Deactivation and Regeneration Method for Ni Catalysts by H2S Poisoning in CO2 Methanation Reaction
Abstract
:1. Introduction
2. Results and Discussion
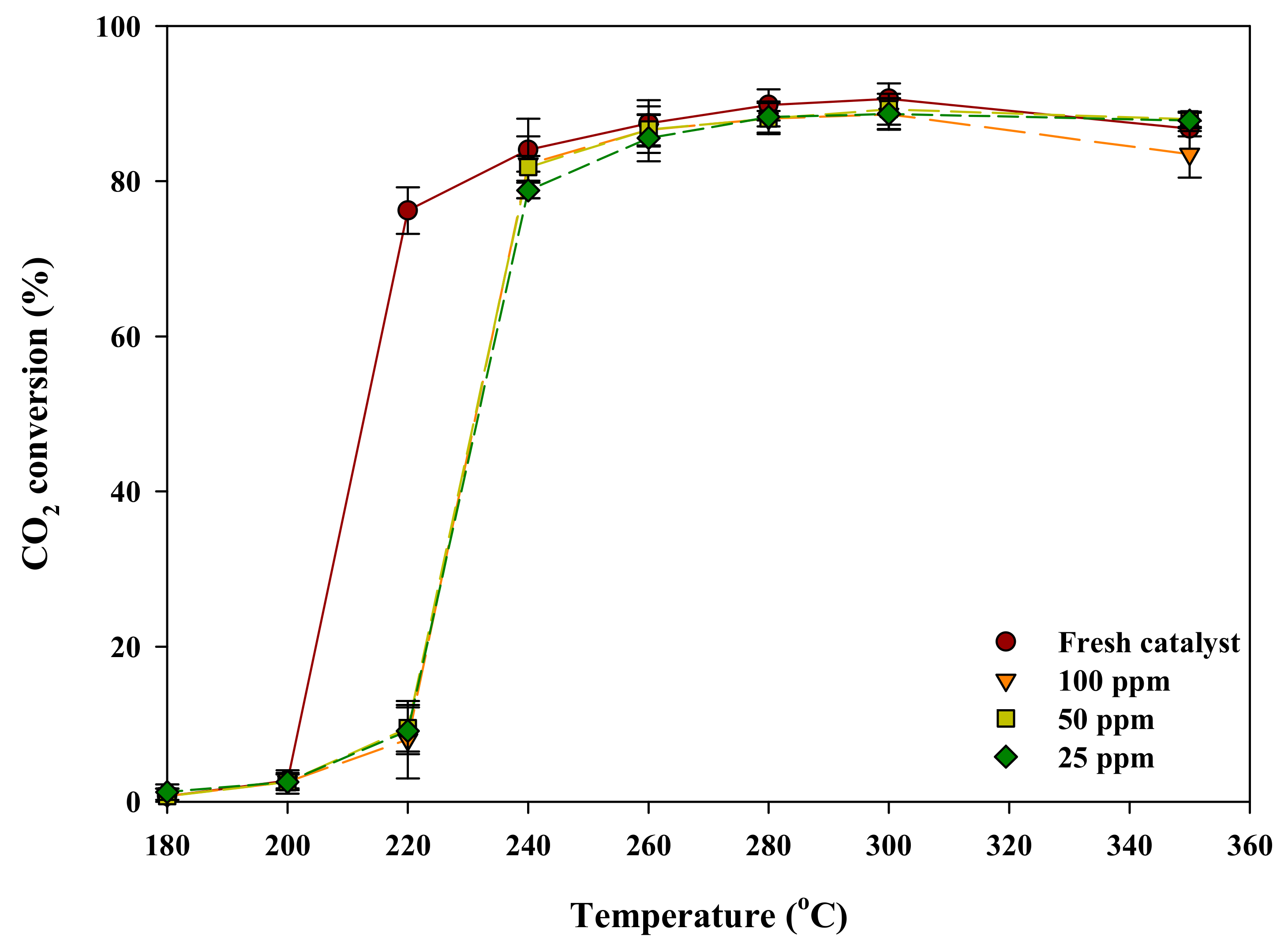
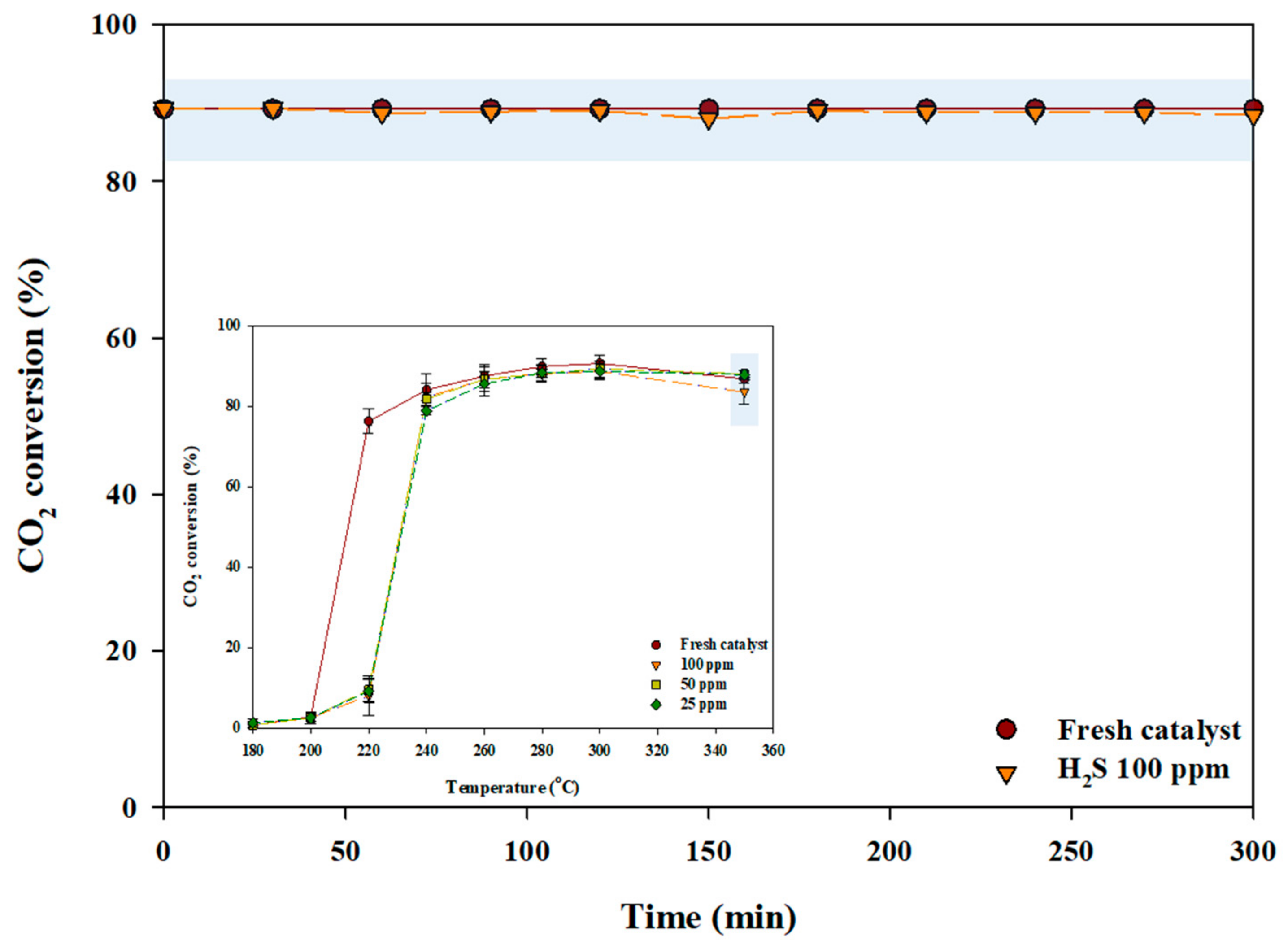
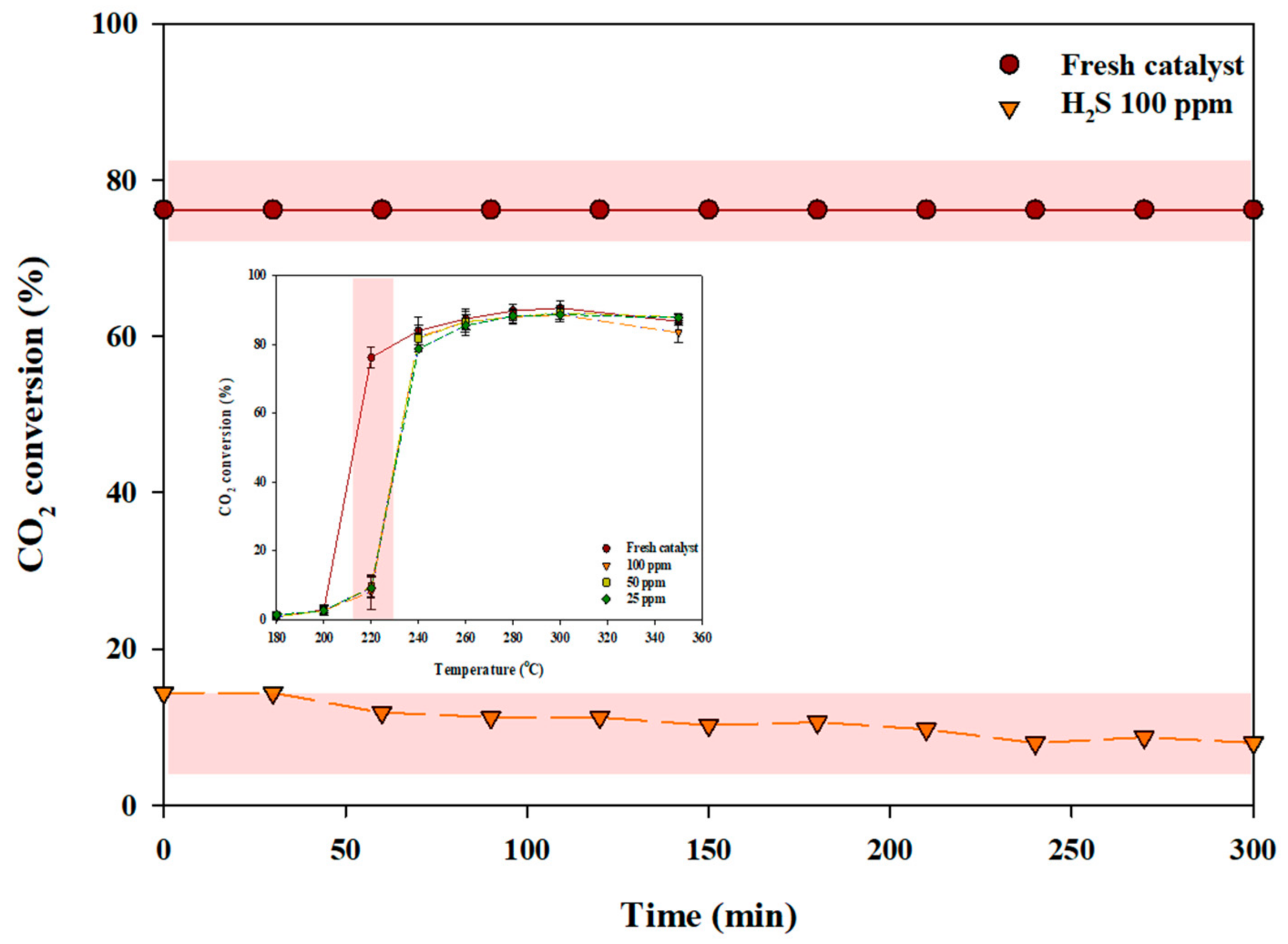
2.1. Activity Evaluation of a Poisoned Catalyst
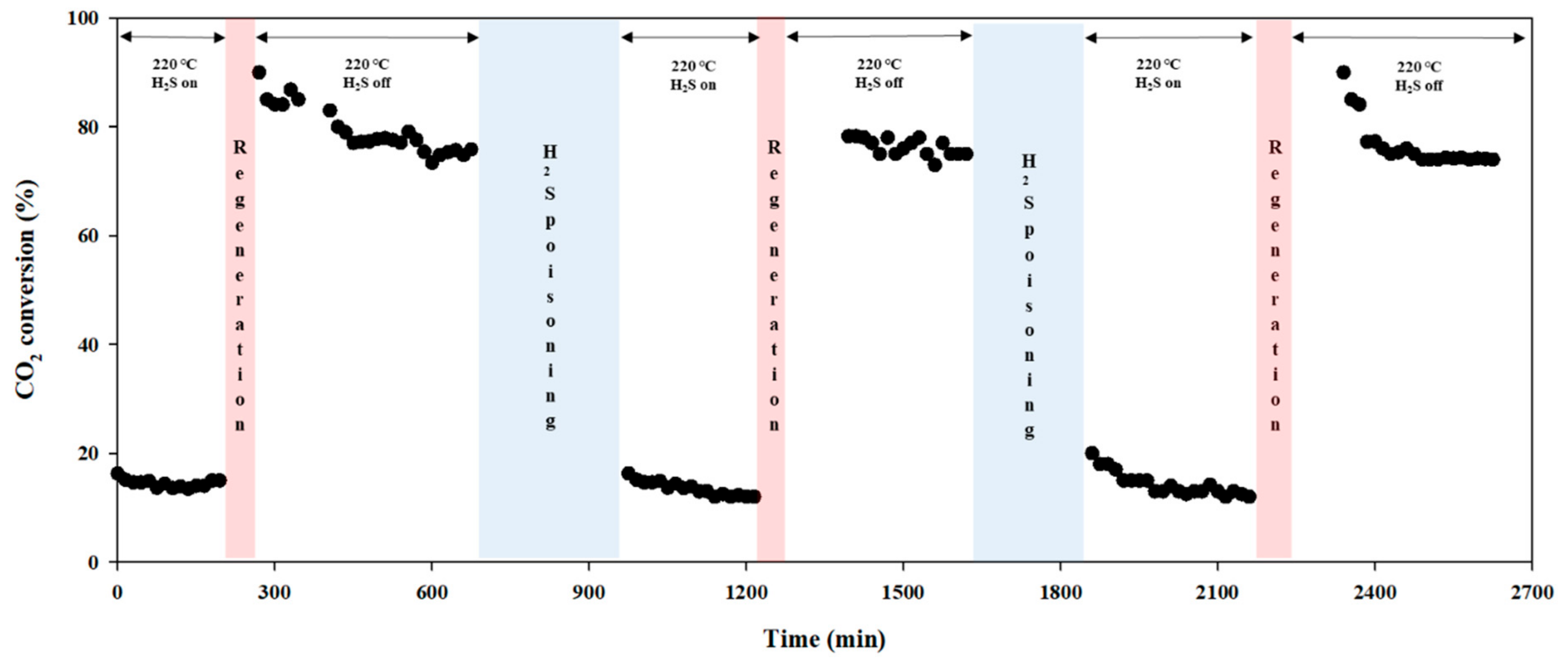
2.2. Regeneration Effects
2.3. Catalyst Characterization
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Experimental Apparatus and Activity Test
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Sixth Assessment Report. AR6 Climate Change 2021: The Physical Science Basis. Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 6 August 2021).
- The European Commission. Fit for 55. Available online: https://ec.europa.eu/info/sites/default/files/chapeau_communication.pdf (accessed on 14 July 2021).
- Christina, W.; Jochen, L.; Petra, Z. Review of power-to-gas projects in Europe. Energy Procedia 2018, 155, 367–378. [Google Scholar]
- Alessandra, P.; Linda, M.; Giorgio, F.; Giuseppe, S.; Laura, C.; Marco, D. SNG Generation via Power to Gas Technology: Plant Design and Annual Performance Assessment. Appl. Sci. 2020, 23, 8443. [Google Scholar]
- Manuel, G.; Amy Mc Daniel, K.; Frank, G. State of the art and perspectives of CO2 methanation process concepts for power-to-gas applications. In International Gas Union Research Conference; International Gas Union: Fornebu, Norway, 2014; Volume 13. [Google Scholar]
- Frédéric David, M.; Frédéric-Paul, P.; Suren, E. Power-to-gas through CO2 methanation: Assessment of the carbon balance regarding EU directives. J. Energy Storage 2017, 11, 16–24. [Google Scholar]
- Irene, C.M. Carbon Dioxide Methanation for Intensified Reactors. Aalto Univ. Sch. Chem. Technol. Degree Progr. Chem. Technol. 2015, 7, 83. [Google Scholar]
- Beatrice, C.; Alberto, M.G.; Elena, M.; Benedetto, N.; Andrea, P.; Mirko, F.; Andrea, N.; Federico, R. Experimental Investigation on CO2 Methanation Process for Solar Energy Storage Compared to CO2-Based Methanol Synthesis. Energies 2017, 10, 855. [Google Scholar]
- Ahn, J.; Chang, S.; Lee, S.; Kim, S.; Chung, W.; Lee, J.; Cho, Y.; Shin, K.; Moon, D.; Dinh, D.N. Developing Ni-based honeycomb-type catalysts using different binary oxide-supported species for synergistically enhanced CO2 methanation activity. Fuel 2019, 250, 277–284. [Google Scholar] [CrossRef]
- Kristian, K.; Dori, K.; Hailong, L.; Zhixin, Y. CO2 methanation: The effect of catalysts and reaction conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar]
- Garbarino, G.; Bellotti, D.; Finocchio, E.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3: Catalytic activity and infrared study. Catal. Today 2016, 277, 21–28. [Google Scholar] [CrossRef]
- Dreyer, J.A.H.; Li, P.; Zhang, L.; Beh, G.K.; Zhang, R.; Sit, P.H.L.; Teoh, W.Y. Influence of the oxide support reducibility on the CO2 methanation over Ru-based catalysts. Appl. Catal. B Environ. 2017, 219, 715–726. [Google Scholar] [CrossRef]
- Arandiyan, H.; Kani, K.; Wang, Y.; Jiang, B.; Kim, J.; Yoshino, M.; Rezaei, M.; Rowan, A.E.; Dai, H.; Yamauchi, Y. Highly selective reduction of carbon dioxide to methane on novel mesoporous Rh catalysts. ACS Appl. Mater. Interfaces 2018, 10, 24963–24968. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Ryuji, K. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 527–5531. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, Y.; Wang, S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci. Total Environ. 2018, 625, 686–695. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Zhuo, Y.; Zhu, Y.; Wnag, S. Enhancement of CO2 methanation over La-Modified Ni/SBA-15 catalysts prepared by different doping methods. ACS Sustain. Chem. Eng. 2019, 17, 14647–14660. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Li, W.; Mu, M.; Ding, F.; Liu, Z.; Guo, X.; Song, C. Organic acid-assisted preparation of highly dispersed Co/ZrO2 catalysts with superior activity for CO2 methanation. Appl. Catal. B Environ. 2019, 254, 531–540. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Amir, I.A.; Mei, Y.O.; Saifuddin, N.; Kit, W.C.; Pau, L.S. Technologies for biogas upgrading to bio methane: A review. Bioengineering 2019, 6, 92. [Google Scholar]
- Gerda, R.; Johannes, L. Evaluating CO2 sources for power-to-gas applications–A case study for Austria. J. CO2 Util. 2015, 10, 40–49. [Google Scholar]
- Victor, S.; Claudia, U.; Ximena, G. A CFD Design Approach for Industrial Size Tubular Reactors for SNG Production from Biogas (CO2 Methanation). Energies 2017, 14, 6175. [Google Scholar]
- David, M.M.; Laura, B.V.; Jesús, M.R.; José, F.C. A study of deactivation by H2S and regeneration of a Ni catalyst supported on Al2O3, during methanation of CO2. Effect of the promoters Co, Cr, Fe and Mo. RSC Adv. 2020, 10, 16551–16564. [Google Scholar]
- Nam, J.; Shin, J.L.; Hong, S.; Hahm, H.; Park, W.; So, K. Biomethanol conversion from biogas produced by anaerobic digestion. J. Korea Org. Resour. Recycl. Assoc. 2006, 14, 93–103. [Google Scholar]
- Wojciech, G.; Witold, Z.; Marek, R.; Grzegorz, S.; Magdalena, G. CO2 Methanation in the Presence of Ce-Promoted Alumina Supported Nickel Catalysts: H2S Deactivation Studies. Top. Catal. 2019, 62, 524–534. [Google Scholar]
- Wan, A.A.B.; Mohd, O.; Rusmidah, A.; Ching, Y.; Susilawati, T. The investigation of active sites on nickel oxide-based catalysts towards the in-situ reactions of methanation and desulfurization. Mod. Appl. Sci. 2009, 3, 36–44. [Google Scholar]
- Bakar, W.A.W.A.; Ali, R.; Toemen, S. Catalytic methanation reaction over supported nickel-ruthenium oxide base for purification of simulated natural gas. Sci. Iran. 2012, 19, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Kim, H.; Ro, Y.; Kim, J.; Chung, W.; Chang, S. Development of Pilot-Scale CO2 Methanation Using Pellet-Type Catalysts for CO2 Recycling in Sewage Treatment Plants and Its Validation through Computational Fluid Dynamics (CFD) Modeling. Catalysts 2021, 11, 1005. [Google Scholar] [CrossRef]



| Element | Fresh (before Exposure) | Spent (after Exposure) | Regeneration | |
|---|---|---|---|---|
| 300 °C | 220 °C | |||
| wt.% | wt.% | wt.% | wt.% | |
| C | 9 | 8 | 9.4 | 13.9 |
| O | 8.1 | 6.1 | 3.3 | 10 |
| Ni | 61.4 | 61.1 | 59.3 | 53.3 |
| Zr | 5.2 | 6.5 | 2.4 | 5.6 |
| Ce | 16.3 | 18.4 | 5.4 | 17.3 |
| S | - | - | 20.2 | |
| Ni/Ce/Zr | BET (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Fresh | 7.04 | 0.035 | 19.8 |
| Spent | 6.89 | 0.024 | 13.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Chung, W.; Chang, S. Deactivation and Regeneration Method for Ni Catalysts by H2S Poisoning in CO2 Methanation Reaction. Catalysts 2021, 11, 1292. https://doi.org/10.3390/catal11111292
Ahn J, Chung W, Chang S. Deactivation and Regeneration Method for Ni Catalysts by H2S Poisoning in CO2 Methanation Reaction. Catalysts. 2021; 11(11):1292. https://doi.org/10.3390/catal11111292
Chicago/Turabian StyleAhn, Jeongyoon, Woojin Chung, and Soonwoong Chang. 2021. "Deactivation and Regeneration Method for Ni Catalysts by H2S Poisoning in CO2 Methanation Reaction" Catalysts 11, no. 11: 1292. https://doi.org/10.3390/catal11111292
APA StyleAhn, J., Chung, W., & Chang, S. (2021). Deactivation and Regeneration Method for Ni Catalysts by H2S Poisoning in CO2 Methanation Reaction. Catalysts, 11(11), 1292. https://doi.org/10.3390/catal11111292








