Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio
Abstract
:1. Introduction
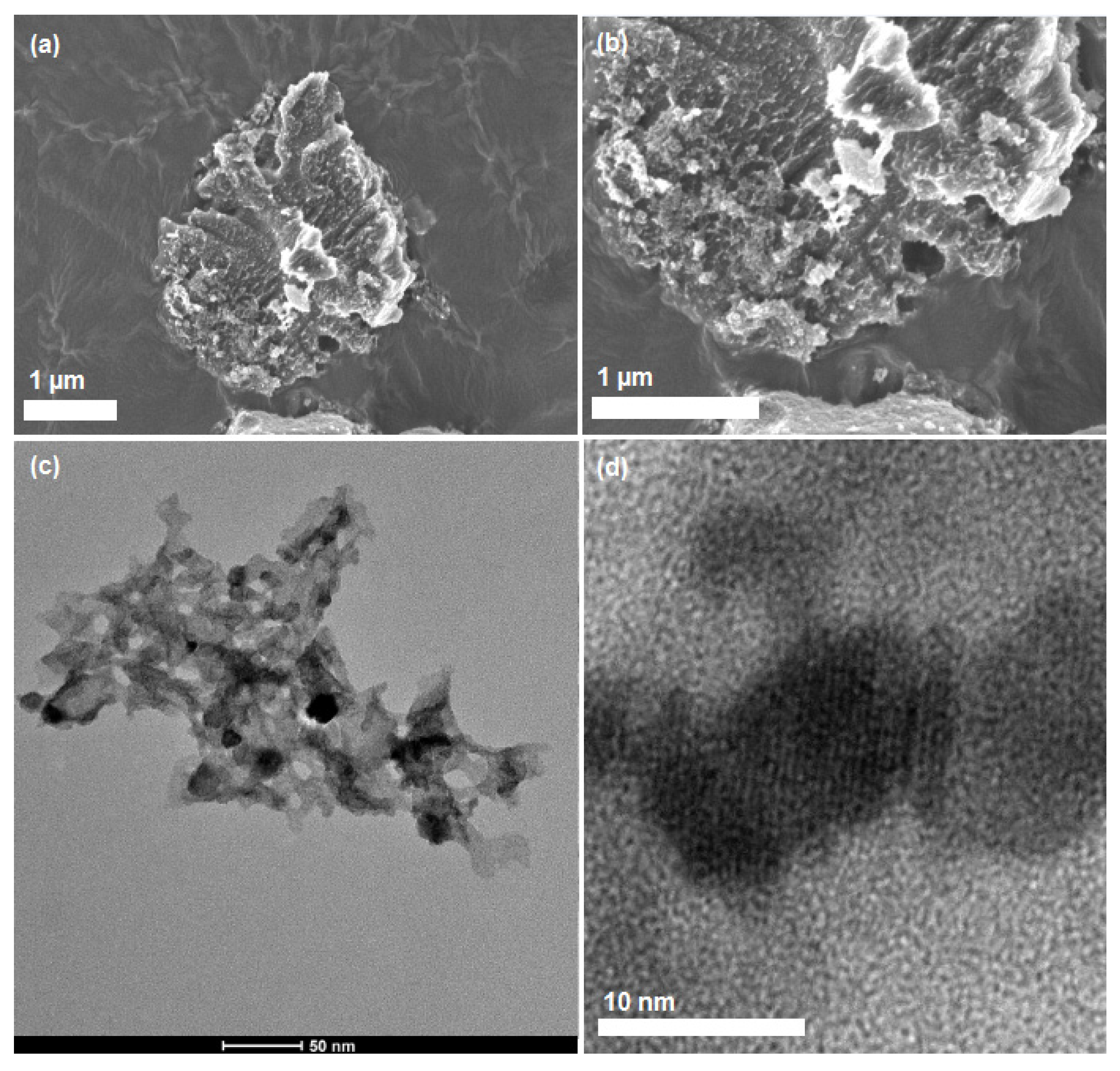
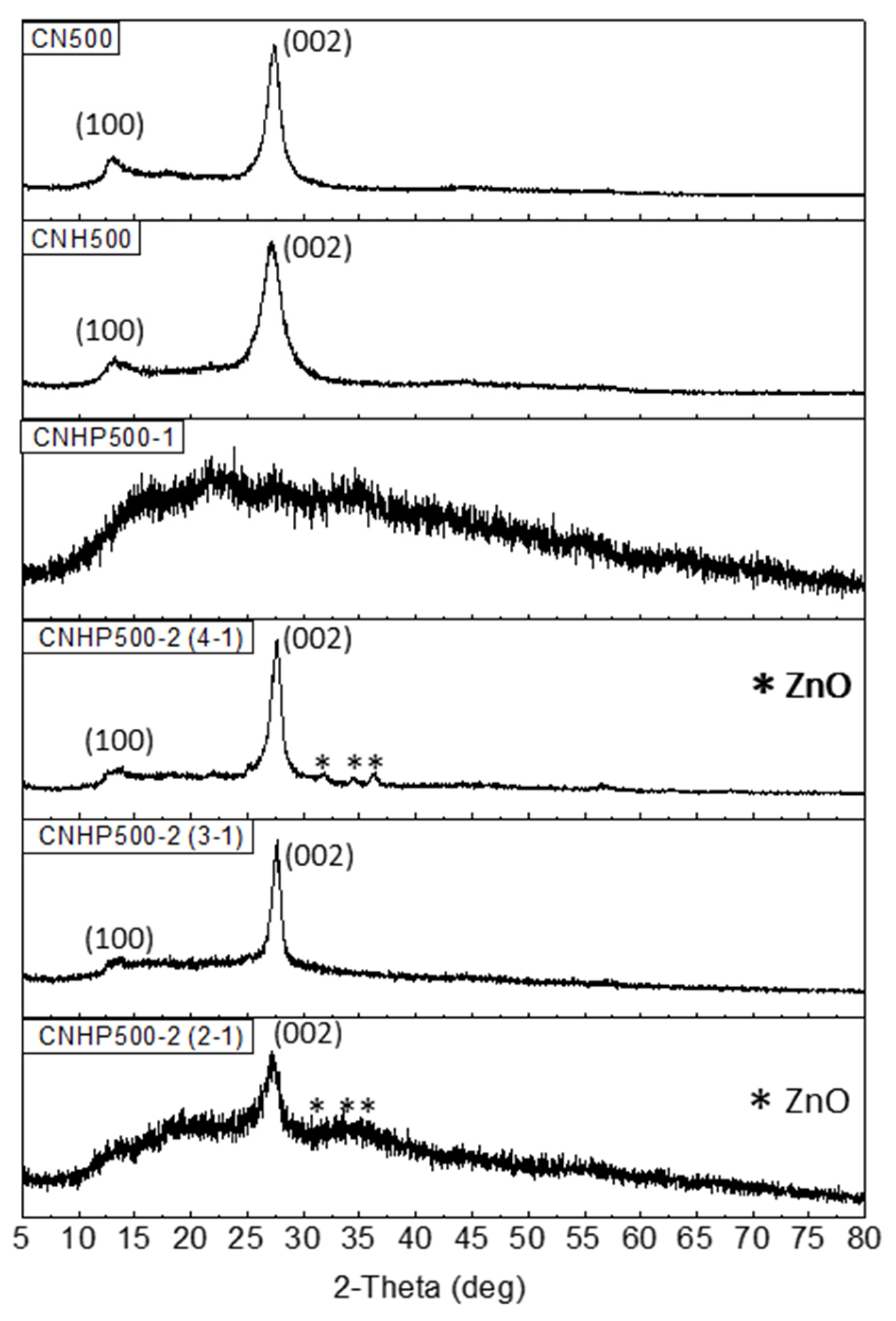
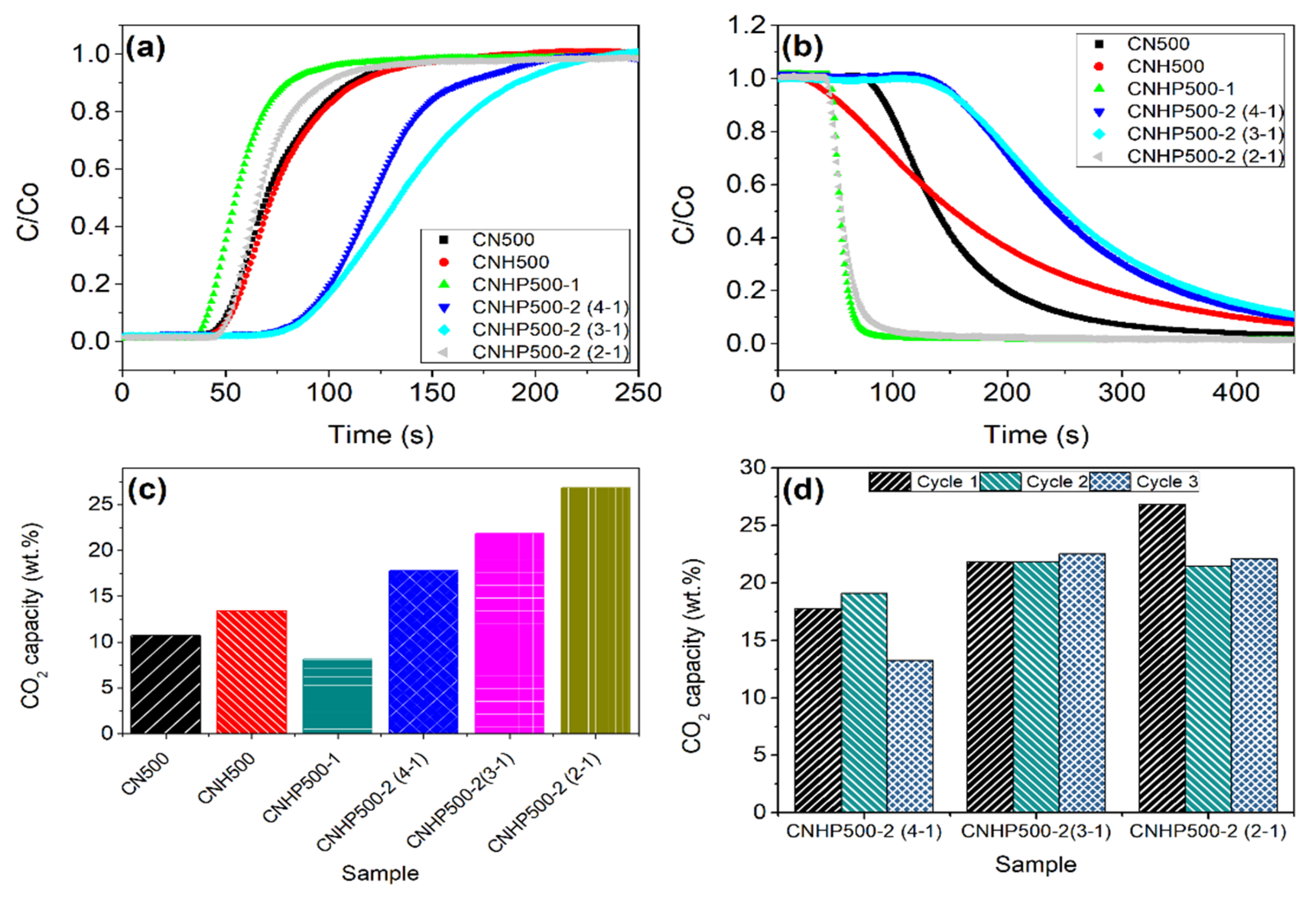
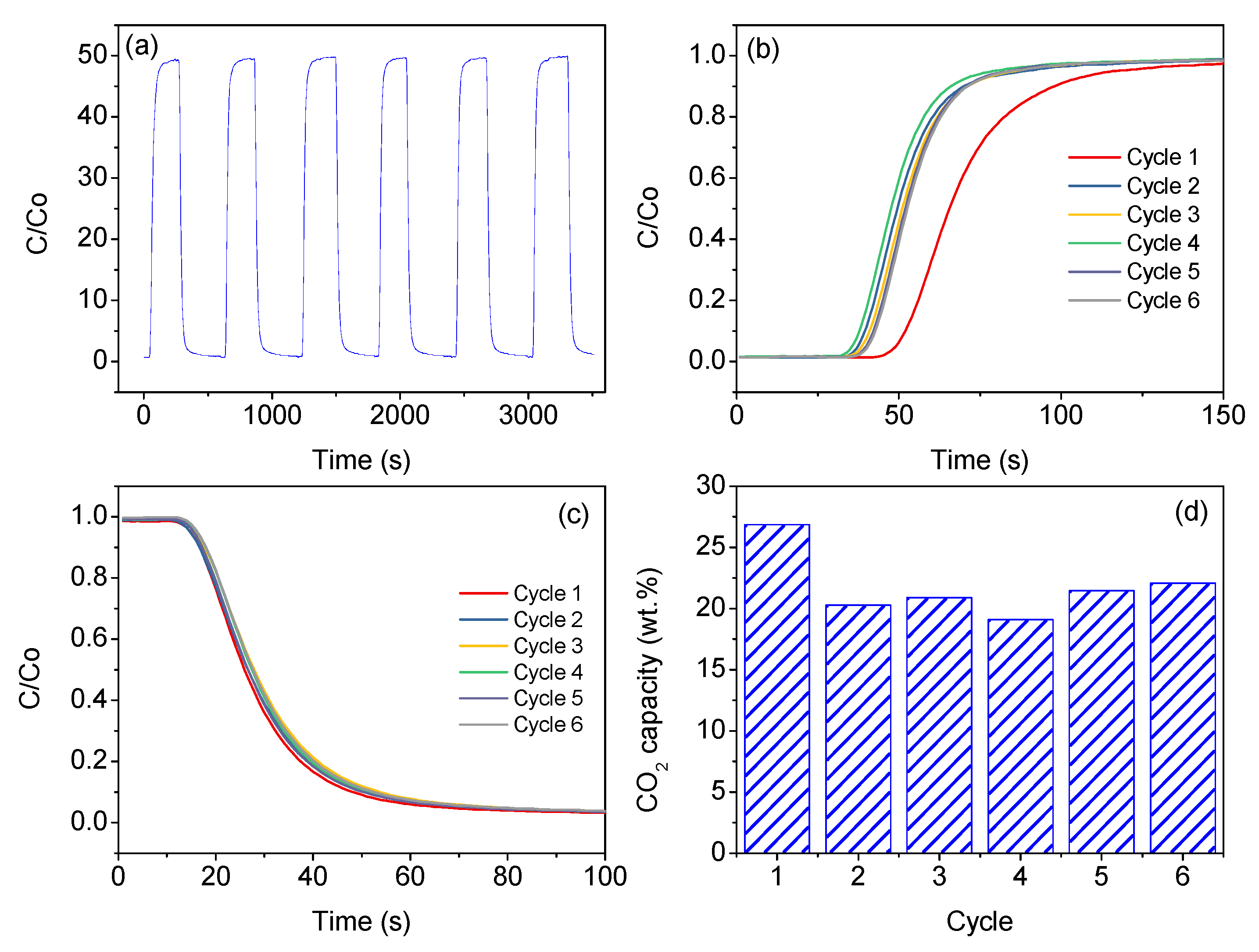
2. Results and Discussion
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Synthesis of Adsorbents
3.3. Characterization
3.4. CO2 Capture Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Xu, K.; Fleischer, C.; Liu, X.; Grandcolas, M.; Strandbakke, R.; Bjørheim, T.S.; Norby, T.; Chatzitakis, A. Earth-abundant electrocatalysts in proton exchange membrane electrolyzers. Catalysts 2018, 8, 657. [Google Scholar] [CrossRef] [Green Version]
- Ohi, J.M.; Vanderborgh, N.; Voecks, G. Hydrogen Fuel Quality Specifications for Polymer Electrolyte Fuel Cells in Road Vehicles: Report to the Safety, Codes and Standards Program; US Department of Energy: Washington, DC, USA, 2016.
- Ravi, N.; Anuar, S.A.; Yusuf, N.Y.M.; Isahak, W.N.R.W.; Masdar, M.S. Amine–mixed oxide hybrid materials for carbon dioxide adsorption from CO2/H2 mixture. Mater. Res. Express 2018, 5, 55501. [Google Scholar] [CrossRef]
- Yusuf, N.Y.; Masdar, M.S.; Isahak, W.N.R.W.; Nordin, D.; Husaini, T.; Majlan, E.H.; Rejab, S.A.M.; Chew, C.L. Ionic liquid-impregnated activated carbon for biohydrogen purification in an adsorption unit. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12071. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hsiao, I.-C.; Liu, C.-M.; Mt Yusuf, N.Y.; Wan Isahak, W.N.R.; Masdar, M.S. A novel bio-cellulose membrane and modified adsorption approach in CO2/H2 separation technique for PEM fuel cell applications. Int. J. Hydrogen Energy 2017, 42, 27630–27640. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. Amino functionalized mesostructured SBA-15 silica for CO2 capture: Exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous Mesoporous Mater. 2012, 158, 309–317. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, X.; Sun, F.; Ren, H.; Liu, J.; Zhang, F.; Zhao, N.; Zhu, G. Development of hydrogen-selective CAU-1 MOF membranes for hydrogen purification by ‘dual-metal-source’ approach. Int. J. Hydrogen Energy 2013, 38, 5338–5347. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.V.; Álvarez-Gutiérrez, N.; Martínez, M.; Rubiera, F.; Pevida, C.; Morán, A. Carbon adsorbents for CO2 capture from bio-hydrogen and biogas streams: Breakthrough adsorption study. Chem. Eng. J. 2015, 269, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous Graphitic-C3N4@Carbon Metal-Free Electrocatalysts for Highly Efficient Oxygen Reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef]
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Tahir, A.A.; Ahmad Ludin, N.; Ibrahim, M.A.; Wan Isahak, W.N.R.; Mat Teridi, M.A. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B Environ. 2018, 234, 296–310. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Simple and Large Scale Construction of MoS2-g-C3N4 Heterostructures Using Mechanochemistry for High Performance Electrochemical Supercapacitor and Visible Light Photocatalytic Applications. Sci. Rep. 2017, 7, 43055. [Google Scholar] [CrossRef]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Deng, Q.-F.; Liu, L.; Lin, X.-Z.; Du, G.; Liu, Y.; Yuan, Z.-Y. Synthesis and CO2 capture properties of mesoporous carbon nitride materials. Chem. Eng. J. 2012, 203, 63–70. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.-S.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Lakhi, K.S.; Baskar, A.V.; Zaidi, J.S.M.; Al-Deyab, S.S.; El-Newehy, M.; Choy, J.-H.; Vinu, A. Morphological control of mesoporous CN based hybrid materials and their excellent CO2 adsorption capacity. RSC Adv. 2015, 5, 40183–40192. [Google Scholar] [CrossRef] [Green Version]
- Lakhi, K.S.; Park, D.-H.; Singh, G.; Talapaneni, S.N.; Ravon, U.; Al-Bahily, K.; Vinu, A. Energy efficient synthesis of highly ordered mesoporous carbon nitrides with uniform rods and their superior CO2 adsorption capacity. J. Mater. Chem. A 2017, 5, 16220–16230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gao, Y.; Pfeiffer, H.; Louis, B.; Sun, L.; O’Hare, D.; Wang, Q. Recent advances in lithium containing ceramic based sorbents for high-temperature CO2 capture. J. Mater. Chem. A 2019, 7, 7962–8005. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous calcium oxide–silica and magnesium oxide–silica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2016, 4, 10914–10924. [Google Scholar] [CrossRef]
- Tang, Q.-L.; Luo, Q.-H. Adsorption of CO2 at ZnO: A Surface Structure Effect from DFT+U Calculations. J. Phys. Chem. C 2013, 117, 22954–22966. [Google Scholar] [CrossRef]
- Farias, S.A.S.; Longo, E.; Gargano, R.; Martins, J.B.L. CO2 adsorption on polar surfaces of ZnO. J. Mol. Model. 2013, 19, 2069–2078. [Google Scholar] [CrossRef]
- Kumar, S. The effect of elevated pressure, temperature and particles morphology on the carbon dioxide capture using zinc oxide. J. CO2 Util. 2014, 8, 60–66. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Mäki-Arvela, P.; Aho, A.; Sergiienko, S.A.; Yaremov, P.S.; Eränen, K.; Murzin, D.Y. Melamine-derived graphitic carbon nitride as a new effective metal-free catalyst for Knoevenagel condensation of benzaldehyde with ethylcyanoacetate. Catal. Sci. Technol. 2018, 8, 2928–2937. [Google Scholar] [CrossRef]
- Wan, S.; Zhong, Q.; Ou, M.; Zhang, S. Highly efficient simulated solar-light photocatalytic oxidation of gaseous NO with porous carbon nitride from copolymerization with thymine and mechanistic analysis. RSC Adv. 2016, 6, 101208–101215. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Ahmad, K.N.; Wan Isahak, W.N.R.; Rosli, M.I.; Yusop, M.R.; Kassim, M.B.; Yarmo, M.A. Rare earth metal doped nickel catalysts supported on exfoliated graphitic carbon nitride for highly selective CO and CO2 methanation. Appl. Surf. Sci. 2022, 571, 151321. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Alman, V.; Singh, K.; Bhat, T.; Sheikh, A.; Gokhale, S. Sunlight Assisted improved photocatalytic degradation of rhodamine B using Pd-loaded g-C3N4/WO3 nanocomposite. Appl. Phys. A 2020, 126, 724. [Google Scholar] [CrossRef]
- Nayak, P.K.; Wang, Z.; Anjum, D.H.; Hedhili, M.N.; Alshareef, H.N. Highly stable thin film transistors using multilayer channel structure. Appl. Phys. Lett. 2015, 106, 103505. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, Y.; Gu, X.; Yang, Q.; Zhao, X. Increasing the CO2/N2 Selectivity with a Higher Surface Density of Pyridinic Lewis Basic Sites in Porous Carbon Derived from a Pyridyl-Ligand-Based Metal–Organic Framework. Chem. An Asian J. 2016, 11, 1913–1920. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Wang, C.-C. Surface crystal feature-dependent photoactivity of ZnO–ZnS composite rods via hydrothermal sulfidation. RSC Adv. 2018, 8, 5063–5070. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fan, X.; Zhang, L.; Liu, J.; Wang, B.; Cheng, R.; Li, M.; Tian, J.; Shi, J. Probing the role of O-containing groups in CO2 adsorption of N-doped porous activated carbon. Nanoscale 2017, 9, 17593–17600. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, Z.; Li, C. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. A Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Huang, C.; Wen, Y.; Ma, J.; Dong, D.; Shen, Y.; Liu, S.; Ma, H.; Zhang, Y. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef]
- Ma, D.; Li, X.; Wang, X.; Luo, Y. Research development on graphitic carbon nitride and enhanced catalytic activity on ammonium perchlorate. RSC Adv. 2021, 11, 5729–5740. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Solar light responsive TiO2-ZnO, modified with graphitic carbon nitride nano-sheet for degradation of AB29. J. Chem. Technol. Biotechnol. 2020, 95, 2674–2683. [Google Scholar] [CrossRef]
- Anuar, S.A.; Wan Isahak, W.N.R.; Masdar, M.S. Carbon nanoflake hybrid for biohydrogen CO2 capture: Breakthrough adsorption test. Int. J. Energy Res. 2020, 44, 3148–3159. [Google Scholar] [CrossRef]
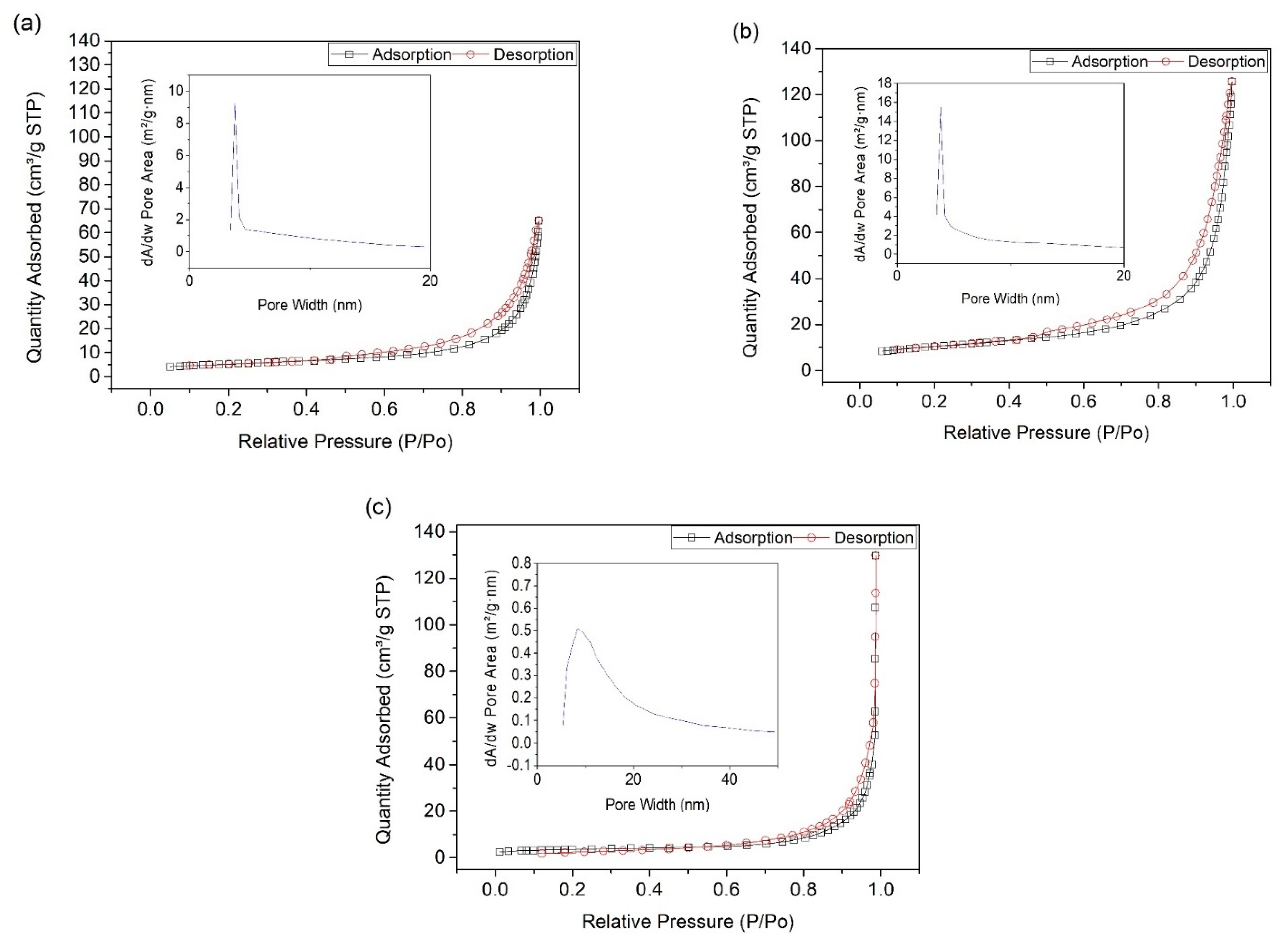


| Sample | BET Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|
| CNHP500-2(2-1) | 12.3 | 42.5 |
| CNHP500-2(3-1) | 36.3 | 18.1 |
| CNHP500-2(4-1) | 18.2 | 17.4 |
| Element | Atomic Percentage (at %) |
|---|---|
| C | 41.8 |
| N | 22.3 |
| O | 22.7 |
| Zn | 13.2 |
| Sample | Method | Amine Source | Amine/Metal Oxide Mass Ratio |
|---|---|---|---|
| CN500 | Dry pyrolysis | Melamine | - |
| CNH500 | Dry pyrolysis | Melamine and urea | - |
| CNHP500-1 | Dry pyrolysis | Melamine and urea | 2:1 |
| CNHP500-2(2-1) | Hydrothermal and dry pyrolysis | Melamine and urea | 2:1 |
| CNHP500-2(3-1) | Hydrothermal and dry pyrolysis | Melamine and urea | 3:1 |
| CNHP500-2(4-1) | Hydrothermal and dry pyrolysis | Melamine and urea | 4:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anuar, S.A.; Ahmad, K.N.; Al-Amiery, A.; Masdar, M.S.; Wan Isahak, W.N.R. Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio. Catalysts 2021, 11, 1253. https://doi.org/10.3390/catal11101253
Anuar SA, Ahmad KN, Al-Amiery A, Masdar MS, Wan Isahak WNR. Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio. Catalysts. 2021; 11(10):1253. https://doi.org/10.3390/catal11101253
Chicago/Turabian StyleAnuar, Siti Aishah, Khairul Naim Ahmad, Ahmed Al-Amiery, Mohd Shahbudin Masdar, and Wan Nor Roslam Wan Isahak. 2021. "Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio" Catalysts 11, no. 10: 1253. https://doi.org/10.3390/catal11101253
APA StyleAnuar, S. A., Ahmad, K. N., Al-Amiery, A., Masdar, M. S., & Wan Isahak, W. N. R. (2021). Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio. Catalysts, 11(10), 1253. https://doi.org/10.3390/catal11101253









