Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pellet Catalysts for Tar Conversion
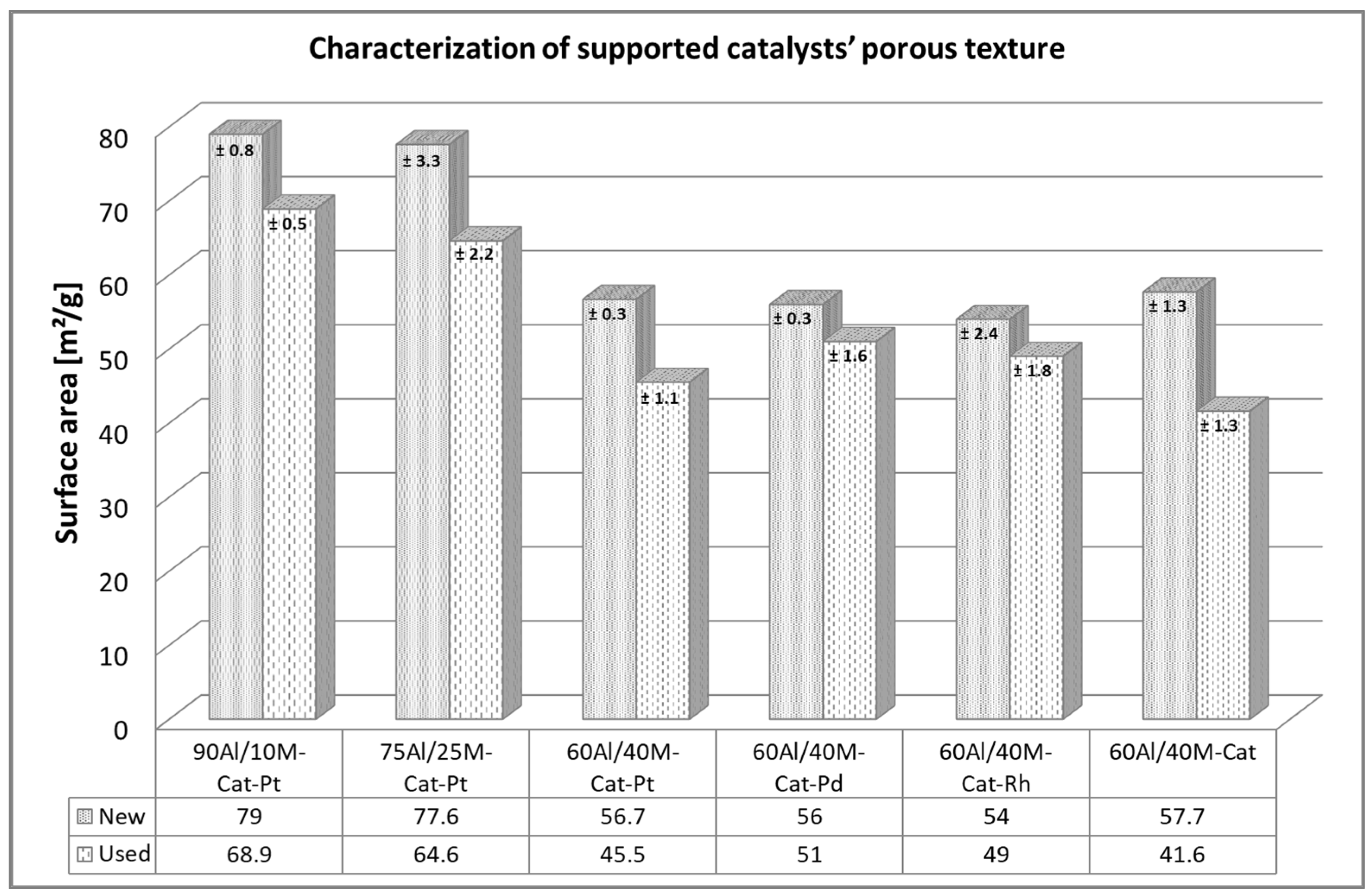
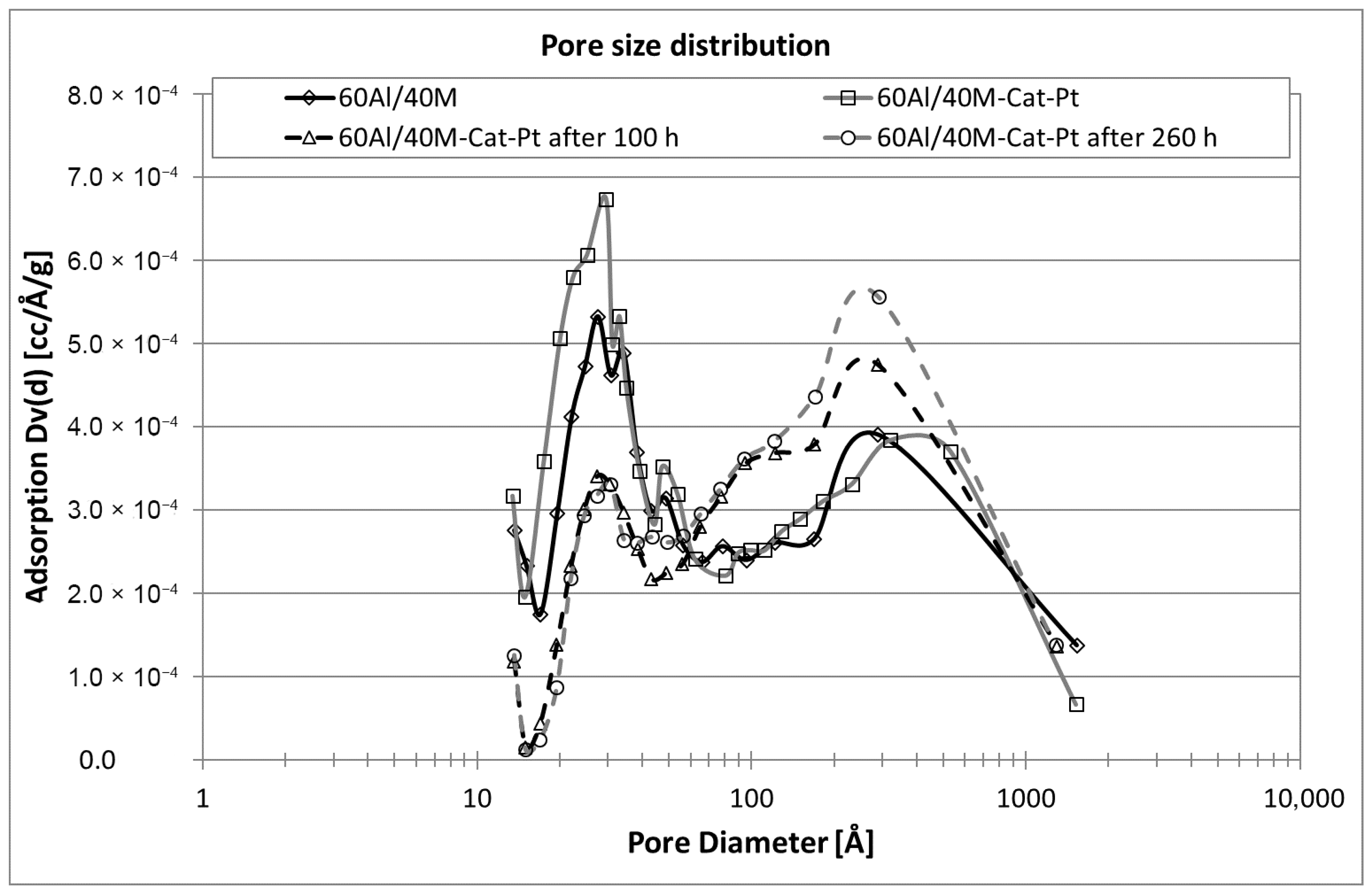
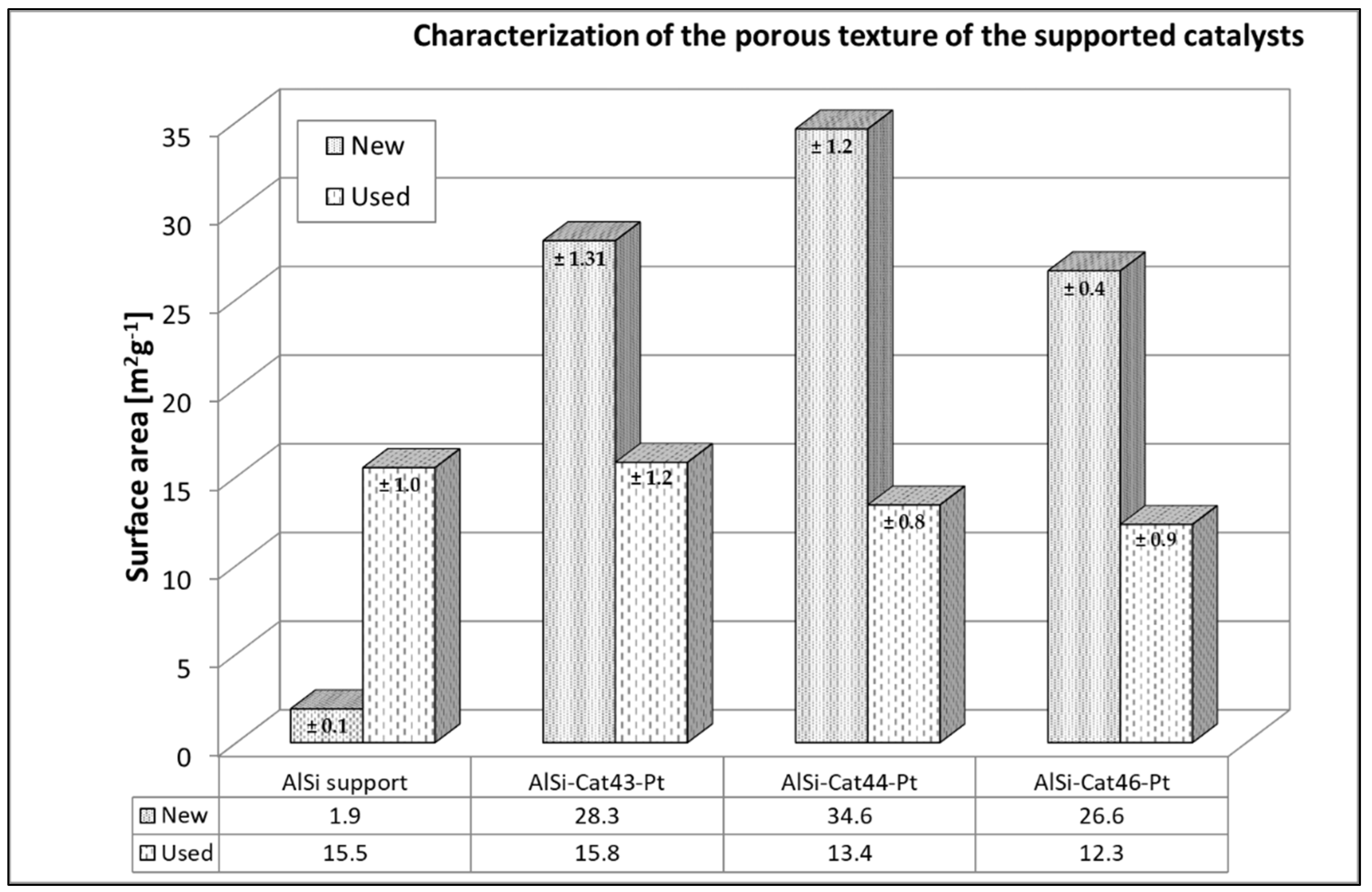
2.1.1. Porous Texture Analysis
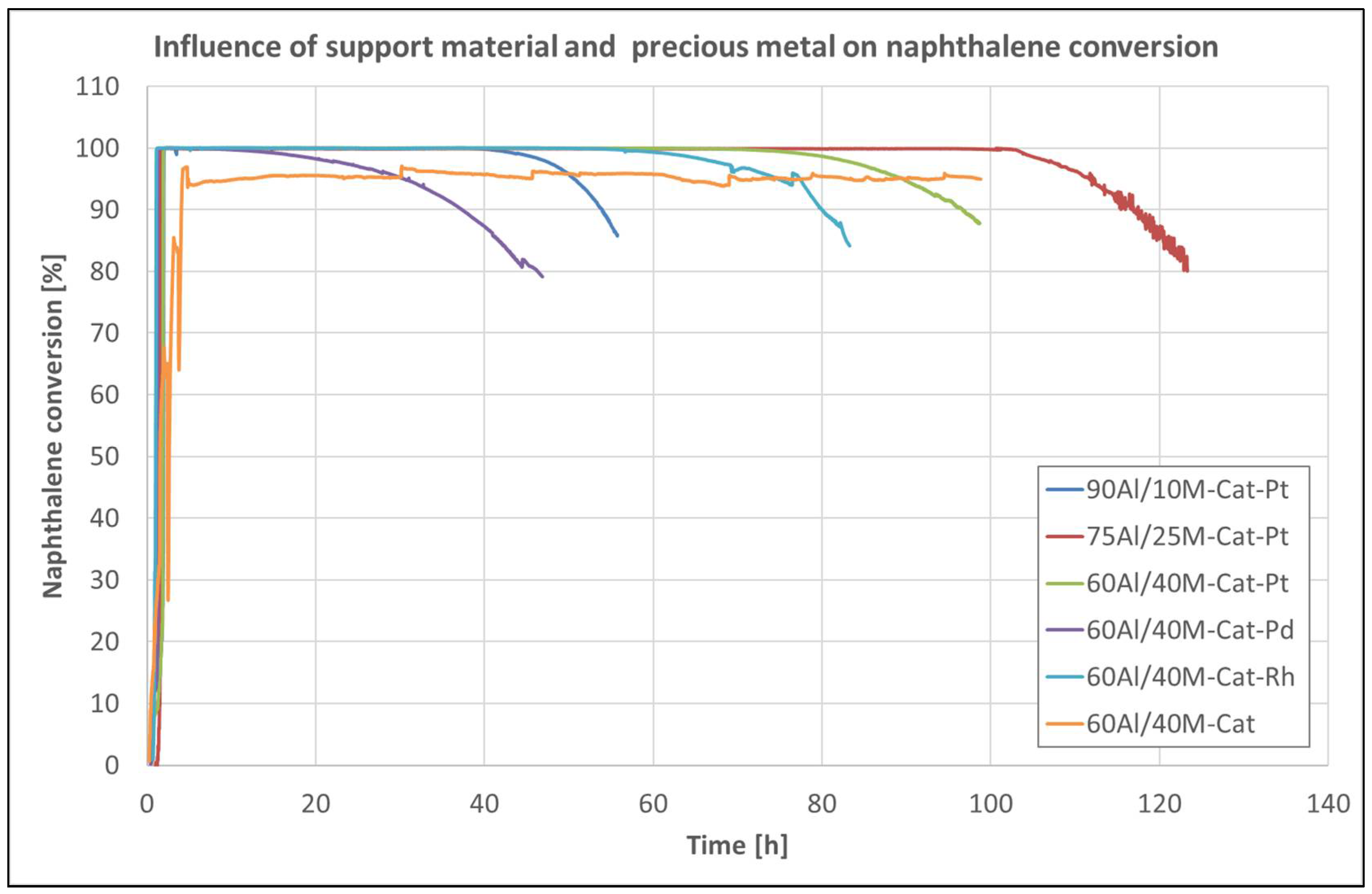
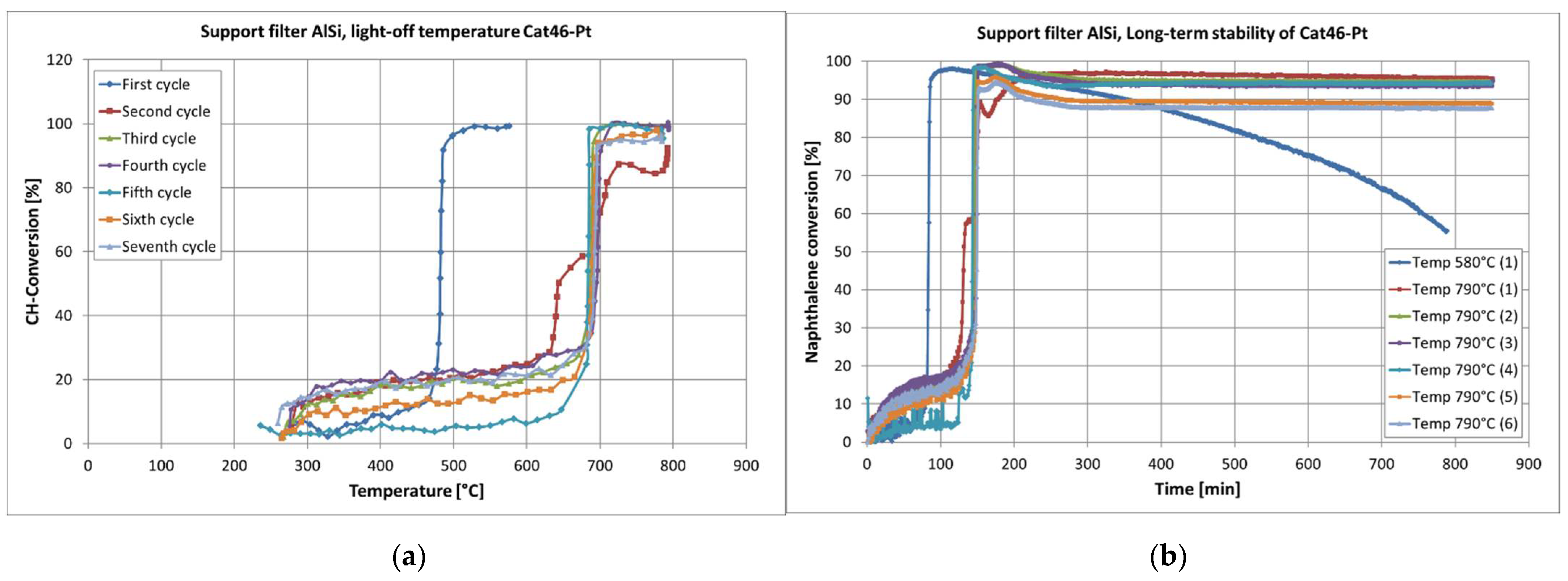
2.1.2. Catalytic Activity
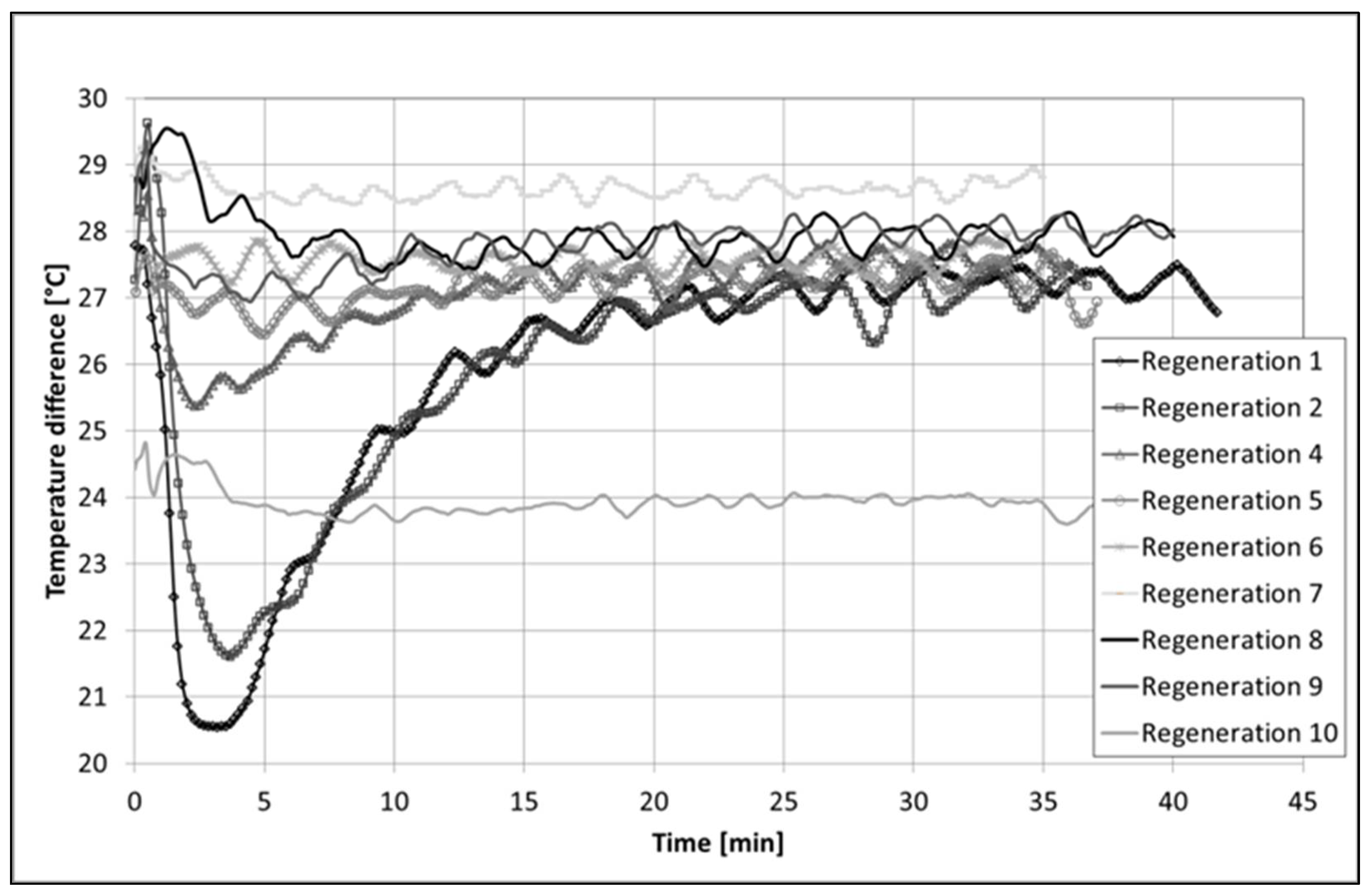
2.1.3. In Situ Regeneration
2.2. Catalytic Ceramic Filter Material for Tar Conversion
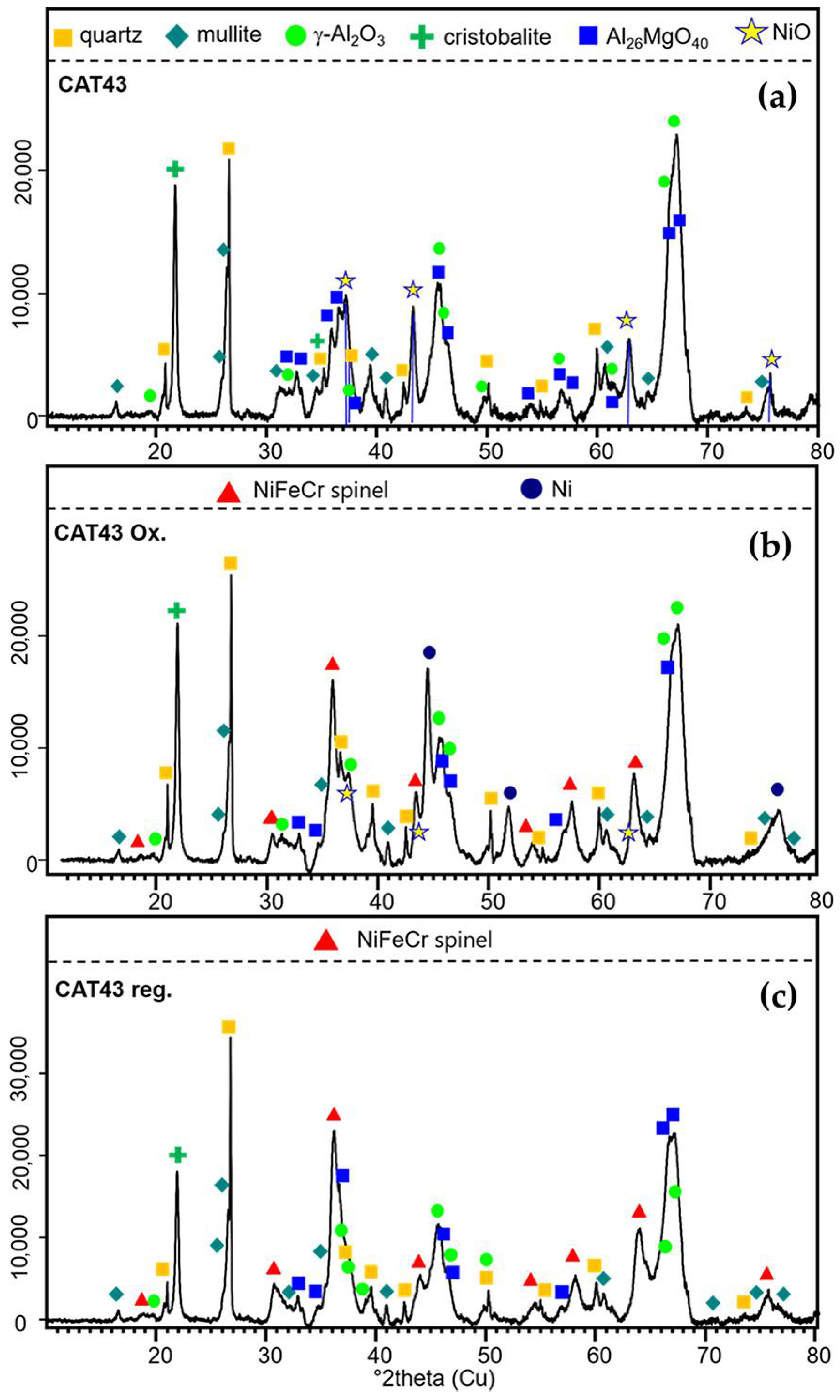
2.2.1. Catalytic Filter Material Morphology
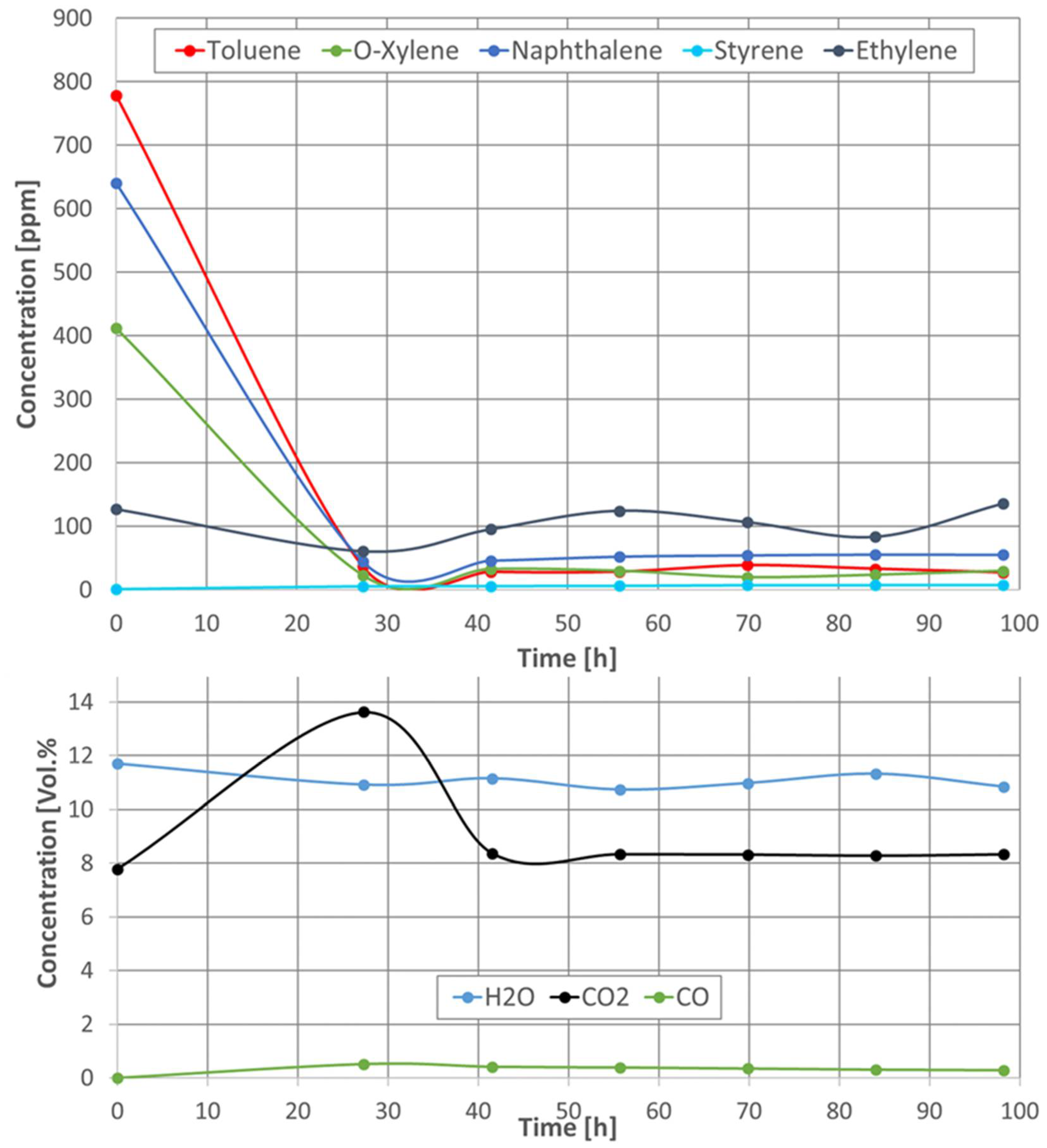
2.2.2. Catalytic Activity of the Catalytic Filter Material
2.3. Mechanism of Naphthalene Conversion and Role of the Support Material
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Pellet Catalysts
3.1.2. Catalytic Filter Material
3.2. Catalyst Characterization
3.3. Catalyst Activity
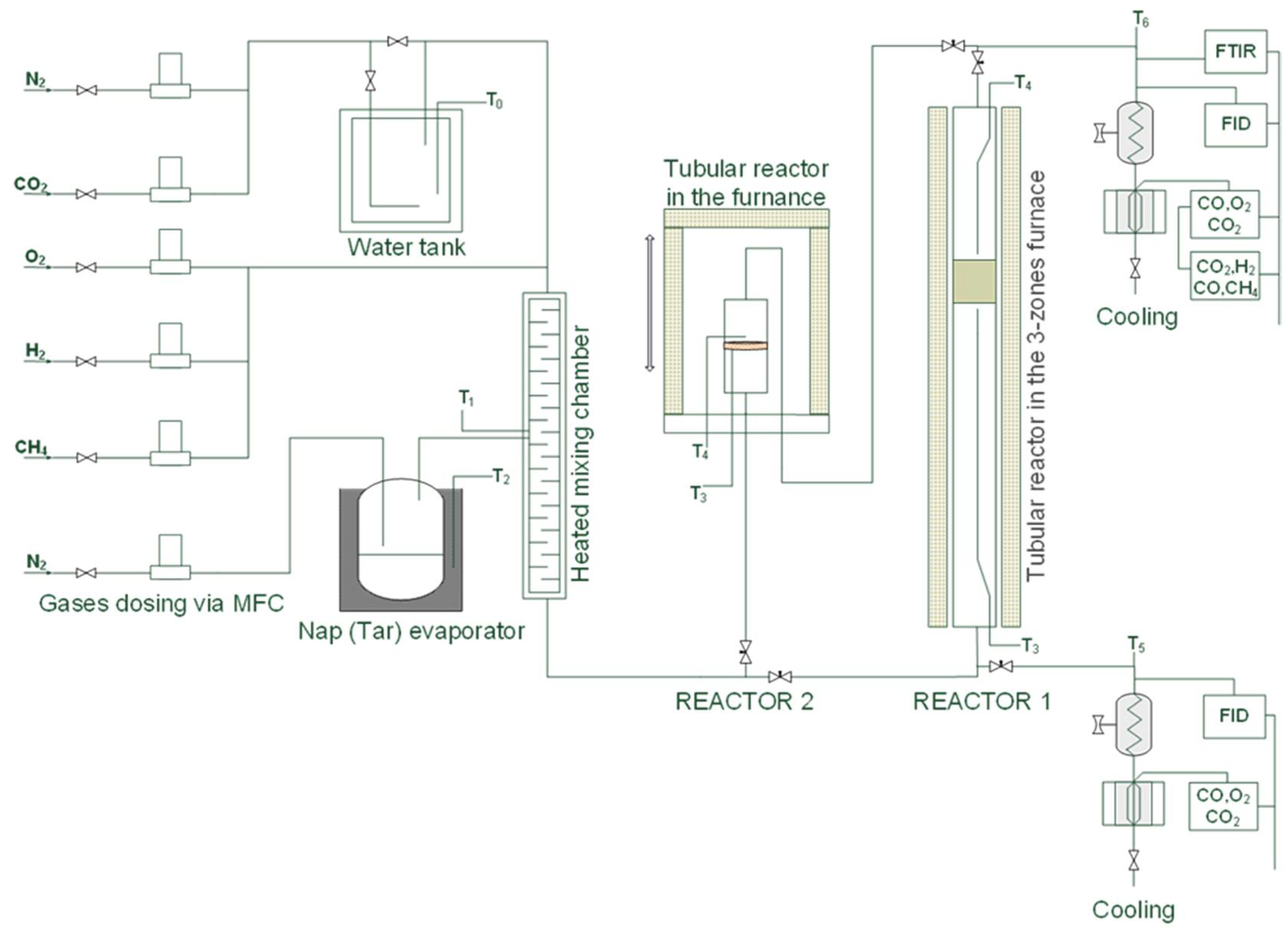
3.4. Catalytic Test Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hasler, P.; Nussbaumer, T. Gas cleaning for IC engine applications from fixed bed biomass gasifications. Biomass Bioenergy 1999, 16, 385–395. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Ul Hai, I.; Neubauer, Y.; Schröder Ph Oldenburg, H.; Seilkopf, A.; Kölling, A. Gas cleaning strategies for biomass gasification product gas. Int. J. Low-Carbon Technol. 2012, 7, 69–74. [Google Scholar] [CrossRef]
- Zwart, R.W.R. Gas Cleaning Downstream Biomass Gasification; Status Report; (ECN-E-08-078); Energy Research Center of the Netherlands (ECN): Petten, The Netherlands, 2009; pp. 1–65. [Google Scholar]
- Myren, C.; Hörnell Ch Björnbom, E.; Sjöström, K. Catalytic tar decomposition of biomass pyrolysis gas with a combination of dolomite and silica. Biomass Bioenergy 2002, 23, 217–227. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.; Janssen, F.; van Paasen, S.; Bergman, P.; Kiel, J. Catalytic decomposition of biomass tars: Use of dolomite and untreated olivine. Renew. Energy 2005, 30, 565–587. [Google Scholar] [CrossRef]
- Devi, L.; Craje, M.; Thüne, P.; Ptasinski, K.J.; Janssen, F.J.J.G. Olivine as tar removal catalyst for biomass gasifiers: Catalyst characterization. Appl. Catal. A Gen. 2005, 294, 68–79. [Google Scholar] [CrossRef]
- Rapagn, S.; Jand, N.; Kiennemann, A.; Foscolo, P.U. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Swierczynski, D.; Libs, S.; Courson, C.; Kiennemann, A. Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl. Catal. B Environ. 2007, 74, 211–222. [Google Scholar] [CrossRef]
- Wang, T.J.; Chang, J.; Wu, C.Z.; Fu, Y.; Chen, Y. The steam reforming of naphthalene over a nickel–dolomite cracking catalyst. Biomass Bioenergy 2005, 28, 508–514. [Google Scholar] [CrossRef]
- Yang, X.; Xu, S.; Xu, H.; Liu, X.; Liu, C. Nickel supported on modified olivine catalysts for steam reforming of biomass gasification tar. Catal. Commun. 2010, 11, 383–386. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Koike, M.; Nakagawa, Y.; Tomishige, K. Steam reforming of tar from pyrolysis of biomass over Ni/Mg/Al catalysts prepared from hydrotalcite-like precursors. Appl. Catal. B Environ. 2011, 102, 528–538. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kimura, T.; Nishikawa, J.; Kado, S.; Kunimori, K.; Tomishige, K. Catalytic performance of supported Ni catalysts in partial oxidation and steam reforming of tar derived from the pyrolysis of wood biomass. Catal. Today 2006, 115, 254–262. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.H.; Sohn, J.M.; Park, J.; Jeon, J.-K.; Kim, S.-S.; Park, Y.-K. Steam reforming of biomass gasification tar using benzene as a model compound over various Ni supported metal oxide catalysts. Bioresour. Technol. 2010, 101, S101–S103. [Google Scholar] [CrossRef]
- Tomishige, K.; Miyazawa, T.; Asadullah, M.; Ito, S.; Kunimori, K. Catalyst performance in reforming of tar derived from biomass over noble metal catalyst. Green Chem. 2003, 5, 399–403. [Google Scholar] [CrossRef]
- Diehl, F.; Barbier Jr, J.; Duprez, D.; Guibard, I.; Mabilion, G. Catalytic oxidation of heavy hydrocarbons over Pt/Al2O3. Influence of the structure of the molecule on its reactivity. Appl. Catal. B Environ. 2010, 95, 217–227. [Google Scholar] [CrossRef]
- Nacken, M.; Ma, L.; Engelen, K.; Heidenreich, S.; Baron, G.V. Development of a Tar Reforming Catalyst for Integration in a Ceramic Filter Element and Use in Hot Gas Cleaning. Ind. Eng. Chem. Res. 2007, 46, 1945–1951. [Google Scholar] [CrossRef]
- Engelen, K.; Zhang, Y.; Baron, G.V. Development of a Catalytic Candle Filter for One-Step Tar and Particle Removal in Biomass Gasification Gas. Int. J. Chem. React. Eng. 2003, 1, 1–11. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Brillis, A.A.; Manos, G. Catalyst deactivation during catalytic cracking of n-octane, isobutene and 1-octane over USHY Zeolite at mild conditions and short time on stream. Stud. Surf. Catal. 2001, 139, 255–269. [Google Scholar]
- Aguero, F.N.; Barbero, B.P.; Pereira, M.F.; Figueiredo, J.L.; Cadus, L.E. Mixed platinum-manganese oxide catalysts for combustion of volatile organic compounds. Ind. Eng. Chem. Res. 2009, 48, 2795–2800. [Google Scholar] [CrossRef]
- Mann, R. Catalyst deactivation by coke deposition: Approaches based on interactions of coke laydown with pore structure. Catal. Today 1997, 37, 331–349. [Google Scholar] [CrossRef]
- Cheah, K.Y.; Chiaranussati, N.; Hollewand, M.P.; Gladden, L.F. Coke profiles in deactivated alumina pellets studied by NMR imaging. Appl. Catal. A Gen. 1994, 115, 147–155. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Huber, G.W.; Boudart, M. Part 1. Introduction, Ch. 1.1 Principles of Heterogeneous Catalysis. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1–15. [Google Scholar]
- Hayek, K.; Kramer, R.; Paal, Z. Metal-support boundary sites in catalysis. Appl. Catal. A Gen. 1997, 162, 1–15. [Google Scholar] [CrossRef]
- Jenness, G.R.; Schmidt, J.R. Unraveling the role of metal-support interactions in heterogeneous catalysis: Oxygenate selectivity in Fischer-Tropsch synthesis. ACS Catal. 2013, 3, 2881–2890. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanism of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Rhodes, C.N.; Brown, D.R. Catalytic activity of acid-treated montmorillonite in polar and non-polar reaction media. Catal. Lett. 1994, 24, 285–291. [Google Scholar] [CrossRef]
- Wallis, P.J.; Gates, W.P.; Patti, A.F.; Scott, J.; Teoh, L.E. Assessing and improving the catalytic activity of K-10 montmorillonite. Green Chem. 2007, 9, 980–986. [Google Scholar] [CrossRef]
- Huskić, M.; Žagar, E. Catalytic activity of mineral montmorillonite on the reaction of phenol with formaldehyde. Appl. Clay Sci. 2017, 136, 158–163. [Google Scholar] [CrossRef]
- Hansen, T.W.; Delariva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; van Engen, P.G.; Jongebreur, R. Magneto-optical properties of metallic ferromagnetic materials. J. Magn. Magn. Mater. 1983, 38, 1–22. [Google Scholar] [CrossRef]
- Lee, S.; Keskar, G.; Liu Ch Schwartz, W.R.; McEnally, C.S.; Kim, J.-Y.; Pfefferle, L.D.; Haller, G.L. Deactivation characteristics of Ni/CeO2-Al2O3 catalyst for cyclic regeneration in a portable steam reformer. Appl. Catal. B Environ. 2012, 111–112, 157–164. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Parvari, M. Deactivation and regeneration of Nickel-based catalysts for steam-Methane reforming. Chin. J. Catal. 2011, 32, 273–279. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al Angari, Y.M. Effect of chromium ion substitution on the electromagnetic properties of nickel ferrite. Mater. Chem. Phys. 2009, 118, 153–160. [Google Scholar]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Keil, F.J. Complexities in modeling of heterogeneous catalytic reactions. Comput. Math. Appl. 2013, 65, 1674–1697. [Google Scholar] [CrossRef]
- Yeh, T.; Linic, S.; Savage, P.E. Deactivation of Pt Catalysts during Hydrothermal Decarboxylation of Butyric Acid. Sustain. Chem. Eng. 2014, 2, 2399–2406. [Google Scholar] [CrossRef]
- Stöcklmayer, C.; Höflinger, W. Simulation of the filtration behavior of dust filters. Simul. Pract. Theory 1998, 6, 281–296. [Google Scholar] [CrossRef]
- Ide, M.S.; Falcone, D.D.; Davis, R.J. On the deactivation of supported platinum catalysts for selective oxidation of alcohols. J. Catal. 2014, 311, 295–305. [Google Scholar] [CrossRef]
- Takanabe, K.; Aika, K.; Seshan, K.; Lefferts, L. Catalyst deactivation during steam reforming of acetic acid over Pt/ZrO2. Chem. Eng. J. 2006, 120, 133–137. [Google Scholar] [CrossRef]
- Caballero, M.A.; Corella, J.; Aznar, M.-P.; Gil, J. Biomass Gasification with Air in Fluidized Bed. Hot Gas Cleanup with Selected Commercial and Full-Size Nickel-Based Catalysts. Ind. Eng. Chem. Res. 2000, 39, 1143–1154. [Google Scholar] [CrossRef]
- Sato, K.; Shinoda, T.; Fujimoto, K. New Nickel-Based Catalyst for Tar Reforming, with Superior Resistance to Coking and Sulfur Poisoning in Biomass Gasification Processes. J. Chem. Eng. Jpn. 2007, 40, 860–868. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Anuradha, R.; Park, S.D.; Park, T.S.; Cha, W.S.; Jang, H.T. Effect of Bimetallic Pt-Rh and Trimetallic Pt-Pd-Rh Catalysts for Low Temperature Catalytic Combustion of Methane. Bull. Korean Chem. Soc. 2010, 31, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Trinh, Q.T.; Nguyen, A.V.; Huynh, D.C.; Pham, T.H.; Mushrif, S.H. Mechanistic insights into the catalytic elimination of tar an the promotional effect of boron on it: First-principles study using toluene as a model compound. Catal. Sci. Technol. 2016, 6, 5871–5883. [Google Scholar] [CrossRef]
- Adnan, M.A.; Muraza, O.; Razzak, S.A.; Hossain, M.M.; de Lasa, H.I. Iron oxide over silica-doped alumina catalyst for catalytic steam reforming of toluene as a surrogate tar biomass species. Energy Fuel 2017, 31, 7471–7481. [Google Scholar] [CrossRef]












| Catalyst | Temperature (°C) |
|---|---|
| 90Al/10M-Cat-Pt | 537 |
| 75Al/25M-Cat-Pt | 530 |
| 60Al/40M-Cat-Pt | 464 |
| 60Al/40M-Cat-Pd | 480 |
| 60Al/40M-Cat-Rh | 440 |
| 60Al/40M-Cat | 780 |
| Specific Surface Areas (m²/g) | |||
|---|---|---|---|
| 60Al/40M-Cat-Pt | New catalyst | Catalyst after 100 h | Catalyst after 260 h |
| 56.7 ± 0.3 | 45.5 ± 1.1 | 42.1 ± 0.3 | |
| Catalyst | Temperature (°C) |
|---|---|
| AlSi-Cat43-Pt | 449 → 653 |
| AlSi-Cat44-Pt | 462 → 670 |
| AlSi-Cat46-Pt | 481 → 692 |
| AlSi-Cat43-PtO | 450 → 670 |
| AlSi-Cat43-PtRu | 454 → 678 |
| AlSi-Cat43-PtORu | 420 → 702 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straczewski, G.; Mai, R.; Gerhards, U.; Garbev, K.; Leibold, H. Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems. Catalysts 2021, 11, 1231. https://doi.org/10.3390/catal11101231
Straczewski G, Mai R, Gerhards U, Garbev K, Leibold H. Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems. Catalysts. 2021; 11(10):1231. https://doi.org/10.3390/catal11101231
Chicago/Turabian StyleStraczewski, Grazyna, Robert Mai, Uta Gerhards, Krassimir Garbev, and Hans Leibold. 2021. "Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems" Catalysts 11, no. 10: 1231. https://doi.org/10.3390/catal11101231
APA StyleStraczewski, G., Mai, R., Gerhards, U., Garbev, K., & Leibold, H. (2021). Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems. Catalysts, 11(10), 1231. https://doi.org/10.3390/catal11101231







