Homogeneous Catalyzed Valorization of Furanics: A Sustainable Bridge to Fuels and Chemicals
Abstract
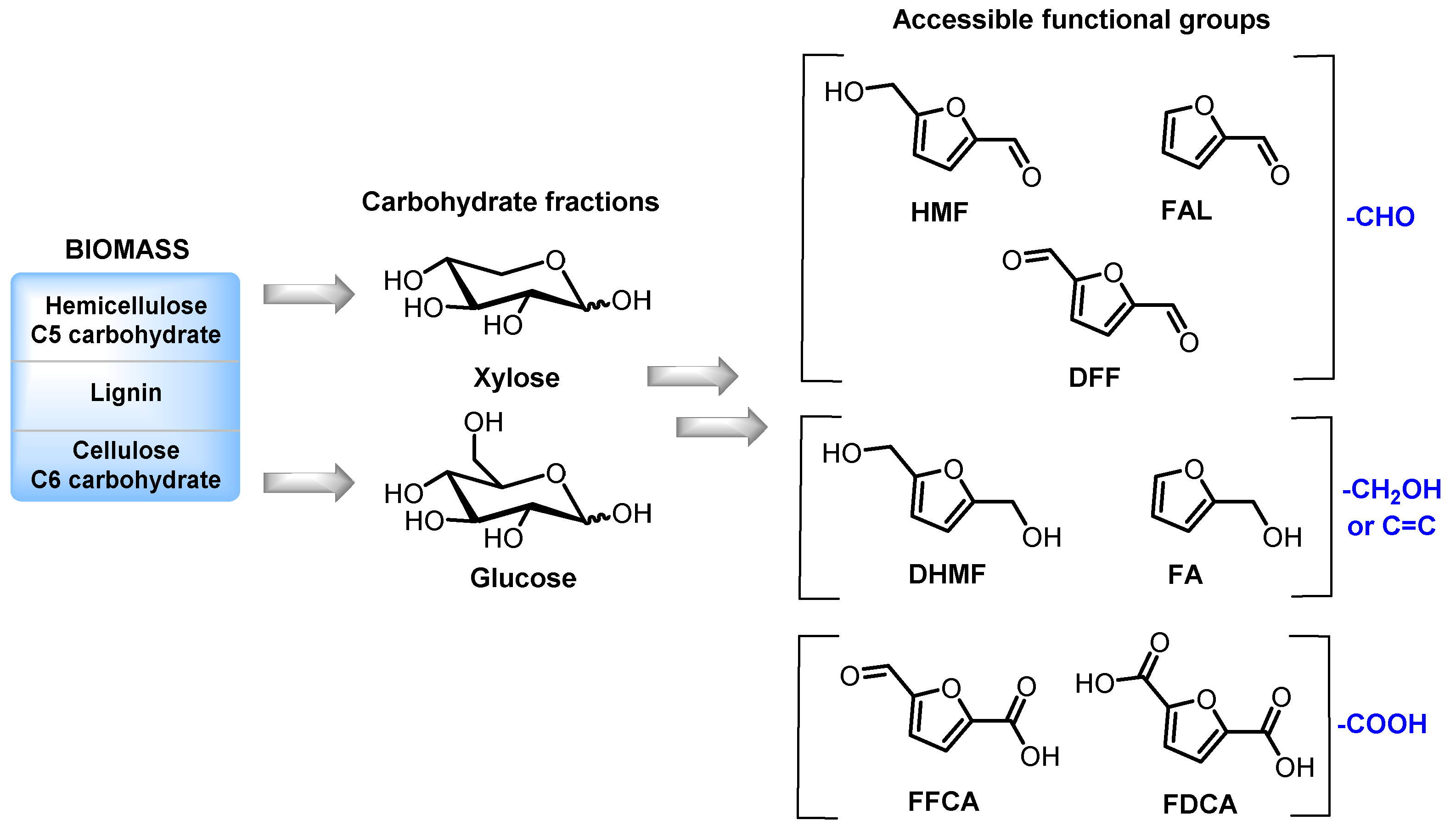
:1. Introduction
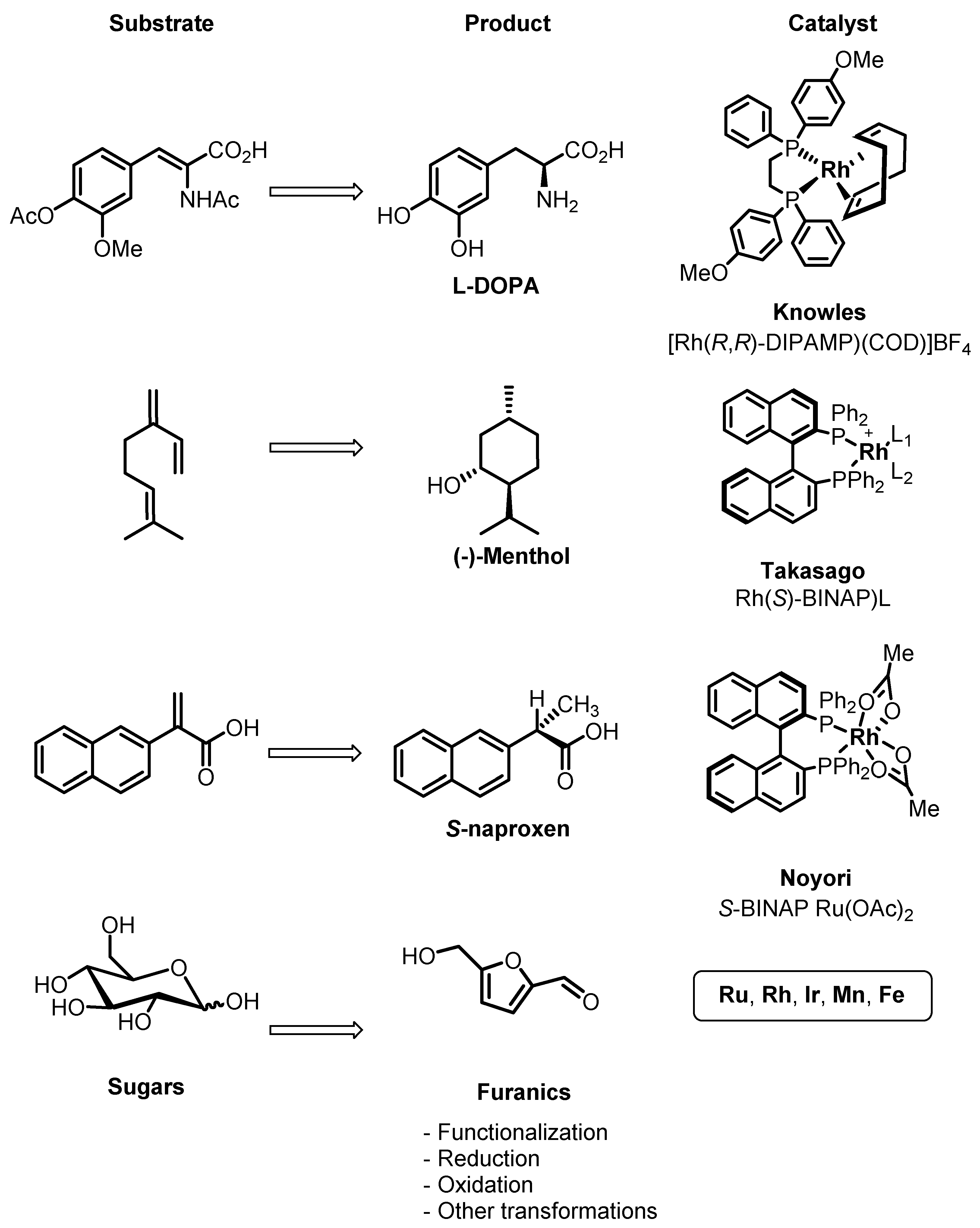
2. Homogeneous Catalysts as a Toolbox for Biorefining
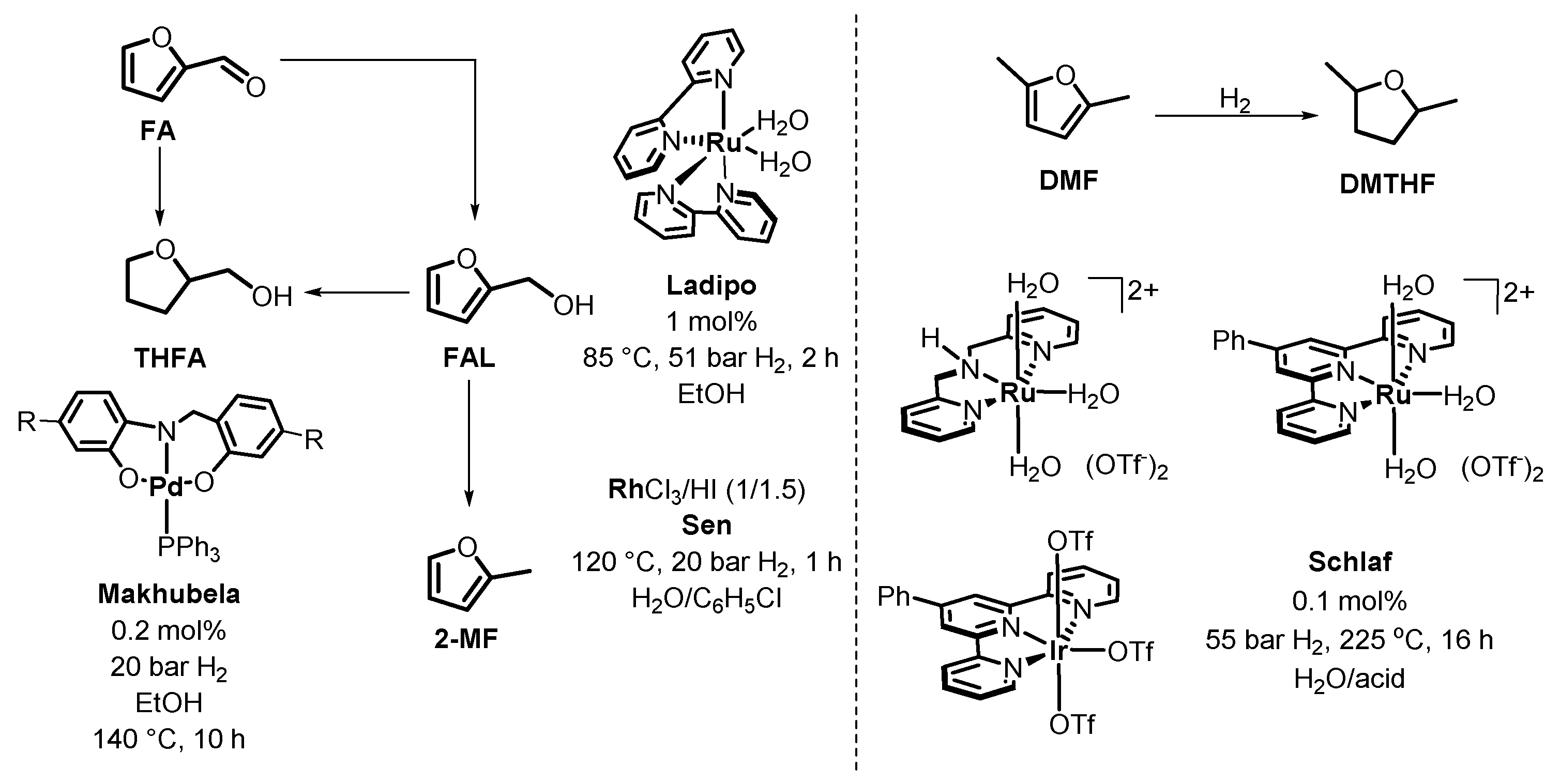
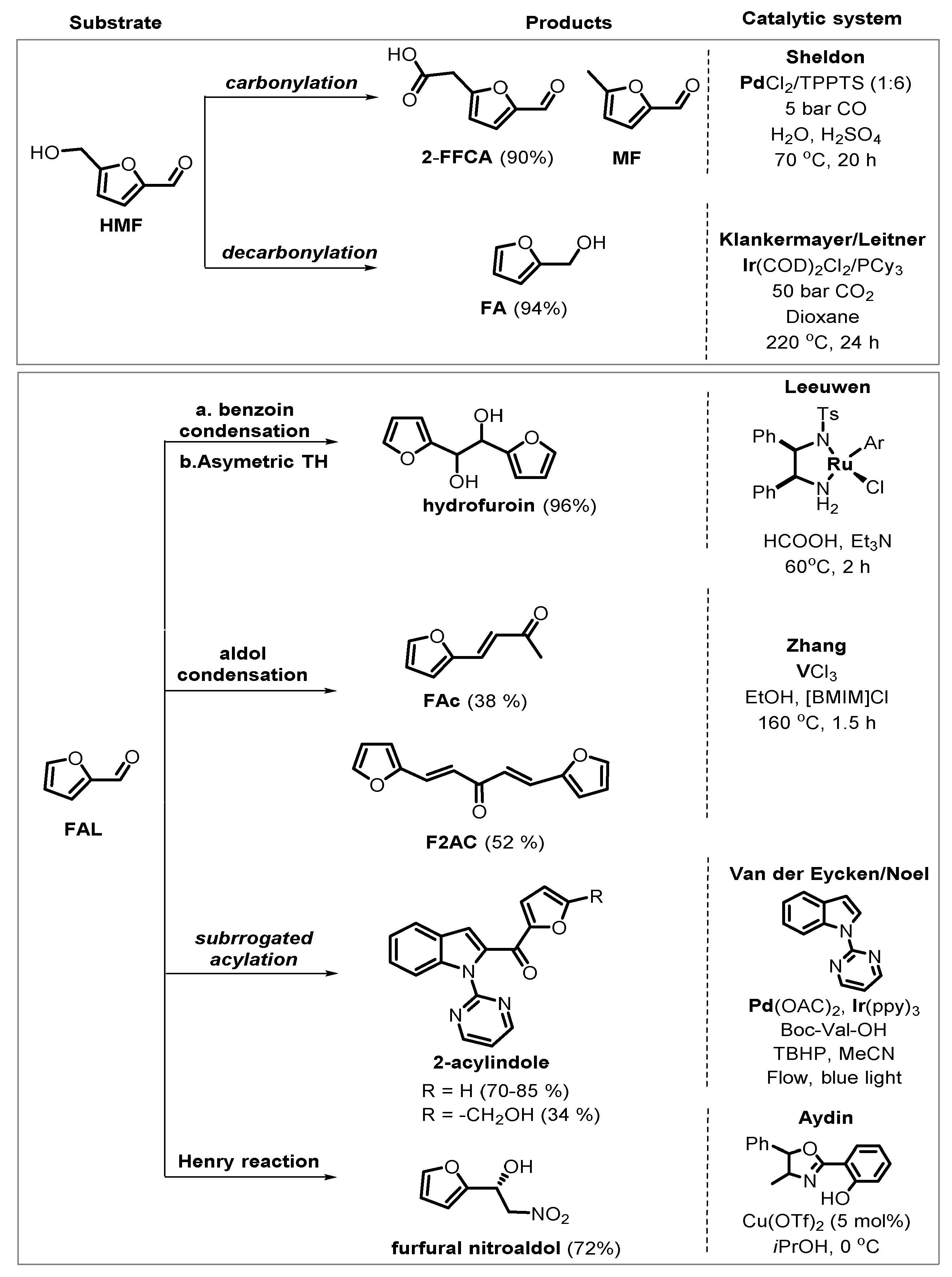
3. Homogeneous Catalysts for the Production of FAL and HMF
4. Catalytic Reduction of Furanics
Hydrodeoxygenation of Furanics
5. Oxidative Transformations
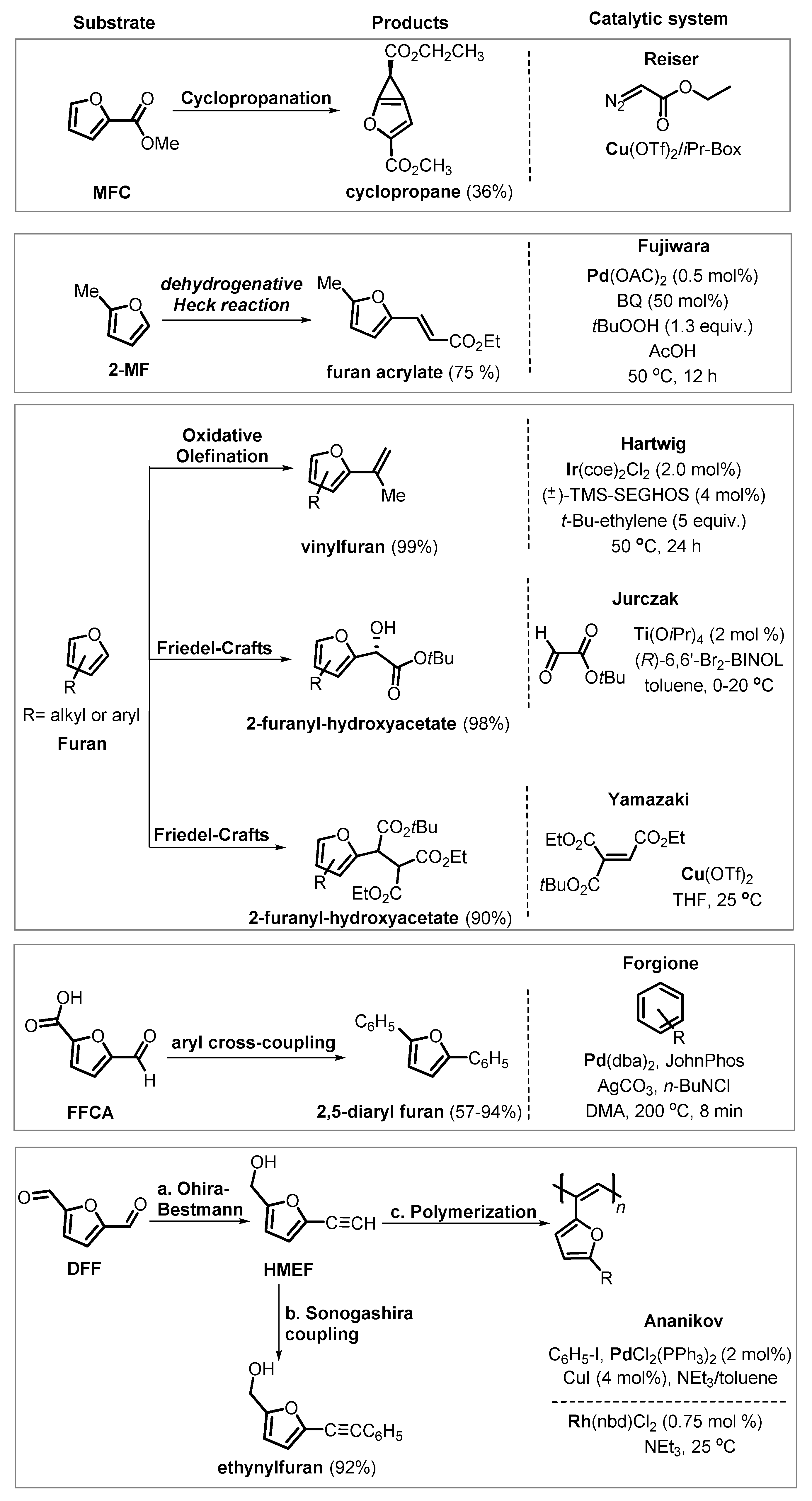
6. Other Chemical Transformation of Furanics
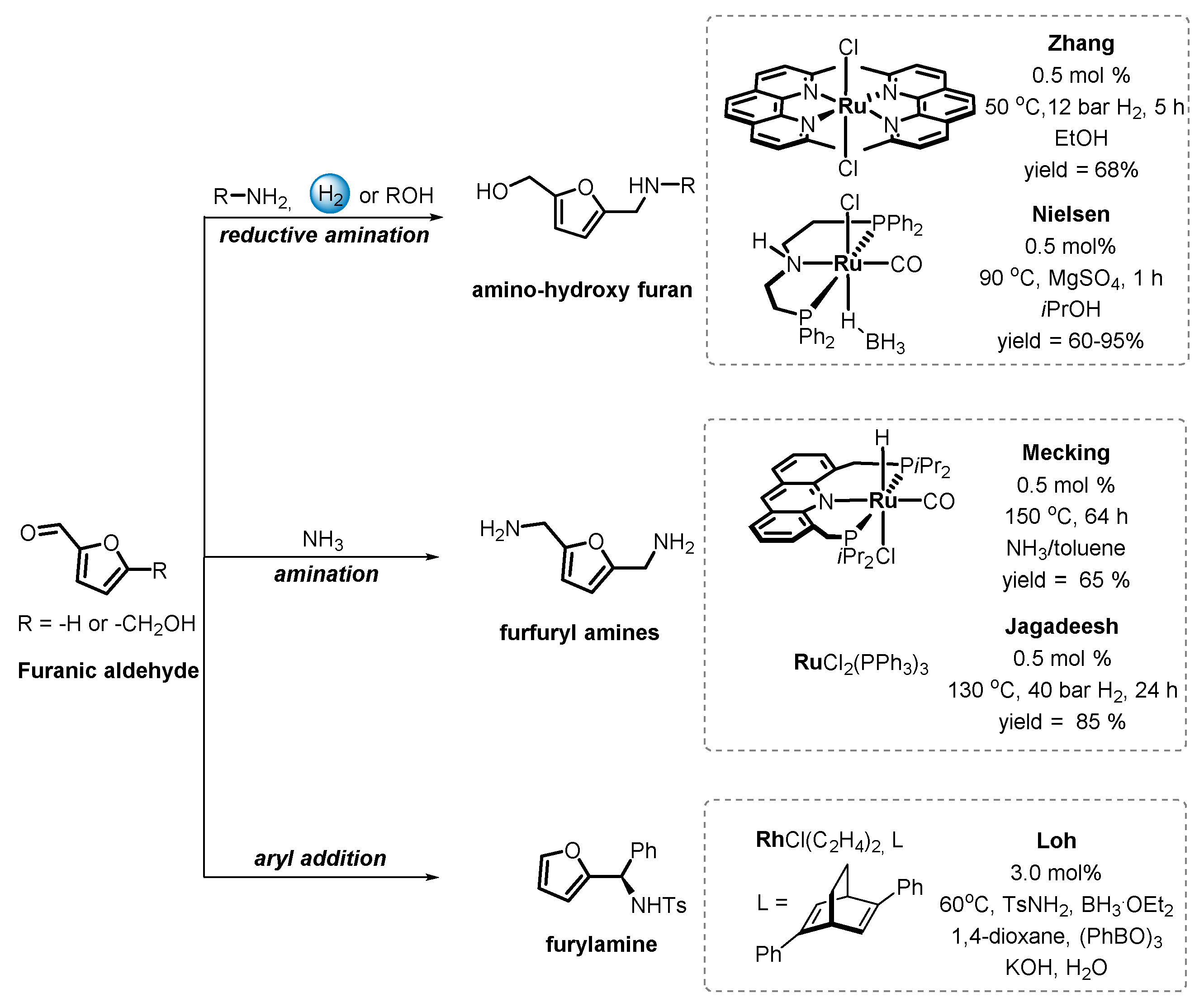
6.1. Catalytic Carbonyl Coupling of Furanics with Nucleophilic Nitrogen Sources
6.2. Functionalization of Furanics and C–C Bond Formation
6.3. Undirected C–H Activation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, L.; Wang, X.-C.; Dai, M.; Chen, B.; Qiao, Y.; Deng, H.; Zhang, D.; Zhang, Y.; Villas Bôas de Almeida, C.M.; Chiu, A.S.F.; et al. Shifting from Fossil-Based Economy to Bio-Based Economy: Status Quo, Challenges, and Prospects. Energy 2021, 228, 120533. [Google Scholar] [CrossRef]
- Nelson, A.E. Fundamentals of Industrial Catalytic Processes, 2nd Edition. C. H. Bartholomew and Robert J. Farrauto John Wiley and Sons, Hoboken, NJ, 966 pp., 2006. Can. J. Chem. Eng. 2008, 85, 127–128. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. Catalyst Design for Biorefining. Philo. Trans. R. Soc. A 2016, 374, 20150081. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Raspolli Galletti, A.M. New Intensification Strategies for the Direct Conversion of Real Biomass into Platform and Fine Chemicals: What Are the Main Improvable Key Aspects? Catalysts 2020, 10, 961. [Google Scholar] [CrossRef]
- Lin, L.; Han, X.; Han, B.; Yang, S. Emerging Heterogeneous Catalysts for Biomass Conversion: Studies of the Reaction Mechanism. Chem. Soc. Rev. 2021, 50, 11270–11292. [Google Scholar] [CrossRef]
- Walker, T.W.; Motagamwala, A.H.; Dumesic, J.A.; Huber, G.W. Fundamental Catalytic Challenges to Design Improved Biomass Conversion Technologies. J. Catal. 2019, 369, 518–525. [Google Scholar] [CrossRef]
- Deuss, P.J.; Barta, K.; de Vries, J.G. Homogeneous Catalysis for the Conversion of Biomass and Biomass-Derived Platform Chemicals. Catal. Sci. Technol. 2014, 4, 1174–1196. [Google Scholar] [CrossRef] [Green Version]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, L.; Lobo Justo Pinheiro, D.; Nielsen, M. Recent Progress with Pincer Transition Metal Catalysts for Sustainability. Catalysts 2020, 10, 773. [Google Scholar] [CrossRef]
- Crabtree, R.H. An Organometallic Future in Green and Energy Chemistry? Organometallics 2011, 30, 17–19. [Google Scholar] [CrossRef]
- Dixneuf, P.H. Organometallics for Green Catalysis, 1st ed.; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-10954-7. [Google Scholar]
- Bender, T.A.; Dabrowski, J.A.; Gagné, M.R. Homogeneous Catalysis for the Production of Low-Volume, High-Value Chemicals from Biomass. Nat. Rev. Chem. 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Dahiya, S.; Katakojwala, R.; Ramakrishna, S.; Mohan, S.V. Biobased Products and Life Cycle Assessment in the Context of Circular Economy and Sustainability. Mater. Circ. Econ. 2020, 2, 7. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Du, J.; Liu, K.; Wang, T.; Liu, L. Biocatalytic Production of 2,5-Furandicarboxylic Acid: Recent Advances and Future Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 527–543. [Google Scholar] [CrossRef]
- Long, J.; Xu, Y.; Zhao, W.; Li, H.; Yang, S. Heterogeneous Catalytic Upgrading of Biofuranic Aldehydes to Alcohols. Front. Chem. 2019, 7, 529. [Google Scholar] [CrossRef]
- Nilges, P.; Schröder, U. Electrochemistry for Biofuel Generation: Production of Furans by Electrocatalytic Hydrogenation of Furfurals. Energy Environ. Sci. 2013, 6, 2925. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The Role of Biomass and Bioenergy in a Future Bioeconomy: Policies and Facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery-Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery Roadmap Based on Catalytic Production and Upgrading 5-Hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Li, S.; Ma, Q.; Zhong, W.; Zhao, X.; Wei, X.; Zhang, X.; Liu, Q.; Wang, C.; Ma, L.; Zhang, Q. One-Pot Hydrodeoxygenation of Bioderived Furans into Octane at Low Temperatures via an Octanediol Route. Green Chem. 2021, 23, 4741–4752. [Google Scholar] [CrossRef]
- Dutta, S. Hydro(Deoxygenation) Reaction Network of Lignocellulosic Oxygenates. ChemSusChem 2020, 13, 2894–2915. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Q.; Deng, W.; Zhang, Q.; Wang, Y. Catalytic valorization of biomass and bioplatforms to chemicals through deoxygenation. In Advances in Catalysis; Elsevier: Amherst, MA, USA, 2020; Volume 66, pp. 1–108. ISBN 978-0-12-820369-9. [Google Scholar]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation Processes: Advances on Catalytic Transformations of Biomass-Derived Platform Chemicals into Hydrocarbon Fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandarias, I.; Luis, P. Hydrotreating Catalytic Processes for Oxygen Removal in the Upgrading of Bio-Oils and Bio-Chemicals. In Liquid, Gaseous and Solid Biofuels—Conversion Techniques; Fang, Z., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1050-7. [Google Scholar]
- Rogers, K.A.; Zheng, Y. Selective Deoxygenation of Biomass-Derived Bio-Oils within Hydrogen-Modest Environments: A Review and New Insights. ChemSusChem 2016, 9, 1750–1772. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butler, I.S.; Xu, L.; Song, H.; Wei, X. Catalytic Conversion of 5-Hydroxymethylfurfural to Some Value-Added Derivatives. Green Chem. 2018, 20, 3657–3682. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Thomas, J.M. The Societal Significance of Catalysis and the Growing Practical Importance of Single-Site Heterogeneous Catalysts. Proc. R. Soc. A 2012, 468, 1884–1903. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Delidovich, I.; Palkovits, R. Catalytic versus Stoichiometric Reagents as a Key Concept for Green Chemistry. Green Chem. 2016, 18, 590–593. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A. Concepts in Homogeneous Catalysis: The Industrial View. J. Catal. 2003, 216, 23–31. [Google Scholar] [CrossRef]
- Jess, A.; Wasserscheid, P. Chemical Technology: From Principles to Products, 2nd ed.; Wiley: Weinheim, Germany, 2020; ISBN 978-3-527-34421-5. [Google Scholar]
- Knowles, W.S.; Sabacky, M.J. Catalytic Asymmetric Hydrogenation Employing a Soluble, Optically Active, Rhodium Complex. Chem. Commun. 1968, 1445–1446. [Google Scholar] [CrossRef]
- Knowles, W.S. Asymmetric Hydrogenations (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 1998–2007. [Google Scholar] [CrossRef]
- Knowles, W.S.; Sabacky, M.J.; Vineyard, B.D. US Patent 4 005.127, 1977. US Patent 4 005.127, 1977. [Google Scholar]
- Noyori, R. Asymmetric Catalysis: Science and Opportunities (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 2008–2022. [Google Scholar] [CrossRef]
- Federsel, H.-J. Asymmetry on Large Scale: The Roadmap to Stereoselective Processes. Nat. Rev. Drug Discov. 2005, 4, 685–697. [Google Scholar] [CrossRef]
- Pan, J.; Dang, N.-D.; Zheng, G.-W.; Cheng, B.; Ye, Q.; Xu, J.-H. Efficient Production of L-Menthol in a Two-Phase System with SDS Using an Immobilized Bacillus Subtilis Esterase. Bioresour. Bioprocess. 2014, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Knowles, W.S.; Sabacky, M.J.; Vineyard, B.D.; Weinkauff, D.J. Asymmetric Hydrogenation with a Complex of Rhodium and a Chiral Bisphosphine. J. Am. Chem. Soc. 1975, 97, 2567–2568. [Google Scholar] [CrossRef]
- Wolfson, A.; Vankelecom, I.F.J.; Geresh, S.; Jacobs, P.A. The Role of the Solvent in the Asymmetric Hydrogenation of β-Keto Esters with Ru-BINAP. J. Mol. Catal. A Chem. 2003, 198, 39–45. [Google Scholar] [CrossRef]
- Kumobayashi, H.; Miura, T.; Sayo, N.; Saito, T.; Zhang, X. Recent Advances of BINAP Chemistry in the Industrial Aspects. Synlett 2001, 2001, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.H. Green Chemistry for the Second Generation Biorefinery—Sustainable Chemical Manufacturing Based on Biomass. J. Chem. Technol. Biotechnol. 2007, 82, 603–609. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent Advances in Catalytic Transformation of Biomass-Derived 5-Hydroxymethylfurfural into the Innovative Fuels and Chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel Strategies for the Production of Fuels, Lubricants, and Chemicals from Biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Jimenez-Sanchidrian, C. Heterogeneous Catalysis in the Meerwein-Ponndorf-Verley Reduction of Carbonyl Compounds. COC 2007, 11, 1113–1125. [Google Scholar] [CrossRef]
- Meng, Q.; Zheng, H.; Zhu, Y.; Li, Y. Study on the Reaction Pathway in Decarbonylation of Biomass-Derived 5-Hydroxymethylfurfural over Pd-Based Catalyst. J. Mol. Catal. A Chem. 2016, 421, 76–82. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.-P.; Su, F.; Yan, Z.; Kusema, B.T.; Streiff, S.; Huang, Y.; Pera-Titus, M.; Shi, F. Reductive Amination of Furanic Aldehydes in Aqueous Solution over Versatile NiyAlOx Catalysts. ACS Omega 2019, 4, 2510–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, P.; Tejero, T.; Delso, J.I.; Matute, R. Furan Oxidations in Organic Synthesis: Recent Advances and Applications. COC 2007, 11, 1076–1091. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G.; Natalino, R. Highly Selective Synthesis under Benign Reaction Conditions of Furfural Dialkyl Acetal Using SnCl2 as a Recyclable Catalyst. New J. Chem. 2019, 43, 8606–8612. [Google Scholar] [CrossRef]
- Xu, M.; Richard, F.; Corbet, M.; Marion, P.; Clacens, J.-M. Upgrading of Furfural by Knoevenagel Condensation over Functionalized Carbonaceous Basic Catalysts. Catal. Commun. 2019, 130, 105777. [Google Scholar] [CrossRef]
- Kumar, P.; Dutta, S.; Kumar, S.; Bahadur, V.; Van der Eycken, E.V.; Vimaleswaran, K.S.; Parmar, V.S.; Singh, B.K. Aldehydes: Magnificent Acyl Equivalents for Direct Acylation. Org. Biomol. Chem. 2020, 18, 7987–8033. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(Hydroxymethyl)Furfural. ChemistryOpen 2020, 9, 1135–1148. [Google Scholar] [CrossRef]
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current Status, Challenges, and Future Direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar] [CrossRef]
- Catoire, L.; Yahyaoui, M.; Osmont, A.; Gökalp, I.; Brothier, M.; Lorcet, H.; Guénadou, D. Thermochemistry of Compounds Formed during Fast Pyrolysis of Lignocellulosic Biomass. Energy Fuels 2008, 22, 4265–4273. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis Goes Bio: From Carbohydrates and Sugar Alcohols to Platform Chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Bhaumik, P.; Dhepe, P.L. Solid Acid Catalyzed Synthesis of Furans from Carbohydrates. Cataly. Rev. 2016, 58, 36–112. [Google Scholar] [CrossRef]
- Zhang, Z.C. Chapter 3—Emerging Catalysis for 5-HMF Formation from Cellulosic Carbohydrates. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 53–71. ISBN 978-0-444-53878-9. [Google Scholar]
- Thoma, C.; Konnerth, J.; Sailer-Kronlachner, W.; Solt, P.; Rosenau, T.; Herwijnen, H.W.G. Current Situation of the Challenging Scale-Up Development of Hydroxymethylfurfural Production. ChemSusChem 2020, 13, 3544–3564. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gartner, C.A.; Dumesic, J.A. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754. [Google Scholar] [CrossRef]
- Scapin, E.; Rambo, M.K.D.; Viana, G.C.C.; Marasca, N.; Lacerda, G.E.; Rambo, M.C.D.; Fernandes, R.d.M.N. Sustainable Production of Furfural and 5-Hidroximetilfurfural from Rice Husks and Soybean Peel by Using Ionic Liquid. Food Sci. Technol. 2020, 40, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Kläusli, T. AVA Biochem: Commercialising Renewable Platform Chemical 5-HMF. Green Process. Synth. 2014, 3, 235–236. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, Properties and Catalytic Hydrogenation of Furfural to Fuel Additives and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Simeonov, S.P.; Lazarova, H.I.; Marinova, M.K.; Popova, M.D. Achmatowicz Rearrangement Enables Hydrogenolysis-Free Gas-Phase Synthesis of Pentane-1,2,5-Triol from Furfuryl Alcohol. Green Chem. 2019, 21, 5657–5664. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, G. Catalytic Transformation of the Furfural Platform into Bifunctionalized Monomers for Polymer Synthesis. ACS Catal. 2021, 11, 10058–10083. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Marzo, M. Absence of Expected Side-Reactions in the Dehydration Reaction of Fructose to HMF in Water over Niobic Acid Catalyst. Catal. Commun. 2011, 12, 1122–1126. [Google Scholar] [CrossRef]
- Song, J.; Fan, H.; Ma, J.; Han, B. Conversion of Glucose and Cellulose into Value-Added Products in Water and Ionic Liquids. Green Chem. 2013, 15, 2619. [Google Scholar] [CrossRef]
- Seri, K.; Inoue, Y.; Ishida, H. Catalytic Activity of Lanthanide(III) Ions for the Dehydration of Hexose to 5-Hydroxymethyl-2-Furaldehyde in Water. BCSJ 2001, 74, 1145–1150. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Lin, L. Efficient Conversion of Glucose into 5-Hydroxymethylfurfural by Chromium(III) Chloride in Inexpensive Ionic Liquid. Ind. Eng. Chem. Res. 2012, 51, 1099–1104. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhao, Z.K. Direct Conversion of Glucose and Cellulose to 5-Hydroxymethylfurfural in Ionic Liquid under Microwave Irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Jiang, Y.; Wang, X.; He, A.; Song, J.; Xu, J.; Zhou, S.; Zhao, Y.; Xu, J. Recent Advances in Catalytic and Autocatalytic Production of Biomass-Derived 5-Hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2020, 134, 110317. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Dunn, E.F.; Liu, D.; Chen, E.Y.-X. Role of N-Heterocyclic Carbenes in Glucose Conversion into HMF by Cr Catalysts in Ionic Liquids. Appl. Catal. A Gen. 2013, 460–461, 1–7. [Google Scholar] [CrossRef]
- Enslow, K.R.; Bell, A.T. SnCl4-Catalyzed Isomerization/Dehydration of Xylose and Glucose to Furanics in Water. Catal. Sci. Technol. 2015, 5, 2839–2847. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Audemar, M.; De Oliveira Vigier, K.; Cartigny, D.; Clacens, J.-M.; Costa Gomes, M.F.; Pádua, A.A.H.; De Campo, F.; Jérôme, F. Selectivity Enhancement in the Aqueous Acid-Catalyzed Conversion of Glucose to 5-Hydroxymethylfurfural Induced by Choline Chloride. Green Chem. 2013, 15, 3205. [Google Scholar] [CrossRef]
- Yan, L.; Ma, R.; Wei, H.; Li, L.; Zou, B.; Xu, Y. Ruthenium Trichloride Catalyzed Conversion of Cellulose into 5-Hydroxymethylfurfural in Biphasic System. Bioresour. Technol. 2019, 279, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, Y.; Thushara, D.; Liu, Y.; Ren, Q. Novel Dehydration of Carbohydrates to 5-Hydroxymethylfurfural Catalyzed by Ir and Au Chlorides in Ionic Liquids. J. Taiwan Inst. Chem. Eng. 2011, 42, 363–370. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Zhao, Z.K. Microwave-Assisted Catalytic Conversion of Cellulose into 5-Hydroxymethylfurfural in Ionic Liquids. Chem. Eng. J. 2013, 215–216, 517–521. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Alam, M.I.; Abu-Omar, M.M.; Saha, B. Direct Conversion of Cellulose and Lignocellulosic Biomass into Chemicals and Biofuel with Metal Chloride Catalysts. J. Catal. 2012, 288, 8–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Zhao, Z.K. Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Hafnium(IV) Chloride in Ionic Liquids. Starch Stärke 2012, 64, 770–775. [Google Scholar] [CrossRef]
- Mittal, N.; Nisola, G.M.; Chung, W.-J. Facile Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural by Niobium Pentachloride. Tetrahedron Lett. 2012, 53, 3149–3155. [Google Scholar] [CrossRef]
- Beckerle, K.; Okuda, J. Conversion of Glucose and Cellobiose into 5-Hydroxymethylfurfural (HMF) by Rare Earth Metal Salts in N,N′-Dimethylacetamide (DMA). J. Mol. Catal. A Chem. 2012, 356, 158–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; Qian, X.; Chen, E.Y.-X. Ionic Liquid−Water Mixtures: Enhanced Kw for Efficient Cellulosic Biomass Conversion. Energy Fuels 2010, 24, 2410–2417. [Google Scholar] [CrossRef]
- Wrigstedt, P.; Keskiväli, J.; Leskelä, M.; Repo, T. The Role of Salts and Brønsted Acids in Lewis Acid-Catalyzed Aqueous-Phase Glucose Dehydration to 5-Hydroxymethylfurfural. ChemCatChem 2015, 7, 501–507. [Google Scholar] [CrossRef]
- Yadav, K.K.; Ahmad, S.; Chauhan, S.M.S. Elucidating the Role of Cobalt Phthalocyanine in the Dehydration of Carbohydrates in Ionic Liquids. J. Mol. Catal. A Chem. 2014, 394, 170–176. [Google Scholar] [CrossRef]
- Wang, F.; Shi, A.-W.; Qin, X.-X.; Liu, C.-L.; Dong, W.-S. Dehydration of Fructose to 5-Hydroxymethylfurfural by Rare Earth Metal Trifluoromethanesulfonates in Organic Solvents. Carbohydr. Res. 2011, 346, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural Using Lewis and Brønsted Acid Catalysts in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Marcotullio, G.; De Jong, W. Chloride Ions Enhance Furfural Formation from D-Xylose in Dilute Aqueous Acidic Solutions. Green Chem. 2010, 12, 1739. [Google Scholar] [CrossRef]
- Danon, B.; Marcotullio, G.; de Jong, W. Mechanistic and Kinetic Aspects of Pentose Dehydration towards Furfural in Aqueous Media Employing Homogeneous Catalysis. Green Chem. 2014, 16, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wyman, C.E. The Enhancement of Xylose Monomer and Xylotriose Degradation by Inorganic Salts in Aqueous Solutions at 180 °C. Carbohydr. Res. 2006, 341, 2550–2556. [Google Scholar] [CrossRef]
- Hart, D. Hydrogen, End Uses and Economics. In Encyclopedia of Energy; Elsevier: London, UK, 2004; pp. 231–239. ISBN 978-0-12-176480-7. [Google Scholar]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Ralphs, K.; McCourt, É.; Ormandy, C.; Carneiro de Souza, T.A.; Nockemann, P.; Jacquemin, J.; Manyar, H.G. Highly Selective Reduction of α, β-Unsaturated Aldehydes and Ketones under Ambient Conditions Using Tetraalkylphosphonium-based Ionic Liquids. ChemistrySelect 2018, 3, 11706–11711. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, T.; Tang, X.; Peng, L.; Wei, J.; Lin, L. Methods in the synthesis and conversion of 2,5-Bis-(hydroxylmethyl)furan from bio-derived 5-hydroxymethylfurfural and its great potential in polymerization. BioResources 2018, 13, 7137–7154. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xie, W.; Tang, Y.; Guo, D.; Ni, Y. Catalytic Transfer Hydrogenation of Biobased HMF to 2,5-Bis-(Hydroxymethyl)Furan over Ru/Co3O4. Catalysts 2017, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Supported Metal Catalysts for Total Hydrogenation of Furfural and 5-Hydroxymethylfurfural. J. Jpn. Pet. Inst. 2017, 60, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Koley, P.; Rao, B.S.; Sabri, Y.M.; Bhargava, S.K.; Tardio, J.; Lingaiah, N. Selective Conversion of Furfural into Tetrahydrofurfuryl Alcohol Using a Heteropoly Acid-Based Material as a Hydrogenation Catalyst. Sustain. Energy Fuels 2020, 4, 4768–4779. [Google Scholar] [CrossRef]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Brønsted Acids on Catalytic Hydrothermal Decomposition of Hexose to Levulinic Acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Jansen, E.; Jongbloed, L.S.; Tromp, D.S.; Lutz, M.; de Bruin, B.; Elsevier, C.J. Ligand Effects on the Hydrogenation of Biomass-Inspired Substrates with Bifunctional Ru, Ir, and Rh Complexes. ChemSusChem 2013, 6, 1737–1744. [Google Scholar] [CrossRef]
- Cadu, A.; Sekine, K.; Mormul, J.; Ohlmann, D.M.; Schaub, T.; Hashmi, A.S.K. Homogeneous Catalysed Hydrogenation of HMF. Green Chem. 2018, 20, 3386–3393. [Google Scholar] [CrossRef]
- Pasini, T.; Solinas, G.; Zanotti, V.; Albonetti, S.; Cavani, F.; Vaccari, A.; Mazzanti, A.; Ranieri, S.; Mazzoni, R. Substrate and Product Role in the Shvo’s Catalyzed Selective Hydrogenation of the Platform Bio-Based Chemical 5-Hydroxymethylfurfural. Dalton Trans. 2014, 43, 10224–10234. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.; Koranchalil, S.; Nielsen, M. Efficient and Selective Catalytic Hydrogenation of Furanic Aldehydes Using Well Defined Ru and Ir Pincer Complexes. Green Chem. 2020, 22, 6767–6772. [Google Scholar] [CrossRef]
- Dach, R.; Song, J.J.; Roschangar, F.; Samstag, W.; Senanayake, C.H. The Eight Criteria Defining a Good Chemical Manufacturing Process. Org. Process. Res. Dev. 2012, 16, 1697–1706. [Google Scholar] [CrossRef]
- Elangovan, S.; Topf, C.; Fischer, S.; Jiao, H.; Spannenberg, A.; Baumann, W.; Ludwig, R.; Junge, K.; Beller, M. Selective Catalytic Hydrogenations of Nitriles, Ketones, and Aldehydes by Well-Defined Manganese Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 8809–8814. [Google Scholar] [CrossRef] [PubMed]
- Falco, G.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. FA Polymerization Disruption by Protic Polar Solvents. Polymers 2018, 10, 529. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Li, W.; Lu, Q.; Zhu, X. Homogeneous Catalytic Hydrogenation of Bio-Oil and Related Model Aldehydes with RuCl2(PPh3)3. Chem. Eng. Technol. 2010, 33, 2082–2088. [Google Scholar] [CrossRef]
- Bhor, M.D.; Panda, A.G.; Nandurkar, N.S.; Bhanage, B.M. Synthesis of Alkyl Iodides/Nitriles from Carbonyl Compounds Using Novel Ruthenium Tris(2,2,6,6-Tetramethyl-3,5-Heptanedionate) as Catalyst. Tetrahedron Lett. 2008, 49, 6475–6479. [Google Scholar] [CrossRef]
- Strassberger, Z.; Mooijman, M.; Ruijter, E.; Alberts, A.H.; de Graaff, C.; Orru, R.V.A.; Rothenberg, G. A Facile Route to Ruthenium–Carbene Complexes and Their Application in Furfural Hydrogenation. Appl. Organomet. Chem. 2009, 24, 142–146. [Google Scholar] [CrossRef]
- Strassberger, Z.; Mooijman, M.; Ruijter, E.; Alberts, A.H.; Maldonado, A.G.; Orru, R.V.A.; Rothenberg, G. Finding Furfural Hydrogenation Catalysts via Predictive Modelling. Adv. Synth. Catal. 2010, 352, 2201–2210. [Google Scholar] [CrossRef] [Green Version]
- Tukacs, J.M.; Bohus, M.; Dibó, G.; Mika, L.T. Ruthenium-Catalyzed Solvent-Free Conversion of Furfural to Furfuryl Alcohol. RSC Adv. 2017, 7, 3331–3335. [Google Scholar] [CrossRef] [Green Version]
- Gowda, A.S.; Parkin, S.; Ladipo, F.T. Hydrogenation and Hydrogenolysis of Furfural and Furfuryl Alcohol Catalyzed by Ruthenium(II) Bis(Diimine) Complexes: Ru(II)-Catalyzed Hydrogenation and Hydrogenolysis of Furfural. Appl. Organomet. Chem. 2012, 26, 86–93. [Google Scholar] [CrossRef]
- Wu, W.-P.; Xu, Y.-J.; Chang, S.-W.; Deng, J.; Fu, Y. PH-Regulated Aqueous Catalytic Hydrogenation of Biomass Carbohydrate Derivatives by Using Semisandwich Iridium Complexes. ChemCatChem 2016, 8, 3375–3380. [Google Scholar] [CrossRef]
- Garhwal, S.; Maji, B.; Semwal, S.; Choudhury, J. Ambient-Pressure and Base-Free Aldehyde Hydrogenation Catalyst Supported by a Bifunctional Abnormal NHC Ligand. Organometallics 2018, 37, 4720–4725. [Google Scholar] [CrossRef]
- Christie, F.; Zanotti-Gerosa, A.; Grainger, D. Hydrogenation and Reductive Amination of Aldehydes Using Triphos Ruthenium Catalysts. ChemCatChem 2018, 10, 1012–1018. [Google Scholar] [CrossRef]
- Moyo, P.S.; Matsinha, L.C.; Makhubela, B.C.E. Pd(II) and Pt(II) Catalysed Selective Synthesis of Furfuryl Alcohol: Solvent Effects and Insights into the Mechanism. J. Organomet. Chem. 2020, 922, 121362. [Google Scholar] [CrossRef]
- Valdebenito, G.; Parra-Melipán, S.; López, V.; Aranda, B.; García, E.; Vega, A.; Negrete-Vergara, C.; Moya, S.A.; Aguirre, P. Selective Hydrogenation of Furfural to Furfuryl Alcohol Catalysed by Ruthenium Complexes Containing Phosphorus-nitrogen Ligands. Appl. Organomet. Chem. 2021, 35, e6382. [Google Scholar] [CrossRef]
- Bullock, R.M. Abundant Metals Give Precious Hydrogenation Performance. Science 2013, 342, 1054–1055. [Google Scholar] [CrossRef]
- Wienhöfer, G.; Westerhaus, F.A.; Junge, K.; Ludwig, R.; Beller, M. A Molecularly Defined Iron-Catalyst for the Selective Hydrogenation of α,β-Unsaturated Aldehydes. Chem. Eur. J. 2013, 19, 7701–7707. [Google Scholar] [CrossRef]
- Gorgas, N.; Stöger, B.; Veiros, L.F.; Kirchner, K. Highly Efficient and Selective Hydrogenation of Aldehydes: A Well-Defined Fe(II) Catalyst Exhibits Noble-Metal Activity. ACS Catal. 2016, 6, 2664–2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S.; Brünig, J.; Zeindlhofer, V.; Schröder, C.; Stöger, B.; Limbeck, A.; Kirchner, K.; Bica, K. Selective Hydrogenation of Aldehydes Using a Well-Defined Fe(II) PNP Pincer Complex in Biphasic Medium. ChemCatChem 2018, 10, 4386–4394. [Google Scholar] [CrossRef] [PubMed]
- Csendes, Z.; Brünig, J.; Yigit, N.; Rupprechter, G.; Bica-Schröder, K.; Hoffmann, H.; Kirchner, K. Influence of the Ionic Liquid on the Activity of a Supported Ionic Liquid Phase Fe II Pincer Catalyst for the Hydrogenation of Aldehydes: Influence of the Ionic Liquid on the Activity of a Supported Ionic Liquid Phase Fe II Pincer Catalyst for the Hydrogenation of Aldehydes. Eur. J. Inorg. Chem. 2019, 3503–3510. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Milstein, D. Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes. ACS Catal. 2018, 8, 11435–11469. [Google Scholar] [CrossRef]
- Yang, W.; Chernyshov, I.Y.; van Schendel, R.K.A.; Weber, M.; Müller, C.; Filonenko, G.A.; Pidko, E.A. Robust and Efficient Hydrogenation of Carbonyl Compounds Catalysed by Mixed Donor Mn(I) Pincer Complexes. Nat. Commun 2021, 12, 12. [Google Scholar] [CrossRef]
- Glatz, M.; Stöger, B.; Himmelbauer, D.; Veiros, L.F.; Kirchner, K. Chemoselective Hydrogenation of Aldehydes under Mild, Base-Free Conditions: Manganese Outperforms Rhenium. ACS Catal. 2018, 8, 4009–4016. [Google Scholar] [CrossRef] [PubMed]
- Klomp, D.; Hanefeld, U.; Peters, J.A. Transfer Hydrogenation Including the Meerwein-Ponndorf-Verley Reduction. In The Handbook of Homogeneous Hydrogenation; de Vries, J.G., Elsevier, C.J., Eds.; Wiley: Weinheim, Germany, 2006; pp. 585–630. ISBN 978-3-527-61938-2. [Google Scholar]
- Gladiali, S.; Taras, R. Reduction of Carbonyl Compounds by Hydrogen Transfer. In Modern Reduction Methods; Andersson, P.G., Munslow, I.J., Eds.; Wiley: Weinheim, Germany, 2008; pp. 135–157. ISBN 978-3-527-62211-5. [Google Scholar]
- Gliński, M.; Ulkowska, U. Reactivity of Alcohols in Chemoselective Transfer Hydrogenation of Acrolein over Magnesium Oxide as the Catalyst. Catal. Lett. 2011, 141, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)Furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef]
- Baldino, S.; Facchetti, S.; Nedden, H.G.; Zanotti-Gerosa, A.; Baratta, W. Chemoselective Transfer Hydrogenation of Aldehydes with HCOONH4 Catalyzed by RuCl(CNNPh)(PP) Pincer Complexes. ChemCatChem 2016, 8, 3195–3198. [Google Scholar] [CrossRef]
- Townsend, T.M.; Kirby, C.; Ruff, A.; O’Connor, A.R. Transfer Hydrogenation of Aromatic and Linear Aldehydes Catalyzed Using Cp*Ir(Pyridinesulfonamide)Cl Complexes under Base-Free Conditions. J. Organomet. Chem. 2017, 843, 7–13. [Google Scholar] [CrossRef]
- Aboo, A.H.; Bennett, E.L.; Deeprose, M.; Robertson, C.M.; Iggo, J.A.; Xiao, J. Methanol as Hydrogen Source: Transfer Hydrogenation of Aromatic Aldehydes with a Rhodacycle. Chem. Commun. 2018, 54, 11805–11808. [Google Scholar] [CrossRef]
- Farrar-Tobar, R.A.; Wei, Z.; Jiao, H.; Hinze, S.; de Vries, J.G. Selective Base-Free Transfer Hydrogenation of α,β-Unsaturated Carbonyl Compounds Using i PrOH or EtOH as Hydrogen Source. Chem. Eur. J. 2018, 24, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Jana, N.C.; Panda, S.; Bagh, B. Transfer Hydrogenation of Aldehydes and Ketones in Air with Methanol and Ethanol by an Air-Stable Ruthenium–Triazole Complex. ACS Sustain. Chem. Eng. 2021, 9, 4903–4914. [Google Scholar] [CrossRef]
- Oklu, N.K.; Makhubela, B.C.E. Chemoselective and Efficient Catalytic Hydrogenation of Furfural by Iridium and Ruthenium Half-Sandwich Complexes. New J. Chem. 2020, 44, 9382–9390. [Google Scholar] [CrossRef]
- Gorgas, N.; Ilic, A.; Kirchner, K. Chemoselective Transfer Hydrogenation of Aldehydes in Aqueous Media Catalyzed by a Well-Defined Iron(II) Hydride Complex. Monatsh. Chem. 2019, 150, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Shipman, M.A.; Onabule, D.Y.; Sproules, S.; Symes, M.D. Selective Aldehyde Reductions in Neutral Water Catalysed by Encapsulation in a Supramolecular Cage. Chem. Sci. 2021, 12, 5082–5090. [Google Scholar] [CrossRef]
- Stadler, B.M.; Puylaert, P.; Diekamp, J.; van Heck, R.; Fan, Y.; Spannenberg, A.; Hinze, S.; de Vries, J.G. Inexpensive Ruthenium NNS-Complexes as Efficient Ester Hydrogenation Catalysts with High C=O vs. C=C Selectivities. Adv. Synth. Catal. 2018, 360, 1151–1158. [Google Scholar] [CrossRef]
- Junge, K.; Wendt, B.; Jiao, H.; Beller, M. Iridium-Catalyzed Hydrogenation of Carboxylic Acid Esters. ChemCatChem 2014, 6, 2810–2814. [Google Scholar] [CrossRef]
- Farrar-Tobar, R.A.; Wozniak, B.; Savini, A.; Hinze, S.; Tin, S.; de Vries, J.G. Base-Free Iron Catalyzed Transfer Hydrogenation of Esters Using EtOH as Hydrogen Source. Angew. Chem. Int. Ed. 2019, 58, 1129–1133. [Google Scholar] [CrossRef]
- Elangovan, S.; Garbe, M.; Jiao, H.; Spannenberg, A.; Junge, K.; Beller, M. Hydrogenation of Esters to Alcohols Catalyzed by Defined Manganese Pincer Complexes. Angew. Chem. Int. Ed. 2016, 55, 15364–15368. [Google Scholar] [CrossRef]
- Widegren, M.B.; Harkness, G.J.; Slawin, A.M.Z.; Cordes, D.B.; Clarke, M.L. A Highly Active Manganese Catalyst for Enantioselective Ketone and Ester Hydrogenation. Angew. Chem. Int. Ed. 2017, 56, 5825–5828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tang, Y.; Han, Z.; Ding, K. Lutidine-Based Chiral Pincer Manganese Catalysts for Enantioselective Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2019, 58, 4973–4977. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Matsumura, Y.; Hamaguchi, H. Easy Synthesis of 2-Hydroxy-3-Methylcyclopent-2-Enone. J. Chem. Soc. Chem. Commun. 1977, 712–713. [Google Scholar] [CrossRef]
- Gupta, K.; Tyagi, D.; Dwivedi, A.D.; Mobin, S.M.; Singh, S.K. Catalytic Transformation of Bio-Derived Furans to Valuable Ketoacids and Diketones by Water-Soluble Ruthenium Catalysts. Green Chem. 2015, 17, 4618–4627. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, P.; Xu, W.; Liu, X.; Xia, Z.; Chung, B.; Jia, S.; Zhang, Z.C. Hydrogenation/Hydrolytic Ring Opening of 5-HMF by Cp*-Iridium(III) Half-Sandwich Complexes for Bioketones Synthesis. ACS Catal. 2015, 5, 788–792. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, P.; Li, H.; Liu, K.; Liu, X.; Jia, S.; Zhang, Z.C. Active Cp*Iridium(III) Complex with Ortho -Hydroxyl Group Functionalized Bipyridine Ligand Containing an Electron-Donating Group for the Production of Diketone from 5-HMF. ACS Catal. 2016, 6, 3784–3788. [Google Scholar] [CrossRef]
- Wozniak, B.; Spannenberg, A.; Li, Y.; Hinze, S.; de Vries, J.G. Cyclopentanone Derivatives from 5-Hydroxymethylfurfural via 1-Hydroxyhexane-2,5-Dione as Intermediate. ChemSusChem 2018, 11, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, Economic, and Energetic Costs and Benefits of Biodiesel and Ethanol Biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated Furfural Production as a Renewable Fuel and Chemical Platform from Lignocellulosic Biomass: Furfural Production from Lignocellulosic Biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Vinod, N.; Dutta, S. Energy Densification of Biomass-Derived Furfurals to Furanic Biofuels by Catalytic Hydrogenation and Hydrodeoxygenation Reactions. Sustain. Chem. 2021, 2, 521–549. [Google Scholar] [CrossRef]
- Makhubela, B.C.E.; Darkwa, J. The Role of Noble Metal Catalysts in Conversion of Biomass and Bio-Derived Intermediates to Fuels and Chemicals. Johns. Matthey Technol. Rev. 2018, 62, 4–31. [Google Scholar] [CrossRef]
- Tian, G.; Daniel, R.; Li, H.; Xu, H.; Shuai, S.; Richards, P. Laminar Burning Velocities of 2,5-Dimethylfuran Compared with Ethanol and Gasoline. Energy Fuels 2010, 24, 3898–3905. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Lucas, N.; Chilukuri, S.V. Efficient Preparation of Liquid Fuel 2,5-Dimethylfuran from Biomass-Derived 5-Hydroxymethylfurfural over Ru–NaY Catalyst. ACS Sustain. Chem. Eng. 2015, 3, 2909–2916. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of Dimethylfuran for Liquid Fuels from Biomass-Derived Carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Sezer, İ. Thermodynamic, Performance and Emission Investigation of a Diesel Engine Running on Dimethyl Ether and Diethyl Ether. Int. J. Therm. Sci. 2011, 50, 1594–1603. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R. Upgrading of Carbohydrates to the Biofuel Candidate 5-Ethoxymethylfurfural (EMF). Int. J. Chem. Eng. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Yan, G.; Chen, G.; Feng, Y.; Zeng, X.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Recent Progress in the Development of Advanced Biofuel 5-Ethoxymethylfurfural. BMC Energy 2020, 2, 2. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Wu, K.; Bi, C.; Cui, Q. Conversion of Fructose into 5-Hydroxymethylfurfural (HMF) and Its Derivatives Promoted by Inorganic Salt in Alcohol. Carbohydr. Res. 2012, 350, 20–24. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Liu, B.; Zhou, Q.; Wang, S.; Deng, K. Catalytic Conversion of Fructose into Furans Using FeCl3 as Catalyst. J. Ind. Eng. Chem. 2014, 20, 644–649. [Google Scholar] [CrossRef]
- Lee, R.; Vanderveen, J.R.; Champagnffe, P.; Jessop, P.G. CO2-Catalysed Aldol Condensation of 5-Hydroxymethylfurfural and Acetone to a Jet Fuel Precursor. Green Chem. 2016, 18, 5118–5121. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on Catalytic Hydrodeoxygenation of Pyrolysis Oil and Its Model Compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Schlaf, M. Homogeneous Catalysts for the Hydrodeoxygenation of Biomass-Derived Carbohydrate Feedstocks. In Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion II; Schlaf, M., Zhang, Z.C., Eds.; Green Chemistry and Sustainable Technology; Springer: Singapore, 2016; pp. 13–38. ISBN 978-981-287-768-0. [Google Scholar]
- Dedsuksophon, W.; Champreda, V.; Laosiripojana, N. Study of Liquid Alkanes Production from Biomass-Derived Carbohydrates by Aldol-Condensation and Hydrogenation Processes. EJ 2010, 14, 1–10. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Latifi, E.; Chung, B.K.-M.; Soldatov, D.V.; Schlaf, M. Hydrodeoxygenation of 2,5-Hexanedione and 2,5-Dimethylfuran by Water-, Air-, and Acid-Stable Homogeneous Ruthenium and Iridium Catalysts. ACS Catal. 2014, 4, 4116–4128. [Google Scholar] [CrossRef]
- Yang, W.; Grochowski, M.R.; Sen, A. Selective Reduction of Biomass by Hydriodic Acid and Its In Situ Regeneration from Iodine by Metal/Hydrogen. ChemSusChem 2012, 5, 1218–1222. [Google Scholar] [CrossRef]
- Mishra, D.K.; Cho, J.K.; Kim, Y.J. Facile Production of 2,5-Diformylfuran from Base-Free Oxidation of 5-Hydroxymethyl Furfural over Manganese–Cobalt Spinels Supported Ruthenium Nanoparticles. J. Ind. Eng. Chem. 2018, 60, 513–519. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Z.; Liu, B.; Chen, S.; Zhang, Z. Catalytic Oxidation of Biomass Derived 5-Hydroxymethylfurfural (HMF) over Ru III -Incorporated Zirconium Phosphate Catalyst. J. Ind. Eng. Chem. 2016, 38, 181–185. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Pandey, A.; Sangwan, R.S. Biomass-Derived HMF Oxidation with Various Oxidants. In Biofuels; Agarwal, A.K., Agarwal, R.A., Gupta, T., Gurjar, B.R., Eds.; Green Energy and Technology; Springer: Singapore, 2017; pp. 51–67. ISBN 978-981-10-3790-0. [Google Scholar]
- Navarro, O.C.; Canós, A.C.; Chornet, S.I. Chemicals from Biomass: Aerobic Oxidation of 5-Hydroxymethyl-2-Furaldehyde into Diformylfurane Catalyzed by Immobilized Vanadyl-Pyridine Complexes on Polymeric and Organofunctionalized Mesoporous Supports. Top. Catal 2009, 52, 304–314. [Google Scholar] [CrossRef]
- Jia, X.; Ma, J.; Wang, M.; Du, Z.; Lu, F.; Wang, F.; Xu, J. Promoted Role of Cu(NO3)2 on Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran over VOSO4. Appl. Catal. A Gen. 2014, 482, 231–236. [Google Scholar] [CrossRef]
- Ma, J.; Du, Z.; Xu, J.; Chu, Q.; Pang, Y. Efficient Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran, and Synthesis of a Fluorescent Material. ChemSusChem 2011, 4, 51–54. [Google Scholar] [CrossRef]
- Dai, J. Synthesis of 2,5-Diformylfuran from Renewable Carbohydrates and Its Applications: A Review. Green Energy Environ. 2021, 6, 22–32. [Google Scholar] [CrossRef]
- Dessbesell, L.; Souzanchi, S.; Venkateswara Rao, K.T.; Carrillo, A.A.; Bekker, D.; Hall, K.A.; Lawrence, K.M.; Tait, C.L.J.; Xu, C. (Charles) Production of 2,5-furandicarboxylic Acid (FDCA) from Starch, Glucose, or High-fructose Corn Syrup: Techno-economic Analysis. Biofuels Bioprod. Bioref. 2019, 13, 1234–1245. [Google Scholar] [CrossRef]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent Advances in Catalytic Conversion of Biomass to 2,5-Furandicarboxylic Acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Meraldo, A. Introduction to Bio-based Polymers. In Multilayer Flexible Packaging, 2nd ed.; Wagner, J.R., Ed.; Academic Press: New York, NY, USA, 2016; pp. 47–52. [Google Scholar] [CrossRef]
- Verdeguer, P.; Merat, N.; Gaset, A. Oxydation Catalytique Du HMF En Acide 2,5-Furane Dicarboxylique. J. Mol. Catal. 1993, 85, 327–344. [Google Scholar] [CrossRef]
- Hong, M.; Min, J.; Wu, S.; Cui, H.; Zhao, Y.; Li, J.; Wang, S. Metal Nitrate Catalysis for Selective Oxidation of 5-Hydroxymethylfurfural into 2,5-Diformylfuran under Oxygen Atmosphere. ACS Omega 2019, 4, 7054–7060. [Google Scholar] [CrossRef] [PubMed]
- Partenheimer, W.; Grushin, V.V. Synthesis of 2,5-Diformylfuran and Furan-2,5-Dicarboxylic Acid by Catalytic Air-Oxidation of 5-Hydroxymethylfurfural. Unexpectedly Selective Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde with Metal/Bromide Catalysts. Adv. Synth. Catal. 2001, 343, 102–111. [Google Scholar] [CrossRef]
- Saha, B.; Dutta, S.; Abu-Omar, M.M. Aerobic Oxidation of 5-Hydroxylmethylfurfural with Homogeneous and Nanoparticulate Catalysts. Catal. Sci. Technol. 2012, 2, 79–81. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Green, D.; McMillan, E. Efficient Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran Using Mn(III)–Salen Catalysts. Catal. Commun. 2008, 9, 286–288. [Google Scholar] [CrossRef]
- Hansen, T.S.; Sádaba, I.; García-Suárez, E.J.; Riisager, A. Cu Catalyzed Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran and 2,5-Furandicarboxylic Acid under Benign Reaction Conditions. Appl. Catal. A Gen. 2013, 456, 44–50. [Google Scholar] [CrossRef]
- Song, S.; Wu, G.; Guan, N.; Li, L. Upgrading of Biomass-Derived Furans into Value-Added Chemicals. In Production of Biofuels and Chemicals with Bifunctional Catalysts; Fang, Z., Smith, R.L., Li, H., Eds.; Biofuels and Biorefineries; Springer: Singapore, 2017; Volume 8, pp. 273–303. ISBN 978-981-10-5136-4. [Google Scholar]
- Du, Z.; Ma, J.; Wang, F.; Liu, J.; Xu, J. Oxidation of 5-Hydroxymethylfurfural to Maleic Anhydride with Molecular Oxygen. Green Chem. 2011, 13, 554. [Google Scholar] [CrossRef]
- Guo, H.; Yin, G. Catalytic Aerobic Oxidation of Renewable Furfural with Phosphomolybdic Acid Catalyst: An Alternative Route to Maleic Acid. J. Phys. Chem. C 2011, 115, 17516–17522. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, R.; Santarelli, F.; Paul, S.; Dumeignil, F.; Cavani, F.; Gonçalves, R.V. Recent Developments in Maleic Acid Synthesis from Bio-Based Chemicals. Sustain. Chem Process. 2015, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Guo, H.; Su, Y.; Hiraga, Y.; Fang, Z.; Hensen, E.J.M.; Watanabe, M.; Smith, R.L. N-Formyl-Stabilizing Quasi-Catalytic Species Afford Rapid and Selective Solvent-Free Amination of Biomass-Derived Feedstocks. Nat. Commun. 2019, 10, 699. [Google Scholar] [CrossRef] [Green Version]
- Petri, A.; Masia, G.; Piccolo, O. Biocatalytic Conversion of 5-Hydroxymethylfurfural: Synthesis of 2,5-Bis(Hydroxymethyl)Furan and 5-(Hydroxymethyl)Furfurylamine. Catal. Commun. 2018, 114, 15–18. [Google Scholar] [CrossRef]
- Feriani, A.; Gaviraghi, G.; Toson, G.; Mor, M.; Barbieri, A.; Grana, E.; Boselli, C.; Guarneri, M.; Simoni, D.; Manfredini, S. Cholinergic Agents Structurally Related to Furtrethonium. 2. Synthesis and Antimuscarinic Activity of a Series of N-[5-[(1’-Substituted-Acetoxy)Methyl]-2-Furfuryl]Dialkylamines. J. Med. Chem. 1994, 37, 4278–4287. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Cukalovic, A.; Stevens, C.V. Production of Biobased HMF Derivatives by Reductive Amination. Green Chem. 2010, 12, 1201. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarov, V.I. Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor. Molecules 2020, 25, 4771. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, P.; Xu, W.; Jia, S.; Xia, Z.; Chung, B.; Zhang, Z.C. Direct Reductive Amination of 5-Hydroxymethylfurfural with Primary/Secondary Amines via Ru-Complex Catalyzed Hydrogenation. RSC Adv. 2014, 4, 59083–59087. [Google Scholar] [CrossRef]
- Pingen, D.; Schwaderer, J.B.; Walter, J.; Wen, J.; Murray, G.; Vogt, D.; Mecking, S. Diamines for Polymer Materials via Direct Amination of Lipid- and Lignocellulose-Based Alcohols with NH3. ChemCatChem 2018, 10, 3027–3033. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Yan, P.; Liu, K.; Wan, L.; Xu, W.; Li, H.; Liu, X.; Zhang, Z.C. Synthesis of Bis(Hydroxylmethylfurfuryl)Amine Monomers from 5-Hydroxymethylfurfural. ChemSusChem 2016, 9, 1255–1258. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Liu, S.; Song, K.; Yang, S.; Riisager, A. Sustainable Access to Renewable N-Containing Chemicals from Reductive Amination of Biomass-Derived Platform Compounds. Green Chem. 2020, 22, 6714–6747. [Google Scholar] [CrossRef]
- Senthamarai, T.; Murugesan, K.; Schneidewind, J.; Kalevaru, N.V.; Baumann, W.; Neumann, H.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Simple Ruthenium-Catalyzed Reductive Amination Enables the Synthesis of a Broad Range of Primary Amines. Nat. Commun. 2018, 9, 4123. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, D.; Nielsen, M. Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes. Catalysts 2021, 11, 558. [Google Scholar] [CrossRef]
- Koh, P.-F.; Wang, P.; Huang, J.-M.; Loh, T.-P. Biomass Derived Furfural-Based Facile Synthesis of Protected (2S)-Phenyl-3-Piperidone, a Common Intermediate for Many Drugs. Chem. Commun. 2014, 50, 8324–8327. [Google Scholar] [CrossRef] [Green Version]
- Hazra, C.K.; Gandhamsetty, N.; Park, S.; Chang, S. Borane Catalysed Ring Opening and Closing Cascades of Furans Leading to Silicon Functionalized Synthetic Intermediates. Nat. Commun. 2016, 7, 13431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Du, H.; Xu, J. Regiospecific Synthesis of Polysubstituted Furans with Mono- to Tricarboxylates from Various Sulfonium Acylmethylides and Acetylenic Esters. RSC Adv. 2019, 9, 25034–25038. [Google Scholar] [CrossRef] [Green Version]
- Papadogianakis, G.; Maat, L.; Sheldon, R.A. Catalytic Conversions in Water: A Novel Carbonylation Reaction Catalysed by Palladium Trisulfonated Triphenylphosphine Complexes. J. Chem. Soc. Chem. Commun. 1994, 2659–2660. [Google Scholar] [CrossRef]
- Geilen, F.M.A.; vom Stein, T.; Engendahl, B.; Winterle, S.; Liauw, M.A.; Klankermayer, J.; Leitner, W. Highly Selective Decarbonylation of 5-(Hydroxymethyl)Furfural in the Presence of Compressed Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 6831–6834. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Shao, S.; Hu, X.; Cai, Y. Aldol Condensation/Hydrogenation for Jet Fuel from Biomass-Derived Ketone Platform Compound in One Pot. Fuel Process. Technol. 2021, 215, 106768. [Google Scholar] [CrossRef]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of Fuels and Chemicals from Biomass: Condensation Reactions and Beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, E.; Soto, R.; Bringué, R.; Iborra, M.; Tejero, J. Catalytic Hydroxyalkylation/Alkylation of 2-Methylfuran with Butanal to Form a Biodiesel Precursor Using Acidic Ion-Exchange Resins. Ind. Eng. Chem. Res. 2020, 59, 20676–20685. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, X.; Pan, L.; Wang, M.; Zou, J.-J. One-Pot Production of Branched Decalins as High-Density Jet Fuel from Monocyclic Alkanes and Alcohols. Chem. Eng. Sci. 2018, 180, 64–69. [Google Scholar] [CrossRef]
- Anbu, N.; Maheswari, R.; Elamathi, V.; Varalakshmi, P.; Dhakshinamoorthy, A. Chitosan as a Biodegradable Heterogeneous Catalyst for Knoevenagel Condensation between Benzaldehydes and Cyanoacetamide. Catal. Commun. 2020, 138, 105954. [Google Scholar] [CrossRef]
- Liu, D.; Chen, E.Y.-X. Integrated Catalytic Process for Biomass Conversion and Upgrading to C12 Furoin and Alkane Fuel. ACS Catal. 2014, 4, 1302–1310. [Google Scholar] [CrossRef]
- Tao, L.; Yan, T.-H.; Li, W.; Zhao, Y.; Zhang, Q.; Liu, Y.-M.; Wright, M.M.; Li, Z.-H.; He, H.-Y.; Cao, Y. Toward an Integrated Conversion of 5-Hydroxymethylfurfural and Ethylene for the Production of Renewable p-Xylene. Chem 2018, 4, 2212–2227. [Google Scholar] [CrossRef] [Green Version]
- Kabro, A.; Escudero-Adán, E.C.; Grushin, V.V.; van Leeuwen, P.W.N.M. Biomass Conversion to High Value Chemicals: From Furfural to Chiral Hydrofuroins in Two Steps. Org. Lett. 2012, 14, 4014–4017. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Z.; Yan, P.; Zhang, Z.C. A Catalytic Aldol Condensation System Enables One Pot Conversion of Biomass Saccharides to Biofuel Intermediates. Green Chem. 2017, 19, 1751–1756. [Google Scholar] [CrossRef]
- Sharma, U.K.; Gemoets, H.P.L.; Schröder, F.; Noël, T.; Van der Eycken, E.V. Merger of Visible-Light Photoredox Catalysis and C–H Activation for the Room-Temperature C-2 Acylation of Indoles in Batch and Flow. ACS Catal. 2017, 7, 3818–3823. [Google Scholar] [CrossRef]
- Ebru Aydin, A.; Yuksekdanaci, S. Asymmetric Henry Reactions Catalyzed by Metal Complexes of Chiral Oxazoline Based Ligands. Tetrahedron Asymmetry 2013, 24, 14–22. [Google Scholar] [CrossRef]
- Böhm, C.; Schinnerl, M.; Bubert, C.; Zabel, M.; Labahn, T.; Parisini, E.; Reiser, O. A new strategy for the stereoselective synthesis of 1,2,3-trisubstituted cyclopropanes. Eur. J. Org. Chem. 2000, 2000, 2955–2965. [Google Scholar] [CrossRef]
- Asano, R.; Moritani, I.; Fujiwara, Y.; Teranishi, S. Aromatic Substitution of Olefins. XIX. Reaction of Five-Membered Heterocyclic Aromatic Compounds with Styrene. BCSJ 1973, 46, 663–664. [Google Scholar] [CrossRef] [Green Version]
- Sevov, C.S.; Hartwig, J.F. Iridium-Catalyzed Oxidative Olefination of Furans with Unactivated Alkenes. J. Am. Chem. Soc. 2014, 136, 10625–10631. [Google Scholar] [CrossRef] [Green Version]
- Majer, J.; Kwiatkowski, P.; Jurczak, J. Highly Enantioselective Synthesis of 2-Furanyl-Hydroxyacetates from Furans via the Friedel−Crafts Reaction. Org. Lett. 2008, 10, 2955–2958. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Kashima, S.; Kuriyama, T.; Iwata, Y.; Morimoto, T.; Kakiuchi, K. Enantioselective Friedel–Crafts Reactions of Ethenetricarboxylates and Substituted Pyrroles and Furans and Intramolecular Reaction of Benzene Derivatives. Tetrahedron Asymmetry 2009, 20, 1224–1234. [Google Scholar] [CrossRef]
- Chacón-Huete, F.; Mangel, D.; Ali, M.; Sudano, A.; Forgione, P. High-Value Biomass-Derived 2,5-Furandicarboxylic Acid Derivatives Obtained by a Double Decarboxylative Cross-Coupling. ACS Sustain. Chem. Eng. 2017, 5, 7071–7076. [Google Scholar] [CrossRef]
- Chacón-Huete, F.; Lasso, J.D.; Szavay, P.; Covone, J.; Forgione, P. Synthesis of 2,5-Diaryl Nonsymmetric Furans C6-Platform Chemicals via Catalytic Conversion of Biomass and the Formal Synthesis of Dantrolene. J. Org. Chem. 2021, 86, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T. Substituted Polyacetylenes: Synthesis, Properties, and Functions. Polym. Rev. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Romashov, L.V.; Ananikov, V.P. Alkynylation of Bio-Based 5-Hydroxymethylfurfural to Connect Biomass Processing with Conjugated Polymers and Furanic Pharmaceuticals. Chem. Asian J. 2017, 12, 2652–2655. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla, R.; Koranchalil, S.; Nielsen, M. Homogeneous Catalyzed Valorization of Furanics: A Sustainable Bridge to Fuels and Chemicals. Catalysts 2021, 11, 1371. https://doi.org/10.3390/catal11111371
Padilla R, Koranchalil S, Nielsen M. Homogeneous Catalyzed Valorization of Furanics: A Sustainable Bridge to Fuels and Chemicals. Catalysts. 2021; 11(11):1371. https://doi.org/10.3390/catal11111371
Chicago/Turabian StylePadilla, Rosa, Sakhitha Koranchalil, and Martin Nielsen. 2021. "Homogeneous Catalyzed Valorization of Furanics: A Sustainable Bridge to Fuels and Chemicals" Catalysts 11, no. 11: 1371. https://doi.org/10.3390/catal11111371
APA StylePadilla, R., Koranchalil, S., & Nielsen, M. (2021). Homogeneous Catalyzed Valorization of Furanics: A Sustainable Bridge to Fuels and Chemicals. Catalysts, 11(11), 1371. https://doi.org/10.3390/catal11111371






