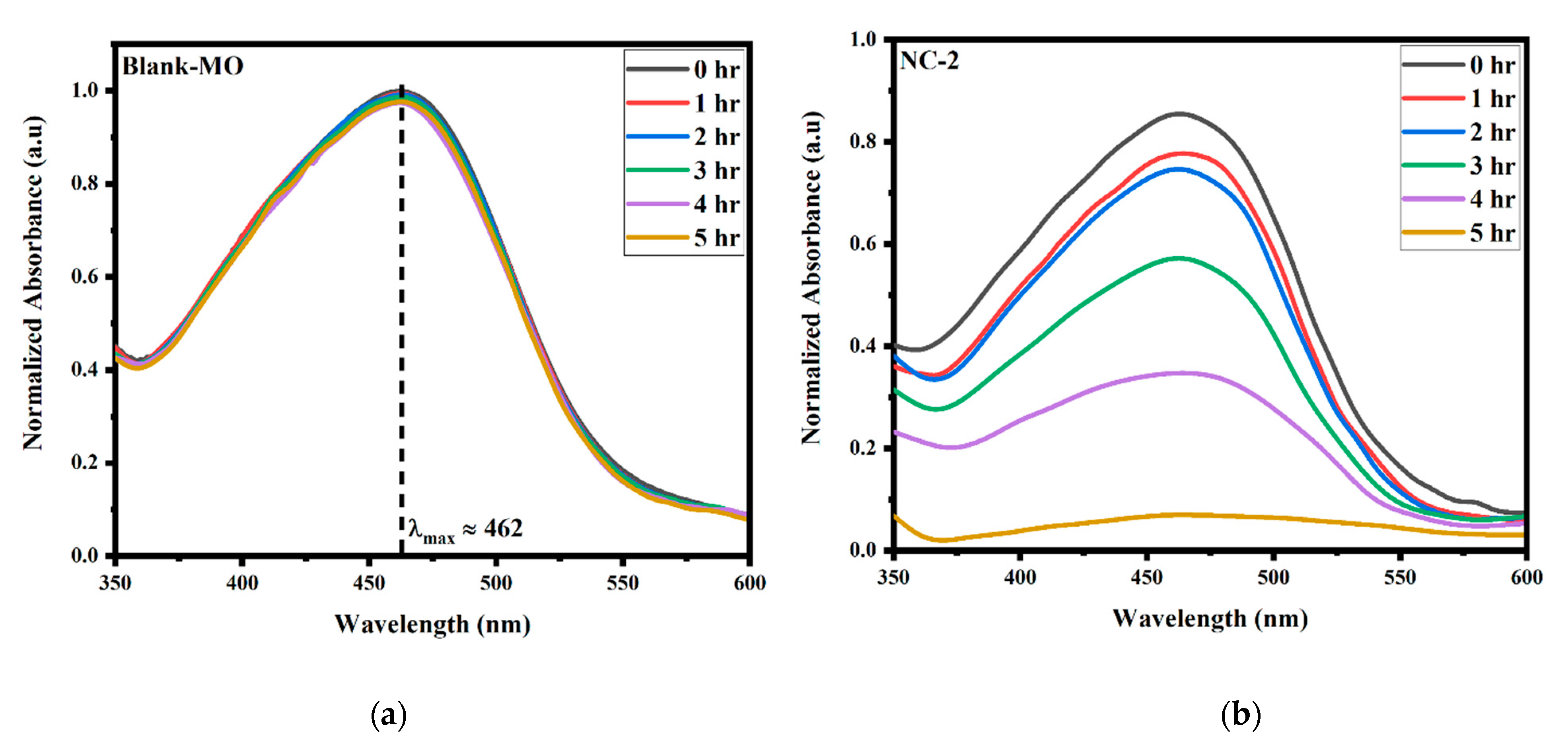
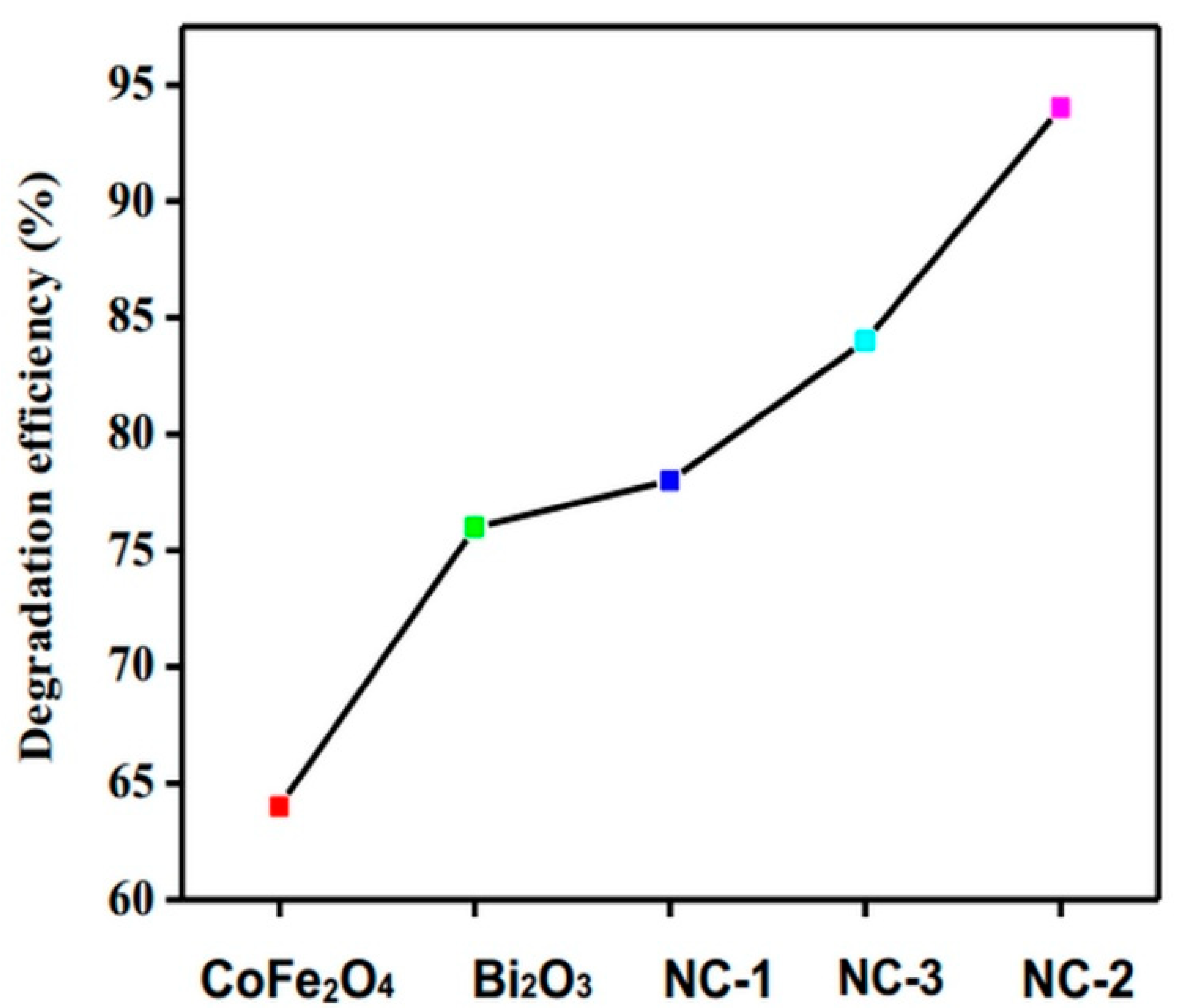
2.5. Photodegradation Activity
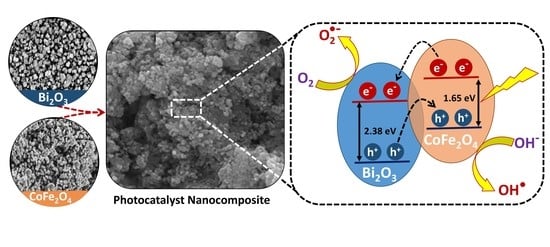
2.6. Proposed Photodegradation Scheme
3. Materials and Methods
3.1. Synthesis of Bi2O3 Nanoparticles
3.2. Synthesis of CoFe2O4 Nanoparticles
3.3. Synthesis of Bi2O3/CoFe2O4 Nanocomposites
3.4. Degradation Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, Q.; Xu, J.; Bu, X.-H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.R. Removal of heavy metals from wastewater using black tea waste. Arab. J. Sci. Eng. 2012, 37, 1505–1520. [Google Scholar] [CrossRef]
- Manaa, Z.; Chebli, D.; Bouguettoucha, A.; Atout, H.; Amrane, A. Low-Cost Photo-Fenton-Like Process for the Removal of Synthetic Dye in Aqueous Solution at Circumneutral pH. Arab. J. Sci. Eng. 2019, 44, 9859–9867. [Google Scholar] [CrossRef]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; de Moraes, L.A.B.; Fiore, M.F.; Monteiro, R.T.R. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.; Lim, L.H. Batch adsorption studies on the removal of acid blue 25 from aqueous solution using Azolla pinnata and soya bean waste. Arab. J. Sci. Eng. 2016, 41, 2453–2464. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. Rsc. Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Moo-Young, H.K. Pulp and paper effluent management. JSTOR 2007, 79, 1733–1741. [Google Scholar] [CrossRef]
- Maleš, L.; Fakin, D.; Bračič, M.; Gorgieva, S. Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials 2020, 10, 642. [Google Scholar] [CrossRef] [Green Version]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Rauwel, P.; Uhl, W.; Rauwel, E. Editorial for the Special Issue on ‘Application and Behavior of Nanomaterials in Water Treatment’; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2019. [Google Scholar]
- Coleman, H.M.; Eggins, B.R.; Byrne, J.A.; Palmer, F.L.; King, E. Photocatalytic degradation of 17-β-oestradiol on immobilised TiO2. Appl. Catal. B Environ. 2000, 24, L1–L5. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Chauhan, N.; Singh, V.; Kumar, S.; Dhiman, R. Influence of Nickel, Silver, and Sulphur Doping on the Photocatalytic Efficiency of Mesoporous ZnO Nanoparticles. Arab. J. Sci. Eng. 2020, 45, 249–259. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ismail, N.J.; Othman, M.H.D.; Kamaludin, R.; Esham, M.I.M.; Ali, N.A.; Rahman, M.A.; Jaafar, J.; Bakar, S.A. Characterization of Bauxite as a Potential Natural Photocatalyst for Photodegradation of Textile Dye. Arab. J. Sci. Eng. 2019, 44, 10031–10040. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Narayanan, V.; Stephen, A. Comparative study on photocatalytic activity of ZnO prepared by different methods. J. Mol. Liq. 2013, 181, 133–141. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Boudjemaa, A.; Popescu, I.; Juzsakova, T.; Kebir, M.; Helaili, N.; Bachari, K.; Marcu, I.C. M-Substituted (M=Co, Ni and Cu) Zinc Ferrite Photo-Catalysts for Hydrogen Production by Water Photo-Reduction. Int. J. Hydrog. Energy 2016, 41, 11108–11118. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, X.; Luo, J.; Wang, Z.; Tang, J. Magnetic Recyclable CoFe2O4@ Ppy Prepared by in Situ Fenton Oxidization Polymerization with Advanced Photo-Fenton Performance. RSC Adv. Tang 2020, 10, 1858–1869. [Google Scholar] [CrossRef] [Green Version]
- Dutta, V.; Sheetal, S.; Pankaj, R.; Ahmad, H.B.; Vinod, K.G.; Pardeep, J. Review on Augmentation in Photocatalytic Activity of CoFe2O4 Via Heterojunction Formation for Photocatalysis of Organic Pollutants in Water. J. Saudi Chem. Soc. Singh 2019, 23, 1119–1136. [Google Scholar]
- Habibi, M.H.; Janan, J. Cobalt Ferrite Nano-Composite Coated on Glass by Doctor Blade Method for Photo-Catalytic Degradation of an Azo Textile Dye Reactive Red 4: Xrd, Fesem and Drs Investigations. Spectrochim. Acta Part A Mol. Parhizkar Biomol. Spectrosc. 2015, 150, 879–885. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, V.; Bansal, S.; Singhal, S. Boosting the Catalytic Performance of Pristine CoFe2O4 with Yttrium (Y3+) Inclusion in the Spinel Structure. Mater. Res. Bull. Singhal 2017, 90, 94–103. [Google Scholar] [CrossRef]
- Devi, L.G.; Srinivas, M. Hydrothermal synthesis of reduced graphene oxide-CoFe2O4 heteroarchitecture for high visible light photocatalytic activity: Exploration of efficiency, stability and mechanistic pathways. J. Environ. Chem. Eng. 2017, 5, 3243–3255. [Google Scholar] [CrossRef]
- Yamani, Z. Magnetic Properties and Photocatalytic Degradation Performance of MFe2O4 (M=Co, Ni)/Biocl Composites Catalysts under Uv Light Irradiation. Arab. J. Sci. Eng. 2018, 43, 383–388. [Google Scholar] [CrossRef]
- Fan, H.; Pan, S.; Teng, X.; Ye, C.; Li, G.; Zhang, L. δ-Bi2O3 thin films prepared by reactive sputtering: Fabrication and characterization. Thin Solid Film. 2006, 513, 142–147. [Google Scholar] [CrossRef]
- Shan, D.; Zhang, J.; Xue, H.-G.; Zhang, Y.-C.; Cosnier, S.; Ding, S.-N. Polycrystalline bismuth oxide films for development of amperometric biosensor for phenolic compounds. Biosens. Bioelectron. 2009, 24, 3671–3676. [Google Scholar] [CrossRef]
- Shafi, K.V.; Gedanken, A.; Prozorov, R.; Balogh, J. Sonochemical preparation and size-dependent properties of nanostructured CoFe2O4 particles. Chem. Mater. 1998, 10, 3445–3450. [Google Scholar] [CrossRef]
- Zi, Z.; Sun, Y.; Zhu, X.; Yang, Z.; Dai, J.; Song, W. Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1251–1255. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, S.; Hanif, M.A.; Shahid, S.A. Nanoscience for environmental remediation: A review. Int. J. Chem. Biochem. Sci. 2012, 2, 60–77. [Google Scholar]
- Dong, F.; Guo, S.; Wang, H.; Li, X.; Wu, Z. Enhancement of the visible light photocatalytic activity of C-doped TiO2 nanomaterials prepared by a green synthetic approach. J. Phys. Chem. C 2011, 115, 13285–13292. [Google Scholar] [CrossRef]
- Narayana, R.L.; Matheswaran, M.; Aziz, A.A.; Saravanan, P. Photocatalytic decolourization of basic green dye by pure and Fe, Co doped TiO2 under daylight illumination. Desalination 2011, 269, 249–253. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Deac, I.G.; Neag, E.; Cadar, O. Influence of Cu2+, Ni2+, and Zn2+ Ions Doping on the Structure, Morphology, and Magnetic Properties of Co-Ferrite Embedded in SiO2 Matrix Obtained by an Innovative Sol-Gel Route. Nanomaterials 2020, 10, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serpone, N.; Emeline, A. Semiconductor Photocatalysis-Past, Present, and Future Outlook; ACS Publications: Washington, DC, USA, 2012. [Google Scholar]
- Chen, S.; Hu, Y.; Ji, L.; Jiang, X.; Fu, X. Preparation and characterization of direct Z-scheme photocatalyst Bi2O3/NaNbO3 and its reaction mechanism. Appl. Surf. Sci. 2014, 292, 357–366. [Google Scholar] [CrossRef]
- Kang, I.-C.; Zhang, Q.; Yin, S.; Sato, T.; Saito, F. Improvement in photocatalytic activity of TiO2 under visible irradiation through addition of N-TiO2. Environ. Sci. Technol. 2008, 42, 3622–3626. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Luo, R.; Li, D.; Chen, A.; Liu, C.-C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MoO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Rakass, S.; Al Wadaani, F.T.; Al-Ghamdi, K.J.; Omer, A.; Messali, M.; Abboudi, M. Synthesis, characterization and photocatalytic activity of α-Bi2O3 nanoparticles. J. Taibah Univ. Sci. 2015, 9, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Mallahi, M.; Shokuhfar, A.; Vaezi, M.; Esmaeilirad, A.; Mazinani, V. Synthesis and characterization of bismuth oxide nanoparticles via sol-gel method. AJER 2014, 3, 162–165. [Google Scholar]
- Cote, L.J.; Teja, A.S.; Wilkinson, A.P.; Zhang, Z.J. Continuous hydrothermal synthesis of CoFe2O4 nanoparticles. Fluid Phase Equilibria 2003, 210, 307–317. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic degradation of methyl orange by TiO2-coated activated carbon and kinetic study. Water Res. 2006, 40, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Xu, B.-Q.; Zhao, J.; Mai, B.; Sheng, G.; Fu, J. Enhanced photocatalytic performance of nanosized coupled ZnO/SnO2 photocatalysts for methyl orange degradation. J. Photochem. Photobiol. A Chem. 2004, 168, 47–52. [Google Scholar] [CrossRef]
- Mendelson, M.I. Average grain size in polycrystalline ceramics. J. Am. Ceram. Soc. 1969, 52, 443–446. [Google Scholar] [CrossRef]
- Medernach, J.W.; Snyder, R.L. Powder diffraction patterns and structures of the bismuth oxides. J. Am. Ceram. Soc. 1978, 61, 494–497. [Google Scholar] [CrossRef]
- Khan, M.S.; Ashiq, M.N.; Ehsan, M.F.; He, T.; Ijaz, S. Controlled synthesis of cobalt telluride superstructures for the visible light photo-conversion of carbon dioxide into methane. Appl. Catal. A Gen. 2014, 487, 202–209. [Google Scholar] [CrossRef]
- Bera, K.K.; Majumdar, R.; Chakraborty, M.; Bhattacharya, S.K. Phase control synthesis of α, β and α/β Bi2O3 hetero-junction with enhanced and synergistic photocatalytic activity on degradation of toxic dye, Rhodamine-B under natural sunlight. J. Hazard. Mater. 2018, 352, 182–191. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Xie, M.; Xu, H.; He, M.; Xia, J.; Huang, L.; Li, H. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 71–80. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Y.; Chang, B.; Xi, F.; Dong, X. BiOBr–carbon nitride heterojunctions: Synthesis, enhanced activity and photocatalytic mechanism. J. Mater. Chem. 2012, 22, 21159–21166. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A review on UV/TiO2 photocatalytic oxidation process (Journal Review). Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- He, G.; Ding, J.; Zhang, J.; Hao, Q.; Chen, H. One-step ball-milling preparation of highly photocatalytic active CoFe2O4–reduced graphene oxide heterojunctions for organic dye removal. Ind. Eng. Chem. Res. 2015, 54, 2862–2867. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Ala’a, H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]


| Sample | Average Crystallite Size (nm) |
|---|---|
| Bi2O3 | 32 |
| CoFe2O4 | 26.6 |
| NC-1 | 19 |
| NC-2 | 21 |
| NC-3 | 30 |
| Sample | Elemental Weight Percentage | |||
|---|---|---|---|---|
| Co | Fe | Bi | O | |
| CoFe2O4 | 17.43 | 30.48 | - | 52.09 |
| Bi2O3 | - | - | 23.06 | 76.94 |
| NC-1 | 21.20 | 44.19 | 19.97 | 14.63 |
| NC-2 | 13.13 | 28.14 | 25.42 | 33.31 |
| NC-3 | 10.73 | 17.74 | 47.89 | 23.63 |
| Sample | Absorbance | |||||
|---|---|---|---|---|---|---|
| Time (h) | 0 | 1 | 2 | 3 | 4 | 5 |
| MO-Blank | 1.00 | 0.998 | 0.995 | 0.991 | 0.986 | 0.980 |
| CoFe2O3 | 0.99 | 0.97 | 0.94 | 0.86 | 0.67 | 0.37 |
| Bi2O3 | 0.95 | 0.91 | 0.89 | 0.82 | 0.53 | 0.24 |
| NC-I | 0.85 | 0.79 | 0.76 | 0.63 | 0.32 | 0.15 |
| NC-II | 0.82 | 0.77 | 0.74 | 0.58 | 0.34 | 0.06 |
| NC-III | 0.88 | 0.84 | 0.82 | 0.67 | 0.48 | 0.19 |
| Wt.% of Bi2O3 | Wt.% of CoFe2O4 | |
|---|---|---|
| NC-1 | 25 | 75 |
| NC-2 | 50 | 50 |
| NC-3 | 75 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveed, A.B.; Riaz, F.; Mahmood, A.; Shahid, A.; Aqeel, S. A Facile Synthesis of Bi2O3/CoFe2O4 Nanocomposite with Improved Synergistic Photocatalytic Potential for Dye Degradation. Catalysts 2021, 11, 1180. https://doi.org/10.3390/catal11101180
Naveed AB, Riaz F, Mahmood A, Shahid A, Aqeel S. A Facile Synthesis of Bi2O3/CoFe2O4 Nanocomposite with Improved Synergistic Photocatalytic Potential for Dye Degradation. Catalysts. 2021; 11(10):1180. https://doi.org/10.3390/catal11101180
Chicago/Turabian StyleNaveed, Abdul Basit, Fakhira Riaz, Azhar Mahmood, Ammara Shahid, and Saman Aqeel. 2021. "A Facile Synthesis of Bi2O3/CoFe2O4 Nanocomposite with Improved Synergistic Photocatalytic Potential for Dye Degradation" Catalysts 11, no. 10: 1180. https://doi.org/10.3390/catal11101180
APA StyleNaveed, A. B., Riaz, F., Mahmood, A., Shahid, A., & Aqeel, S. (2021). A Facile Synthesis of Bi2O3/CoFe2O4 Nanocomposite with Improved Synergistic Photocatalytic Potential for Dye Degradation. Catalysts, 11(10), 1180. https://doi.org/10.3390/catal11101180