Kinetic Study on CO-Selective Methanation over Nickel-Based Catalysts for Deep Removal of CO from Hydrogen-Rich Reformate
Abstract
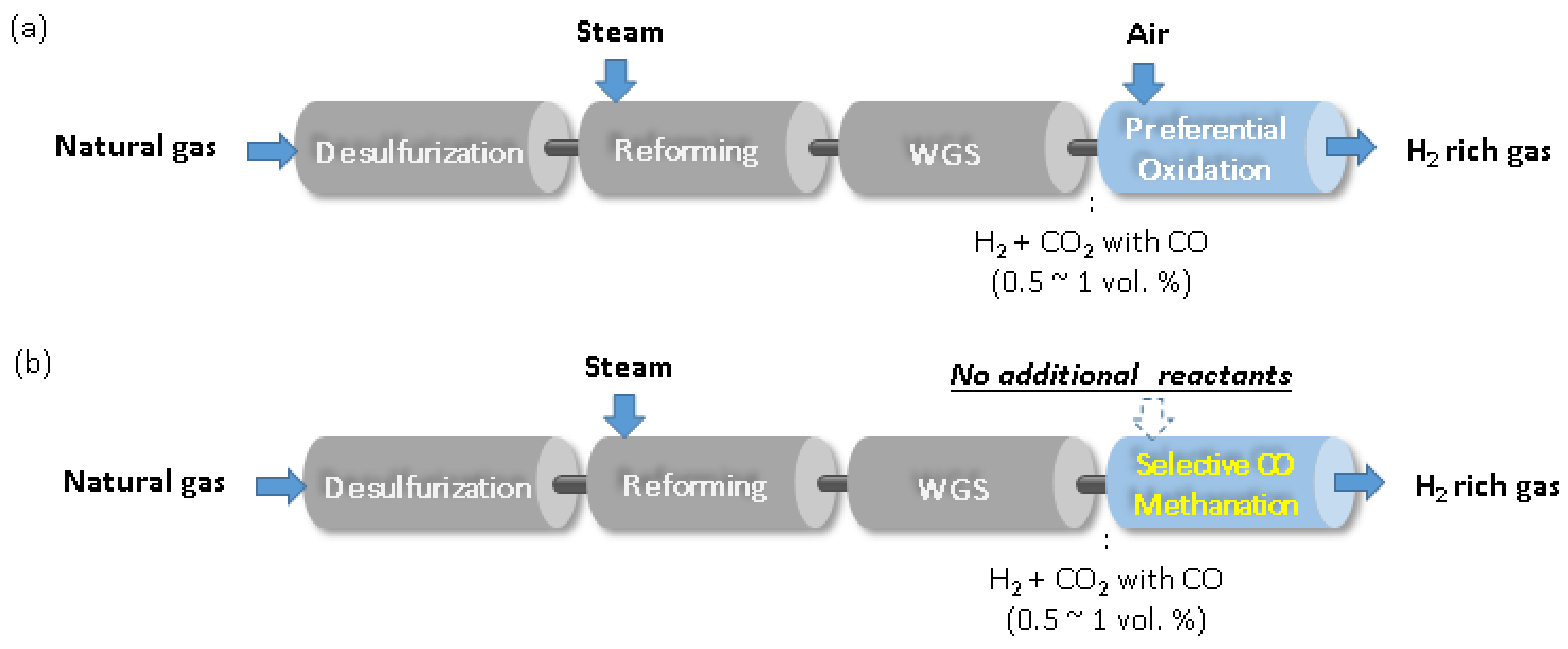
:1. Introduction
2. Results and Discussion
2.1. Mechanistic Reaction Model and Kinetic Parameter Estimation Method
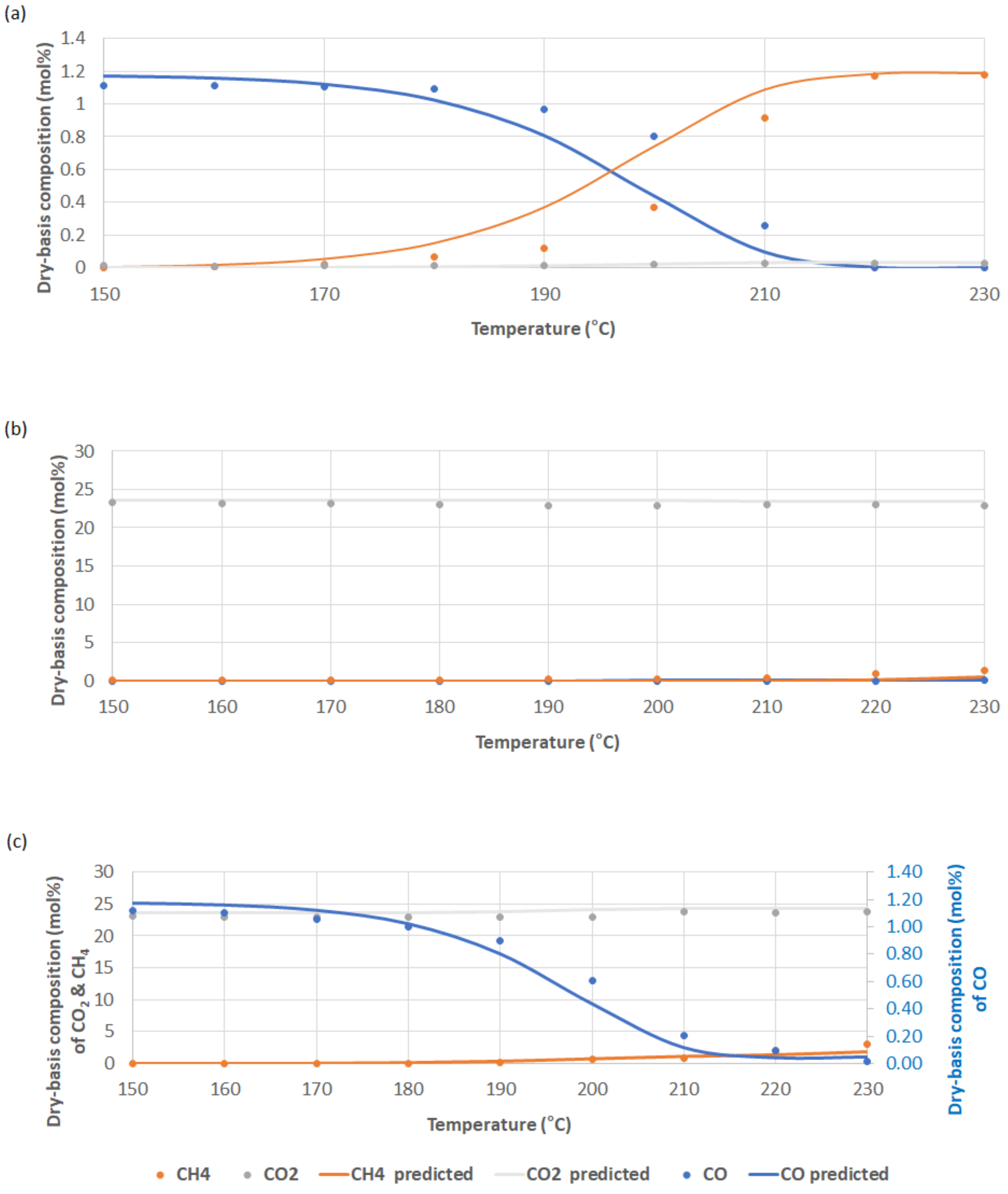
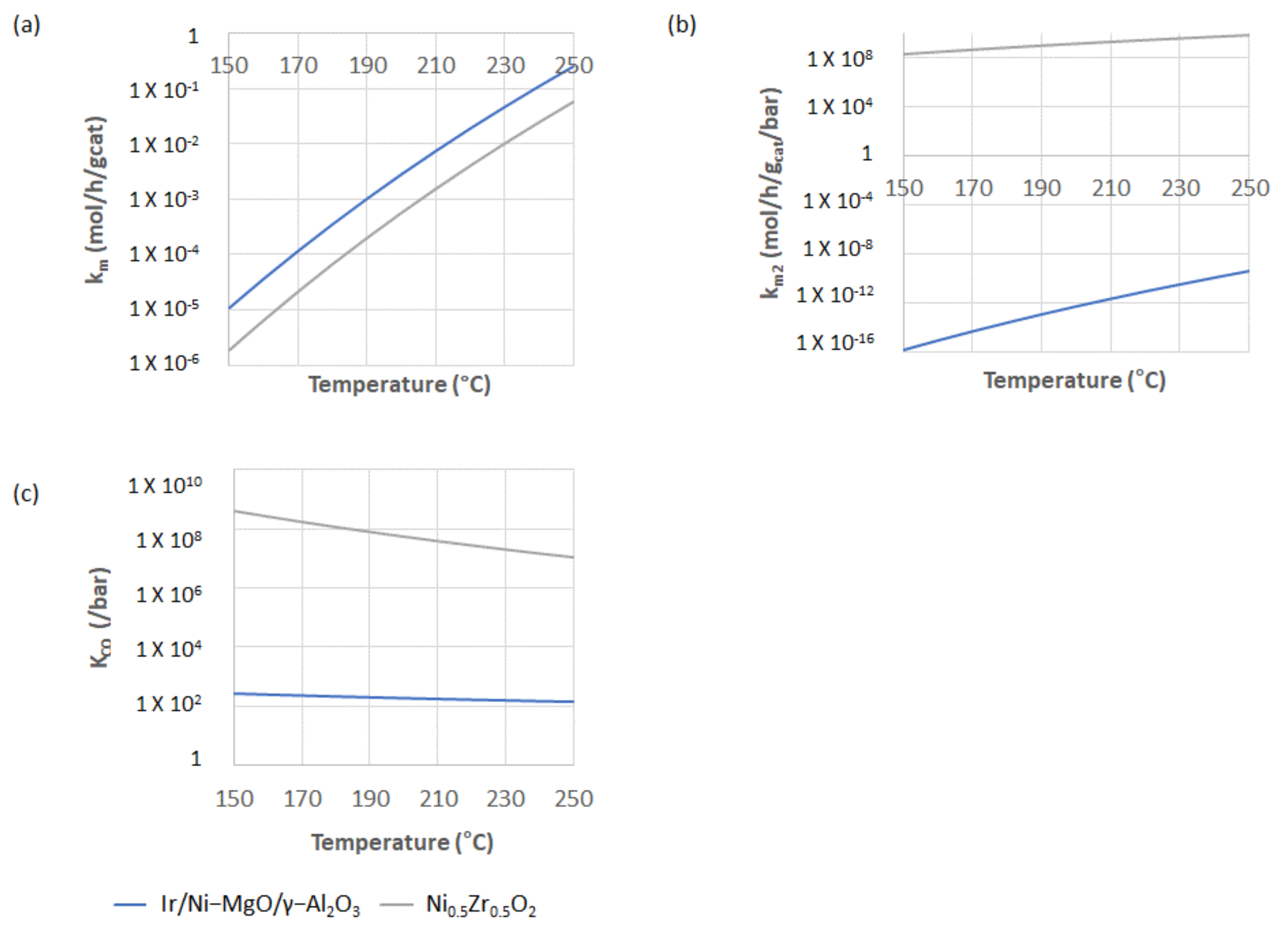
2.2. Parameter Estimation Results
2.2.1. Ir/Ni-MgO/γ-Alumina and Ni0.5Zr0.5O2 Catalyst
2.2.2. Comparisons of the Kinetic Parameters with Other Ni-Based Catalysts
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Speight, J.G.; Loyalka, S.K. Handbook of alternative fuel technologies. In Fuel Cells, 2nd ed.; Chapter 16; CRC Press: Boca Raton, FL, USA, 2007; pp. 493–523. [Google Scholar]
- Kolb, G. Fuel Processing; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Ghenciu, A.F. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Jung, U.H.; Kim, W.; Koo, K.Y.; Yoon, W.L. Genuine design of compact natural gas fuel processor for 1-kWe class residential proton exchange membrane fuel cell systems. Fuel Process. Technol. 2014, 121, 32–37. [Google Scholar] [CrossRef]
- Kim, W.; Koo, K.Y.; Lee, H.; Shul, Y.G.; Yoon, W.L. Highly dispersed nickel catalyst promoted by precious metals for CO selective methanation. Int. J. Hydrogen Energy 2015, 40, 10033–10040. [Google Scholar] [CrossRef]
- Chen, A.; Miyao, T.; Higashiyama, K.; Yamashita, H.; Watanabe, M. High catalytic performance of ruthenium-doped mesoporous nickel-aluminum oxides for selective CO methanation. Angew. Chem. 2010, 122, 10091–10094. [Google Scholar] [CrossRef]
- Takenaka, S.; Shimizu, T.; Otsuka, K. Complete removal of carbon monoxide in hydrogen-rich gas stream through methanation over supported metal catalysts. Int. J. Hydrogen Energy 2004, 29, 1065–1073. [Google Scholar] [CrossRef]
- Gao, Z.; Cui, L.; Ma, H. Selective methanation of CO over Ni/Al2O3 catalyst: Effects of preparation method and Ru addition. Int. J. Hydrogen Energy 2016, 41, 5484–5493. [Google Scholar] [CrossRef]
- Chen, A.; Miyao, T.; Higashiyama, K.; Watanabe, M. High catalytic performance of mesoporous zirconia supported nickel catalysts for selective CO methanation. Catal. Sci. Technol. 2014, 4, 2508–2511. [Google Scholar] [CrossRef]
- Dagle, R.A.; Wang, Y.; Xia, G.; Strohm, J.J.; Holladay, J.; Palo, D.R. Selective CO methanation catalysts for fuel processing applications. Appl. Catal. A 2007, 326, 213–218. [Google Scholar] [CrossRef]
- Galletti, C.; Specchia, S.; Saracco, G.; Specchia, V. CO-selective methanation over Ru-Al2O3 catalysts in H2-rich gas for PEM FC applications. Chem. Eng. Sci. 2010, 65, 590–596. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Mo, X.; Lin, W. Selective catalytic methanation of CO in hydrogen-rich gases over Ni/ZrO2 catalyst. J. Nat. Gas Chem. 2008, 17, 268–272. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Snytnikov, P.V.; Gulyaev, R.V.; Amosov, Y.I.; Boronin, A.I.; Sobyanin, V.A. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas. Chem. Eng. J. 2014, 238, 189–197. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported noble metal catalysts: Effects of the nature of the metallic phase on catalytic performance. Appl. Catal. A 2008, 344, 45–54. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported Ru catalysts. Appl. Catal. B 2009, 88, 470–478. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R.; Urasaki, K.; Satokawa, S. Effect of reduction pretreatment and support materials on selective CO methanation over supported Ru catalysts. Appl. Catal. A 2011, 404, 149–154. [Google Scholar] [CrossRef]
- Urasaki, K.; Endo, K.; Takahiro, T.; Kikuchi, R.; Kojima, T.; Satokawa, S. Effect of support materials on the selective methanation of CO over Ru catalysts. Top. Catal. 2010, 53, 707–711. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R. Preparation of Ru nanoparticles on TiO2 using selective deposition method and their application to selective CO methanation. Catal. Sci. Technol. 2014, 4, 26–29. [Google Scholar] [CrossRef]
- Eckle, S.; Anfang, H.-G.; Behm, R.J. What drives the selectivity for CO methanation in the methanation of CO2-rich reformate gases on supported Ru catalysts? Appl. Catal. A 2011, 391, 325–333. [Google Scholar] [CrossRef]
- Park, E.D.; Lee, D.; Lee, H.C. Recent progress in selective CO removal in a H2-rich stream. Catal. Today 2009, 139, 280–290. [Google Scholar] [CrossRef]
- Mohaideen, K.K.; Kim, W.; Yoon, W.L. Highly efficient non-noble metal based nanostructured catalysts for selective CO methanation. Catal. Commun. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, K.; Iwasa, N.; Fujita, S.; Arai, M. Selective methanation of CO in H2-rich gas stream by synthetic nickel-containing smectite based catalysts. Appl. Catal. B 2015, 162, 93–97. [Google Scholar] [CrossRef]
- Konishchev, M.V.; Potemkin, D.I.; Snytnikov, P.V.; Zyryanov, M.M.; Pakharukova, V.P.; Simonov, P.A.; Sobyanin, V.A. Selective CO methanation in H2-rich stream over Ni-, Co- and Fe/CeO2: Effect of metal and precursor nature. Int. J. Hydrogen Energy 2015, 40, 14058–14063. [Google Scholar] [CrossRef]
- van Herwijnen, T.; van Doesburg, H.; de Jong, W.A. Kinetics of the methanation of CO and CO2 on a nickel catalyst. J. Catal. 1973, 28, 391–402. [Google Scholar] [CrossRef]
- Klose, J.; Baerns, M. Kinetics of the methanation of carbon monoxide on an alumina-supported nickel catalyst. J. Catal. 1984, 85, 105–116. [Google Scholar] [CrossRef]
- Sehested, J.; Dahl, S.; Jacobsen, J.; Rostrup-Nielsen, J.R. Methanation of CO over nickel: Mechanism and kinetics at high H2/CO ratios. J. Phys. Chem. B 2005, 109, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Loc, L.C.; Huan, N.M.; Gaidai, N.A.; Thoang, H.S.; Agafonov, Y.A.; Nekrasov, N.V.; Lapidus, A.L. Kinetics of carbon monoxide methanation on nickel catalysts. Kinet. Catal. 2012, 53, 384–394. [Google Scholar] [CrossRef]
- Alstrup, I. On the kinetics of CO Methanation on nickel surfaces. J. Catal. 1995, 151, 216–225. [Google Scholar] [CrossRef]
- Lim, J.Y.; McGregor, J.; Sederman, A.J.; Dennis, J.S. Kinetic studies of CO2 methanation over a Ni/γ-Al2O3 catalyst using a batch reactor. Chem. Eng. Sci. 2016, 141, 28–45. [Google Scholar] [CrossRef] [Green Version]
- Rönsch, S.; Köchermann, J.; Schneider, J.; Matthischke, S. Global reaction kinetics of CO and CO2 methanation for dynamic process modeling. Chem. Eng. Technol. 2016, 39, 208–2018. [Google Scholar] [CrossRef]
- Hubble, R.A.; Lim, J.Y.; Dennis, J.S. Kinetic studies of CO2 methanation over a Ni/γ-Al2O3 catalyst. Faraday Discuss. 2016, 192, 529–544. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/gamma-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B 2012, 113, 237–249. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R. Mechanistic study and catalyst development for selective carbon monoxide methanation. Catal. Sci. Technol. 2015, 5, 3061–3070. [Google Scholar] [CrossRef]
- Miyao, T.; Shen, W.; Chen, A.; Higashiyama, K.; Watanabe, M. Mechanistic study of the effect of chlorine on selective CO methanation over Ni alumina-based catalysts. Appl. Catal. A 2014, 486, 187–192. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: I. Intrinsic Kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Twigg, M.V. Catalyst Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Moe, J.M. Design of water-gas shift reactors. Chem. Eng. Prog. 1962, 58, 33–36. [Google Scholar]
- Kim, W.; Yun, C.; Kim, Y.; Park, J.; Park, S.; Jung, K.T.; Lee, Y.H.; Kim, S.H. Modeling of a Tubular Reactor Producing Epichlorohydrin with Consideration of Reaction Kinetics and Deactivation of Titanium Silicate-1 Catalyst. Ind. Eng. Chem. Res. 2011, 50, 1187–1195. [Google Scholar] [CrossRef]
- Ferreiro, A.I.; Rabaçal, M.; Costa, M. A combined genetic algorithm and least squares fitting procedure for the estimation of the kinetic parameters of the pyrolysis of agricultural residues. Energ. Convers. Manag. 2016, 125, 290–3300. [Google Scholar] [CrossRef]
- The MathWorks, Inc. Optimization ToolboxTM User’s Guide. 2017. Available online: https://kr.mathworks.com/help/pdf_doc/optim/optim_tb.pdf (accessed on 3 November 2021).
- Kim, W.; Mohaideen, K.K.; Yoon, W.L. Kinetic study of CO selective methanation on nickel-based catalysts. In Proceedings of the 21st World Hydrogen Energy Conference, Zaragoza, Spain, 13–16 June 2016. [Google Scholar]


| Experiment | GHSV (/h) | Temperature (°C) | Gas Composition (vol.%) |
|---|---|---|---|
| Case 1 | 8400/h | 150–250 | 1% CO, 0% CO2, 78.9% H2, 15.2% steam and 4.9% N2 |
| Case 2 | 0% CO, 20% CO2, 59.9% H2, 15.2% steam and 4.9% N2 | ||
| Case 3 | 1% CO, 20% CO2, 58.9% H2, 15.2% steam and 4.9% N2 |
| Number of Populations | Fitness Scaling | Selection | Reproduction | Mutation | Crossover | Hybrid Function |
|---|---|---|---|---|---|---|
| 200 | Rank | Stochastic uniform | Elite count: 4 Crossover fraction: 0.8 | Adaptive feasible | Constraint dependent | fmincon |
| Catalyst | Parameters of the Arrhenius Equation | |||||
|---|---|---|---|---|---|---|
| Am (×1015) | Awgs (×1015) | Am2 (×1015) | Eam (kJ/mol) | Eawgs (kJ/mol) | Eam2 (kJ/mol) | |
| Ir/Ni-MgO/γ-alumina | 881.042 | 452.152 | 632.149 | 185.647 | 16.967 | 272.479 |
| Ni0.5Zr0.5O2 | 522.722 | 3.213 | 46.657 | 189.647 | 19.083 | 67.834 |
| Ni/γ-alumina [24] | 2.09 × 10−10 | 1.36 × 10−3 | 226.2 | 106.3 | ||
| Ni catalyst [28] | 105.9 | |||||
| Ni/alumina [30] | 4.8 × 10−6 | 7.83 × 10−9 | 103 | 21 | ||
| Catalyst | Adsorption Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| BCO | BH2 | BCO2 | BH2O (×103) | ΔHCO (kJ/mol) | ΔHH2 (kJ/mol) | ΔHCO2 (kJ/mol) | ΔHH2O (kJ/mol) | |
| Ir/Ni-MgO/γ-alumina | 8.515 | 2.227 | 6.412 | 3.792 | −12.057 | −74.309 | −65.430 | 1.000 |
| Ni0.5Zr0.5O2 | 2.702 | 0.002 | 0.010 | 99.789 | −65.841 | −53.603 | −79.935 | 1.306 |
| Ni/γ-alumina [24] | 4.56 × 10−4 | −52.1 | ||||||
| Ni catalyst [28] | ||||||||
| Ni/alumina [30] | 8.23 × 10−5 | 6.12 × 10−9 | 177 | −70.7 * | 82.9 * | 88.7 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Kamal, K.M.; Seo, D.J.; Yoon, W.L. Kinetic Study on CO-Selective Methanation over Nickel-Based Catalysts for Deep Removal of CO from Hydrogen-Rich Reformate. Catalysts 2021, 11, 1429. https://doi.org/10.3390/catal11121429
Kim W, Kamal KM, Seo DJ, Yoon WL. Kinetic Study on CO-Selective Methanation over Nickel-Based Catalysts for Deep Removal of CO from Hydrogen-Rich Reformate. Catalysts. 2021; 11(12):1429. https://doi.org/10.3390/catal11121429
Chicago/Turabian StyleKim, Woohyun, Khaja Mohaideen Kamal, Dong Joo Seo, and Wang Lai Yoon. 2021. "Kinetic Study on CO-Selective Methanation over Nickel-Based Catalysts for Deep Removal of CO from Hydrogen-Rich Reformate" Catalysts 11, no. 12: 1429. https://doi.org/10.3390/catal11121429
APA StyleKim, W., Kamal, K. M., Seo, D. J., & Yoon, W. L. (2021). Kinetic Study on CO-Selective Methanation over Nickel-Based Catalysts for Deep Removal of CO from Hydrogen-Rich Reformate. Catalysts, 11(12), 1429. https://doi.org/10.3390/catal11121429






