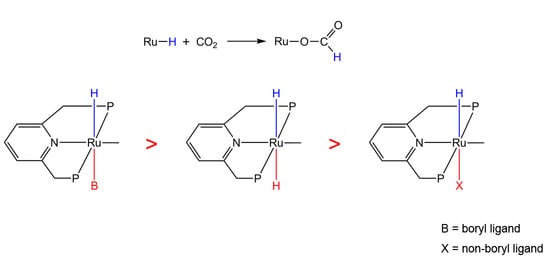
Trans Influence of Boryl Ligands in CO2 Hydrogenation on Ruthenium Complexes: Theoretical Prediction of Highly Active Catalysts for CO2 Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Trans Influence of Non-Boryl and Boryl Ligands
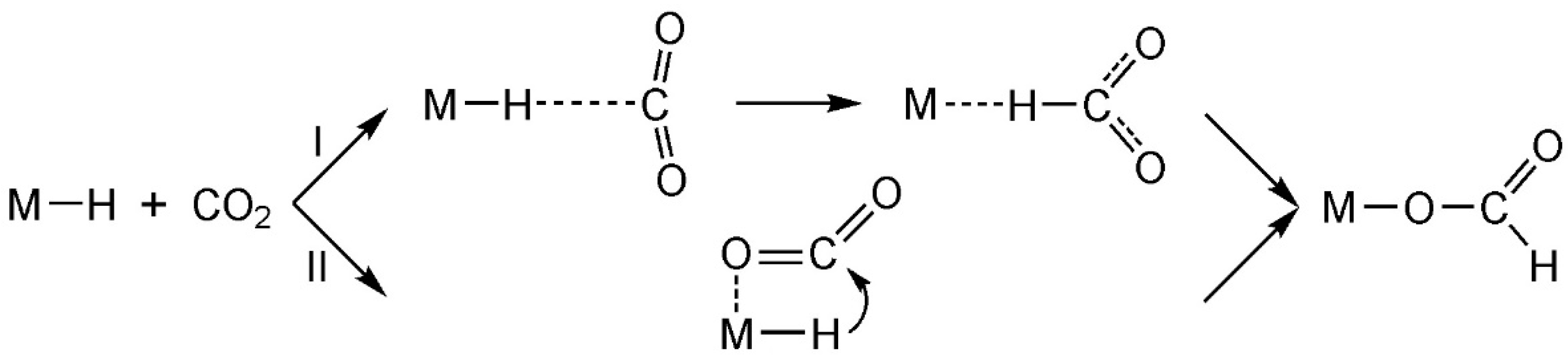
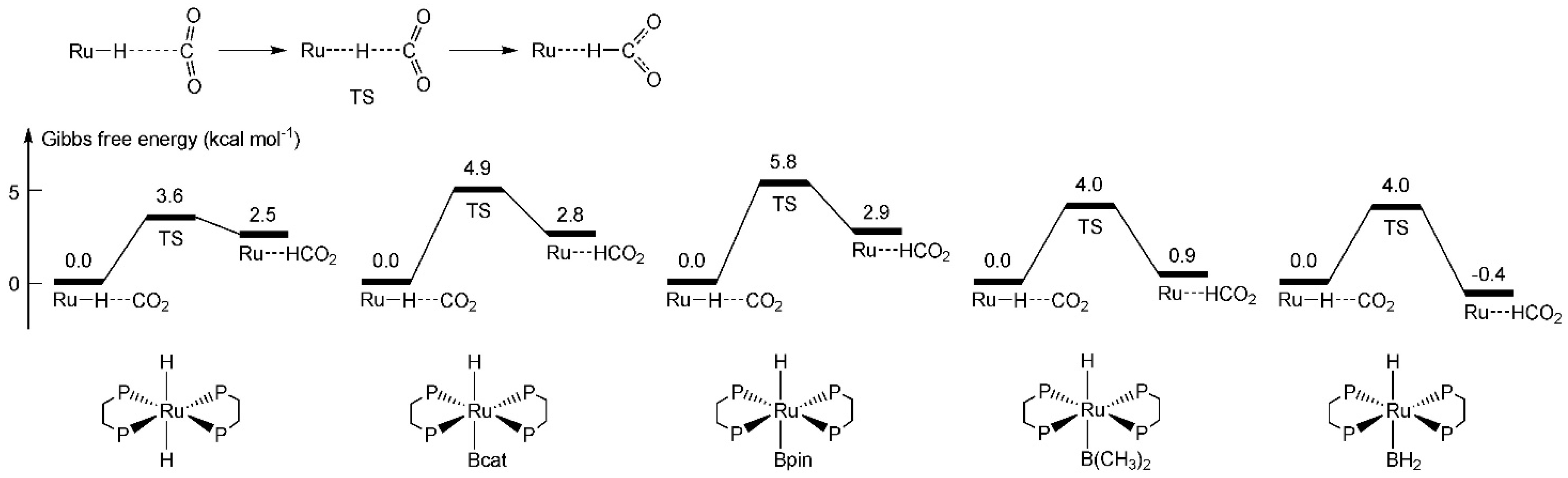
2.2. Activity of the Hydride under Trans Influence
2.3. Trans Influence of B Ligands on Trans-[Ru(dmpe)2HX] Complexes
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jessop, P.G.; Ikariya, T.; Noyori, R. Homogeneous Hydrogenation of Carbon Dioxide. Chem. Rev. 1995, 95, 259–272. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, X. How to Make the Most of Carbon Dioxide. Nature 2015, 526, 628–630. [Google Scholar] [CrossRef] [Green Version]
- Bernskoetter, W.H.; Hazari, N. Reversible Hydrogenation of Carbon Dioxide to Formic Acid and Methanol: Lewis Acid Enhancement of Base Metal Catalysts. Acc. Chem. Res. 2017, 50, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Sordakis, K.; Tang, C.H.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef]
- Chu, W.Y.; Culakova, Z.; Wang, B.T.; Goldberg, K.I. Acid-Assisted Hydrogenation of CO2 to Methanol in a Homogeneous Catalytic Cascade System. ACS Catal. 2019, 9, 9317–9326. [Google Scholar] [CrossRef]
- Sen, R.; Goeppert, A.; Kar, S.; Prakash, G.K.S. Hydroxide Based Integrated CO2 Capture from Air and Conversion to Methanol. J. Am. Chem. Soc. 2020, 142, 4544–4549. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)−Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Wattanavinin, N.; Kusumoto, S.; Nozaki, K. Development of Highly Active Ir–PNP Catalysts for Hydrogenation of Carbon Dioxide with Organic Bases. Bull. Chem. Soc. Jpn. 2016, 89, 113–124. [Google Scholar] [CrossRef]
- Takaoka, S.; Eizawa, A.; Kusumoto, S.; Nakajima, K.; Nishibayashi, Y.; Nozaki, K. Hydrogenation of Carbon Dioxide with Organic Base by PCIIP-Ir Catalysts. Organometallics 2018, 37, 3001–3009. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filonenko, G.A.; van Putten, R.; Schulpen, E.N.; Hensen, E.J.M.; Pidko, E.A. Highly Efficient Reversible Hydrogenation of Carbon Dioxide to Formates Using a Ruthenium PNP-Pincer Catalyst. ChemCatChem 2014, 6, 1526–1530. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Hensen, E.J.M.; Pidko, E.A. Mechanism of CO2 hydrogenation to formates by homogeneous Ru-PNP pincer catalyst: From a theoretical description to performance optimization. Catal. Sci. Technol. 2014, 4, 3474–3485. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, J.; Ruiz-Castaneda, M.; Garcia-Herbosa, G.; Carbayo, A.; Jalon, F.A.; Rodriguez, A.M.; Manzano, B.R.; Espino, G. Versatile Rh- and Ir-Based Catalysts for CO2 Hydrogenation, Formic Acid Dehydrogenation, and Transfer Hydrogenation of Quinolines. Inorg. Chem. 2018, 57, 14186–14198. [Google Scholar] [CrossRef]
- Ahlquist, M.S.G. Iridium catalyzed hydrogenation of CO2 under basic conditions—Mechanistic insight from theory. J. Mol. Catal. A Chem. 2010, 324, 3–8. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Chung, L.W.; Morokuma, K.; Nozaki, K. Mechanistic Studies on the Reversible Hydrogenation of Carbon Dioxide Catalyzed by an Ir-PNP Complex. Organometallics 2011, 30, 6742–6750. [Google Scholar] [CrossRef]
- Li, J.; Yoshizawa, K. Catalytic Hydrogenation of Carbon Dioxide with a Highly Active Hydride on Ir(III)–Pincer Complex: Mechanism for CO2 Insertion and Nature of Metal–Hydride Bond. Bull. Chem. Soc. Jpn. 2011, 84, 1039–1048. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Conley, M.P.; Copéret, C.; Lutz, M.; Hensen, E.J.M.; Pidko, E.A. The impact of Metal–Ligand Cooperation in Hydrogenation of Carbon Dioxide Catalyzed by Ruthenium PNP Pincer. ACS Catal. 2013, 3, 2522–2526. [Google Scholar] [CrossRef]
- Praveen, C.S.; Comas-Vives, A.; Copéret, C.; Vande Vondele, J. Role of Water, CO2, and Noninnocent Ligands in the CO2 Hydrogenation to Formate by an Ir(III) PNP Pincer Catalyst Evaluated by Static-DFT and ab Initio Molecular Dynamics under Reaction Conditions. Organometallics 2017, 36, 4908–4919. [Google Scholar] [CrossRef]
- Sawatlon, B.; Wodrich, M.D.; Corminboeuf, C. Unraveling Metal/Pincer Ligand Effects in the Catalytic Hydrogenation of Carbon Dioxide to Formate. Organometallics 2018, 37, 4568–4575. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Lu, X. Theoretical Study of the Mechanism for Direct Addition of Hydride to CO2 on Ruthenium Complexes: Nature of Ru–H Bond and Effect of Hydrogen Bonding. Bull. Chem. Soc. Jpn. 2016, 89, 905–910. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, W.; Zhu, Y.; Lu, X. Simple Ligand Modifications to Modulate the Activity of Ruthenium Catalysts for CO2 Hydrogenation: Trans Influence of Boryl Ligands and Nature of Ru–H Bond. Acta Phys.-Chim. Sin. 2018, 34, 1097–1105. [Google Scholar] [CrossRef]
- Urakawa, A.; Jutz, F.; Laurenczy, G.; Baiker, A. Carbon dioxide hydrogenation catalyzed by a ruthenium dihydride: A DFT and high-pressure spectroscopic investigation. Chem.-Eur. J. 2007, 13, 3886–3899. [Google Scholar] [CrossRef]
- Pidcock, A.; Richards, R.E.; Venanzi, L.M. 195Pt–31P nuclear spin coupling constants and the nature of the trans-effect in platinum complexes. J. Chem. Soc. A 1966, 1707–1710. [Google Scholar] [CrossRef]
- Greif, A.H.; Hrobárik, P.; Hrobáriková, V.; Arbuznikov, A.V.; Autschbach, J.; Kaupp, M. A Relativistic Quantum-Chemical Analysis of the trans Influence on 1H NMR Hydride Shifts in Square-Planar Platinum(II) Complexes. Inorg. Chem. 2015, 54, 7199–7208. [Google Scholar] [CrossRef]
- Sajith, P.K.; Suresh, C.H. Quantification of the Trans Influence in Hypervalent Iodine Complexes. Inorg. Chem. 2012, 51, 967–977. [Google Scholar] [CrossRef]
- Kovács, A.; Konings, R.J.M. A Theoretical Study of the Structure and Bonding of UOX4 (X=F, Cl, Br, I) Molecules: The Importance of Inverse Trans Influence. ChemPhysChem 2006, 7, 455–462. [Google Scholar] [CrossRef]
- Lewis, A.J.; Mullane, K.C.; Nakamaru-Ogiso, E.; Carroll, P.J.; Schelter, E.J. The Inverse Trans Influence in a Family of Pentavalent Uranium Complexes. Inorg. Chem. 2014, 53, 6944–6953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lin, Z.; Marde, T.B. Trans Influence of Boryl Ligands and Comparison with C, Si, and Sn Ligands. Inorg. Chem. 2005, 44, 9384–9390. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Lin, Z.; Marder, T.B. Boryl ligands and their roles in metal-catalysed borylation reactions. Chem. Commun. 2009, 27, 3987–3995. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Damme, A.; Kupfer, T. Evidence for a Strong trans Influence of the Diboran(4)yl Ligand. Chem.-Eur. J. 2012, 18, 15927–15931. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.P.; Peters, J.C. Boryl−Metal Bonds Facilitate Cobalt/Nickel-Catalyzed Olefin Hydrogenation. J. Am. Chem. Soc. 2014, 136, 13672–13683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, B.; Bag, R.; Ghorai, S.; Bakthavachalam, K.; Jemmis, E.D.; Ghosh, S. Synthesis, Structure, Bonding, and Reactivity of Metal Complexes Comprising Diborane(4) and Diborene(2): [{Cp*Mo(CO)2}2{μ-η2:η2-B2H4}] and [{Cp*M(CO)2}2B2H2M(CO)4], M=Mo, W. Angew. Chem. Int. Ed. 2018, 57, 8079–8083. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Nakao, Y.; Sato, H.; Sakaki, S. Ruthenium(II)-Catalyzed Hydrogenation of Carbon Dioxide to Formic Acid. Theoretical Study of Significant Acceleration by Water Molecules. Organometallics 2006, 25, 3352–3363. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision A.02); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Dolg, M. Effective Core Potentials. In Modern Methods and Algorithms of Quantum Chemistry; Grotendorst, J., Ed.; Johnvon Neumann Institute for Computing: Jülich, Germany, 2000; Volume 1, pp. 479–508. [Google Scholar]
- Martin, J.M.L.; Sundermann, A. Correlation consistent valence basis sets for use with the Stuttgart-Dresden-Bonn relativistic effective core potentials: The atoms Ga-Kr and In-Xe. J. Chem. Phys. 2001, 114, 3408–3420. [Google Scholar] [CrossRef] [Green Version]
- Woon, D.E.; Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The Atoms Aluminum through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef] [Green Version]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Weinhold, F. NBO 5.9; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2009. [Google Scholar]
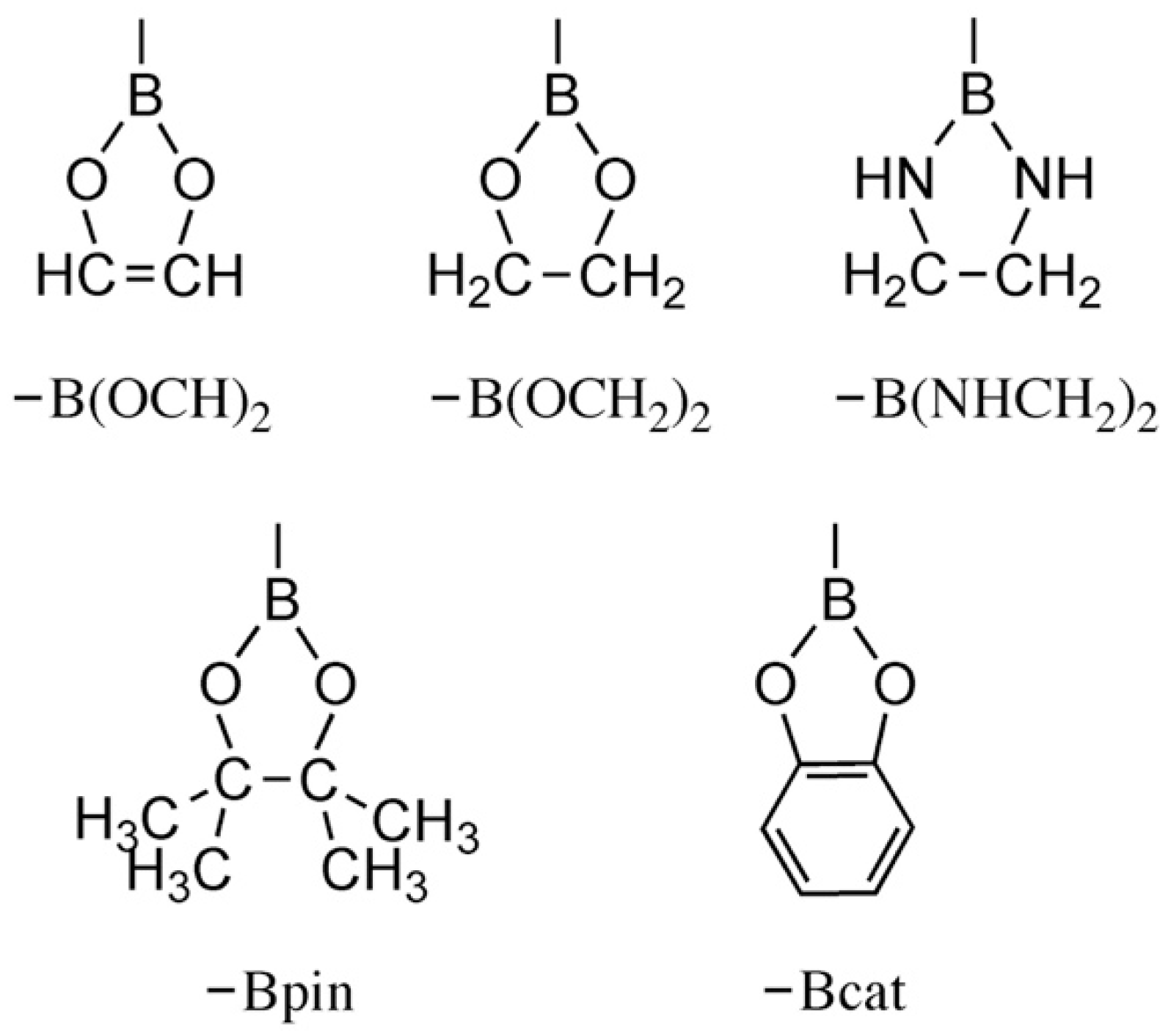


| Trans Ligand | Distance of Ru–H Bond (Å) | Trans Ligand | Distance of Ru–H Bond (Å) |
|---|---|---|---|
| –H | 1.697 | –H | 1.697 |
| –Et | 1.683 | –BBr2 | 1.711 |
| –Me | 1.682 | –BCl2 | 1.713 |
| –Ph | 1.678 | –B(OCH)2 | 1.713 |
| –SiH3 | 1.676 | –Bcat | 1.714 |
| –CN | 1.662 | –B(OCH2)2 | 1.715 |
| –NH2 | 1.656 | –B(OH)2 | 1.715 |
| –NO2 | 1.637 | –Bpin | 1.716 |
| –OH | 1.637 | –B(NHCH2)2 | 1.717 |
| –OMe | 1.636 | –B(OCH3)2 | 1.720 |
| –F | 1.621 | –B(CH3)2 | 1.726 |
| –ONO | 1.621 | –BH2 | 1.739 |
| –SCN | 1.616 | ||
| –Cl | 1.611 | ||
| –Br | 1.609 | ||
| –NO3 | 1.609 |
| Trans Ligand | Distance of Ru–H Bond (Å) | Coefficients/Hybrids of Ru–H Bond Orbital | Activation Free Energy for Hydride Addition to CO2 (kcal mol−1) |
|---|---|---|---|
| –H | 1.697 | 0.706 Ru(sd2.11) + 0.709 H(s) | 7.0 |
| –Bcat | 1.714 | 0.702 Ru(sd1.83) + 0.712 H(s) | 5.9 |
| –Bpin | 1.716 | 0.699 Ru(sd1.80) + 0.715 H(s) | 5.6 |
| –B(NHCH2)2 | 1.717 | 0.699 Ru(sd1.76) + 0.715 H(s) | 5.2 |
| –B(OCH3)2 | 1.720 | 0.686 Ru(sd1.63) + 0.728 H(s) | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, Z.; Tang, L.; Li, J.; Yang, Z. Trans Influence of Boryl Ligands in CO2 Hydrogenation on Ruthenium Complexes: Theoretical Prediction of Highly Active Catalysts for CO2 Reduction. Catalysts 2021, 11, 1356. https://doi.org/10.3390/catal11111356
Liu T, Liu Z, Tang L, Li J, Yang Z. Trans Influence of Boryl Ligands in CO2 Hydrogenation on Ruthenium Complexes: Theoretical Prediction of Highly Active Catalysts for CO2 Reduction. Catalysts. 2021; 11(11):1356. https://doi.org/10.3390/catal11111356
Chicago/Turabian StyleLiu, Tian, Zhangyong Liu, Lipeng Tang, Jun Li, and Zhuhong Yang. 2021. "Trans Influence of Boryl Ligands in CO2 Hydrogenation on Ruthenium Complexes: Theoretical Prediction of Highly Active Catalysts for CO2 Reduction" Catalysts 11, no. 11: 1356. https://doi.org/10.3390/catal11111356