Synthesis and Characterization of Sn/Ni Single Doped and Co–Doped Anatase/Rutile Mixed–Crystal Nanomaterials and Their Photocatalytic Performance under UV–Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure
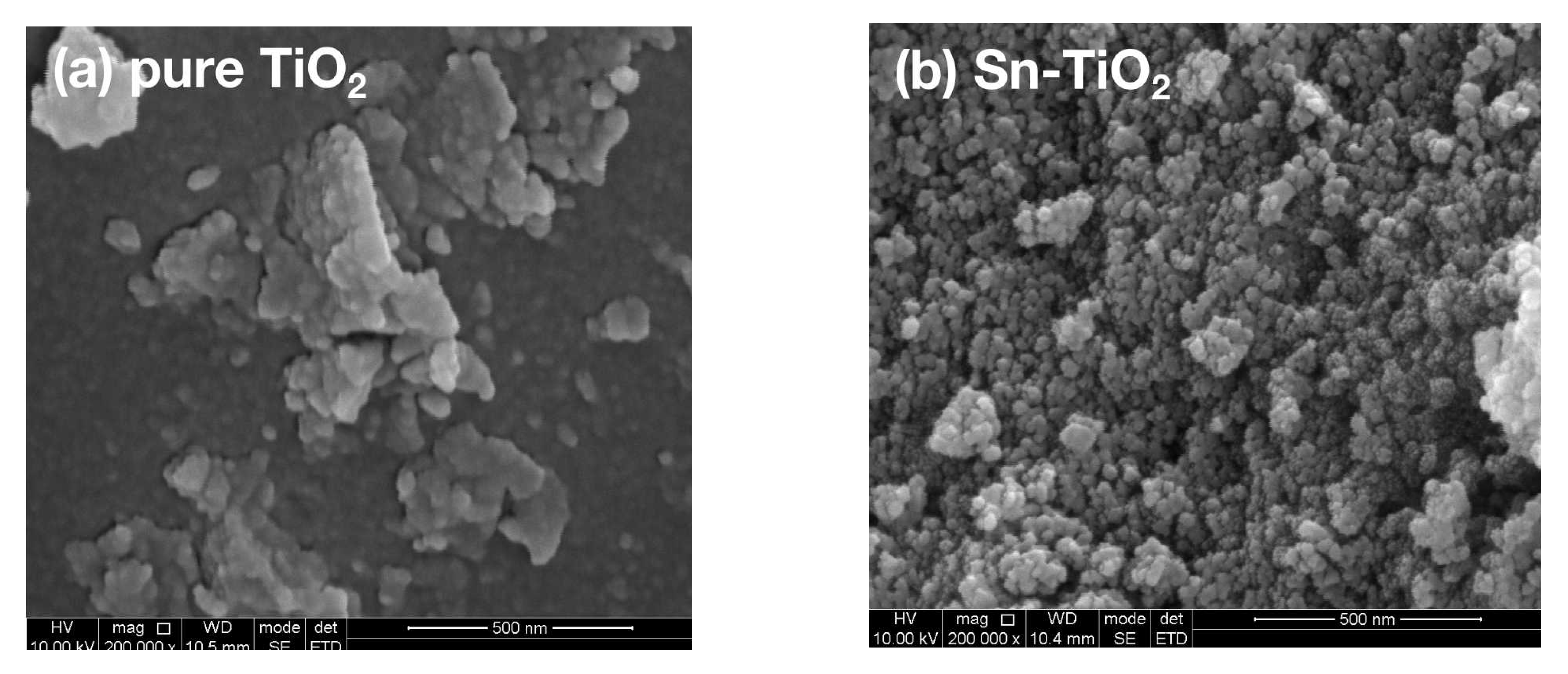
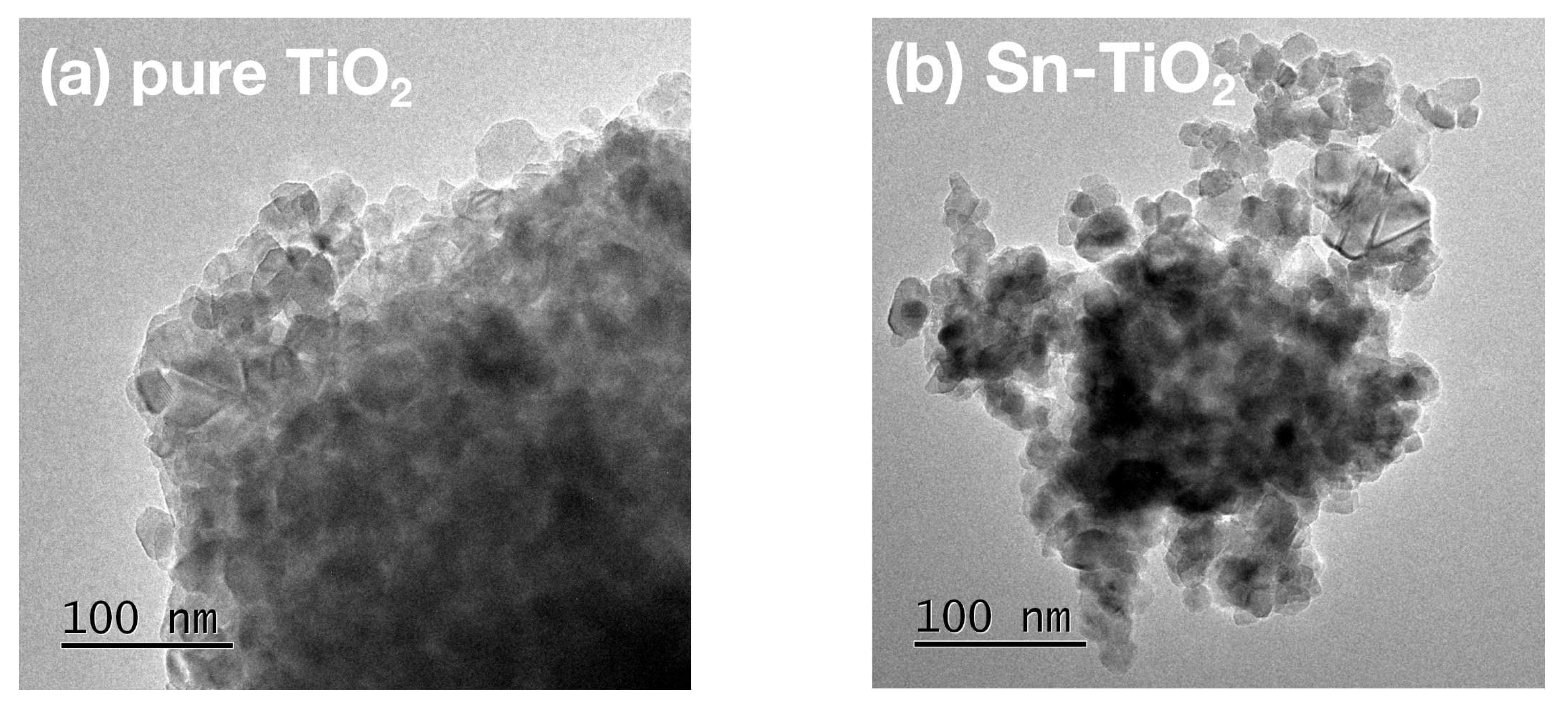
2.2. Morphology
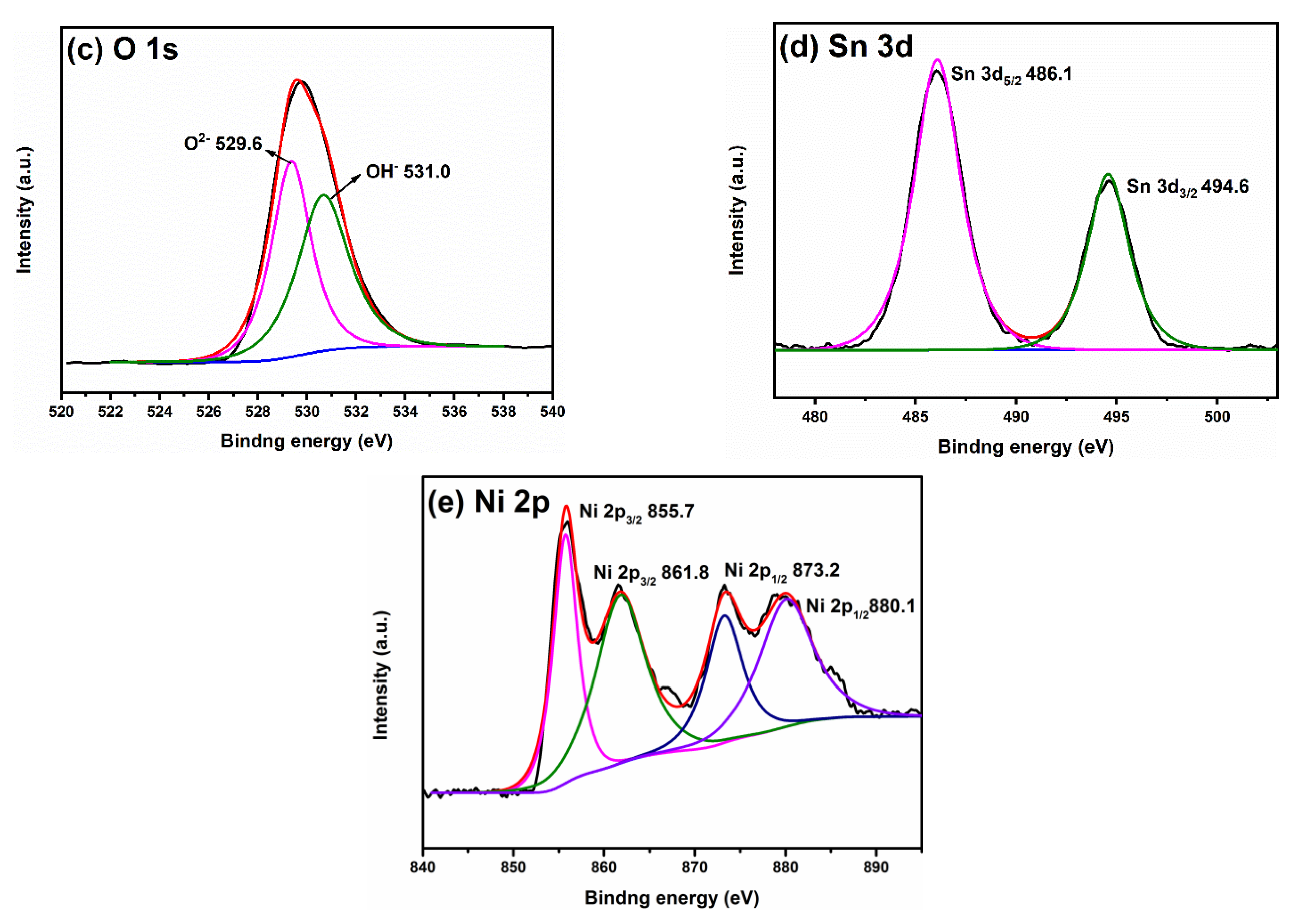
2.3. Element Composition and State
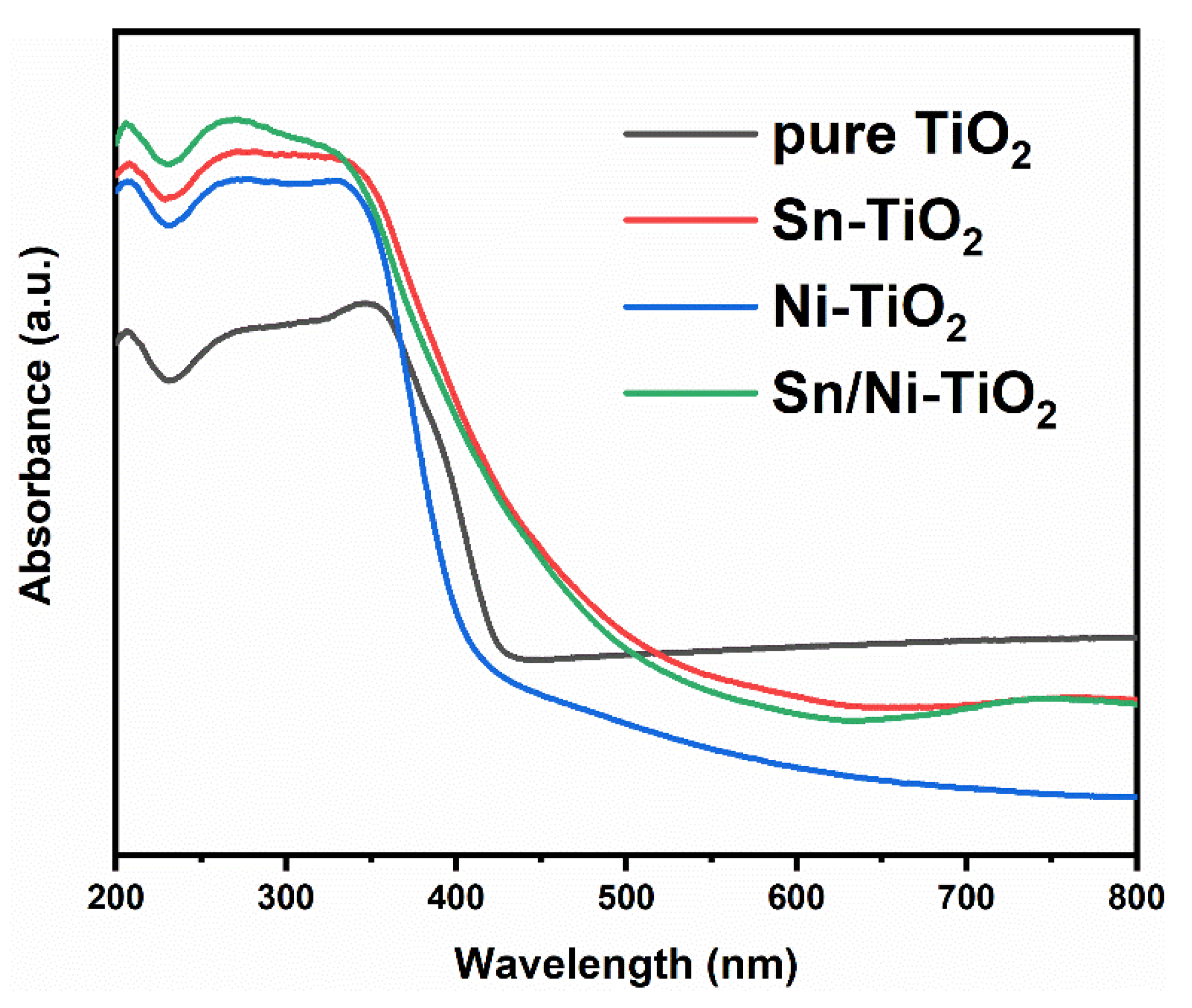
2.4. Optical Property
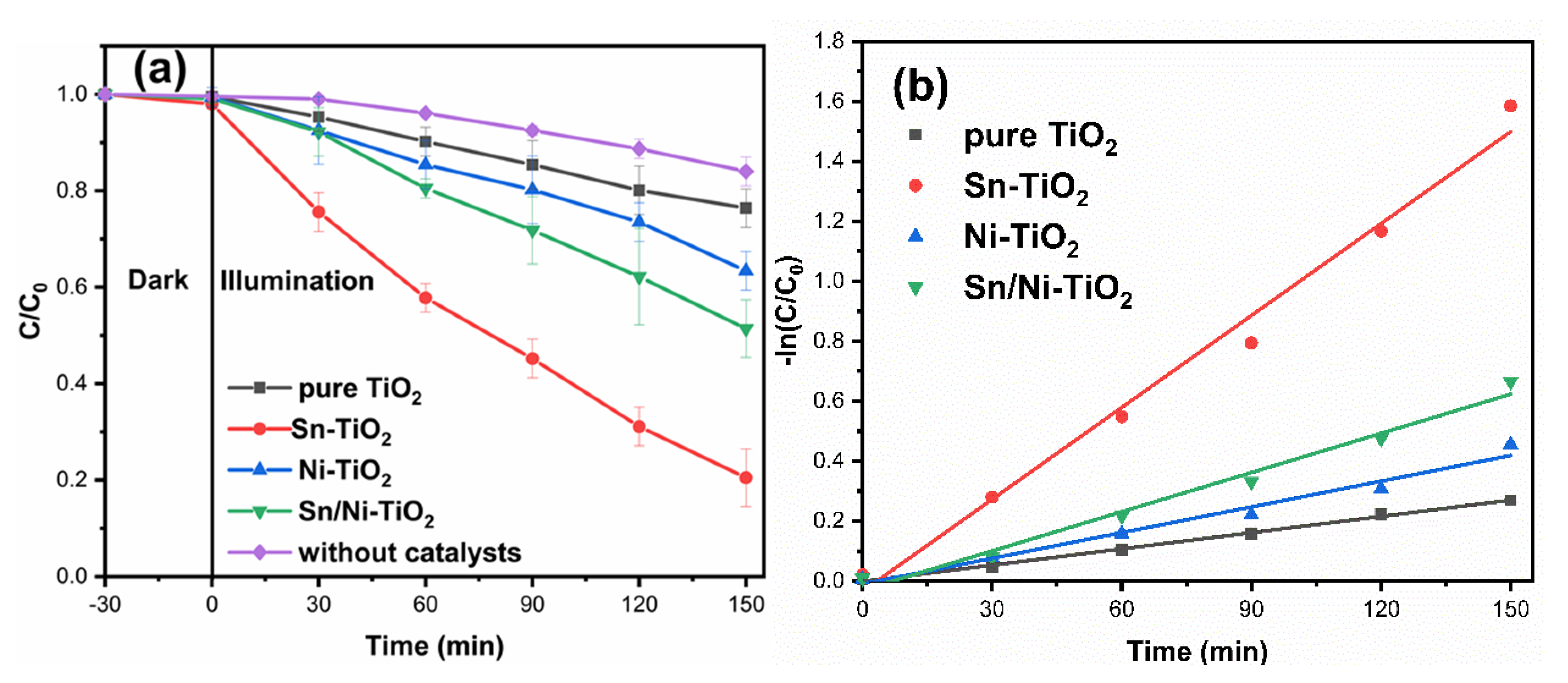
2.5. Photocatalytic Activity
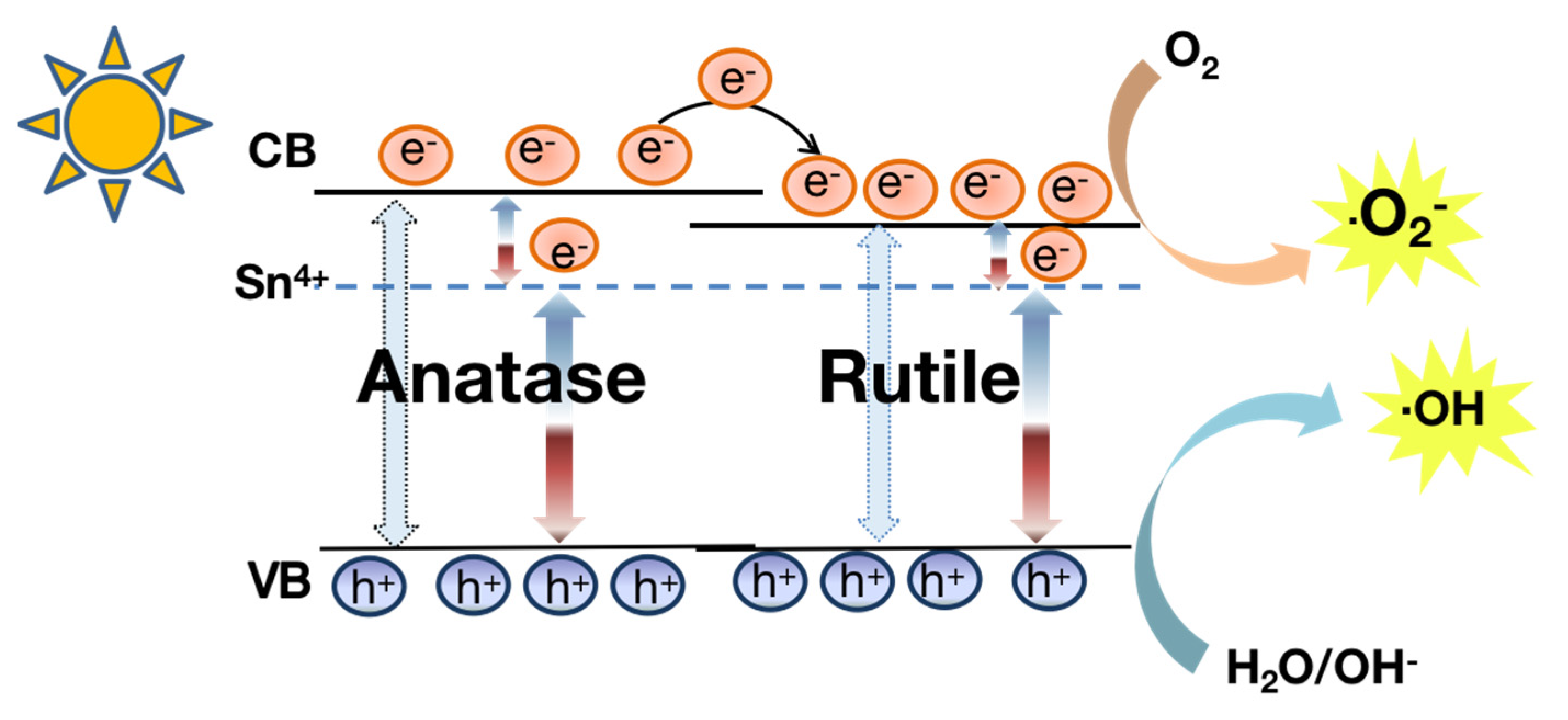
2.6. Mechanism of Photocatalytic
3. Experimental
3.1. Sample Preparation
3.2. Sample Characterization
3.3. Photocatalysis Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cherrak, R.; Hadjel, M.; Benderdouche, N.; Adjdir, M.; Mokhtar, A.; Khaldi, K.; Sghier, A.; Weidler, P.G. Preparation of nano-TiO2/diatomite composites by non-hydrolytic sol-gel process and its application in photocatalytic degradation of crystal violet. Silicon 2020, 12, 927–935. [Google Scholar] [CrossRef]
- Singh, J.; Manna, A.K.; Soni, R.K. Bifunctional Au-TiO2 thin films with enhanced photocatalytic activity and SERS based multiplexed detection of organic pollutant. J. Mater. Sci. Mater. Electron. 2019, 30, 16478–16493. [Google Scholar] [CrossRef]
- Gerasimova, T.V.; Evdokimova, O.L.; Kraev, A.S.; Ivanov, V.K.; Agafonov, A.V. Micro–mesoporous anatase TiO2 nanorods with high specific surface area possessing enhanced adsorption ability and photocatalytic activity. Microporous Mesoporous Mater. 2016, 235, 185–194. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Nagaraju, G.; Dupont, J. Photocatalytic activity of Li–doped TiO2 nanoparticles: Synthesis via ionic liquid-assisted hydrothermal route. Mater. Res. Bull. 2016, 78, 103–111. [Google Scholar] [CrossRef]
- Zhu, X.D.; Han, S.H.; Zhu, D.Z.; Chen, S.H.; Feng, W.; Kong, Q.Q. Preparation and characterisation of Ag modified rutile titanium dioxide and its photocatalytic activity under simulated solar light. Micro Nano Lett. 2019, 14, 757–760. [Google Scholar] [CrossRef]
- Kundu, A.; Mondal, A. Structural, optical, physio-chemical properties and photodegradation study of methylene blue using pure and iron-doped anatase titania nanoparticles under solar-light irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 3244–3256. [Google Scholar] [CrossRef]
- Yang, K.; Li, X.X.; Yu, C.L.; Zeng, D.B.; Chen, F.Y.; Zhang, K.L.; Huang, W.Y.; Ji, H.B. Review on heterophase/homophase junctions for efficient photocatalysis: The case of phase transition construction. Chin. J. Catal. 2019, 40, 796–818. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Boobas, S.; Jayaprakash, J.; Rajaboopathi, M.; Han, B.; Louhi-Kultanen, M. Effect of Cu doping on TiO2 nanoparticles and its photocatalytic activity under visible light. J. Mater. Sci. Mater. Electron. 2016, 27, 7438–7447. [Google Scholar] [CrossRef]
- Umar, K.; Ibrahim, M.N.M.; Ahmad, A.; Rafatullah, M. Synthesis of Mn–doped TiO2 by novel route and photocatalytic mineralization/intermediate studies of organic pollutants. Res. Chem. Intermed. 2019, 45, 2927–2945. [Google Scholar] [CrossRef]
- Zhu, X.D.; Song, H.J.; Feng, W.; Wen, G.L.; Li, H.Y.; Zhou, J. Photocurrent response and photocatalytic activity of Nd–doped TiO2 thin films prepared by sol-gel method. J. Adv. Oxid. Technol. 2017, 20, 20160190. [Google Scholar] [CrossRef]
- Ali, I.; Kim, S.R.; Kim, S.P.; Kim, J.O. Anodization of bismuth doped TiO2 nanotubes composite for photocatalytic degradation of phenol in visible light. Catal. Today 2017, 282, 31–37. [Google Scholar] [CrossRef]
- Crisşan, M.; Răileanu, M.; Drăgan, N.; Crişan, D.; Ianculescu, A.; Nitoi, I.; Oancea, P.; Somacescu, S.; Stănică, N.; Vasile, B.; et al. Sol–gel iron–doped TiO2 nanopowders with photocatalytic activity. Appl. Catal. A Gen. 2015, 504, 130–142. [Google Scholar] [CrossRef]
- Grujić-Brojčin, M.; Armaković, S.; Tomić, N.; Abramović, B.; Golubović, A.; Stojadinović, B.; Kremenović, A.; Babić, B.; Dohčević-Mitrović, Z.; Šćepanović, M. Surface modification of sol–gel synthesized TiO2 nanoparticles induced by La–doping. Mater. Charact. 2014, 88, 30–41. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Shehata, N.; Betiha, M.A.; Rabie, A.M. Ni-doped and Ni/Cr co–doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2019, 555, 31–41. [Google Scholar] [CrossRef]
- Georgieva, J.; Valova, E.; Armyanov, S.; Tatchev, D.; Sotiropoulos, S.; Avramova, I.; Dimitrova, N.; Hubin, A.; Steenhaut, O. A simple preparation method and characterization of B and N co–doped TiO2 nanotube arrays with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2017, 413, 284–291. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co–doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Zhong, J.S.; Xu, J.R.; Wang, Q.Y. Nitrogen and vanadium co-doped TiO2 mesosponge layers for enhancement in visible photocatalytic activity. Appl. Surf. Sci. 2014, 315, 131–137. [Google Scholar]
- Jung, H.S.; Kim, H. Origin of low photocatalytic activity of rutile TiO2. Electron. Mater. Lett. 2009, 5, 73–76. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.L.; Wang, C.F.; Ni, M.; Leung, M.K.H.; Wang, X.X.; Fu, X.Z. Hydrogen production over titania–based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Chetia, L.; Kalita, D.; Ahmed, G.A. Enhanced photocatalytic degradation by diatom templated mixed phase titania nanostructure. J. Photochem. Photobiol. A Chemistry 2017, 338, 134–145. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mekprasart, W.; Pecharapa, W. Aatase/rutile TiO2 composite prepared via sonochemical process and their photocatalytic activity. Mater. Today Proc. 2017, 4, 6159–6165. [Google Scholar] [CrossRef]
- Likodimos, V.; Chrysi, A.; Calamiotou, M.; Fernández–Rodríguez, C.; Dona-Rodríguez, J.M.; Dionysiou, D.D.; Falaras, P. Microstructure and charge trapping assessment in highly reactive mixed phase TiO2 photocatalysts. Appl. Catal. B Environ. 2016, 192, 242–252. [Google Scholar] [CrossRef]
- Liao, Y.L.; Que, W.X.; Tang, Z.H.; Wang, W.J.; Zhao, W.H. Effects of heat treatment scheme on the photocatalytic activity of TiO2 nanotube powders derived by a facile electrochemical process. J. Alloy. Compd. 2011, 509, 1054–1059. [Google Scholar] [CrossRef]
- Banerjee, D.; Gupta, S.K.; Patra, N.; Raja, S.W.; Pathak, N.; Bhattacharyya, D.; Pujari, P.K.; Thakare, S.V.; Jha, S.N. Unraveling doping induced anatase–rutile phase transition in TiO2 using electron, X–ray and gamma–ray as spectroscopic probes. Phys. Chem. Chem. Phys. 2018, 20, 28699–28711. [Google Scholar] [CrossRef]
- Bai, S.; Gao, C.; Low, J.X.; Xiong, Y.J. Crystal phase engineering on photocatalytic materials for energy and environmental applications. Nano Res. 2019, 12, 2031–2054. [Google Scholar] [CrossRef]
- Liu, R.D.; Li, H.; Duan, L.B.; Shen, H.; Zhang, Q.; Zhao, X.R. Influences of annealing atmosphere on phase transition temperature, optical properties and photocatalytic activities of TiO2 phase-junction microspheres. J. Alloy. Compd. 2019, 789, 1015–1021. [Google Scholar] [CrossRef]
- Elsellami, L.; Dappozze, F.; Fessi, N.; Houas, A.; Guillard, C. Highly photocatalytic activity of nanocrystalline TiO2 (anatase, rutile) powders prepared from TiCl4 by sol–gel method in aqueous solutions. Process. Saf. Environ. Prot. 2018, 113, 109–121. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Ling, P.Y. Heat treatment effects on the characteristics and sonocatalytic performance of TiO2 in the degradation of organic dyes in aqueous solution. J. Hardous Mater. 2010, 173, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, X.J.; Liu, Y.D.; Wang, L.Z.; Lei, J.Y.; Zhang, J.L. Facile phase control for hydrothermal synthesis of anatase–rutile TiO2 with enhanced photocatalytic activity. J. Alloy. Compd. 2015, 646, 380–386. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, Z.; Jiang, P.; Kuang, J.L.; Liu, W.X.; Cao, W.B. Hydrothermal synthesis and enhanced photocatalytic activity of mixed-phase TiO2 powders with controllable anatase/rutile ratio. Solid State Sci. 2018, 77, 14–19. [Google Scholar] [CrossRef]
- Alves, A.K.; Berutti, F.A.; Bergmann, C.P. Visible and UV photocatalytic characterization of Sn–TiO2 electrospun fibers. Catal. Today 2013, 208, 7–10. [Google Scholar] [CrossRef]
- Turkten, N.; Cinar, Z.; Tomruk, A.; Bekbolet, M. Copper-doped TiO2 photocatalysts: Application to drinking water by humic matter degradation. Environ. Sci. Pollut. Res. 2019, 26, 36096–36106. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Z.; Liu, L.; Ma, X.M.; Qi, Z.Z.; He, Y.Z. The influence of alumina dopant on the structural transformation of gel–derived nanometre titania powders. J. Mater. Sci. Lett. 1994, 13, 462–464. [Google Scholar] [CrossRef]
- Song, G.B.; Liang, J.K.; Liu, F.S.; Peng, T.J.; Rao, G.H. Preparation and phase transformation of anatase–rutile crystals in metal doped TiO2/muscovite nanocomposites. Thin Solid Film. 2005, 491, 110–116. [Google Scholar] [CrossRef]
- Li, J.L.; Xu, X.T.; Liu, X.J.; Yu, C.Y.; Yan, D.; Shou, Z.; Pan, L.K. Sn doped TiO2 nanotube with oxygen vacancy for highly efficient visible light photocatalysis. J. Alloy. Compd. 2016, 679, 454–462. [Google Scholar] [CrossRef]
- Wu, M.C.; Wu, P.Y.; Lin, T.H.; Lin, T.F. Photocatalytic performance of Cu–doped TiO2 nanofibers treated by the hydrothermal synthesis and air-thermal treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]
- Duan, Y.D.; Fu, N.Q.; Zhang, Q.; Fang, Y.Y.; Zhou, X.W.; Lin, Y. Influence of Sn source on the performance of dye-sensitized solar cells based on Sn–doped TiO2 photoanodes: A strategy for choosing an appropriate doping source. Electrochim. Acta 2013, 107, 473–480. [Google Scholar] [CrossRef]
- Wang, T.; Wei, J.X.; Shi, H.M.; Zhou, M.; Zhang, Y.; Chen, Q.; Zhang, Z.M. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Physica E 2017, 86, 103–110. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wen, K.; Long, H.Y. Surface doping of TiO2 powders via a gas–melt reaction using thermal plasma as an excitation source. Ceram. Int. 2020, 46, 1577–1585. [Google Scholar] [CrossRef]
- Mech, K. A novel magnetoelectrochemical method of synthesis of photoactive Ni–TiO2 coatings from glycinate electrolytes. Mater. Des. 2019, 182, 108055. [Google Scholar] [CrossRef]
- Zeng, P.; Liu, J.Y.; Wang, J.M.; Peng, T.Y. Fabrication of Ni nanoclusters–modified brookite TiO2 quasi nanocubes and its photocatalytic hydrogen evolution performance. Chin. J. Chem. Phys. 2019, 32, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr(VI) under visible light. Appl. Surf. Sci. 2014, 321, 396–403. [Google Scholar] [CrossRef]
- Fan, X.; Wan, J.; Liu, E.Z.; Sun, L.; Hu, Y.; Li, H.; Hu, X.Y.; Fan, J. High-efficiency photoelectrocatalytic hydrogen generation enabled by Ag deposited and Ce doped TiO2 nanotube arrays. Ceram. Int. 2015, 41, 5107–5116. [Google Scholar] [CrossRef]
- Ke, S.J.; Cheng, X.S.; Wang, Q.H.; Wang, Y.M.; Pan, Z.D. Preparation of a photocatalytic TiO2/ZnTiO3 coating on glazed ceramic tiles. Ceram. Int. 2014, 40, 8891–8895. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K. Fabrication of Ce/N co–doped TiO2/diatomite granule catalyst and its improved visible-light-driven photoactivity. J. Hazard. Mater. 2017, 324, 139–150. [Google Scholar] [CrossRef]
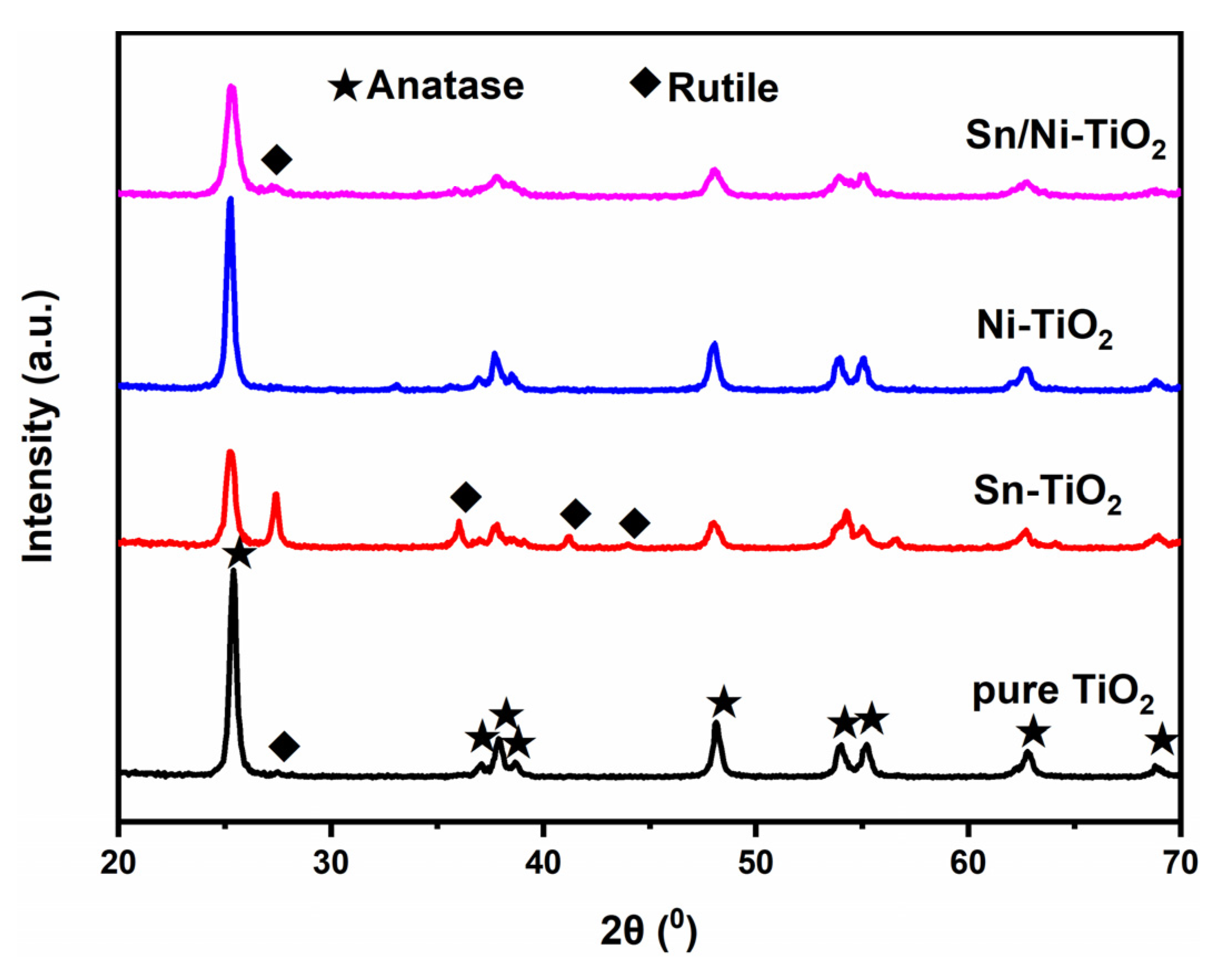




| Samples | Phase Composition (Anatase/Rutile) | D (nm) |
|---|---|---|
| pure TiO2 | 97.9/2.1 | 21.5/26.9 |
| Sn–TiO2 | 58.9/41.1 | 16.9/25.8 |
| Ni–TiO2 | 100.0/0 | 21.7 |
| Sn/Ni–TiO2 | 94.7/5.3 | 14.3/19.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.; Wang, J.; Xia, Y.; Yang, D.; Zhou, Q.; Zhu, X.; Feng, W. Synthesis and Characterization of Sn/Ni Single Doped and Co–Doped Anatase/Rutile Mixed–Crystal Nanomaterials and Their Photocatalytic Performance under UV–Visible Light. Catalysts 2021, 11, 1341. https://doi.org/10.3390/catal11111341
Qin Q, Wang J, Xia Y, Yang D, Zhou Q, Zhu X, Feng W. Synthesis and Characterization of Sn/Ni Single Doped and Co–Doped Anatase/Rutile Mixed–Crystal Nanomaterials and Their Photocatalytic Performance under UV–Visible Light. Catalysts. 2021; 11(11):1341. https://doi.org/10.3390/catal11111341
Chicago/Turabian StyleQin, Qin, Juan Wang, Yangwen Xia, Daixiong Yang, Qin Zhou, Xiaodong Zhu, and Wei Feng. 2021. "Synthesis and Characterization of Sn/Ni Single Doped and Co–Doped Anatase/Rutile Mixed–Crystal Nanomaterials and Their Photocatalytic Performance under UV–Visible Light" Catalysts 11, no. 11: 1341. https://doi.org/10.3390/catal11111341






