Abstract
In this study, we fabricated Er-doped ZnO/CuS/Au core-shell nanowires using two-step wet chemical methods and an ion-sputtering method on a glass substrate as a bifunctional photocatalytic and surface-enhanced Raman scattering (SERS) substrate. The characteristic properties of as-prepared photocatalysts were confirmed by scanning electron microscopy, X-ray diffraction, transmission electron microscopy, energy-dispersive X-ray spectroscopy, DR/UV-Vis spectroscopy, and photoluminescence spectroscopy. Compared with Er-doped ZnO nanowires and Er-doped ZnO/CuS core-shell nanowires, Er-doped ZnO/CuS/Au core-shell nanowires exhibited remarkably photocatalytic activity to degrade acid orange 7 solutions under blue LED light. These results ascribed to the Er-doped ZnO/CuS/Au core-shell nanowires can enhance the visible-light absorbance and the separation efficiency of photogenerated electron-hole pairs, inducing their higher photocatalytic activity under blue LED light. In addition, Er-doped ZnO/CuS/Au core-shell nanowires exhibit high sensitivity, a low detection limit (10−6 M), uniformity, recyclability, and stability of SERS performance for detected acid orange 7.
1. Introduction
Recently, different kinds of metal sulfides have attracted a large amount of attention because of their unique chemical and physical properties [1,2,3,4]. Among them, copper sulfide (CuS) is an essential p-type semiconductor with a narrow bandgap (1.2–2.3 eV), which shows many unique optical, electronic, and other physicochemical properties [5,6,7,8]. In addition, CuS has outstanding potential in variable applications, such as lithium-ion batteries, chemical sensors, solar energy converters, cathode material, photocatalysis, optical filters, and non-linear optical material [9,10,11]. Recently, CuS nanostructures have been synthesized by various methods, such as sonochemical [12], hydrothermal [13], solvothermal [14], microwave-assisted heating [15], and wet chemical methods [16]. The wet chemical method can provide the lowest equipment requirements, a low thermal budget, template-free, and industrial-scale manufacturing potential through low reaction temperature and liquid phase synthesis [17]. In addition, the wet chemical method can also be used to grow different morphologies, such as nanorods [18], nanoparticles [17], nanoflakes [19], and nanotubes [20].
Traditional physical techniques (adsorption on activated carbon, coagulation by chemical agents, ion exchange on synthetic adsorbent resins, etc.) can generally be used efficiently to remove dyes [21,22]. Advanced oxidation processes have been described as efficient procedures for obtaining high oxidation yields from several organic compounds such as photocatalysts [23,24]. Although the CuS nanostructures with a suitable bandgap can be directly applied as a visible-light-driven photocatalyst, they is susceptible to photocorrosion in limited photocatalytic activity [25,26]. In order to overcome this drawback, CuS nanostructures combined with various semiconductor materials can be used to improve their photocatalytic activity [27]. Presently, there are several different kinds of CuS-semiconductor heterostructures synthesized for significantly enhanced photocatalytic efficiency, such as BiOCl/CuS [28], CuS/ZnS [25,29], ZnO/CuS [27,30], CuS/TiO2 [31], PrGO (partially reduced graphene oxide)/CuS [12], and CuS@CuGaS2 [8]. Among them, ZnO/CuS heterostructures have attracted extensive interest because of their special bandgap location, which can inhibit the recombination of photogenerated charge carriers by preventing photocorrosion from enhancing their photocatalytic activity [32]. However, to the best of our knowledge, no literature has ever reported ZnO/CuS heterostructures being further combined with Au nanoparticles and then applied to both photocatalysis and surface-enhanced Raman scattering applications. In addition, metal-doped ZnO has been proven to inhibit the recombination of charge carriers and reduce the bandgap for improved visible-light-driven photocatalytic applications [27].
This study constructed a heterostructure substrate by integrating Er-doped ZnO nanowire with CuS to form a core-shell nanowire using a two-step wet chemical method on the glass substrate. The three-dimensional heterostructures can be beneficial to deposit Au nanoparticles for use as a high-performance photocatalytic and SERS substrate for acid orange 7. Acid orange 7 is generally used for dyeing silk, wool fabrics, leather, and paper [33].
2. Results and Discussion
The surface morphology of Er-doped ZnO nanowires and Er-doped ZnO/CuS core-shell nanowires were characterized by field-emission scanning electron microscopy (FESEM). Figure 1a shows the tilt-view FESEM image of vertical-aligned Er-doped ZnO nanowires directly fabricated on the glass substrate with the ZnO seed layer by a facile wet chemical process at 90 °C for 3 h. Er-doped ZnO nanowires exhibited a sharp surface uniformly with an average diameter and length of 100–200 nm and 3–4 µm, respectively. Then, the equal molar of copper(II) acetate and thioacetamide were selected as the Cu and S precursors to grow CuS nanoparticles on the Er-doped ZnO nanowires via a wet chemical process at 90 °C for 2 h. Figure 1b–e displays the tilt-view FESEM images of Er-doped ZnO/CuS core-shell nanowires which were synthesized at the different concentrations of CuS precursor (2.5, 5, 10, 20 mM). As the concentration of CuS reaction precursors increases, the thickness of the Er-doped ZnO nanowires coated with CuS nanoparticles increases gradually. However, when the concentration of CuS reaction precursor exceeds 20 mM, CuS nanoparticles tend to self-aggregate rather than deposit on the Er-doped ZnO nanowires.
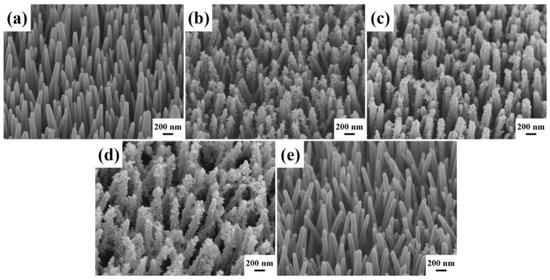
Figure 1.
The tilt-view FESEM images of (a) Er-doped ZnO nanowires and (b–e) Er-doped ZnO/CuS core-shell nanowires with different concentrations of CuS precursor fabricated on the glass substrates. The concentrations of CuS precursor were (b) 2.5, (c) 5, (d) 10, and (e) 20 mM, respectively.
The crystal structure of as-prepared Er-doped ZnO nanowires and Er-doped ZnO/CuS core-shell nanowires were analyzed via an X-ray diffractometer. Figure 2a reveals the XRD pattern of Er-doped ZnO nanowires at diffraction peaks of 31.86°, 34.53°, 36.41°, 47.69°, 56.78°, 63.02°, 68.06°, and 72.98°, corresponding to (100), (002), (102), (101), (102), (110), (103), (112), and (004) crystal planes, confirming the formation of hexagonal ZnO crystal phase (JCPDS Card No. 75-0576). In the XRD pattern (Figure 2b) of Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor), two different diffraction peaks were observed at 2θ = 29.54° and 33.15°, which correspond to (102) and (006) crystal planes, confirming the formation of hexagonal CuS crystal phase (JCPDS Card No. 65-3928). This results can directly prove that CuS has been successfully synthesized on the Er-doped ZnO nanowires.
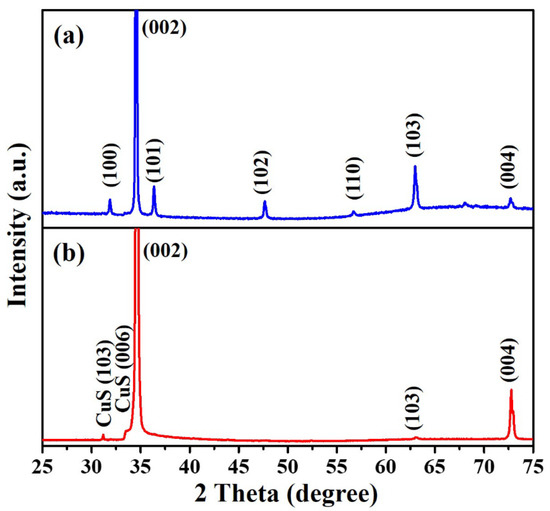
Figure 2.
XRD patterns of (a) Er-doped ZnO nanowires and (b) Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor) prepared on the glass substrates.
Figure 3a,b display the FETEM images of an individual Er-doped ZnO/CuS core-shell nanowire (10 mM CuS precursor), further confirming that CuS nanoparticles have been decorated on the surface of Er-doped ZnO nanowire. The HRTEM image of an individual Er-doped ZnO/CuS core-shell nanowire is shown in Figure 3c. The interlayer spacings of Er-doped ZnO/CuS core-shell nanowire are obtained at 0.261 nm and 0.280 nm, which can be corresponded to the d-spacing of the (002) lattice plane of the hexagonal ZnO crystal (JCPDS Card No. 75-0576) and (102) lattice plane of the hexagonal CuS crystal (JCPDS Card No. 65-3928), respectively. In addition, the EDS mapping analysis of an Er-doped ZnO/CuS core-shell nanowire (Figure 3d) can demonstrate that the core and shell are mainly composed of ZnO and CuS, respectively. Furthermore, the content of Er was about 0.76 at.%. Therefore, it can be observed that the composition and morphology of the Er-doped ZnO/CuS core-shell nanowires are consistent.
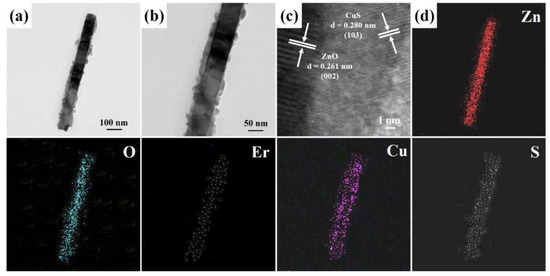
Figure 3.
(a,b) FETEM, (c) HRTEM, and (d) EDS mapping images of an Er-doped ZnO/CuS core-shell nanowire (10 mM CuS precursor).
Recently, Au nanoparticles deposited on the heterostructures can improve their photocatalytic activity by generating the Schottky barrier to suppress the recombination of electron-hole pairs and increase visible light absorption [34,35]. The FETEM image (Figure 4a) of an individual Er-doped ZnO/CuS/Au core-shell nanowire was fabricated at the Au deposition time of 30 s. Figure 4b shows the HRTEM image of an individual Er-doped ZnO/CuS/Au core-shell nanowire, in which the interlayer spacings of Er-doped ZnO/CuS/Au core-shell nanowire are obtained at 0.271 nm and 0.236 nm, which can be corresponded to the d-spacing of the (006) lattice plane of the hexagonal CuS crystal (JCPDS Card No. 65-3928) and (111) lattice plane of the cubic Au crystal (JCPDS Card No. 65-2870), respectively. In addition, the EDS mapping images (Figure 4c) of an Er-doped ZnO/CuS/Au core-shell nanowire can also use to confirm the composition of the core-shell structure by Zn, O, Er, Cu, S, and Au signals.
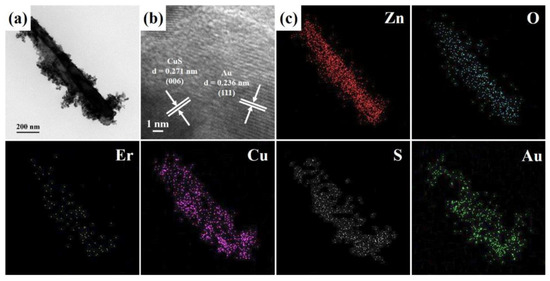
Figure 4.
(a) FETEM, (b) HRTEM, and (c) EDS mapping images of an Er-doped ZnO/CuS/Au core-shell nanowire fabricated at the Au-sputtering time of the 30 s.
The photocatalytic efficiency of as-synthesized photocatalysts with the same area (2.5 cm × 1.5 cm) was evaluated by degrading acid orange 7 (AO7) under blue LED light. Figure 5a shows the plots of C/C0 in the degradation of AO7, where C0 is the initial concentration of AO7 and C is the instantaneous concentrations of the AO7, respectively. Thus, it can be ascertained that the photodegradation rate of AO7 followed the order of Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor) > Er-doped ZnO/CuS core-shell nanowires (5 mM CuS precursor) > Er-doped ZnO/CuS core-shell nanowires (2.5 mM CuS precursor) > Er-doped ZnO/CuS core-shell nanowires (20 mM CuS precursor) > Er-doped ZnO nanowires > ZnO nanowires (without Er-doped) > blank (without photocatalysts) after the irradiation time of 3 h. The plots of C/C0 vs. t reveal that the photocatalytic degradation follows pseudo-first-order reaction in the as-synthesized photocatalysts, as shown in Figure 5b. From the plot of −ln (C/C0) vs. t, rate constant (k) can be determined. The rate constant (k) was calculated to be 0.0000326 (blank), 0.000483 (ZnO nanowires), 0.000665 (Er-doped ZnO nanowires), 0.00626 (Er-doped ZnO/CuS core-shell nanowires (2.5 mM CuS precursor)), 0.00827 (Er-doped ZnO/CuS core-shell nanowires (5 mM CuS precursor)), 0.01004 (Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor)), and 0.000885 min–1 (Er-doped ZnO/CuS core-shell nanowires (20 mM CuS precursor)), respectively. It is obtained that Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor) exhibit the best photocatalytic activity rather than the above five as-synthesized photocatalysts.

Figure 5.
(a) Photocatalytic activities and (b) kinetic linear simulation curves of ZnO nanowires, Er-doped ZnO nanowires, and Er-doped ZnO/CuS core-shell nanowires with different concentrations of CuS precursor under blue LED light. (c) Photocatalytic activities and (d) kinetic linear simulation curves of Er-doped ZnO/CuS core-shell nanowires and Er-doped ZnO/CuS/Au core-shell nanowires with different Au-sputtering times under blue LED light. (e) The dark absorption spectra of as-prepared photocatalysts.
In order to further enhance the photocatalytic activity of Er-doped ZnO/CuS core-shell nanowires, the ion-sputtering method can be used to deposit Au nanoparticles on the Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor) at the different Au-sputtering times under blue LED light, as shown in Figure 5c. As a result, it can be ascertained that the photodegradation rate of AO7 followed the order of Er-doped ZnO/CuS/Au core-shell nanowires (30 s) > Er-doped ZnO/CuS/Au core-shell nanowires (45 s) > Er-doped ZnO/CuS/Au core-shell nanowires (15 s) > Er-doped ZnO/CuS core-shell nanowires (without Au nanoparticles). Thus, the Au nanoparticles decorated on the Er-doped ZnO/CuS core-shell nanowires at the different Au-sputtering times can exhibit a slightly higher photocatalytic efficiency than that of Er-doped ZnO/CuS core-shell nanowires. Figure 5d shows the plots of −ln (C/C0) vs. t of Er-doped ZnO/CuS core-shell nanowires at the different Au-sputtering times, for which the rate constant (k) was calculated to be 0.01004 (Er-doped ZnO/CuS core-shell nanowires (without Au nanoparticles)), 0.01405 (Er-doped ZnO/CuS/Au core-shell nanowires (15 s)), 0.02087 (Er-doped ZnO/CuS/Au core-shell nanowires (30 s)), and 0.01517 min–1 (Er-doped ZnO/CuS/Au core-shell nanowires (45 s)), respectively. A simple ion-sputtering method to deposit 30 s Au nanoparticles on Er-doped ZnO/CuS core-shell nanowires can provide about a 2.08 times higher rate constant. The increase in photocatalytic activity in the case of Au nanoparticles shall be ascribed to restrict the recombination of electron-hole pairs under blue LED light. The dark reaction/adsorption data of as-prepared photocatalysts (ZnO nanowires, Er-doped ZnO nanowires, Er-doped ZnO/CuS core-shell nanowires (10 mM CuS precursor), and Er-doped ZnO/CuS/Au core-shell nanowires (30 s)) is shown in Figure 5e. The results show that the as-prepared photocatalysts are not evident for the dark adsorption, which may be ascorbic to the photocatalysts directly grown on the glass substrates to reduce the dark adsorption.
The UV-Vis diffuse reflectance spectra (DRS) of the Er-doped ZnO nanowires, Er-doped ZnO/CuS core-shell nanowires, and Er-doped ZnO/CuS/Au core-shell nanowires are compared in Figure 6a. In contrast to Er-doped ZnO nanowires and Er-doped ZnO/CuS core-shell nanowires, the as-prepared Er-doped ZnO/CuS/Au core-shell nanowires exhibit the stronger absorption from the UV- to visible-light regions. This result proved that the sequential deposition of CuS nanoparticles and Au nanoparticles could effectively improve the light absorption ability of Er-doped ZnO nanowires to enhance their photocatalytic activity. Furthermore, photoluminescence (PL) measurement is an efficient and suitable tool to understand the improved charge carriers separation rate of the as-prepared Er-doped ZnO/CuS/Au core-shell nanowires over Er-doped ZnO nanowires and Er-doped ZnO/CuS core-shell nanowires, as shown in Figure 6b. The decrease in PL intensity can enhance the separation efficiency of charge carriers [36]. The weaker UV- and visible-light emission intensity of the Er-doped ZnO/CuS/Au core-shell nanowires indicates a lower recombination rate for the photogenerated electron and hole pair than Er-doped ZnO nanowires and Er-doped ZnO/CuS core-shell nanowires. Therefore, the optical absorption and emission properties for the Er-doped ZnO/CuS/Au core-shell nanowires shall be benefited from visible-light-driven photocatalytic applications.
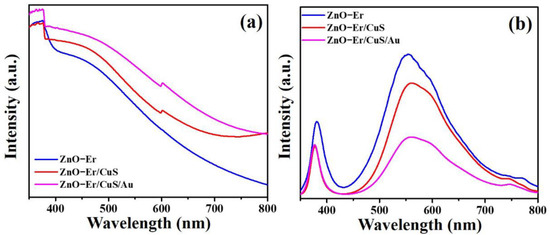
Figure 6.
(a) UV-vis diffuse reflectance spectra and (b) PL spectra of Er-doped ZnO nanowires, Er-doped ZnO/CuS core-shell nanowires, and Er-doped ZnO/CuS/Au core-shell nanowires.
The results prove that the as-prepared Er-doped ZnO/CuS/Au core-shell nanowires have better photocatalytic performance than the nanopowder type of commercially available photocatalysts (ZnO and P25 TiO2) and CuS under blue LED light, as shown in Figure 7a. As a result, it can be ascertained that the photodegradation rate of AO7 followed the order of Er-doped ZnO/CuS/Au core-shell nanowires > CuS nanopowder > P25 TiO2 nanopowder > ZnO nanopowder. Figure 7b shows the plots of −ln (C/C0) vs. t of commercial and as-prepared photocatalysts, for which the rate constant (k) was calculated to be 0.0004878 (ZnO nanopowder), 0.0006895 (P25 TiO2 nanopowder), 0.00325 (CuS nanopowder), and 0.02087 (Er-doped ZnO/CuS/Au core-shell nanowires), respectively. Thus, the Er-doped ZnO/CuS/Au core-shell nanowires reveal a better photodegradation rate of AO7 than commercial P25 TiO2, ZnO, and as-synthesized CuS nanopowders under blue LED light. The excellent photocatalytic activity of Er-doped ZnO/CuS/Au core-shell nanowires might be ascribed to the unique heterostructure, which can benefit the separation of charge carriers and presents better stability, which prevents aggregation from improving the photocatalytic activity. In order to understand the reusability of the as-synthesized Er-doped ZnO/CuS/Au core-shell nanowires under blue LED light, the Er-doped ZnO/CuS/Au core-shell nanowires were reused for the photodegradation of AO7. Figure 7c shows that the photocatalytic efficiency of AO7 after five cycles remained 87.9%. This result can demonstrate that Er-doped ZnO/CuS/Au core-shell nanowires contribute to future applications in actual photocatalysis. The degree of mineralization of AO7 solution with Er-doped ZnO/CuS/Au core-shell nanowires was estimated by determining the decrease in the total organic carbon (TOC) for the reaction solution, as shown in Figure 7d. It can be seen that the TOC has gradually decreased with irradiation time.
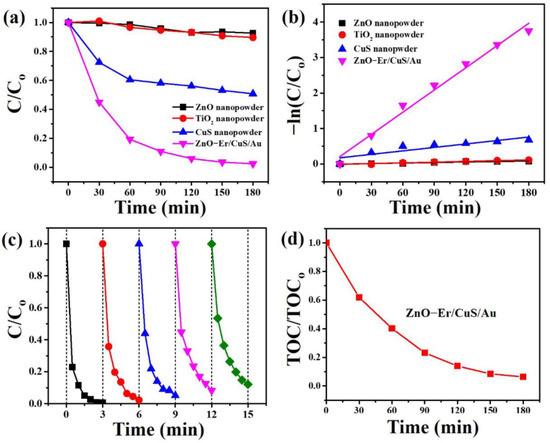
Figure 7.
(a) Photocatalytic activities and (b) kinetic linear simulation curves of as-prepared and commercial photocatalysts under blue LED light. (c) The reusability of the Er-doped ZnO/CuS/Au core-shell nanowires under blue LED light. (d) The total organic carbon of AO7 solution with Er-doped ZnO/CuS/Au core-shell nanowires as a function of irradiation time.
Studying the active species formed in the photocatalytic process is crucial to understand the photocatalytic mechanism of Er-doped ZnO/CuS/Au core-shell nanowires. Therefore, the effects of some scavengers on the photodegradation of AO7 were investigated to evaluate the photocatalytic mechanism. Herein, isopropyl alcohol (IPA), triethanolamine (TEOA), silver nitrate (AgNO3), and L-ascorbic acid (AA) were adopted as quenchers of the hydroxyl radicals (•OH), holes (h+), electrons (e−), and superoxide radical anions (•O2–), respectively [37,38]. Figure 8a shows that the photocatalytic efficiency decreases from 99.2% to 57.5% and 57.2% by adding TEOA and AA. This result demonstrates that •OH and •O2– play as the essential radicals for the photocatalytic degradation of AO7 under blue LED light. As in the above results, the photocatalytic mechanism of Er-doped ZnO/CuS/Au core-shell nanowires is proposed and presented in Figure 8b. For ZnO, the valence and conduction bands were 2.9 and −0.32 eV (vs. NHE), respectively [39]. For CuS, the valence and conduction bands were 1.53 and −0.5 eV (vs. NHE), respectively [40]. Thus, the Er-doped ZnO can create a new electric state band at the bottom of the conduction band of Er-doped ZnO nanowires, which couples with narrowing the bandgap to improve the visible light absorption. As a result, under blue LED light, the photogenerated electrons can be excited from the valance band to the conduction band of ZnO. At the same time, the photogenerated electrons can also be excited from the valance band to the conduction band of CuS. The photogenerated electrons at the conduction band of CuS can transfer to the conduction band of ZnO. The conduction band of ZnO with less than O2/•O2– (−0.046 eV, vs. SHE) can reduce the dissolved molecular oxygen to superoxide radical anions (•O2–). In addition, the photogenerated electrons at the conduction band of CuS can also transfer to Au nanoparticles with stronger electron capture capability and reduce the dissolved molecular oxygen to superoxide radical anions (•O2–). At the same time, the h+ at the valance band of CuS can accept electrons from water or the AO7 adsorbed on the surface of photocatalysts to form hydroxyl radicals (•OH). •O2– and •OH are both main active substances for photodegraded AO7.
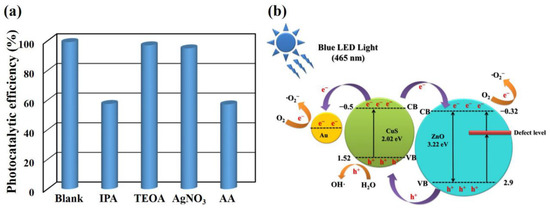
Figure 8.
(a) Photocatalytic activities of Er-doped ZnO/CuS/Au core-shell nanowires with various scavengers under blue LED light. (b) Schematic diagram of the electron transfer mechanism of Er-doped ZnO/CuS/Au core-shell nanowires under blue LED light.
Due to Er-doped ZnO/CuS/Au, core-shell nanowires with unique three-dimensional heterostructures shall be beneficial to use as a high-performance SERS substrate. Herein, we used Er-doped ZnO/CuS/Au core-shell nanowires with different Au-sputtering times to evaluate the optimal SERS substrate. Figure 9a displays the Raman spectra of AO7 molecules deposited on the Er-doped ZnO/CuS/Au core-shell nanowires with the Au-sputtering times of 15, 30, and 45 s, respectively. The prominent characteristic Raman peaks of AO7 are the vibrational peaks at 1098, 1209, 1339, 1420, 1456, and 1602 cm−1 [41]. Furthermore, the Raman intensity of the Er-doped ZnO/CuS/Au core-shell nanowires decreased significantly with increased Au-sputtering times. This result demonstrated that Er-doped ZnO/CuS/Au core-shell nanowires with the Au-sputtering time of 15 s could provide a better hot spot distribution and surface-active sites to enhance the intensity of the Raman signal. Figure 9b displays the Raman spectra of AO7 deposited on the Er-doped ZnO/CuS/Au core-shell nanowires with the Au-sputtering times of 15 s at the different concentrations (10−4 to 10−6 M). The intensity of Raman peaks decreased significantly as the concentration of AO7 solution decreased. The AO7 solution with the lowest detected concentration was 10−6 M. This result proves that the Er-doped ZnO/CuS/Au core-shell nanowires shall be helpful in highly sensitive SERS-base chemical sensing.
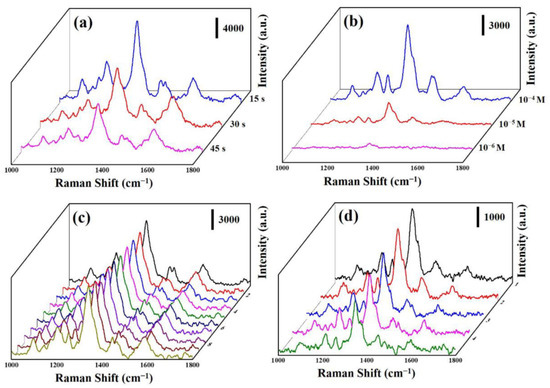
Figure 9.
(a) The Raman spectra of AO7 (10−3 M) on the Er-doped ZnO/CuS/Au core-shell nanowires with different Au-sputtering times. (b) The Raman spectra of AO7 on the Er-doped ZnO/CuS/Au core-shell nanowires from (10−4–10−6 M). (c) The Raman spectra of AO7 (10−4 M) from 10 positions on the Er-doped ZnO/CuS/Au core-shell nanowires. (d) The Raman spectra of AO7 (10−5 M) from five cycles on the Er-doped ZnO/CuS/Au core-shell nanowires.
The uniformity and reusability of the SERS substrate have a profound impact on its practical application. Herein, we measured the Raman spectra (Figure 9c) from 10 random sites to investigate the uniformity of Er-doped ZnO/CuS/Au core-shell nanowires with deposited AO7 solution (10−4 M). This result demonstrates that Er-doped ZnO/CuS/Au core-shell nanowires reveal a relatively consistent Raman intensity. Figure 9d shows the Raman spectra of Er-doped ZnO/CuS/Au core-shell nanowires with deposited AO7 solution (10−5 M) measured during the five recycling processes. The AO7 could be entirely removed by rinsing and being irradiated UV light. Furthermore, the Raman intensity of Er-doped ZnO/CuS/Au core-shell nanowires at 1339 cm–1 for AO7 decreased by less than 25% over five cycles. This result reveals that the Er-doped ZnO/CuS/Au core-shell nanowires exhibit highly efficient reusability.
3. Material and Methods
3.1. Preparation of Er-Doped ZnO Nanowires
The spin coating and thermal annealing processes were used to fabricate the ZnO seed layer onto the glass substrate. A wet chemical method was used to synthesize Er-doped ZnO nanowires on the glass substrate with a ZnO seed layer. In a typical synthesis process, 0.297 g Zn(NO3)2·6H2O, 0.140 g C6H12N4, and 0.016 g Er(NO3)3·5(H2O) were dissolved in 105 mL ultrapure water followed by the addition of 1 mL 1,3-diaminopropane and vigorous stirring for 15 min. The above solution was transferred into a 150 mL sealed bottle in which the glass substrate with a ZnO seed layer was fixed on the wall and heated at 90 °C for 3 h. The Er-doped ZnO nanowires were washed with ultrapure water and ethanol, and dried at 60 °C for 1 h.
3.2. Preparation of Er-Doped ZnO/CuS/Au Core-Shell Nanowires
CuS film was synthesized onto the Er-doped ZnO nanowires through a wet chemical method. In a typical synthesis process, the different concentrations of CuS precursor (equal molar Cu(CH3CO2)2 · H2O and CH3CSNH2) were dissolved in 50 mL ultrapure water by vigorously stirring for 15 min. The above solution was transferred into a 60 mL sealed bottle in which the Er-doped ZnO nanowires were fixed on the wall and heated at 90 °C for 2 h. The Er-doped ZnO/CuS core-shell nanowires were washed with ultrapure water and ethanol, and dried at 60 °C for 1 h. Au nanoparticles were used to decorate the Er-doped ZnO/CuS core-shell nanowires to form Er-doped ZnO/CuS/Au core-shell nanowires by an ion sputtering system (Cressington 108) under the different deposition times.
3.3. Characterization
Field-emission scanning electron microscopy (FE-SEM, Hitachi S-4800, Tokyo, Japan), field-emission transmission electron microscope (FE-TEM, JEOL-2100F, Tokyo, Japan), X-ray diffractometer (XRD, D8 Discover, Bruker, WI, USA), photoluminescence (PL, 325 nm He-Cd laser, Protrustech, Taiwan), and DR/UV-Vis spectroscopy (U-2900, Hitachi, Tokyo, Japan) were used to investigate the growth morphology, crystal structure, composition, and optical properties of the Er-doped ZnO/CuS/Au core-shell nanowires. The Raman spectra were measured using a confocal Raman spectrometer (MRI532S, Protrustech, Taiwan) with an excitation wavelength of 532 nm at a power of 1 mW.
3.4. Photocatalytic Activity Test
The photocatalytic activities of photocatalysts were evaluated via the degradation of acid orange 7 (AO7) solution (0.047 mM) without adjusting the pH value. In a typical photocatalytic process, a blue light LED (λmax = 465 nm, 100 W) lamp was used as a visible light source. The concentration of the AO7 solution was analyzed by using a DR/UV-Vis spectrometer (Hitachi U-2900, Tokyo, Japan) to record the change in the characteristic absorption band (λ = 465 nm). The photocatalytic efficiency of the photocatalysts under blue LED light is defined as C/C0, in which C0 and C are the initial concentration and instantaneous concentration of the AO7 solution, respectively.
4. Conclusions
This study demonstrates that two-step wet chemical and ion-sputtering methods can be used to fabricate Er-doped ZnO/CuS/Au core-shell nanowires as a high-performance photocatalytic and SERS substrate. The effects of different concentrations of CuS precursor and Au-sputtering times on the Er-doped ZnO/CuS/Au core-shell nanowires for photocatalytic performance were systematically investigated. Compared to Er-doped ZnO nanowires or Er-doped ZnO/CuS core-shell nanowires, Er-doped ZnO/CuS/Au core-shell nanowires can be favorable to improve photocatalytic degradation of AO7 solution under blue LED light by increasing the separation of photogenerated electron-hole pairs and their light-harvesting ability. In addition, Er-doped ZnO/CuS/Au core-shell nanowires exhibited good sensitivity, reproducibility, and reusability for detected AO7 solutions. Furthermore, the Er-doped ZnO/CuS/Au core-shell nanowires revealed a low cost and sample approach, high visible-light-induced photocatalytic activity, and high photocatalytic and SERS reusability, which shall be beneficial for different kinds of applications.
Author Contributions
Project administration, resources, supervision, C.-M.C.; formal analysis, investigation, data curation, validation, T.-T.C.; funding acquisition, C.-Y.C. and Y.-C.C.; conceptualization, funding acquisition, methodology, software, resources, writing—review & editing, visualization, Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of Taiwan (MOST 109-2221-E-035-041-MY3) and Taichung Veterans General Hospital (TCVGH-FCU1098203 and TCVGH-1105401C).
Data Availability Statement
Data is contained within the article.
Acknowledgments
This study was supported financially by the Ministry of Science and Technology of Taiwan. The authors appreciate the Precision Instrument Support Center of Feng Chia University in providing the fabrication and measurement facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-Schematic Water Splitting into H2 and O2 Using Metal Sulfide as a Hydrogen-Evolving Photocatalyst and Reduced Graphene Oxide as a Solid-State Electron Mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hakari, Y.; Ikeda, S.; Jia, Q.; Iwase, A.; Kudo, A. Utilization of Metal Sulfide Material of (CuGa)1–xZn2xS2 Solid Solution with Visible Light Response in Photocatalytic and Photoelectrochemical Solar Water Splitting Systems. J. Phys. Chem. Lett. 2015, 6, 1042–1047. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.-J. Recent Progress on Metal Sulfide Composite Nanomaterials for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef]
- Malankowska, A.; Kulesza, D.; Sowik, J.; Cavdar, O.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite. Catalysts 2020, 10, 403. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci.-Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]
- Li, F.; Wu, J.; Qin, Q.; Li, Z.; Huang, X. Controllable synthesis, optical and photocatalytic properties of CuS nanomaterials with hierarchical structures. Powder Technol. 2010, 198, 267–274. [Google Scholar] [CrossRef]
- Hai, Z.; Huang, J.; Remita, H.; Chen, J. Radiolytic synthesis of CuS nanotubes with photocatalytic activity under visible light. Mater. Lett. 2013, 108, 304–307. [Google Scholar] [CrossRef]
- Son, N.; Heo, J.N.; Youn, Y.-S.; Kim, Y.; Do, J.Y.; Kang, M. Enhancement of Hydrogen Productions by Accelerating Electron-Transfers of Sulfur Defects in the CuS@CuGaS2 Heterojunction Photocatalysts. Catalysts 2019, 9, 41. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Chen, H.; Zhang, Y.C.; Le Wu, C. CuS nanostructures prepared by a hydrothermal method. J. Alloys Compd. 2011, 509, 6382–6387. [Google Scholar] [CrossRef]
- Wang, F.; Dong, H.; Pan, J.; Li, J.; Li, Q.; Xu, D. One-Step Electrochemical Deposition of Hierarchical CuS Nanostructures on Conductive Substrates as Robust, High-Performance Counter Electrodes for Quantum-Dot-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 19589–19598. [Google Scholar] [CrossRef]
- Hong, X.; Xu, Z.; Zhang, F.; He, C.; Gao, X.; Liu, Q.; Guo, W.; Liu, X.; Ye, M. Sputtered seed-assisted growth of CuS nanosheet arrays as effective counter electrodes for quantum dot-sensitized solar cells. Mater. Lett. 2017, 203, 73–76. [Google Scholar] [CrossRef]
- Palanisamy, S.; Velmurugan, S.; Yang, T.C.K. One-pot sonochemical synthesis of CuS nanoplates decorated partially reduced graphene oxide for biosensing of dopamine neurotransmitter. Ultrason. Sonochem. 2020, 64, 105043. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Zhang, C.; Zhu, H. 3D Flowerlike Copper Sulfide Nanostructures Synthesized from Copper (I) Oxide Hollow Microspheres. Procedia Eng. 2012, 36, 25–33. [Google Scholar] [CrossRef]
- Du, W.; Qian, X.; Ma, X.; Gong, Q.; Cao, H.; Yin, J. Shape-Controlled Synthesis and Self-Assembly of Hexagonal Covellite (CuS) Nanoplatelets. Chem. Eur. J. 2007, 13, 3241–3247. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Deng, C.; Niu, C.; Le, H. Microwave-assisted controllable synthesis of hierarchical CuS nanospheres displaying fast and efficient photocatalytic activities. J. Mater. Sci. 2018, 53, 14250–14261. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Yu, S.; Zhu, W.; Han, C.; Cao, X. Preparation and electrochemical performance of hierarchical CuS-rGO composite. J. Alloys Compd. 2017, 694, 1067–1072. [Google Scholar] [CrossRef]
- Tailor, J.P.; Chaki, S.H.; Deshpande, M.P. Comparative study between pure and manganese doped copper sulphide (CuS) nanoparticles. Nano Express 2021, 2, 010011. [Google Scholar] [CrossRef]
- Nath, S.K.; Kalita, P.K. Temperature dependent structural, optical and electrical properties of CuS nanorods in aloe vera matrix. Nano-Struct. Nano-Objects 2021, 25, 100651. [Google Scholar] [CrossRef]
- Castillón-Barraza, F.F.; Farías, M.H.; Coronado-López, J.H.; Encinas-Romero, M.A.; Pérez-Tello, M.; Herrera-Urbina, R.; Posada-Amarillas, A. Synthesis and Characterization of Copper Sulfide Nanoparticles Obtained by the Polyol Method. Adv. Sci. Lett. 2011, 4, 596–601. [Google Scholar] [CrossRef]
- Mao, J.; Shu, Q.; Wen, Y.; Yuan, H.; Xiao, D.; Choi, M.M.F. Facile Fabrication of Porous CuS Nanotubes Using Well-Aligned [Cu(tu)]Cl·1/2H2O Nanowire Precursors as Self-Sacrificial Templates. Cryst. Growth Des. 2009, 9, 2546–2548. [Google Scholar] [CrossRef]
- Pandey, S.; Fosso-Kankeu, E.; Redelinghuys, J.; Kim, J.; Kang, M. Implication of biofilms in the sustainability of acid mine drainage and metal dispersion near coal tailings. Sci. Total Environ. 2021, 788, 147851. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Goswami, G.K.; Okoro, H.K.; Fosso-Kankeu, E. Carbon Nanotubes in the 21st Century: An Advancement in Real Time Monitoring and Control of Environmental Water. In Nano and Bio-Based Technologies for Wastewater Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 265–301. [Google Scholar]
- Chen, J.; Xiong, Y.; Duan, M.; Li, X.; Li, J.; Fang, S.; Qin, S.; Zhang, R. Insight into the Synergistic Effect of Adsorption–Photocatalysis for the Removal of Organic Dye Pollutants by Cr-Doped ZnO. Langmuir 2020, 36, 520–533. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Asiri, A.M. Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int. J. Biol. Macromol. 2015, 81, 584–590. [Google Scholar] [CrossRef]
- Thuy, U.T.D.; Liem, N.Q.; Parlett, C.M.A.; Lalev, G.M.; Wilson, K. Synthesis of CuS and CuS/ZnS core/shell nanocrystals for photocatalytic degradation of dyes under visible light. Catal. Commun. 2014, 44, 62–67. [Google Scholar] [CrossRef]
- Qin, N.; Wei, W.; Huang, C.; Mi, L. An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes. Catalysts 2020, 10, 40. [Google Scholar] [CrossRef]
- Basu, M.; Garg, N.; Ganguli, A.K. A type-II semiconductor (ZnO/CuS heterostructure) for visible light photocatalysis. J. Mater. Chem. A 2014, 2, 7517–7525. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Chen, S.; Zhang, K.; Zhang, J.; Zou, W.; Wang, R. One-step fabrication of BiOCl/CuS heterojunction photocatalysts with enhanced visible-light responsive activity. Mater. Chem. Phys. 2015, 158, 67–73. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Ravi, P.; Sathish, M. Cauliflower-like CuS/ZnS nanocomposites decorated g-C3N4 nanosheets as noble metal-free photocatalyst for superior photocatalytic water splitting. Chem. Eng. J. 2019, 360, 1277–1286. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Hachisuka, K.; Katsumata, H.; Suzuki, T.; Funasaka, K.; Kaneco, S. Photocatalytic hydrogen production with CuS/ZnO from aqueous Na2S + Na2SO3 solution. Int. J. Hydrogen Energy 2013, 38, 8625–8630. [Google Scholar] [CrossRef]
- Wang, Q.; An, N.; Bai, Y.; Hang, H.; Li, J.; Lu, X.; Liu, Y.; Wang, F.; Li, Z.; Lei, Z. High photocatalytic hydrogen production from methanol aqueous solution using the photocatalysts CuS/TiO2. Int. J. Hydrogen Energy 2013, 38, 10739–10745. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Feng, Y.; Li, Z. Efficient photocatalytic performance enhancement in Co-doped ZnO nanowires coupled with CuS nanoparticles. Appl. Surf. Sci. 2018, 428, 154–164. [Google Scholar] [CrossRef]
- Kumar Sonwani, R.; Pandey, S.; Kumar Yadav, S.; Shekhar Giri, B.; Katiyar, V.; Sharan Singh, R.; Nath Rai, B. Construction of integrated system for the treatment of Acid orange 7 dye from wastewater: Optimization and growth kinetic study. Bioresour. Technol. 2021, 337, 125478. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, J.-Y.; Chen, C.-M. Double-sided plasmonic Au nanoparticles on Cu-doped ZnO/ZnO heterostructures with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 60–63. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, S.; Barman, D.; De, S.K. Maximization of photocatalytic activity of Bi2S3/TiO2/Au ternary heterostructures by proper epitaxy formation and plasmonic sensitization. Appl. Catal. B 2017, 219, 287–300. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, J.-Y. Double-sided plasmonic silver nanoparticles decorated copper oxide/zinc oxide heterostructured nanomaces with improving photocatalytic performance. J. Photochem. Photobiol. A Chem. 2019, 378, 184–191. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, J.-C.; Chou, C.-M. H2Ti3O7 nanowires as a high-performance photocatalytic and surface-enhanced Raman scattering substrate. J. Photochem. Photobiol. A Chem. 2020, 400, 112666. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrogen Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Wu, S.-H. Bi-functional Al-doped ZnO@SnO2 heteronanowires as efficient substrates for improving photocatalytic and SERS performance. J. Indust. Eng. Chem. 2019, 76, 333–343. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In Situ Construction of CNT/CuS Hybrids and Their Application in Photodegradation for Removing Organic Dyes. Nanomaterials 2020, 10, 178. [Google Scholar] [CrossRef]
- Barnes, A.J.; Majid, M.A.; Stuckey, M.A.; Gregory, P.; Stead, C.V. The resonance Raman spectra of Orange II and Para Red: Molecular structure and vibrational assignment. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 1985, 41, 629–635. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).