Morphology and Catalytic Performance of MoS2 Hydrothermally Synthesized at Various pH Values
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Morphology
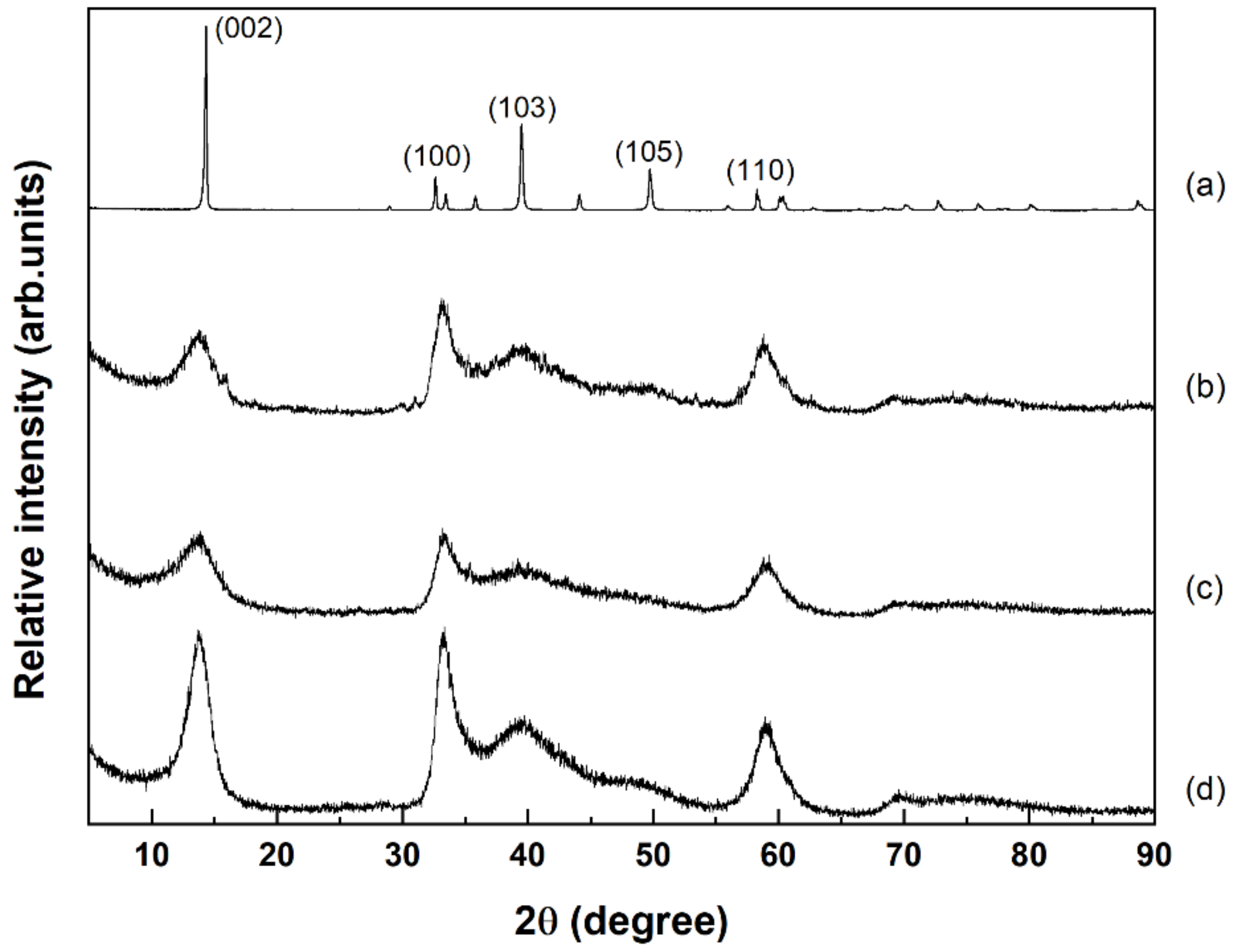
2.2. Crystalline Structure of Catalysts
2.3. Pore Structure of Catalysts
2.4. Surface Composition of Catalysts
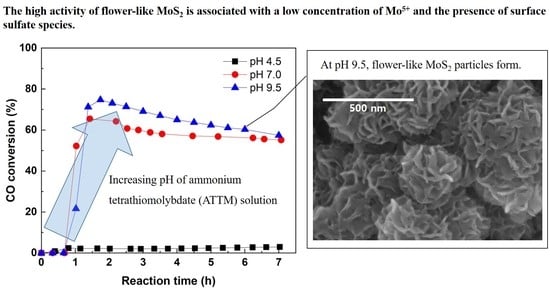
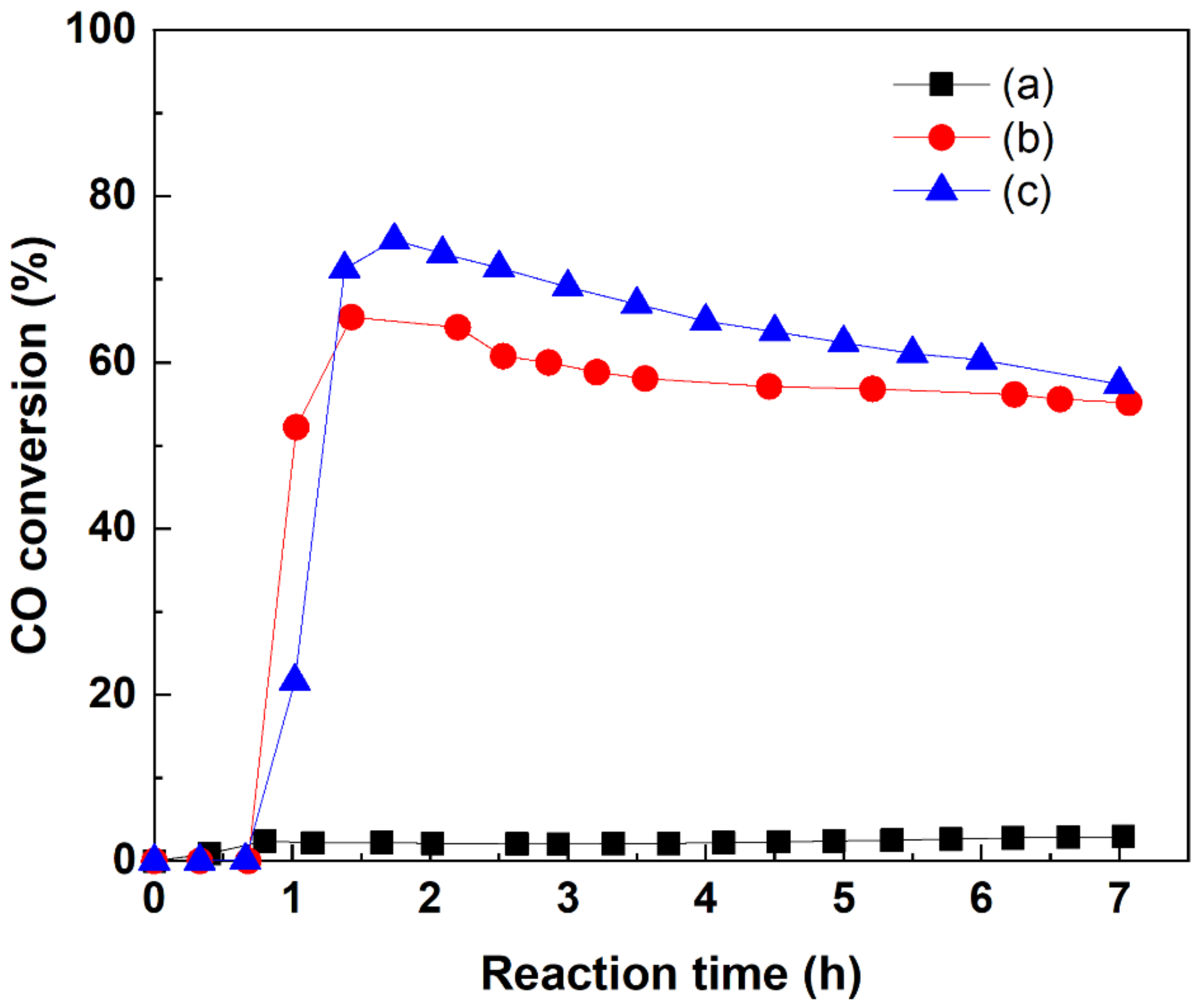
2.5. Catalytic Methanation
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Activity for CO Methanation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, V.; Kumar, R. A comprehensive review on synthesis and applications of molybdenum disulfide (MoS2) material: Past and recent developments. Inorg. Chem. Commun. 2020, 121, 108200. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Singh, K.; Kumar, M.; Kumar, A. A synoptic review of MoS2: Synthesis to applications. Superlattice Microst. 2019, 128, 274–297. [Google Scholar] [CrossRef]
- Tye, C.T.; Smith, K.J. Catalytic activity of exfoliated MoS2 in hydrodesulfurization, hydrodenitrogenation and hydrogenation reactions. Top. Catal. 2006, 37, 129–135. [Google Scholar] [CrossRef]
- Rivera-Muñoz, E.; Lardizabal, D.; Alonso, G.; Aguilar, A.; Siadati, M.H.; Chianelli, R.R. Silica Gel- and MCM-41-supported MoS2 catalysts for HDS reactions. Catal. Lett. 2003, 85, 147–151. [Google Scholar] [CrossRef]
- Jian, M.; Prins, R. The effect of phosphorus on the HDN reaction of piperidine, decahydroquinoline and ortho-propylaniline over Ni-MoS2/Al2O3 catalysts. Catal. Lett. 1995, 35, 193–203. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.; Lee, K.H.; Nam, I.-S.; Chung, J.S.; Kim, Y.G.; Woo, H.C. Role of alkali promoters in K/MoS2 catalysts for CO-H2 reactions. Appl. Catal. A 1994, 110, 11–25. [Google Scholar] [CrossRef]
- Youchang, X.; Naasz, B.N.; Somorjai, G.A. Alcohol synthesis from Co and H2 over molybdenum sulfide. The effect of pressure and promotion by potassium carbonate. Appl. Catal. A 1986, 27, 233–241. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Yuan, H.; Takeshita, H.T.; Sakai, T. Electrochemical hydrogen storage in MoS2 nanotubes. J. Am. Chem. Soc. 2001, 123, 11813–11814. [Google Scholar] [CrossRef]
- Li, X.-L.; Li, Y.-D. MoS2 nanostructures: synthesis and electrochemical Mg2+ intercalation. J. Phys. Chem. B 2004, 108, 13893–13900. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J. Facilitated lithium storage in MoS2 overlayers supported on coaxial carbon nanotubes. J. Phys. Chem. C 2007, 111, 1675–1682. [Google Scholar] [CrossRef]
- Du, G.; Guo, Z.; Wang, S.; Zeng, R.; Chen, Z.; Liu, H. Superior stability and high capacity of restacked molybdenum disulfide as anode material for lithium ion batteries. Chem. Commun. 2010, 46, 1106–1108. [Google Scholar] [CrossRef]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.R.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Chen, W.X.; Tu, J.P.; Xu, Z.D.; Tenne, R.; Rosenstveig, R.; Chen, W.L.; Gan, H.Y. Wear and friction of Ni-P electroless composite coating including inorganic fullerene-WS2 nanoparticles. Adv. Eng. Mater. 2002, 4, 686–690. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Feng, X.; Glans, P.-A.; Guo, J. MoS2 for beyond lithium-ion batteries. APL Mater. 2021, 9, 050903. [Google Scholar] [CrossRef]
- Nawz, T.; Safdar, A.; Hussain, M.; Lee, D.S.; Siyar, M. Graphene to Advanced MoS2: A Review of structure, synthesis, and optoelectronic device application. Crystals 2020, 10, 902. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; Moutaouakil, A.E. A Review on MoS2 properties, synthesis, sensing applications and challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.B.; Guo, Z.; Lou, X.W. Facile synthesis of carbon-coated MoS2 nanorods with enhanced lithium storage properties. Electrochem. Commum. 2012, 20, 7–10. [Google Scholar] [CrossRef]
- Remskar, M.; Mrzel, A.; Virsek, M.; Godec, M.; Krause, M.; Kolitsch, A.; Singh, A.; Seabaugh, A. The MoS2 nanotubes with defect-controlled electric properties. Nanoscale Res. Lett. 2011, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.B.; Bando, Y.; Golberg, D. MoS2 nanoflowers and their field-emission properties. Appl. Phys. Lett. 2003, 82, 1962–1964. [Google Scholar] [CrossRef]
- Wiesel, I.; Popovitz-Biro, R.; Tenne, R. Encapsulation of Mo2C in MoS2 inorganic fullerene-like nanoparticles and nanotubes. Nanoscale 2013, 5, 1499–1502. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.A.; Correa, V.; Torres, B.; Flores, S.; Farias Mancilla, J.R.; Chianelli, R.R. Spherical MoS2 micro particles and their surface dispersion due to addition of cobalt promoters. Rev. Mex. Fis. 2011, 57, 220–223. [Google Scholar]
- Tian, Y.; Zhao, X.; Shen, L.; Meng, F.; Tang, L.; Deng, Y.; Wang, Z. Synthesis of amorphous MoS2 nanospheres by hydrothermal reaction. Mater. Lett. 2006, 60, 527–529. [Google Scholar] [CrossRef]
- Cheon, J.; Gozum, J.E.; Girolami, G.S. Chemical vapor deposition of MoS2 and TiS2 films from the metal−organic precursors Mo(S-t-Bu)4 and Ti(S-t-Bu)4. Chem. Mater. 1997, 9, 1847–1853. [Google Scholar] [CrossRef]
- Li, Q.; Newberg, J.T.; Walter, E.C.; Hemminger, J.C.; Penner, R.M. Polycrystalline molybdenum disulfide (2H−MoS2) nano- and microribbons by electrochemical/chemical synthesis. Nano Lett. 2004, 4, 277–281. [Google Scholar] [CrossRef]
- Ma, L.; Xu, L.-M.; Xu, X.-Y.; Luo, Y.-L.; Chen, W.-X. Synthesis and characterization of flower-like MoS2 microspheres by a facile hydrothermal route. Mater. Lett. 2009, 63, 2022–2024. [Google Scholar] [CrossRef]
- Wei, R.; Yang, H.; Du, K.; Fu, W.; Tian, Y.; Yu, Q.; Liu, S.; Li, M.; Zou, G. A facile method to prepare MoS2 with nanoflower-like morphology. Mater. Chem. Phys. 2008, 108, 188–191. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.-X.; Li, H.; Zheng, Y.-F.; Xu, Z.-D. Ionic liquid-assisted hydrothermal synthesis of MoS2 microspheres. Mater. Lett. 2008, 62, 797–799. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.-X.; Li, H.; Xu, Z.-D. Synthesis and characterization of MoS2 nanostructures with different morphologies via an ionic liquid-assisted hydrothermal route. Mater. Chem. Phys. 2009, 116, 400–405. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Ji, Z.G. Controllable growth of MoS2/C flower-like microspheres with enhanced electrochemical performance for lithium ion batteries. J. Alloys Compd. 2016, 673, 215–219. [Google Scholar] [CrossRef]
- Liu, J.; Wang, E.; Lv, J.; Li, Z.; Wang, B.; Ma, X.; Qin, S.; Sun, Q. Investigation of sulfur-resistant, highly active unsupported MoS2 catalysts for synthetic natural gas production from CO methanation. Fuel Process. Technol. 2013, 110, 249–257. [Google Scholar] [CrossRef]
- Alonso, G.; Berhault, G.; Aguilar, A.; Collins, V.; Ornelas, C.; Fuentes, S.; Chianelli, R.R. Characterization and HDS activity of mesoporous MoS2 catalysts prepared by in situ activation of tetraalkylammonium thiomolybdates. J. Catal. 2002, 208, 359–369. [Google Scholar] [CrossRef]
- Alonso, G.; Del Valle, M.; Cruz, J.; Petranovskii, V.; Licea-Claverie, A.; Fuentes, S. Preparation of MoS2 catalysts by in situ decomposition of tetraalkylammonium thiomolybdates. Catal. Today 1998, 43, 117–122. [Google Scholar] [CrossRef]
- Choi, J.-M.; Kim, S.-H.; Lee, S.-J.; Kim, S.-S. Effects of pressure and temperature in hydrothermal preparation of MoS2 catalyst for methanation reaction. Catal. Lett. 2018, 148, 1803–1814. [Google Scholar] [CrossRef]
- Sen, U.K.; Mitra, S. High-rate and high-energy-density lithium-ion battery anode containing 2D MoS2 nanowall and cellulose binder. ACS Appl. Mater. Interfaces 2013, 5, 1240–1247. [Google Scholar] [CrossRef]
- Poh, C.K.; Onga, S.W.D.; Du, Y.H.; Kamata, H.; Choong, K.S.C.; Chang, J.; Izumi, Y.; Nariai, K.; Mizukami, N.; Chen, L.; et al. Direct methanation with supported MoS2 nano-flakes: Relationship between structure and activity. Catal. Today 2020, 342, 21–31. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Lin, Z.; Huang, F. Formation and self-assembly of cadmium hydroxide nanoplates in molten composite-hydroxide solution. Cryst. Growth Des. 2010, 10, 4285–4291. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L. Synthesis and characterization of composite visible light active photocatalysts MoS2–g-C3N4 with enhanced hydrogen evolution activity. Int. J. Hydrogen Energy 2013, 38, 6960–6969. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Prestridge, E.B.; Pecoraro, T.A.; Deneufville, J.P. Molybdenum Disulfide in the poorly crystalline “rag” structure. Science 1979, 203, 1105–1107. [Google Scholar] [CrossRef]
- Liang, K.S.; Chianelli, R.R.; Chien, F.Z.; Moss, S.C. Structure of poorly crystalline MoS2-A modeling study. J. Non-Cryst. Solids 1986, 79, 251–273. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R.R. Structure-function relations in molybdenum sulfide catalysts: The “rim-edge” model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, H.W.; Skeldon, P.; Thompson, G.E. XPS studies of MoS2 formation from ammonium tetrathiomolybdate solutions. Surf. Coat. Technol. 1997, 91, 200–207. [Google Scholar] [CrossRef]
- Wang, H.W.; Skeldon, P.; Thompson, G.E. Thermogravimetric—Differential thermal analysis of the solid-state decomposition of ammonium tetrathiomolybdate during heating in argon. J. Mater. Sci. 1998, 33, 3079–3083. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis. In Catalysis. Catalysis–Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 11, pp. 1–269. [Google Scholar] [CrossRef]
- Byskov, L.S.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H. DFT Calculations of unpromoted and promoted MoS2-based hydrodesulfurization catalysts. J. Catal. 1999, 187, 109–122. [Google Scholar] [CrossRef]
- Afanasiev, P. On the interpretation of temperature programmed reduction patterns of transition metals sulphides. Appl. Catal. A 2006, 303, 110–115. [Google Scholar] [CrossRef]
- Li, X.S.; Xin, Q.; Guo, X.X.; Grange, P.; Delmon, B. Reversible hydrogen adsorption on MoS2 studied by Temperature-programmed desorption and Temperature-programmed reduction. J. Catal. 1992, 137, 385–393. [Google Scholar] [CrossRef]
- Iwata, Y.; Sato, K.; Yoneda, T.; Miki, Y.; Sugimoto, Y.; Nishijima, A.; Shimada, H. Catalytic functionality of unsupported molybdenum sulfide catalysts prepared with different methods. Catal. Today 1998, 45, 353–359. [Google Scholar] [CrossRef]
- Iwata, Y.; Araki, Y.; Honna, K.; Miki, Y.; Sato, K.; Shimada, H. Hydrogenation active sites of unsupported molybdenum sulfide catalysts for hydroprocessing heavy oils. Catal. Today 2001, 65, 335–341. [Google Scholar] [CrossRef]





| Catalyst Preparation Conditions | Slab Height (nm) | Number of Stacking Layers | |
|---|---|---|---|
| pH | Temperature (°C) | ||
| 4.5 | 350 | 2.7 ± 0.1 | 4.4 |
| 7.0 | 350 | 2.9 ± 0.1 | 4.8 |
| 9.5 | 350 | 3.3 ± 0.1 | 5.3 |
| Catalyst Preparation Conditions | BET Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Size (nm) | |
|---|---|---|---|---|
| pH | Temperature (°C) | |||
| 4.5 | 350 | 83.21 | 0.17 | 8.39 |
| 7.0 | 350 | 167.00 | 0.13 | 3.42 |
| 9.5 | 350 | 158.38 | 0.36 | 6.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Son, Y.-S.; Choi, J.-H.; Kim, S.-S.; Park, S.-Y. Morphology and Catalytic Performance of MoS2 Hydrothermally Synthesized at Various pH Values. Catalysts 2021, 11, 1229. https://doi.org/10.3390/catal11101229
Lee S-J, Son Y-S, Choi J-H, Kim S-S, Park S-Y. Morphology and Catalytic Performance of MoS2 Hydrothermally Synthesized at Various pH Values. Catalysts. 2021; 11(10):1229. https://doi.org/10.3390/catal11101229
Chicago/Turabian StyleLee, Seung-Jae, Yang-Seung Son, Jin-Hoon Choi, Seong-Soo Kim, and Sung-Youl Park. 2021. "Morphology and Catalytic Performance of MoS2 Hydrothermally Synthesized at Various pH Values" Catalysts 11, no. 10: 1229. https://doi.org/10.3390/catal11101229
APA StyleLee, S.-J., Son, Y.-S., Choi, J.-H., Kim, S.-S., & Park, S.-Y. (2021). Morphology and Catalytic Performance of MoS2 Hydrothermally Synthesized at Various pH Values. Catalysts, 11(10), 1229. https://doi.org/10.3390/catal11101229