Promoting Role of Amorphous Carbon and Carbon Nanotubes Growth Modes of Methane Decomposition in One-Pot Catalytic Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystalline Structure—X-ray Diffraction Analysis
2.2. Textural Properties—N2 Physisorption Isotherms Measurement
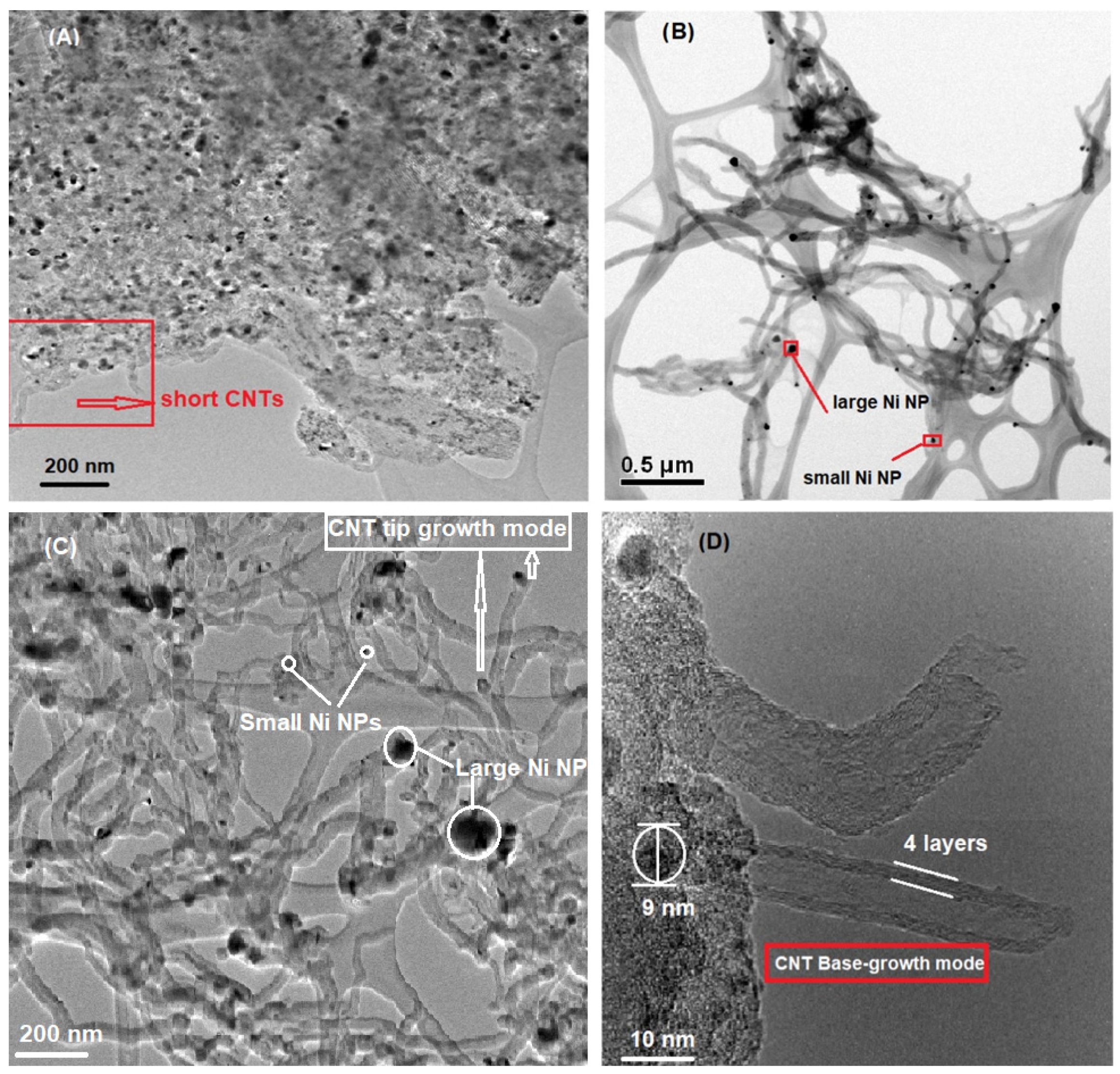
2.3. Morphology–Transmission Electron Microscopy (TEM)
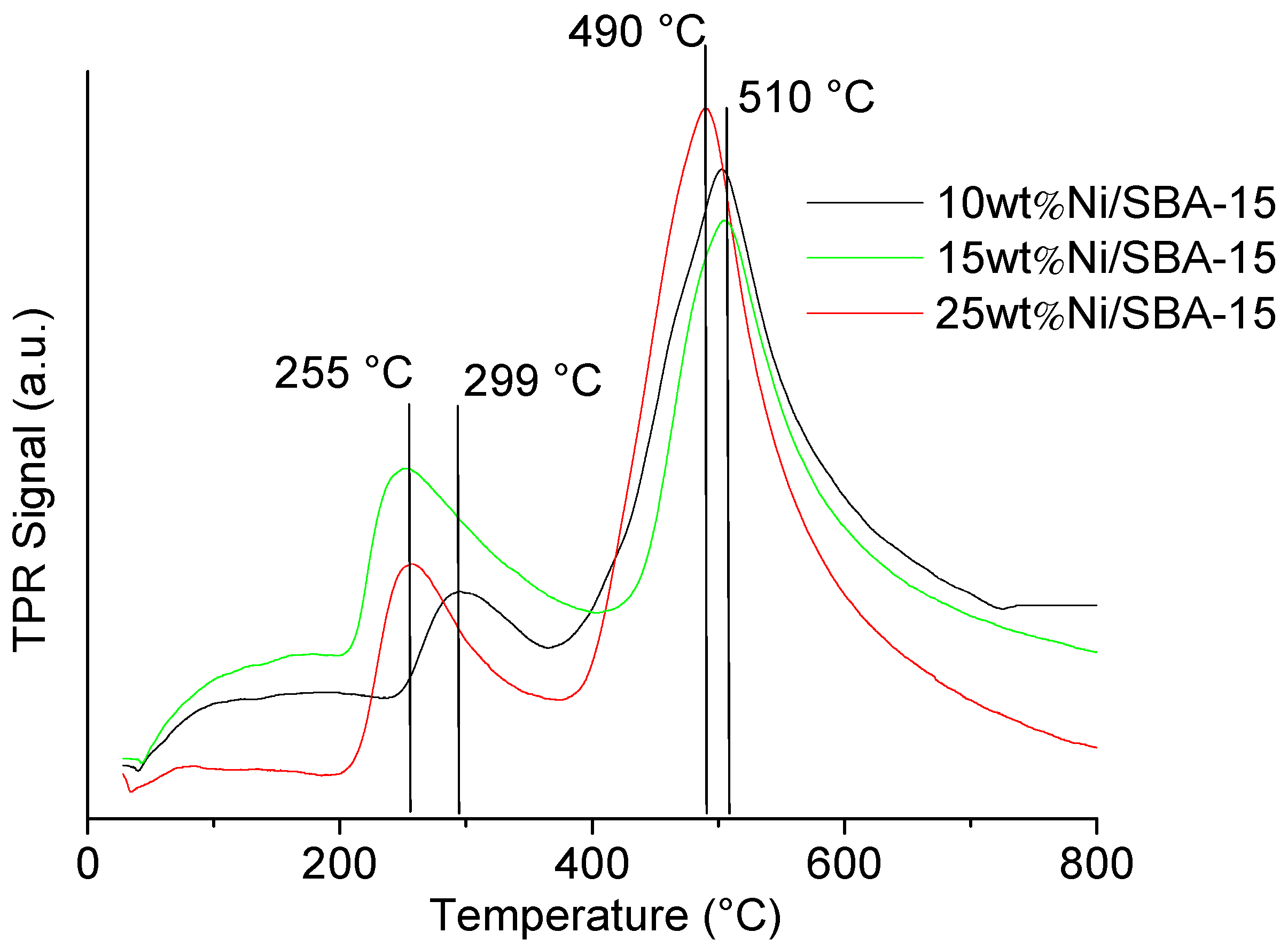
2.4. Oxygen Species Reducibility–Temperature-Programmed Reduction (TPR)
2.5. Catalytic Properties–Methane Catalytic Decomposition
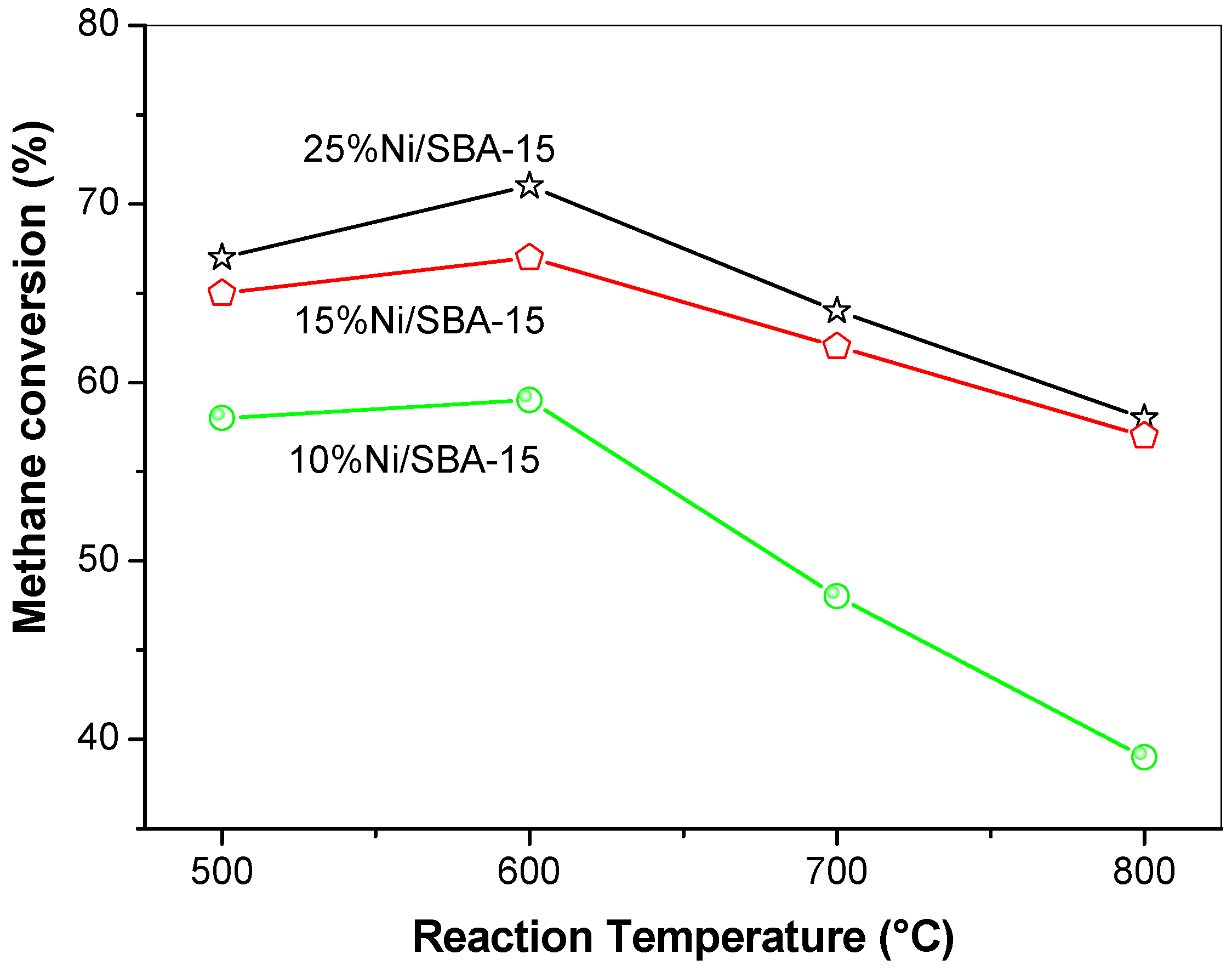
2.5.1. CH4 Conversion as a Function of Reaction Temperature
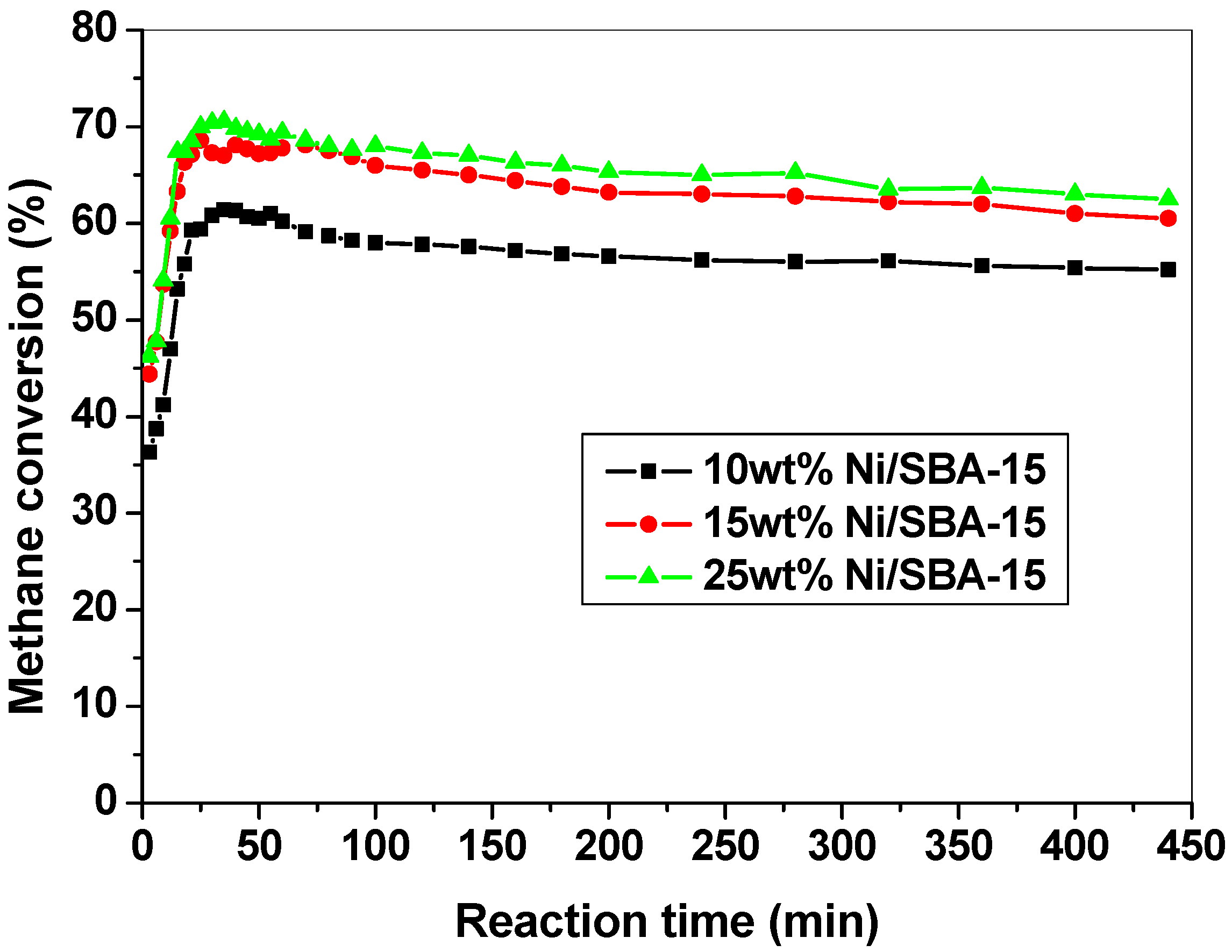
2.5.2. CH4 Conversion as a Function of Reaction Time
2.5.3. Raman Spectra
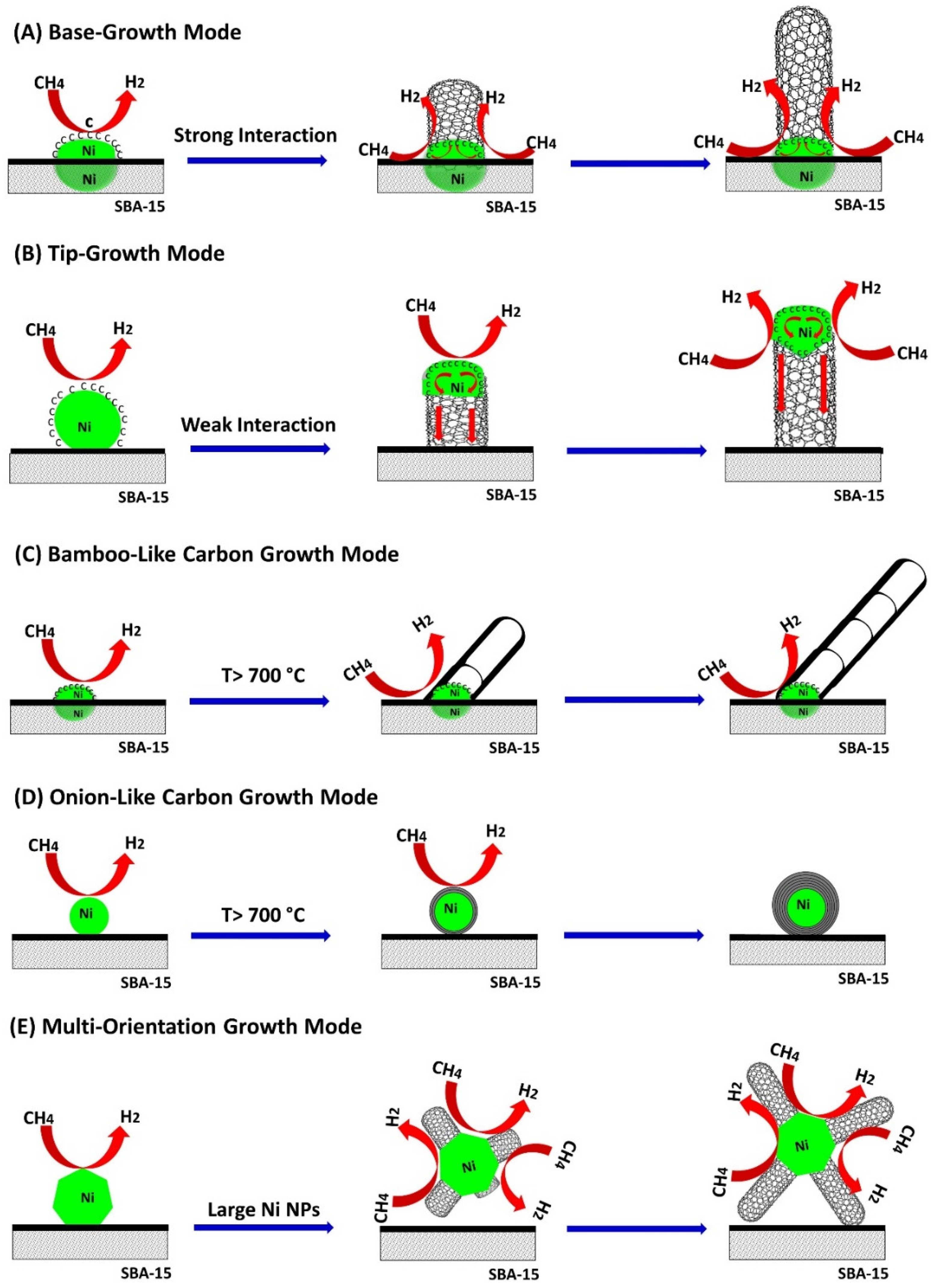
2.5.4. Formation of Nanocarbon and Growth Modes
- 1)
- Some CNTs had Ni NPs on their tip as shown in Figure 9B,C; and they grew following a tip-growth mode;
- 2)
- Some CNTs were formed through a base-growth mode where Ni particles anchored into the catalyst support and CNTs grew based on the surface of Ni NPs, Figure 9D;
- 3)
- Some bamboo-like CNTs were formed at higher temperature, i.e., 800 °C, as shown in Figure 10A,B;
- 4)
- Some metal-encapsulated carbon composites were observed at higher temperature, forming onion-like carbon nanomaterials as shown in Figure 10C,D;
- 5)
- It was observed some large size Ni NPs have several crystal faces where CNTs grew towards different orientations (Figure 10D).
3. Materials and Methods
3.1. Ultrasonic-Assisted Synthesis of SBA-15
3.2. Synthesis of Ni/SBA-15 Catalysts
3.3. Characterization
3.4. Catalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiello, R.; Fiscus, J.E.; Loye, H.-C.Z.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over Ni/SiO2: Catalyst deactivation and regeneration. Appl. Catal. A Gen. 2000, 192, 227–234. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Armor, J. The multiple roles for catalysis in the production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Song, C. Fuel processing for low-temperature and high-temperature fuel cells Challenges, and opportunities for sustainable development in the 21st century. Catal. Today 2002, 77, 17–49. [Google Scholar] [CrossRef]
- McHugh, K. Hydrogen Production Methods; MPR Associates Inc.: Alexandria, VA, USA, 2005; Volume 41, Available online: www.mpr.com/pubs (accessed on 10 September 2015).
- Song, H.; Zhang, L.; Ozkan, U.S. Effect of synthesis parameters on the catalytic activity of Co–ZrO2 for bio-ethanol steam reforming. Green Chem. 2007, 9, 686–694. [Google Scholar] [CrossRef]
- Zhang, Y.; Smith, K.J. CH4 decomposition on Co catalysts: Effect of temperature, dispersion, and the presence of H2 or CO in the feed. Catal. Today 2002, 77, 257–268. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Collins, P.G.; Avouris, P. Nanotubes for Electronics. Sci. Am. 2000, 283, 62–69. [Google Scholar] [CrossRef]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’Ko, Y. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Barone, P.W.; Baik, S.; Heller, D.A.; Strano, M.S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat. Mater. 2004, 4, 86–92. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. BioMed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jhi, S.-H.; Kwon, Y.-K.; Bradley, K.; Gabriel, J.-C.P. Hydrogen storage by physisorption: Beyond carbon. Solid State Commun. 2004, 129, 769–773. [Google Scholar] [CrossRef]
- Peera, S.G.; Maiyalagan, T.; Liu, C.; Ashmath, S.; Lee, T.G.; Jiang, Z.; Mao, S. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int. J. Hydrog. Energy 2021, 46, 3056–3089. [Google Scholar] [CrossRef]
- Camilli, L.; Pisani, C.; Gautron, E.; Scarselli, M.; Castrucci, P.; D’Orazio, F.; Passacantando, M.; Moscone, D.; De Crescenzi, M. A three-dimensional carbon nanotube network for water treatment. Nanotechnology 2014, 25, 065701. [Google Scholar] [CrossRef]
- Venugopal, A.; Kumar, S.N.; Ashok, J.; Prasad, D.H.; Kumari, V.D.; Prasad, K.; Subrahmanyam, M. Hydrogen production by catalytic decomposition of methane over Ni/SiO2. Int. J. Hydrog. Energy 2007, 32, 1782–1788. [Google Scholar] [CrossRef]
- Wang, H.; Baker, R.T.K. Decomposition of Methane over a Ni−Cu−MgO Catalyst to Produce Hydrogen and Carbon Nanofibers. J. Phys. Chem. B 2004, 108, 20273–20277. [Google Scholar] [CrossRef]
- Zaikovskii, V.I.; Chesnokov, V.V.; Buyanov, R.A. The Relationship between the State of Active Species in a Ni/Al2O3Catalyst and the Mechanism of Growth of Filamentous Carbon. Kinet. Catal. 2001, 42, 813–820. [Google Scholar] [CrossRef]
- Salmones, J.; Wang, J.; Valenzuela, M.; Sánchez, E.; García, A. Pore geometry influence on the deactivation behavior of Ni-based catalysts for simultaneous production of hydrogen and nanocarbon. Catal. Today 2009, 148, 134–139. [Google Scholar] [CrossRef]
- Guevara, J.; Wang, J.; Chen, L.; Valenzuela, M.; Salas, P.; García-Ruiz, A.F.; Toledo, J.; Cortes-Jácome, M.; Angeles-Chavez, C.; Novaro, O. Ni/Ce-MCM-41 mesostructured catalysts for simultaneous production of hydrogen and nanocarbon via methane decomposition. Int. J. Hydrog. Energy 2010, 35, 3509–3521. [Google Scholar] [CrossRef]
- Wang, Z.; Navarrete, J. Production of Carbon Nanotubes and Hydrogen Catalyzed with Ni/MCM-41 Catalysts. Green Sustain. Chem. 2012, 2, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Ciuparu, D.; Chen, Y.; Lim, S.; Yang, Y.; Haller, G.L.; Pfefferle, L. Mechanism of Cobalt Cluster Size Control in Co-MCM-41 during Single-Wall Carbon Nanotubes Synthesis by CO Disproportionation. J. Phys. Chem. B 2004, 108, 15565–15571. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Chuvilin, A.; Kuvshinov, G.G. Decomposition of Methane over Iron Catalysts at the Range of Moderate Temperatures: The Influence of Structure of the Catalytic Systems and the Reaction Conditions on the Yield of Carbon and Morphology of Carbon Filaments. J. Catal. 2001, 201, 183–197. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Methane decomposition over Ni, Co and Fe based monometallic catalysts supported on sol gel derived SiO2 microflakes. Chem. Eng. J. 2015, 262, 1009–1021. [Google Scholar] [CrossRef]
- An, W.; Zeng, X.C.; Turner, C.H. First-principles study of methane dehydrogenation on a bimetallic Cu/Ni(111) surface. J. Chem. Phys. 2009, 131, 174702. [Google Scholar] [CrossRef]
- Li, J.; Xiao, C.; Xiong, L.; Chen, X.; Zhao, L.; Dong, L.; Du, Y.; Yang, Y.; Wang, H.; Peng, S. Hydrogen production by methane decomposition over Ni–Cu–SiO2 catalysts: Effect of temperature on catalyst deactivation. RSC Adv. 2016, 6, 52154–52163. [Google Scholar] [CrossRef]
- Lua, A.C.; Wang, H.Y. Hydrogen production by catalytic decomposition of methane over Ni-Cu-Co alloy particles. Appl. Catal. B Environ. 2014, 156–157, 84–93. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Hernández, A.; Noreña-Franco, L.E.; Chen, L.F.; Wang, J.A.; Aguilar, J. Refinery Oil Fraction Fuels Obtained from Polyethylene Catalytic Cracking Employing Heteropolyacid-MCM-41 Materials. Adv. Mater. Res. 2010, 132, 236–245. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Tian, N.; Gong, J.; Liu, J.; Rümmeli, M.H.; Chu, P.K.; Mijiwska, E.; Tang, T. Nanosized Carbon Black Combined with Ni2O3 as “Universal” Catalysts for Synergistically Catalyzing Carbonization of Polyolefin Wastes to Synthesize Carbon Nanotubes and Application for Supercapacitors. Environ. Sci. Technol. 2014, 48, 4048–4055. [Google Scholar] [CrossRef]
- Michaelides, A.; Hu, P. Softened C–H modes of adsorbed methyl and their implications for dehydrogenation: An ab initio study. J. Chem. Phys. 2001, 114, 2523–2526. [Google Scholar] [CrossRef]
- Sharma, A.; Saito, I.; Nakagawa, H.; Miura, K. Effect of carbonization temperature on the nickel crystallite size of a Ni/C catalyst for catalytic hydrothermal gasification of organic compounds. Fuel 2007, 86, 915–920. [Google Scholar] [CrossRef]
- Shaikhutdinov, S.K.; Avdeeva, L.; Goncharova, O.; Kochubey, D.; Novgorodov, B.; Plyasova, L. Coprecipitated Ni-Al and Ni-Cu-Al catalysts for methane decomposition and carbon deposition I. Genesis of Calcined and reduced catalysts. Appl. Catal. A Gen. 1995, 126, 125–139. [Google Scholar] [CrossRef]
- Ganesh, P.; Kent, P.R.C.; Mochalin, V. Formation, characterization, and dynamics of onion-like carbon structures for electrical energy storage from nanodiamonds using reactive force fields. J. Appl. Phys. 2011, 110, 073506. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2015, 4, 3172–3196. [Google Scholar] [CrossRef] [Green Version]
- Lutterotti, L. MAUD Version 2.55. Available online: http://www.maud.radiographema.com (accessed on 15 May 2015).
- Young, R.A.; Sakthivel, A.; Moss, T.S.; Paiva-Santos, C.O. DBWS-9411—An upgrade of the DBWS*.* programs for Rietveld refinement with PC and mainframe computers. J. Appl. Crystallogr. 1995, 28, 366–367. [Google Scholar] [CrossRef]

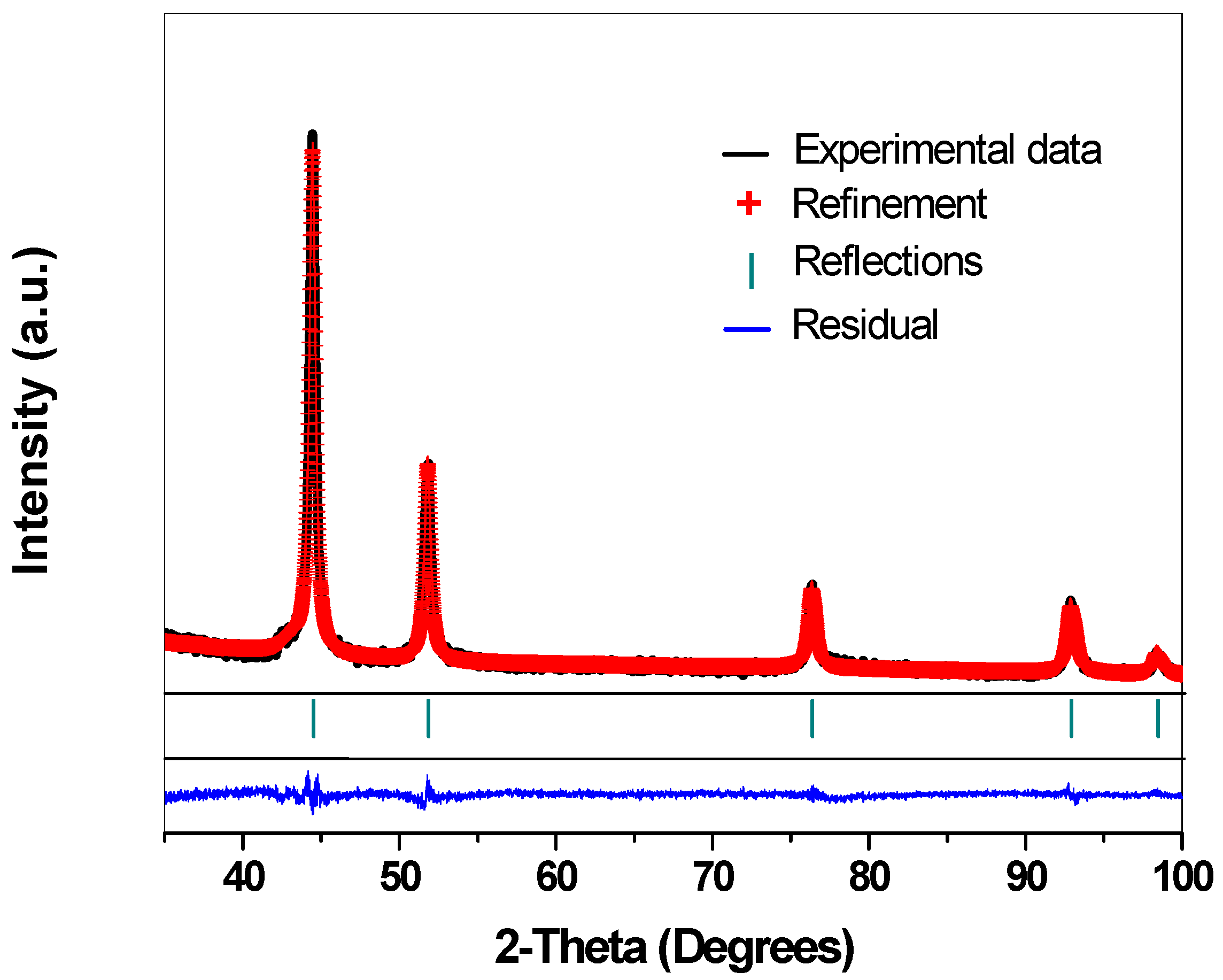




| Catalyst | Crystallite Size (nm) | Cell Parameter a0 (Å) | Rwp (%) |
|---|---|---|---|
| 10 wt%Ni/SBA-15 | 12.23 | 3.5241 | 4.62 |
| 15 wt%Ni/SBA-15 | 25.59 | 3.5360 | 1.06 |
| 25 wt%Ni/SBA-15 | 28.50 | 3.5259 | 0.58 |
| Catalyst | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| SBA-15 | 695.0 | 6.9 | 0.804 |
| 10% Ni/SBA-15 | 627.4 | 6.6 | 0.725 |
| 15% Ni/SBA-15 | 517.7 | 6.0 | 0.649 |
| 25% Ni/SBA-15 | 383.1 | 5.2 | 0.600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Noreña, L.E.; Wang, J.A.; Limas, R.; Arellano, U.; González Vargas, O.A. Promoting Role of Amorphous Carbon and Carbon Nanotubes Growth Modes of Methane Decomposition in One-Pot Catalytic Approach. Catalysts 2021, 11, 1217. https://doi.org/10.3390/catal11101217
Chen L, Noreña LE, Wang JA, Limas R, Arellano U, González Vargas OA. Promoting Role of Amorphous Carbon and Carbon Nanotubes Growth Modes of Methane Decomposition in One-Pot Catalytic Approach. Catalysts. 2021; 11(10):1217. https://doi.org/10.3390/catal11101217
Chicago/Turabian StyleChen, Lifang, Luis Enrique Noreña, Jin An Wang, Roberto Limas, Ulises Arellano, and Oscar Arturo González Vargas. 2021. "Promoting Role of Amorphous Carbon and Carbon Nanotubes Growth Modes of Methane Decomposition in One-Pot Catalytic Approach" Catalysts 11, no. 10: 1217. https://doi.org/10.3390/catal11101217
APA StyleChen, L., Noreña, L. E., Wang, J. A., Limas, R., Arellano, U., & González Vargas, O. A. (2021). Promoting Role of Amorphous Carbon and Carbon Nanotubes Growth Modes of Methane Decomposition in One-Pot Catalytic Approach. Catalysts, 11(10), 1217. https://doi.org/10.3390/catal11101217










