Amperometric Oxygen Sensor Based on Bimetallic Pd-Cu/C Electrocatalysts
Abstract
:1. Introduction
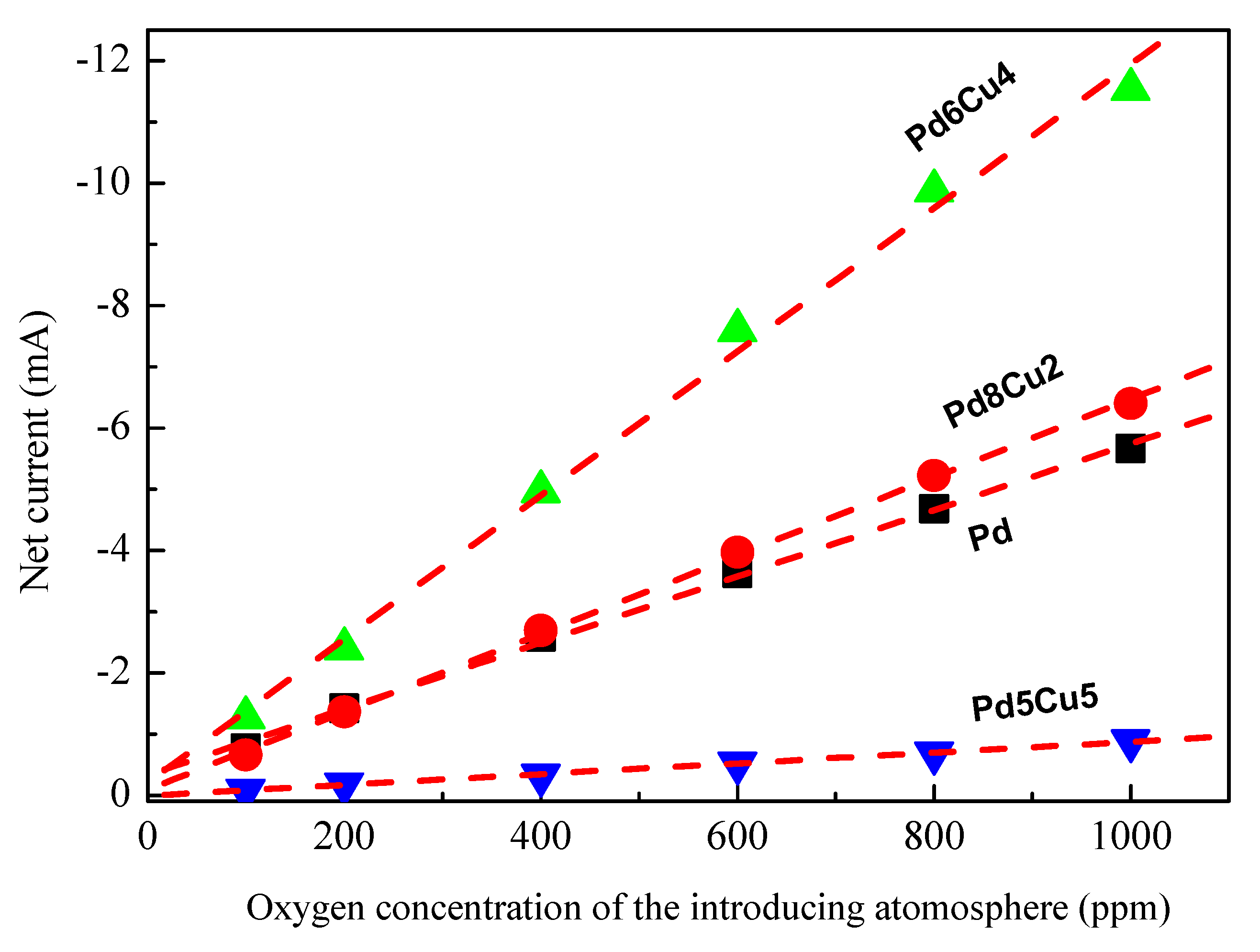
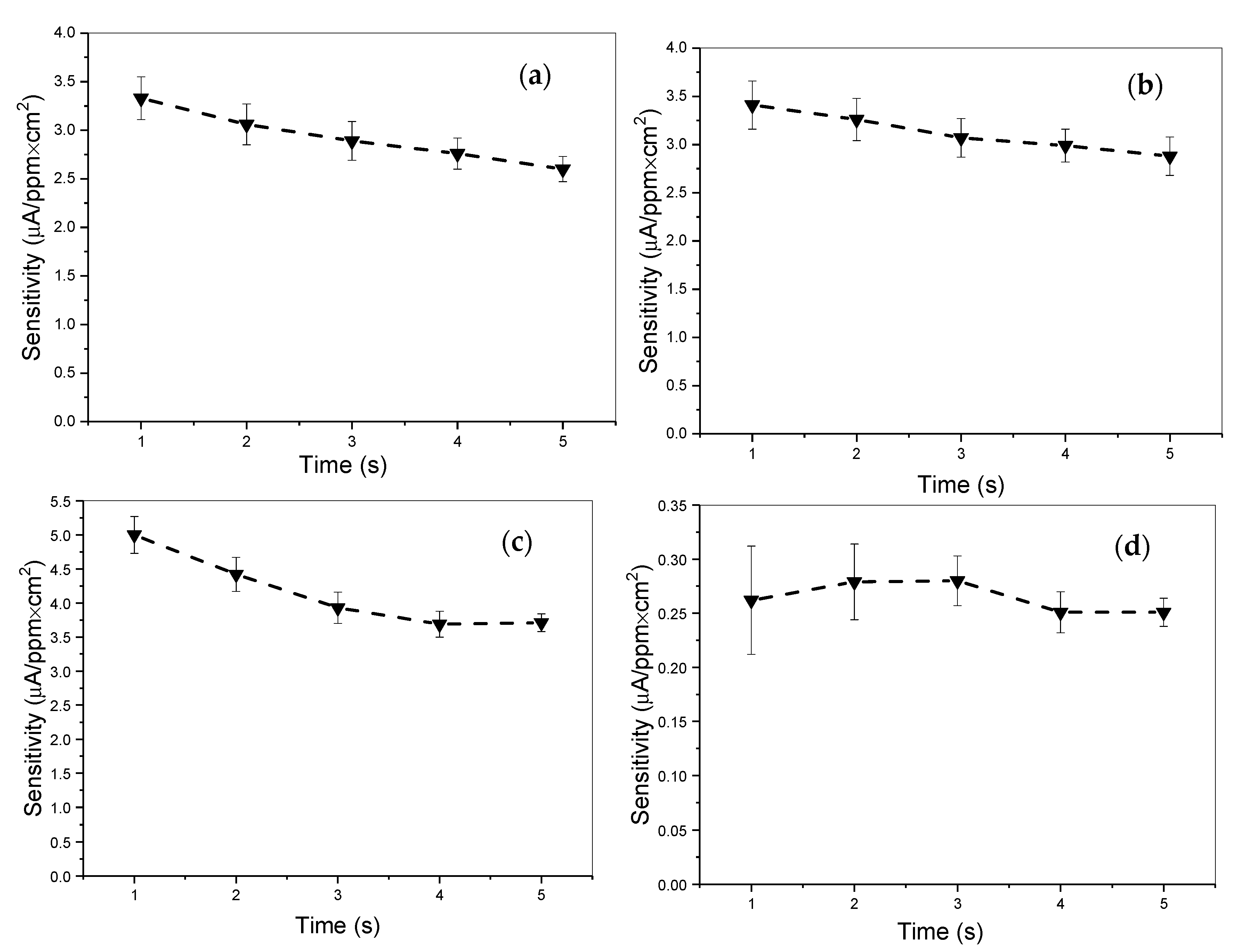
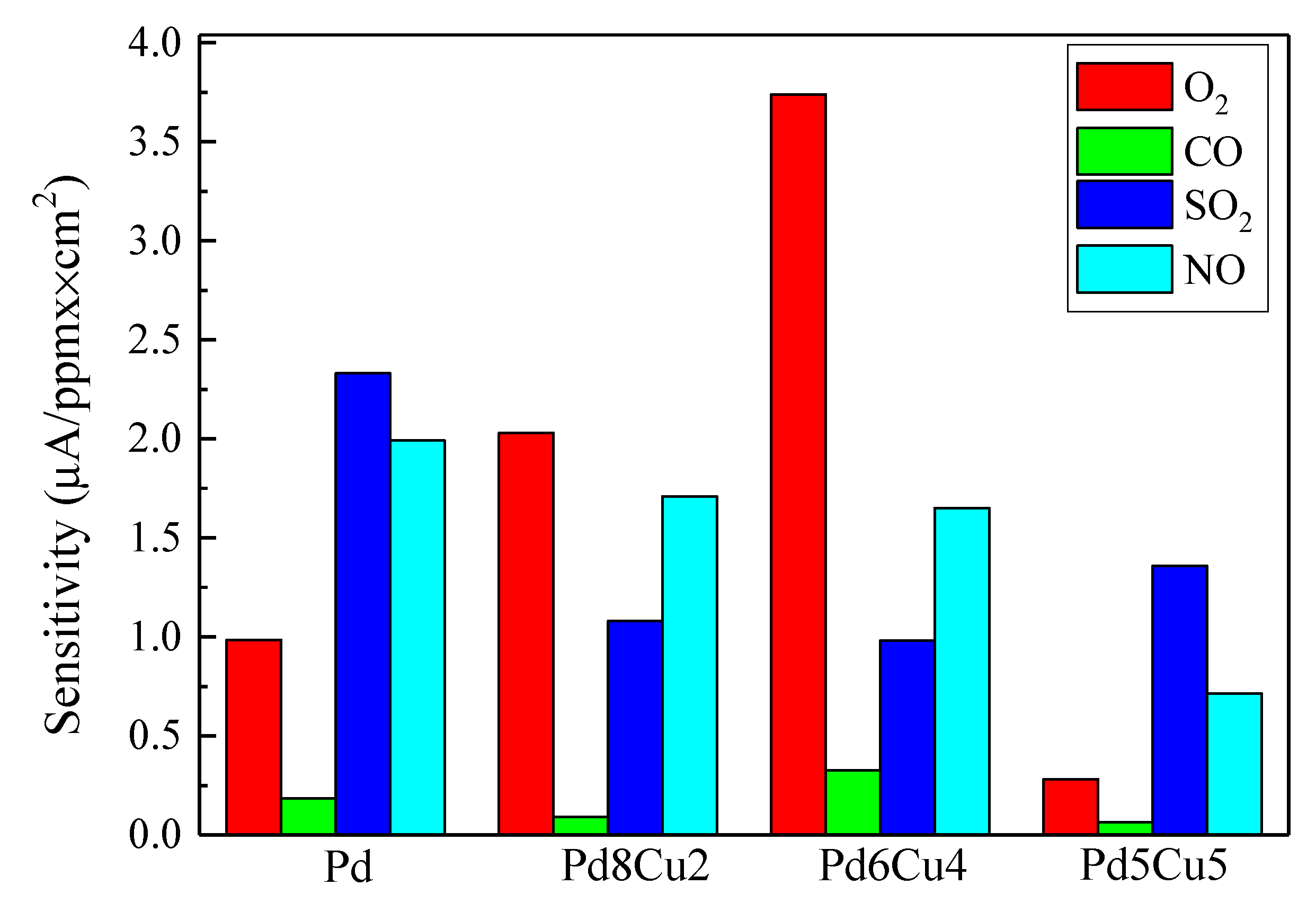
2. Results and Discussion
2.1. Microstructure Investigation of the Electrodes
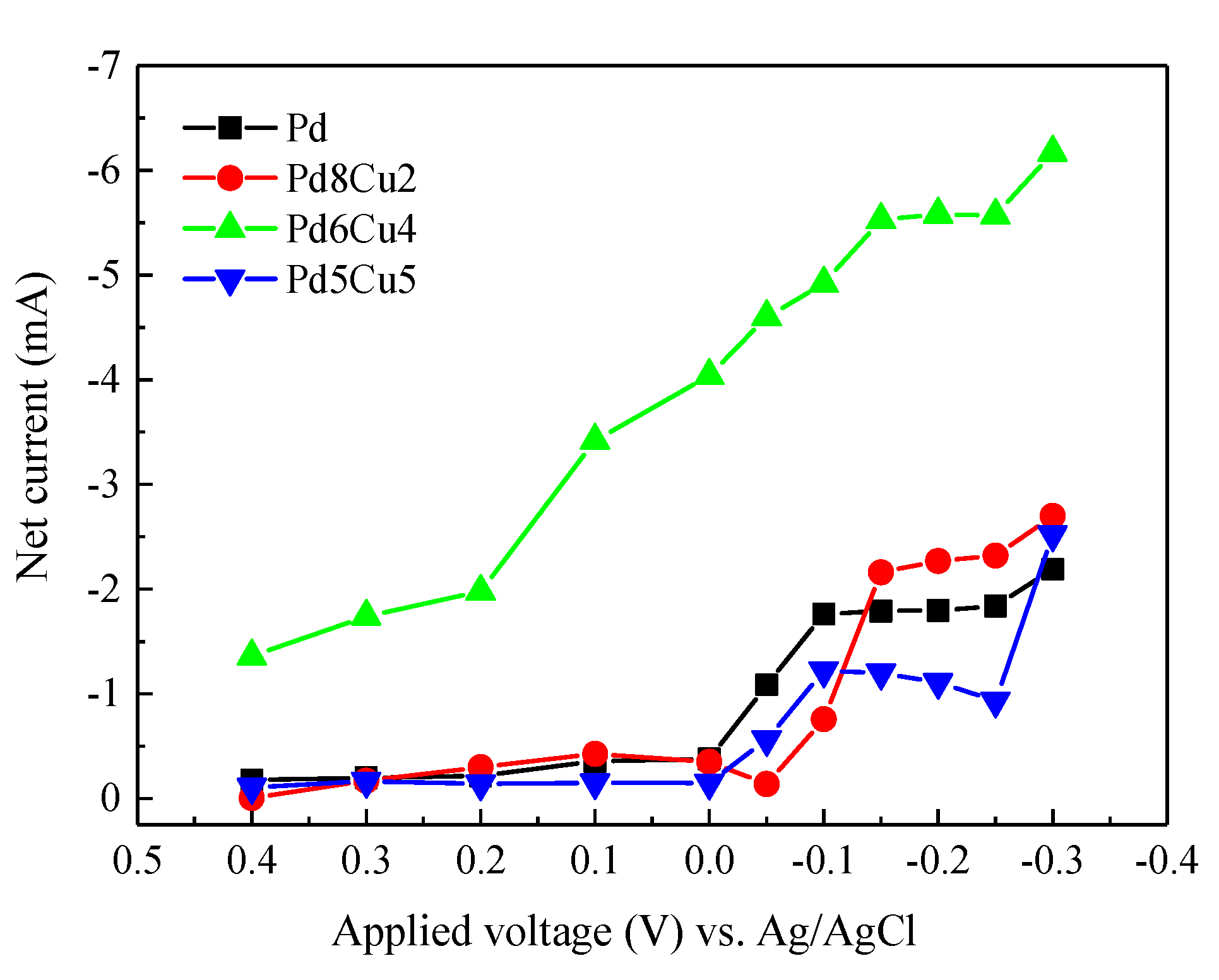
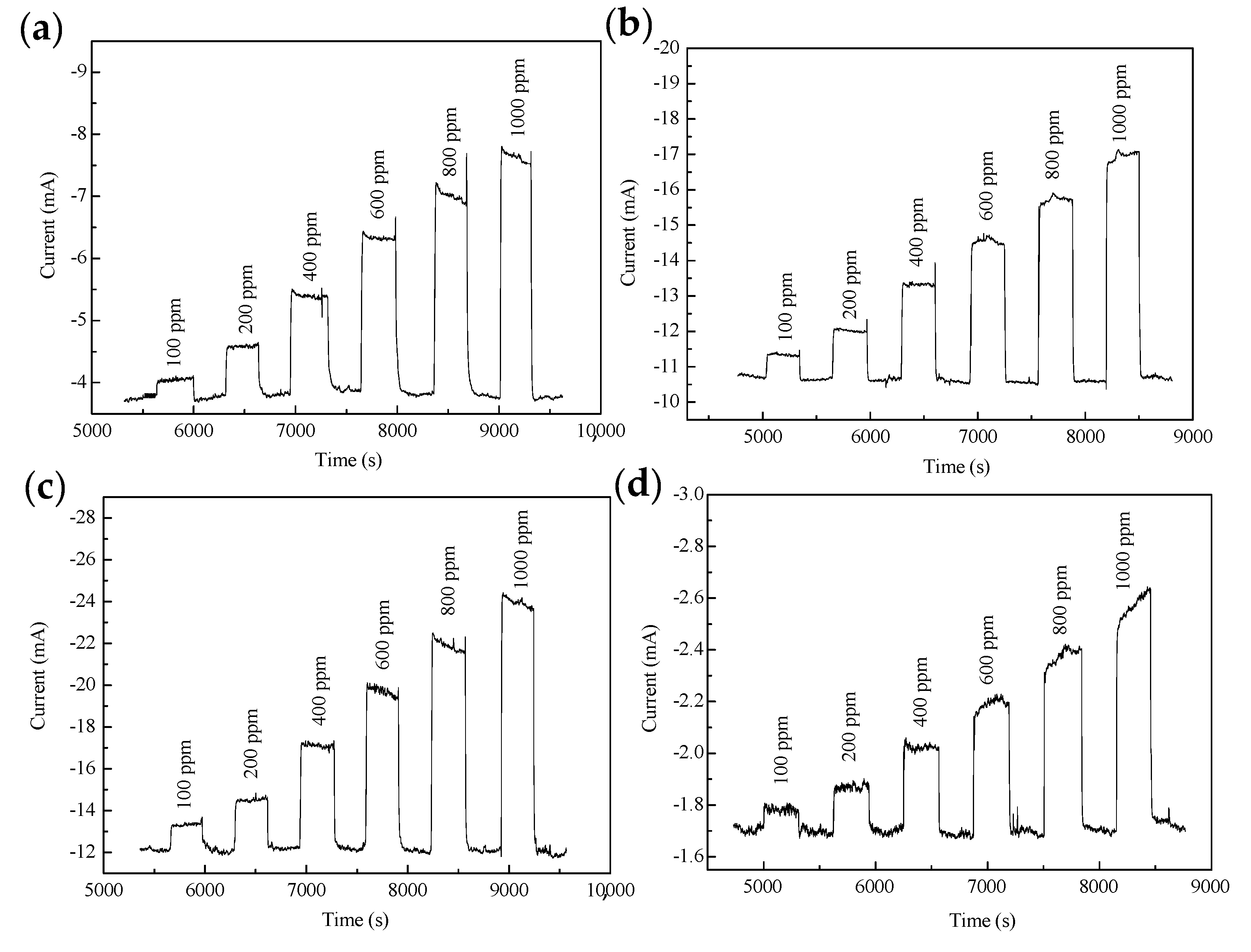
2.2. Electrochemistry
3. Materials and Methods Discussion
3.1. Glassy Carbon Substrate
3.2. Preparation for the Catalyst of Electrochemical Reaction
3.3. Enhancement of the Electrocatalyst
3.4. Structure for O2 Sensing
3.5. Measurements
3.5.1. Preliminary Test for the Optimum Electrochemical Reaction
3.5.2. Investigation of the Electrode Microstructure and Electrochemistry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, T.; Brisard, G.M. Determination of the kinetic parameters of oxygen reduction on copper using a rotating ring single crystal disk assembly (RRDCu(hkl)E). Electrochim. Acta 2007, 52, 4487–4496. [Google Scholar] [CrossRef]
- Trens, P.; Durand, R.; Coq, B.; Coutanceau, C.; Rousseau, S.; Lamy, C. Poisoning of Pt/C catalysts by CO and its consequences over the kinetics of hydrogen chemisorption. Appl. Catal. B 2009, 92, 280–284. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, S.; Strasser, P. Electrocatalysis on Bimetallic Surfaces: Modifying Catalytic Reactivity for Oxygen Reduction by Voltammetric Surface Dealloying. J. Am. Chem. Soc. 2007, 129, 12624. [Google Scholar] [CrossRef]
- Sadjadi, S.; Malmir, M.; Heravi, M.M.; Kahangi, F.G. Biocompatible starch-halloysite hybrid: An efficient support for immobilizing Pd species and developing a heterogeneous catalyst for ligand and copper free coupling reactions. Int. J. Biol. Macromol. B 2018, 18, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Ferna’ndez, J.L.; Walsh, D.A.; Bard, A.J. Thermodynamic Guidelines for the Design of Bimetallic Catalysts for Oxygen Electroreduction and Rapid Screening by Scanning Electrochemical Microscopy. M-Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Barrabe´s, N.; Just, J.; Dafinov, A.; Medina, F.; Fierro, J.L.G.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Catalytic reduction of nitrate on Pt-Cu and Pd-Cu on active carbon using continuous reactor. The effect of copper nanoparticles. Appl. Catal. B 2006, 62, 77–85. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic Reduction of Carbon Dioxide by Hydrous Hydrazine over Au–Cu Alloy Nanoparticles Supported on SrTiO3/TiO2 Coaxial Nanotube Arrays. Angew. Chem. Int. Ed. 2015, 54, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, H.; Saadatinasab, M.; Zolfaghari, A. Hydrogen permeability and sulfur tolerance of a novel dual membrane of PdAg/PdCu layers deposited on porous stainless steel. J. Membr. Sci. 2013, 447, 355–361. [Google Scholar] [CrossRef]
- Gobal, F.; Arab, R. A preliminary study of the electro-catalytic reduction of oxygen on Cu–Pd alloys in alkaline solution. J. Electroanal. Chem. 2010, 647, 66–73. [Google Scholar] [CrossRef]
- Zoski, C.G. Review—Advances in Scanning Electrochemical Microscopy (SECM). J. Electrochem. Soc. 2016, 163, H3088–H3100. [Google Scholar] [CrossRef]
- Wu, J.; Shan, S.; Luo, J.; Joseph, P.; Petkov, V.; Zhong, C.J. PdCu Nanoalloy Electrocatalysts in Oxygen Reduction Reaction: Role of Composition and Phase State in Catalytic Synergy. Appl. Mater. Interfaces 2015, 7, 25906–25913. [Google Scholar] [CrossRef] [PubMed]
- Fouda-Onana, F.; Bah, S.; Savadogo, O. Palladium–copper alloys as catalysts for the oxygen reduction reaction in an acidic media I: Correlation between the ORR kinetic parameters and intrinsic physical properties of the alloys. J. Electroanal. Chem. 2009, 636, 1–9. [Google Scholar] [CrossRef]
- Ruban, A.V.; Skriver, H.L.; Norskov, J.K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59, 15990. [Google Scholar] [CrossRef] [Green Version]
- Peuckert, M. XPS investigation of surface oxidation layers on a platinum electrode in alkaline solution. Electrochim. Acta 1984, 20, 1315–1320. [Google Scholar] [CrossRef]
- Tura, J.M.; Regull, P.; Victorit, L.; Castellar, M.D. XPS and IR (ATR) Analysis of Pd Oxide Films Obtained by Electrochemical Methods. Surf. Interface Anal. 1988, 11, 447–449. [Google Scholar] [CrossRef]
- Shao, M.H.; Huang, T.; Liu, P.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Adzic, R.R. Palladium Monolayer and Palladium Alloy Electrocatalysts for Oxygen Reduction. Langmuir 2006, 22, 10409–10415. [Google Scholar] [CrossRef]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, L.; Henkelman, G. Catalytic Activity of Pd/Cu Random Alloy Nanoparticles for Oxygen Reduction. J. Phys. Chem. Lett. 2011, 2, 1328–1331. [Google Scholar] [CrossRef]
- Wang, X.; Kariuki, N.; Vaughey, J.T.; Goodpaster, J.; Kumar, R.; Myers, D.J. Bimetallic Pd-Cu Oxygen Reduction Electrocatalysts. J. Electrochem. Soc. 2008, 155, B602–B609. [Google Scholar] [CrossRef]
- Ye, H.; Crooks, R.M. Effect of Elemental Composition of PtPd Bimetallic Nanoparticles Containing an Average of 180 Atoms on the Kinetics of the Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2007, 129, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
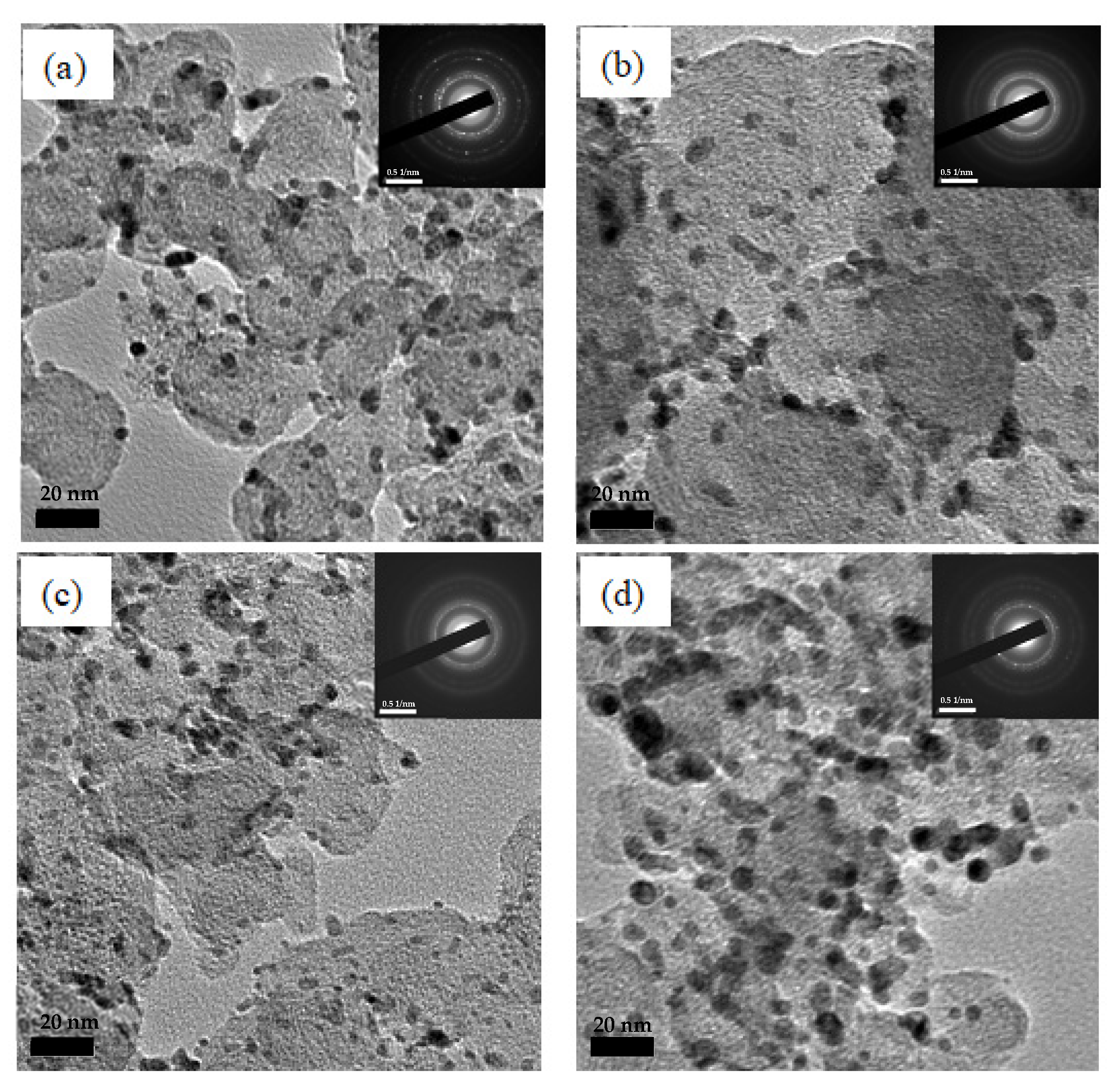

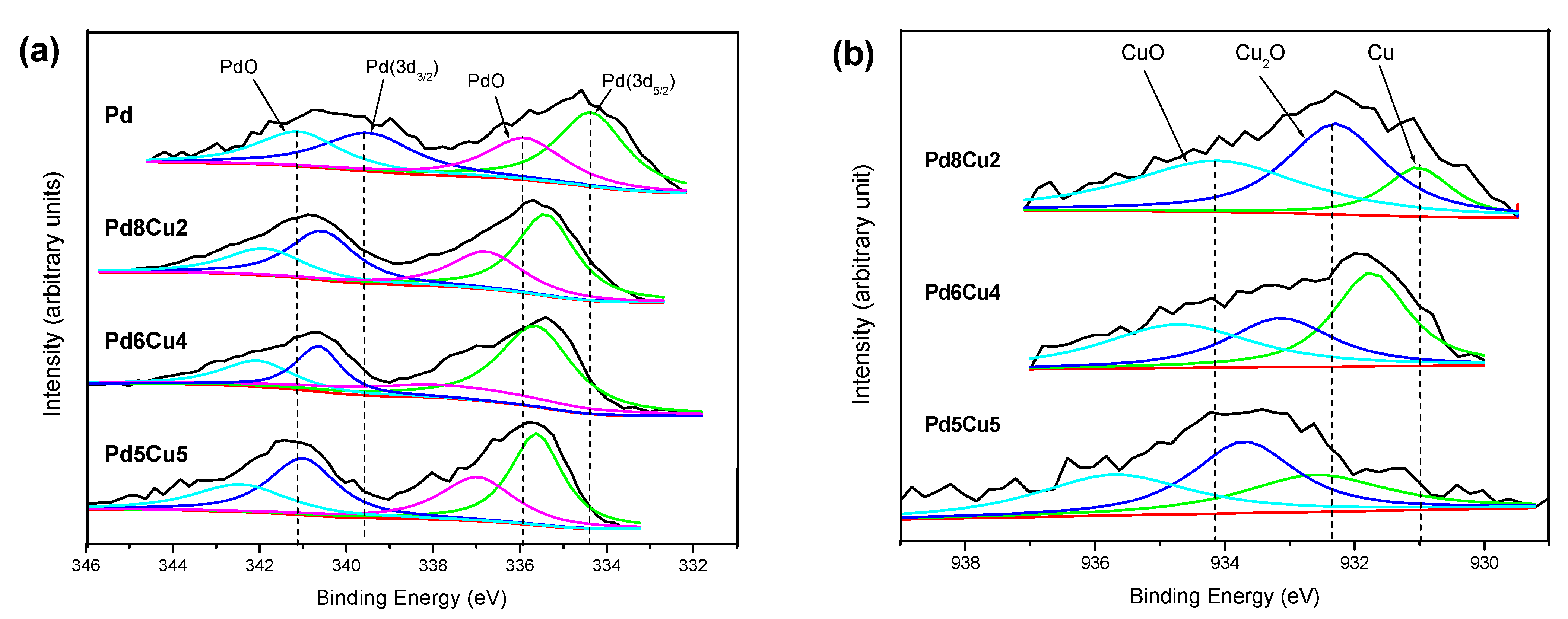


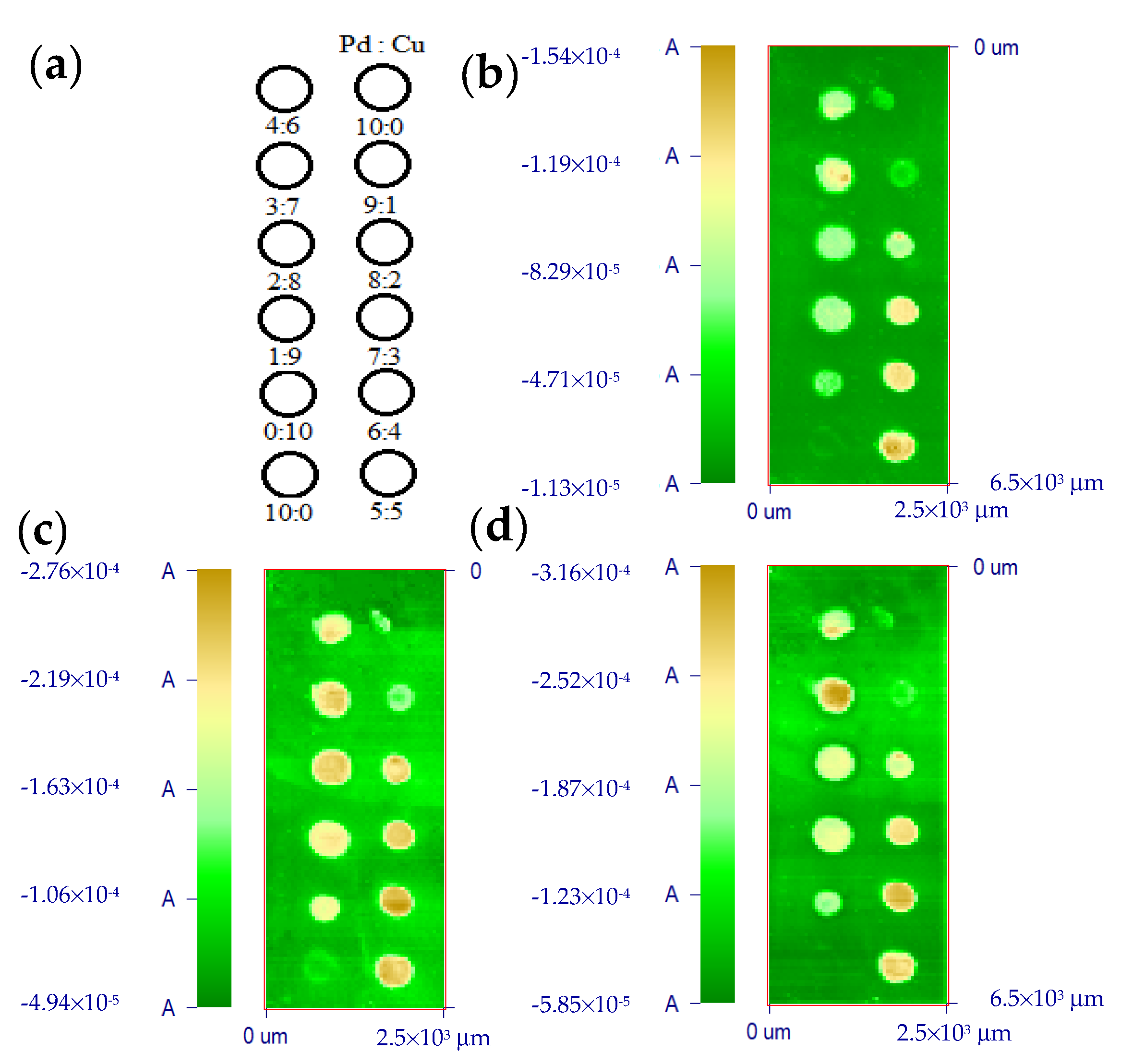
| Catalysts | Composition (atm.%) | Particle Size (nm) | |
|---|---|---|---|
| Pd | Cu | ||
| Pd | 100 | 0 | 5.6 |
| Pd8Cu2 | 85.1 | 14.9 | 4.7 |
| Pd6Cu4 | 71.1 | 28.9 | 4.5 |
| Pd5Cu5 | 60.0 | 40.0 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-G.; Hou, Y.-T.; Weng, Y.-C. Amperometric Oxygen Sensor Based on Bimetallic Pd-Cu/C Electrocatalysts. Catalysts 2021, 11, 1189. https://doi.org/10.3390/catal11101189
Lee Y-G, Hou Y-T, Weng Y-C. Amperometric Oxygen Sensor Based on Bimetallic Pd-Cu/C Electrocatalysts. Catalysts. 2021; 11(10):1189. https://doi.org/10.3390/catal11101189
Chicago/Turabian StyleLee, Yuan-Gee, Ya-Tian Hou, and Yu-Ching Weng. 2021. "Amperometric Oxygen Sensor Based on Bimetallic Pd-Cu/C Electrocatalysts" Catalysts 11, no. 10: 1189. https://doi.org/10.3390/catal11101189
APA StyleLee, Y.-G., Hou, Y.-T., & Weng, Y.-C. (2021). Amperometric Oxygen Sensor Based on Bimetallic Pd-Cu/C Electrocatalysts. Catalysts, 11(10), 1189. https://doi.org/10.3390/catal11101189






