Abstract
Synthesized oleic acid-based wax esters (e.g., cetyl oleate), which can replace spermaceti oil or jojoba oil, have been widely used in the cosmetic, pharmaceutical and other industries. In this work, 4-dodecylbenzenesulfonic acid (DBSA) has been successfully used as an efficient catalyst to synthesize oleic acid-based wax esters through esterification at 40 °C under solvent-free conditions. A 93.6% conversion rate of cetyl alcohol was obtained under optimal conditions: 10 mol% DBSA, a molar ratio of 1.3:1 oleic acid to alcohol, a reaction temperature 40 °C and a reaction time of 4 h. The effect of water content on esterification was investigated, and it was found that the inhibitory effect of water decreased significantly with increasing temperature. Moreover, DBSA-catalyzed esterification could be applied in the production of various oleic acid-based wax esters and excellent conversion (>90%) to esters was obtained under such mild conditions. DBSA-catalyzed low-temperature esterification is an efficient method for the production of liquid wax esters.
1. Introduction
Wax esters are long-chain esters of alcohols and fatty acids with more than 12 carbon atoms [1]. Due to their non-toxic properties, biodegradability and excellent wetting behaviors in interfaces, wax esters (including solid wax esters and liquid wax esters) have been applied in the cosmetic, pharmaceutical, and lubricant industries [2,3,4,5]. Natural sources of liquid wax esters range from animals to plants, such as sperm whales and jojoba oil [6]. After the global ban to stop whaling, jojoba oil became the primary natural source for liquid wax esters [7]. However, the main limitations to the use of jojoba oil are its cost and availability [8]. Therefore, it is highly desirable to synthesize liquid wax esters as jojoba oil analogues for various industry applications.
Generally, wax esters can be prepared through esterification of alcohol with fatty acid in the presence of acid catalysts. Traditional esterification performed using homogeneous catalysts (e.g., sulfuric acid, p-toluenesulfonic acid) or heterogeneous catalysts (e.g., zeolites) [5,9,10]. However, acid catalysts result in many problems, for example, causing side reactions (e.g., oxidation, sulfonation and etherization), and use a high amount of energy to remove water from the reaction [11]. Therefore, efforts have focused on enzymatic catalysis for esterification [2,12]. Though enzymatic catalysis requires mild reaction temperatures (30~70 °C) and produces higher yields, a long reaction time (12–24 h) and the high price of the enzyme limit its large-scale applications [2]. Thus, developing a novel chemical catalysis for wax ester production at mild reaction temperatures (<50 °C) is highly desirable. In fact, a higher reaction temperature (80~160 °C) is required for conventional acid-catalyzed esterification for wax ester production, which can remove the side product water and make esterification a forward reaction [5,9,11]. Thus, removing the generated water from the reaction system at a mild temperature (<50 °C) is crucial to promoting esterification. Alkyl benzenesulfonic acid with a long carbon chain is a type of amphiphilic acid catalyst, such as 4-dodecylbenzenesulfonic acid (DBSA). DBSA is a class of sulfonic acid comprised of a hydrophilic polar head group and a hydrophobic chain [13]. DBSA has been disclosed as an efficient catalyst for direct esterification of carboxylic acids and alcohols in water at 40 °C [14]. Li et al. [15] reported solvent-free esterification of carboxylic acids (C2–C18) with short-chain alcohols (C2–C8) catalyzed by DBSA at room temperature, which required a longer reaction time of 24 h. The above studies suggested that DBSA could form an O/W or W/O emulsion to remove the water from the reaction system [16].
High-oleic sunflower seed oil contains a higher content of oleic acid (~85%) than other kinds of vegetable oils, such as camellia seed oil and olive oil. As a good oleic acid resource, high-oleic sunflower seed oil is much cheaper than camellia seed oil or olive oil. The aim is to develop a method for the large-scale production of liquid wax esters with excellent oxidative stability, and fatty acids prepared from high-oleic sunflower seed oil are suitable for the production of liquid wax esters through esterification.
Thus, the aim of this work is to investigate the synthesis of oleic acid-based wax esters at mild temperatures (<50 °C) in the presence of DBSA. The effects of reaction conditions such as reaction temperature, reaction time, amount of catalyst and the molar ratio of fatty acid to alcohol have been studied. Furthermore, the effect of water content on esterification was also investigated. This work will present very mild reaction conditions for the production of oleic acid-based wax esters, which will be a good substitute to jojoba oil.
2. Results and Discussion
2.1. Fatty Acid Composition of Oleic Acid from High-Oleic Sunflower Seed Oil
The fatty acid composition of fatty acids prepared from high-oleic sunflower seed oil was determined by GC. The results showed that the saturated fatty acid content (palmitic acid plus stearic acid) was only 7.9%. The oleic acid content was as high as 84.4%. In addition, linoleic acid content was only 7.7%. Oleic acid is a monounsaturated fatty acid, which has far better oxidation stability than polyunsaturated fatty acids (e.g., linolenic acid). Additionally, commercial oleic acid reagent with high purity (>95%) is more expensive than fatty acids from high-oleic sunflower seed oil. Thus, oleic acid (purity 84.4%) from high-oleic sunflower seed oil can provide a desirable reactant for the production of liquid wax esters.
2.2. Screening of Catalysts
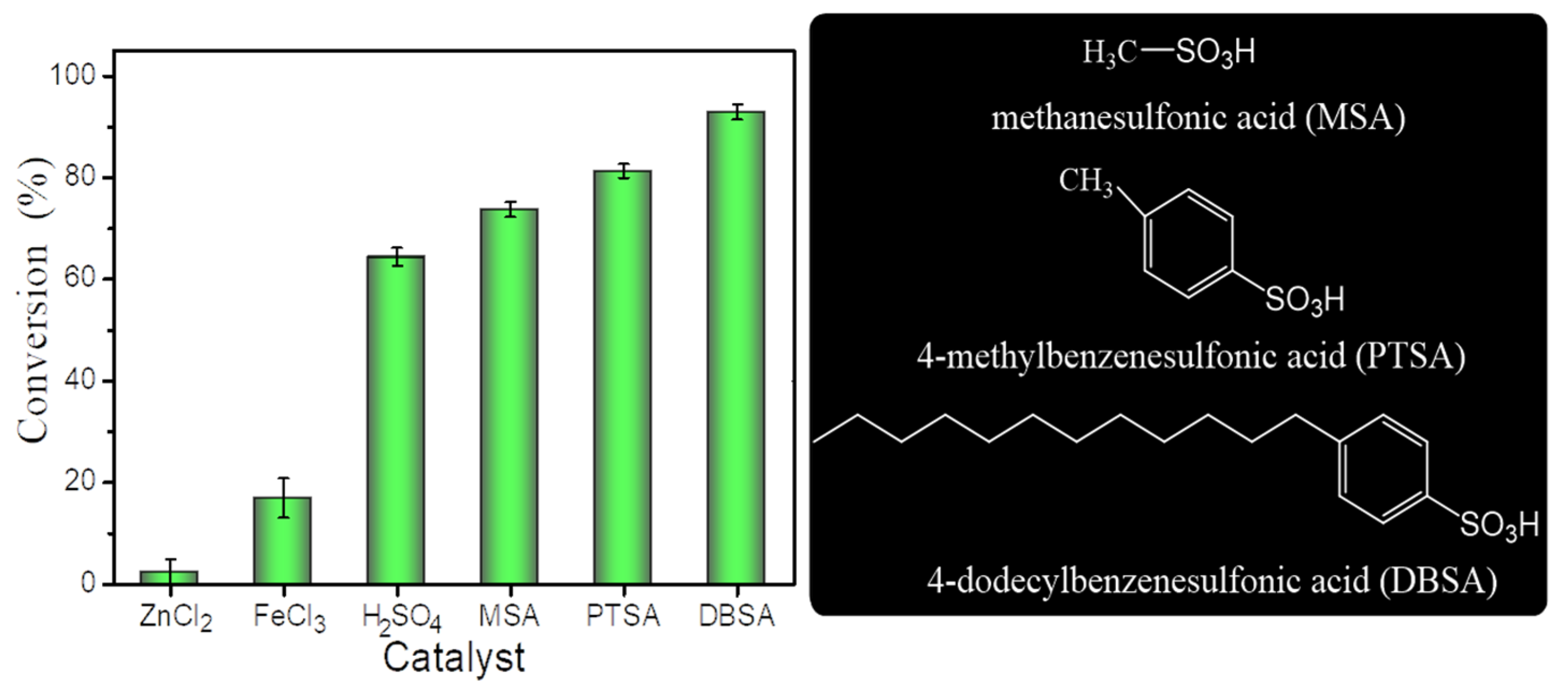
To study the synthesis of unsaturated wax esters (liquid wax esters), esterification of cetyl alcohol with oleic acid (purity 84.4%) prepared from high-oleic sunflower oil was selected as the model reaction and various homogeneous acid catalysts were evaluated (Figure 1). As shown in Figure 2, six acidic homogeneous catalysts were used to promote the esterification of oleic acid and cetyl alcohol to synthesize liquid wax ester (cetyl oleate) at 40 °C. The catalytic efficiency of Lewis acid catalysts (ZnCl2 and FeCl3) was much lower than that of Brønsted acid catalysts, including H2SO4, methanesulfonic acid(MSA), p-toluenesulfonic acid (PTSA), and 4-dodecylbenzenesulfonic acid (DBSA). The different results for the four sulfonic acid catalysts (H2SO4, MSA, PTSA, and DBSA) can be attributed, at least in part, to their molecular structures. Compared with MSA and PTSA, the long hydrophobic aliphatic chains in the molecular structure of DBSA increase its hydrophobicity, so the conversion rate increased significantly at such a mild temperature (40 °C). This observation was consistent with previous reports [13,15].
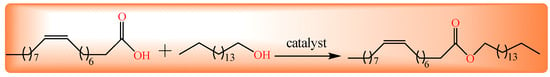
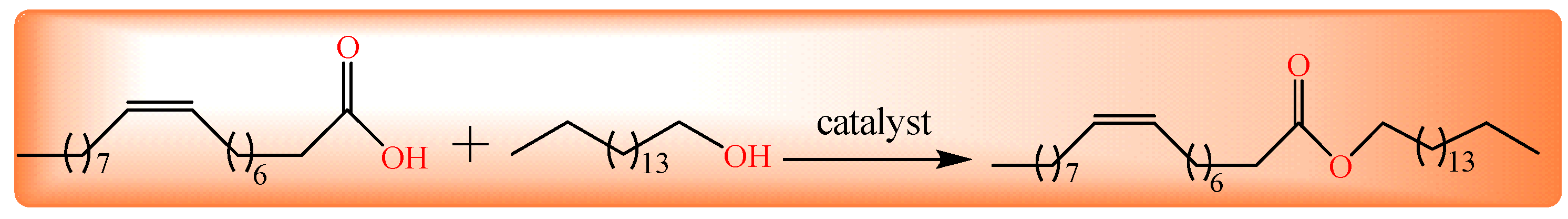
Figure 1.
Synthesis of cetyl oleate through esterification.
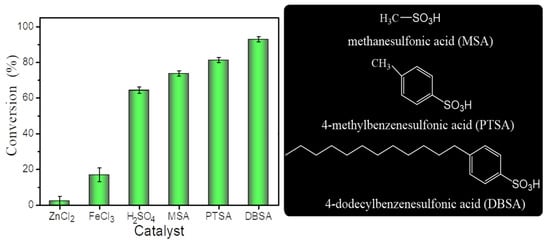
Figure 2.
Effect of catalyst on esterification. Reaction conditions: a reaction temperature of 40 °C, 10 mol% of cetyl alcohol, a molar ratio of 1.5:1 oleic acid to alcohol and a reaction time of 4 h.
2.3. Effect of Reaction Parameters
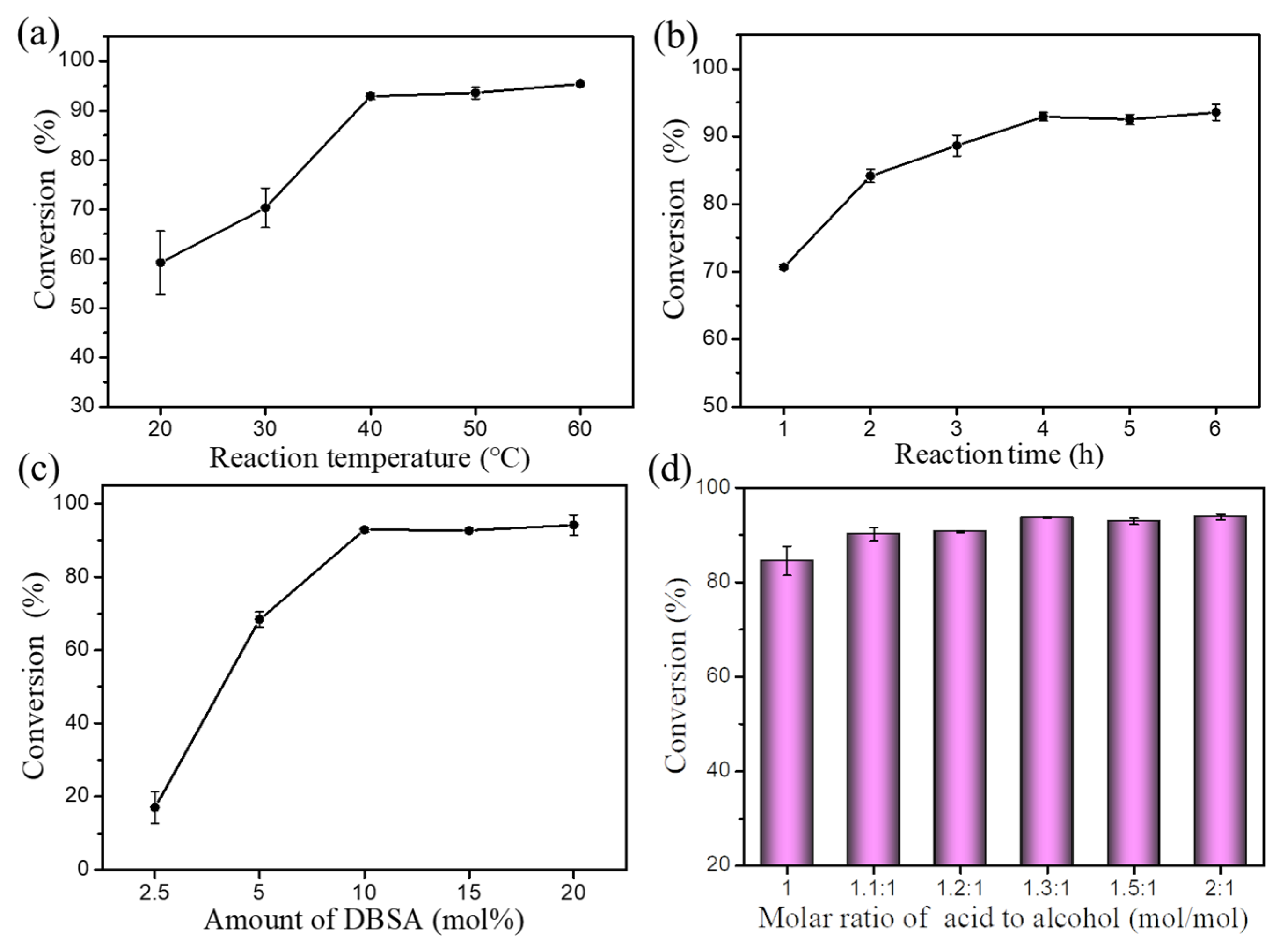
The effects of reaction parameters, such as reaction temperature, reaction time, amount of catalyst (DBSA) and the molar ratio of oleic acid to cetyl alcohol, on esterification were investigated systematically (Figure 3). The reaction temperature is a very important parameter in the esterification of oleic acid and cetyl alcohol. Thus, the effect of reaction temperature was studied and the results are displayed in Figure 3a. It was evident that the conversion of cetyl alcohol increased rapidly (59.2–92.9%) with increasing temperature from 20 to 40 °C. Continuing to increase the reaction temperature (50–60 °C) had no significant effect. Therefore, a reaction temperature of 40 °C was selected for subsequent investigations.
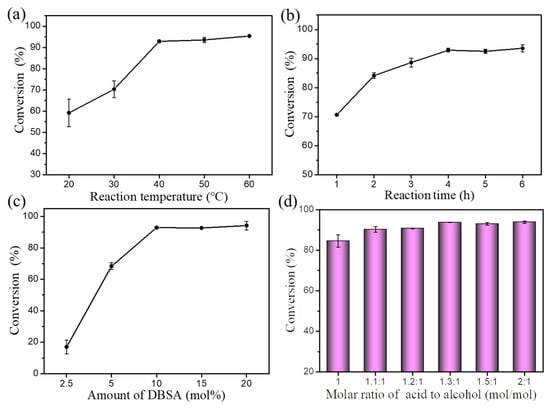
Figure 3.
Effects of (a) reaction temperature, (b) reaction time, (c) amount of DBSA, and (d) molar ratio of oleic acid to alcohol on esterification. Reaction conditions: (a) a molar ratio of 1.5:1 oleic acid to alcohol, 10 mol% DBSA, and a reaction time of 4 h; (b) a reaction temperature of 40 °C, a molar ratio of 1.5:1 oleic acid to alcohol, and 10 mol% DBSA; (c) a reaction temperature of 40 °C, a molar ratio of 1.5:1 oleic acid to alcohol, and a reaction time of 4 h; (d) a reaction temperature of 40 °C, a reaction time of 4 h, and 10 mol% DBSA.
Reaction time is another important parameter that affects esterification. Thus, the effect of reaction time was studied (Figure 3b). The results showed that the conversion of cetyl alcohol increased with prolonged reaction time and a high conversion rate of cetyl alcohol (92.9%) was obtained after 4 h. However, further prolonging the reaction time (5–6 h) had no significant effect on conversion. Therefore, an optimum reaction time of 4 h was used in the following experiments.
Amount of catalyst (DBSA) is also one of the most important factors affecting the conversion rate of cetyl alcohol. Therefore, a suitable amount of catalyst is required for this transformation (Figure 3c). As the amount of DBSA increased (2.5–10 mol%), the conversion of cetyl alcohol increased (17.0–92.9%). However, further increasing the amount of DBSA (15–20 mol%) had no significant effect on the conversion of cetyl alcohol. Thus, 10 mol% of DBSA was selected as the optimum amount of catalyst for esterification.
The molar ratio of substrates also plays an important role in esterification. Experiments were conducted at different molar ratio of substrates (1:1–2:1) to investigate the effect on esterification (Figure 3d). The results showed that the conversion of cetyl alcohol increased (84.5–93.7%) when the molar ratio of oleic acid to cetyl alcohol increased (1:1–1.3:1). However, excess use of oleic acid (1.5:1–2:1) led to no increase in conversion of cetyl alcohol. In detail, the conversion rate of cetyl alcohol reached a maximum (93.6%) when the molar ratio of oleic acid to cetyl alcohol was 1.3:1. Notably, excess oleic acid did not only dilute the concentration of substrates, but also affected subsequent separation and purification of final products. Therefore, 1.3:1 was selected as the optimum molar ratio of oleic acid to cetyl alcohol.
2.4. Effect of the Water Content on Esterification
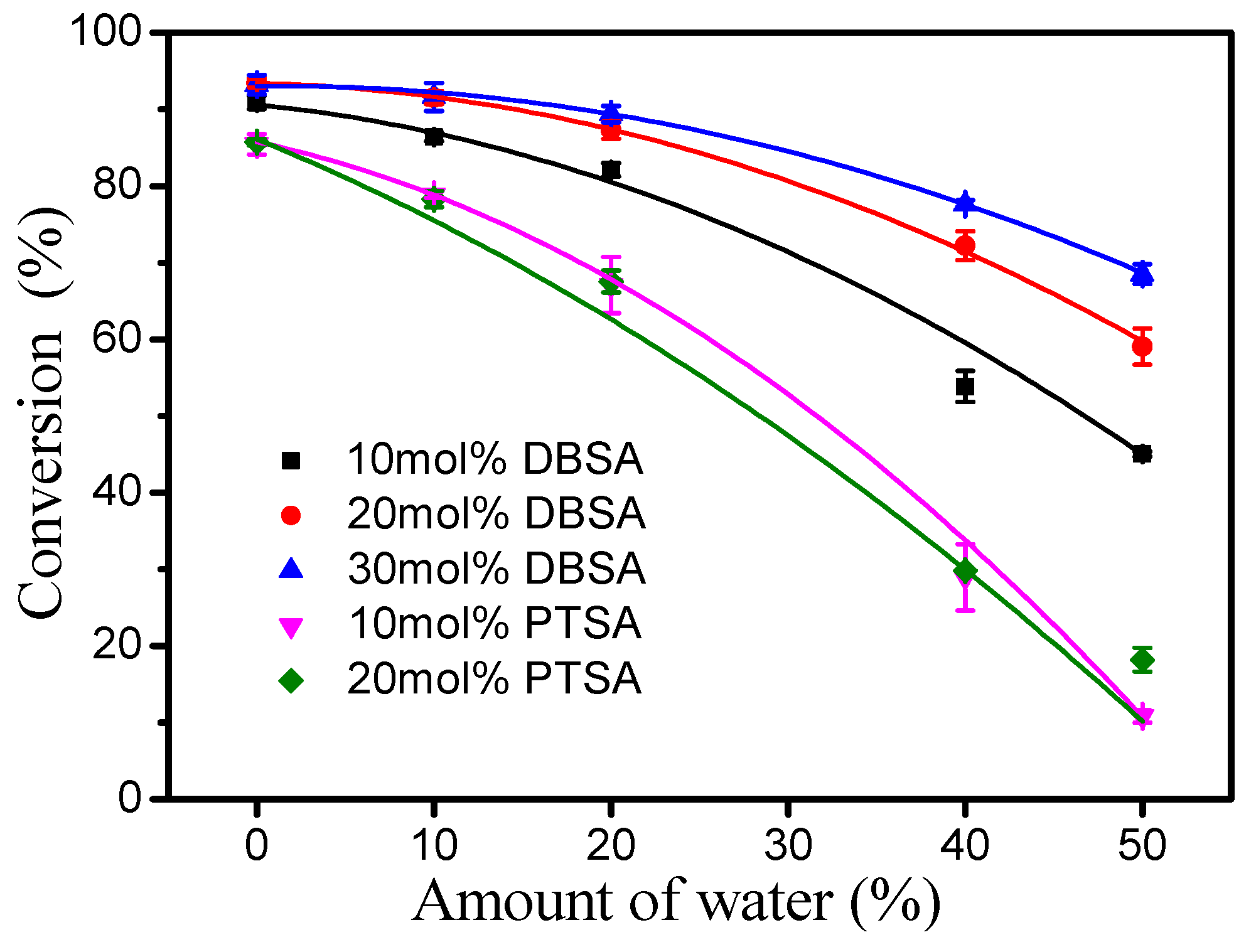
Esterification is a reversible reaction process and one of its products is water, so that water in reactants may affect esterification. Therefore, the effect of water on the esterification of oleic acid (from high-oleic sunflower oil) in the presence of DBSA and PTSA was evaluated under a reaction temperature of 40 °C, a molar ratio of 1.3:1 fatty acid to alcohol and a reaction time of 4 h (Figure 4). In general, the higher the initial water proportion in the reaction mixture, the slower the reaction rate. Interestingly, as the amount of DBSA increased (10–30 mol%), the inhibitory effect of water on the reaction decreased. For comparison, the inhibitory effect of water on the reaction did not increase with an increase in the amount of PTSA from 10 to 20 mol%. This phenomenon may be due to the higher solubility of PTSA in water than DBSA, which is an amphiphilic molecule [17].
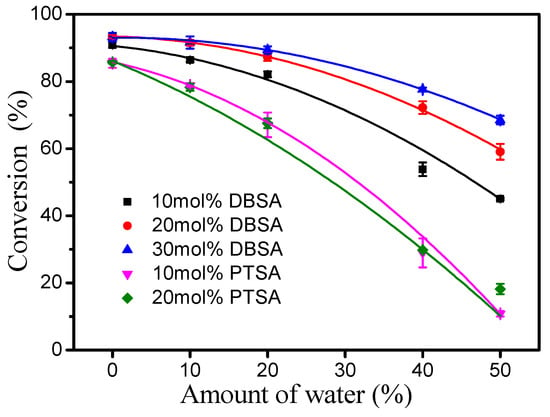
Figure 4.
Effect of water content on the esterification of oleic acid using DBSA or PTSA. Reaction conditions: a reaction temperature of 40 °C, a molar ratio of 1.3:1 oleic acid to alcohol and a reaction time of 4 h.
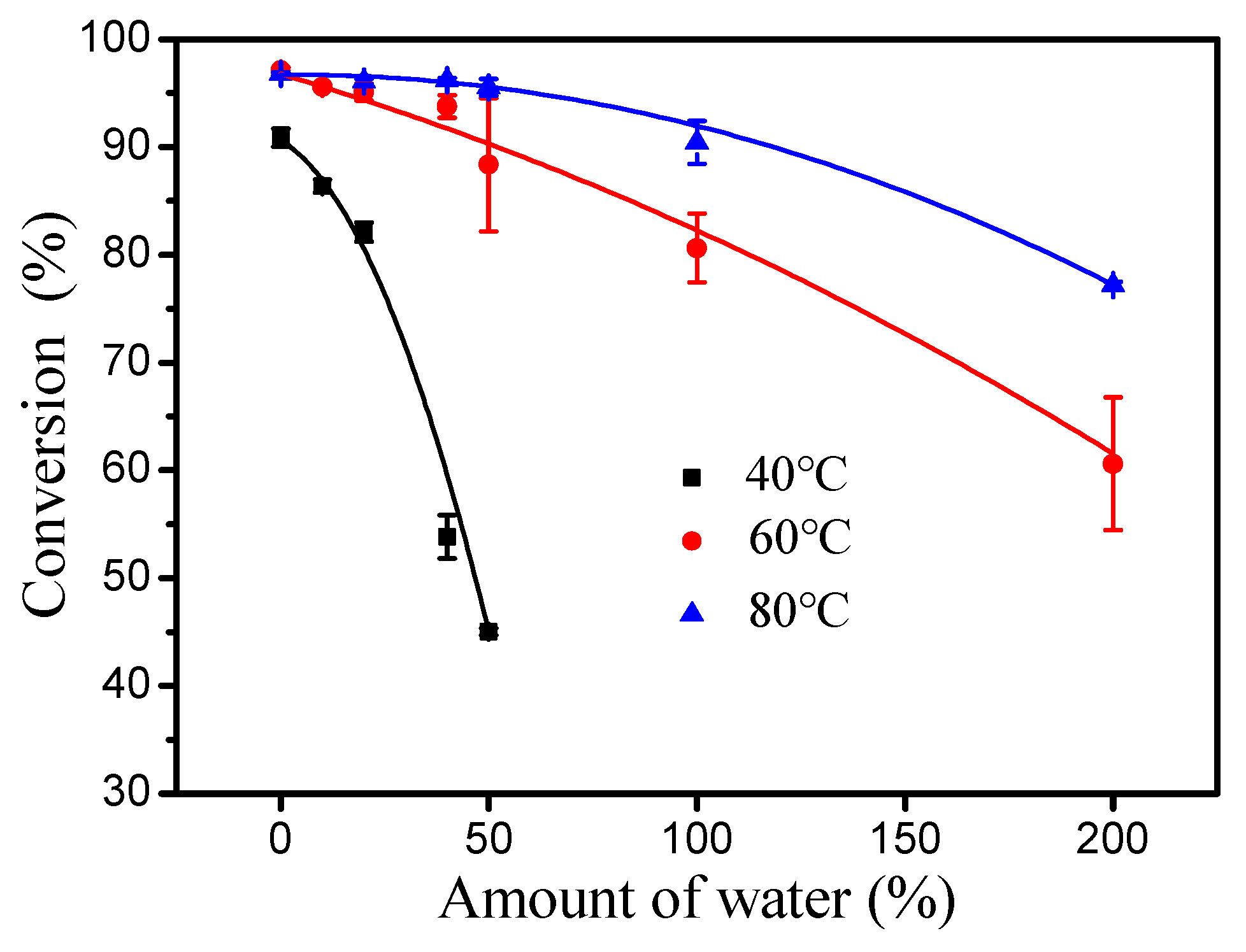
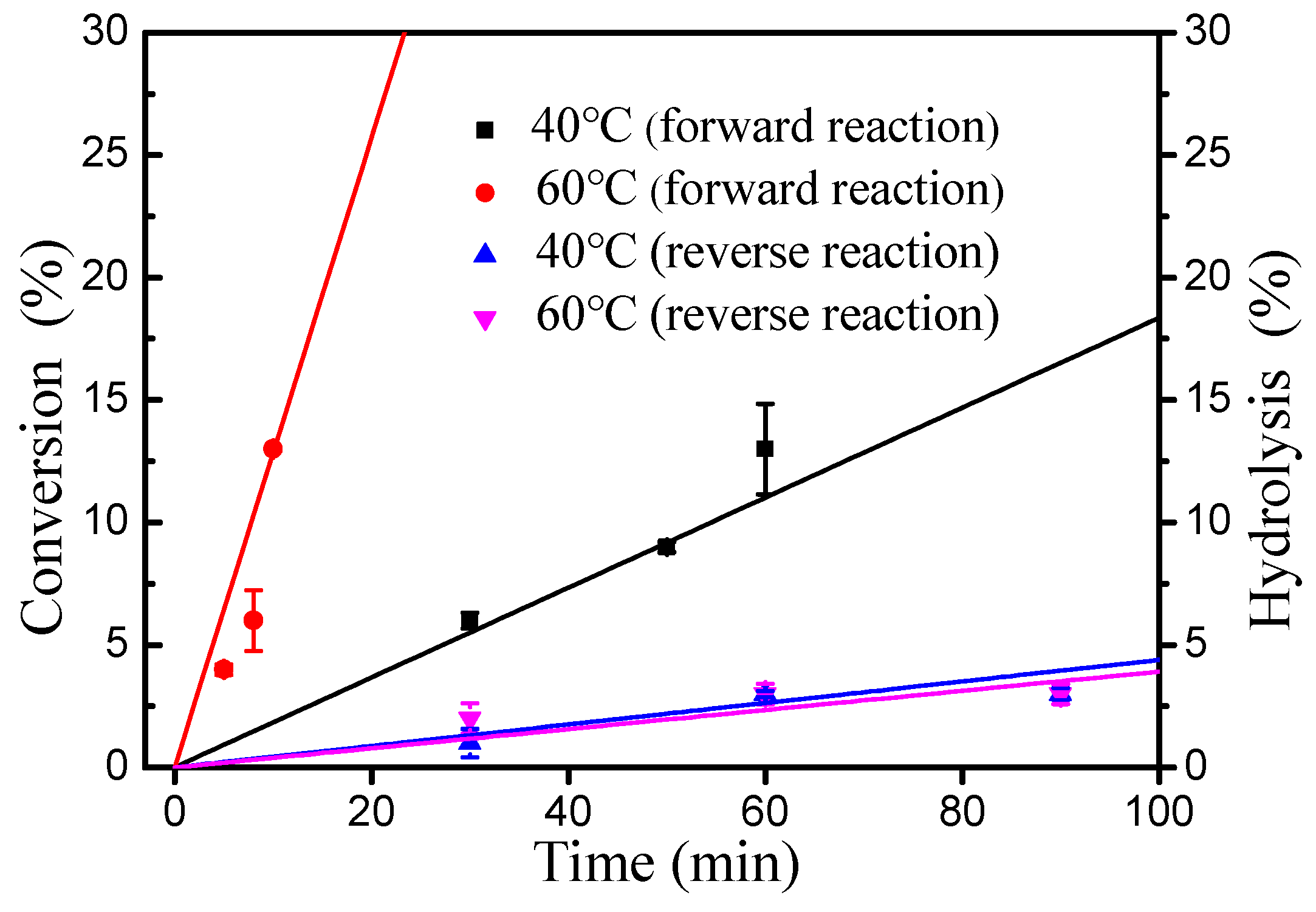
As shown in Figure 5, the inhibitory effect of water to esterification decreased significantly with increasing reaction temperature (40–80 °C). Importantly, introducing an extra 50% water had no effect on the esterification of oleic acid and cetyl alcohol at 80 °C. Thus, the rate of the hydrolysis of wax ester under water is very important for esterification [18]. The initial rates of esterification at 40 and 60 °C were evaluated respectively (Figure 6). At 60 °C, it was found that the forward reaction was 32-fold faster than the reverse reaction (hydrolysis of ester) and 7.1-fold faster than at 40 °C (forward reaction at 60 °C, 1.28 M min−1; reverse reaction at 60 °C, 0.04 M min−1; forward reaction at 40 °C, 0.18 M min−1; reverse reaction at 40 °C, 0.04 M min−1). These results clearly demonstrate that the increase in temperature is favorable for the positive reaction of esterification.
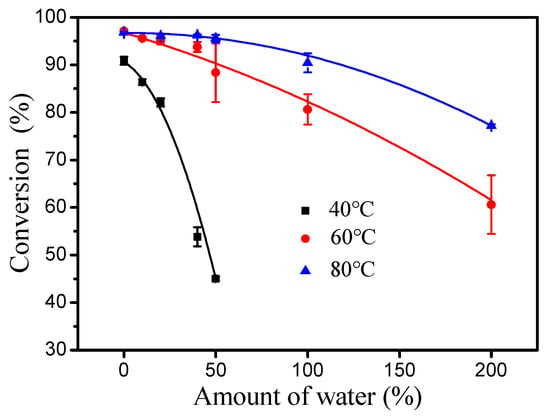
Figure 5.
Effect of water content on esterification with DBSA at different temperatures. Reaction conditions: a molar ratio of 1.3:1 oleic acid to alcohol, a reaction time of 4 h and 10 mol% DBSA of cetyl alcohol.
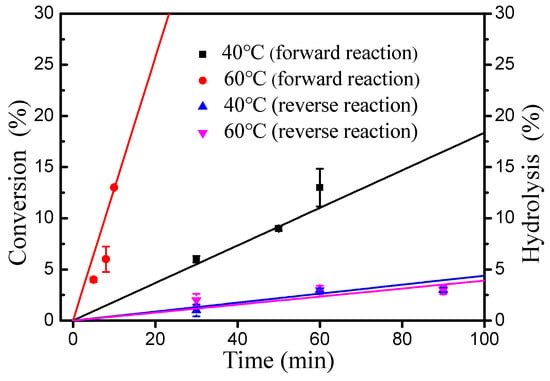
Figure 6.
Initial rates of esterification of oleic acid with DBSA as catalyst for the forward and reverse reactions in water. Reaction conditions: for the forward reaction: a molar ratio of 1.3:1 oleic acid to alcohol, 10 mol% DBSA of cetyl alcohol and 100 wt% water of cetyl alcohol; For reverse reaction: 100 wt% water of wax ester and 10 mol% DBSA of wax esters.
2.5. Mechanism of Wax Esters Synthesis with DBSA Catalyst
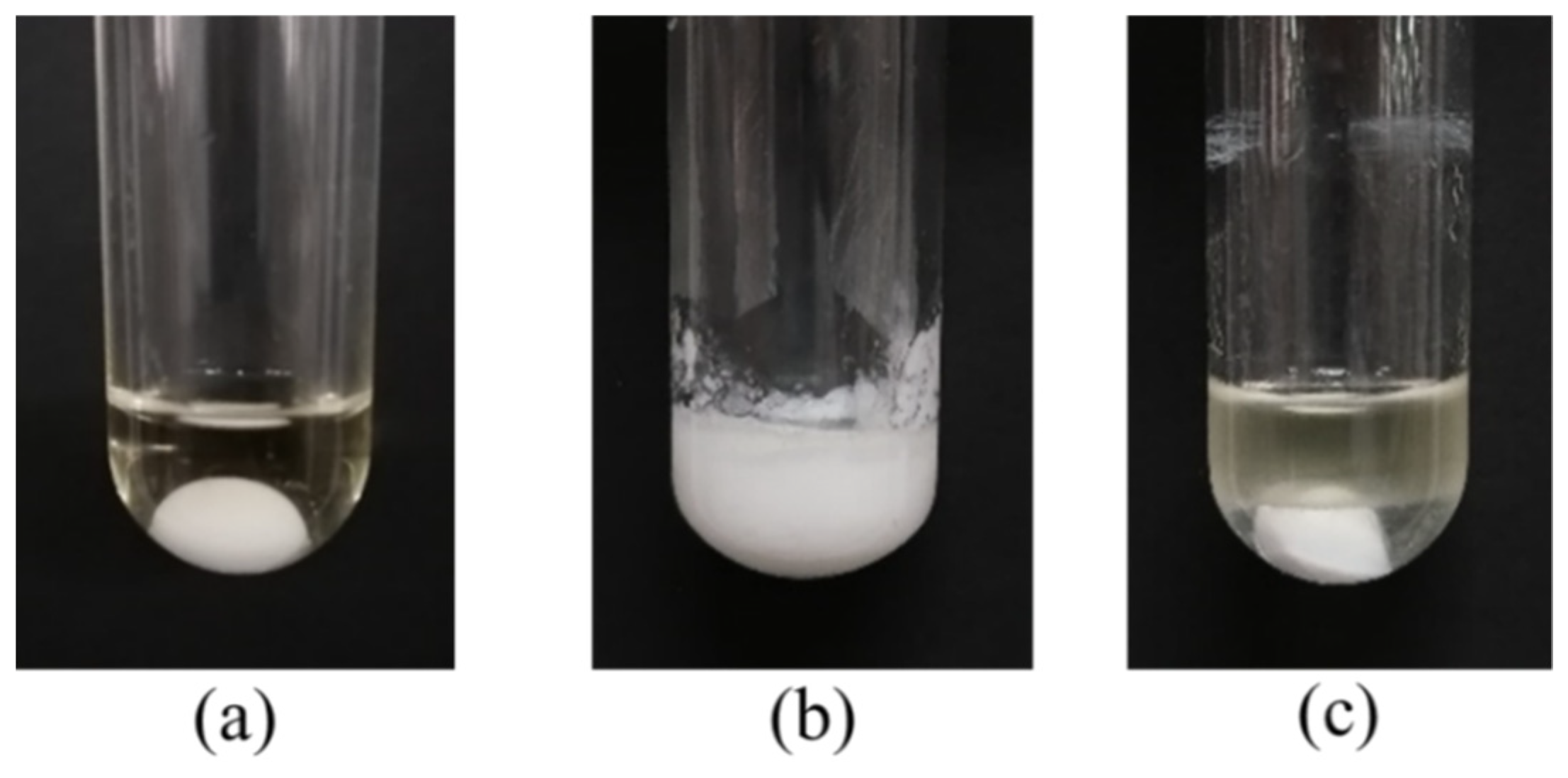
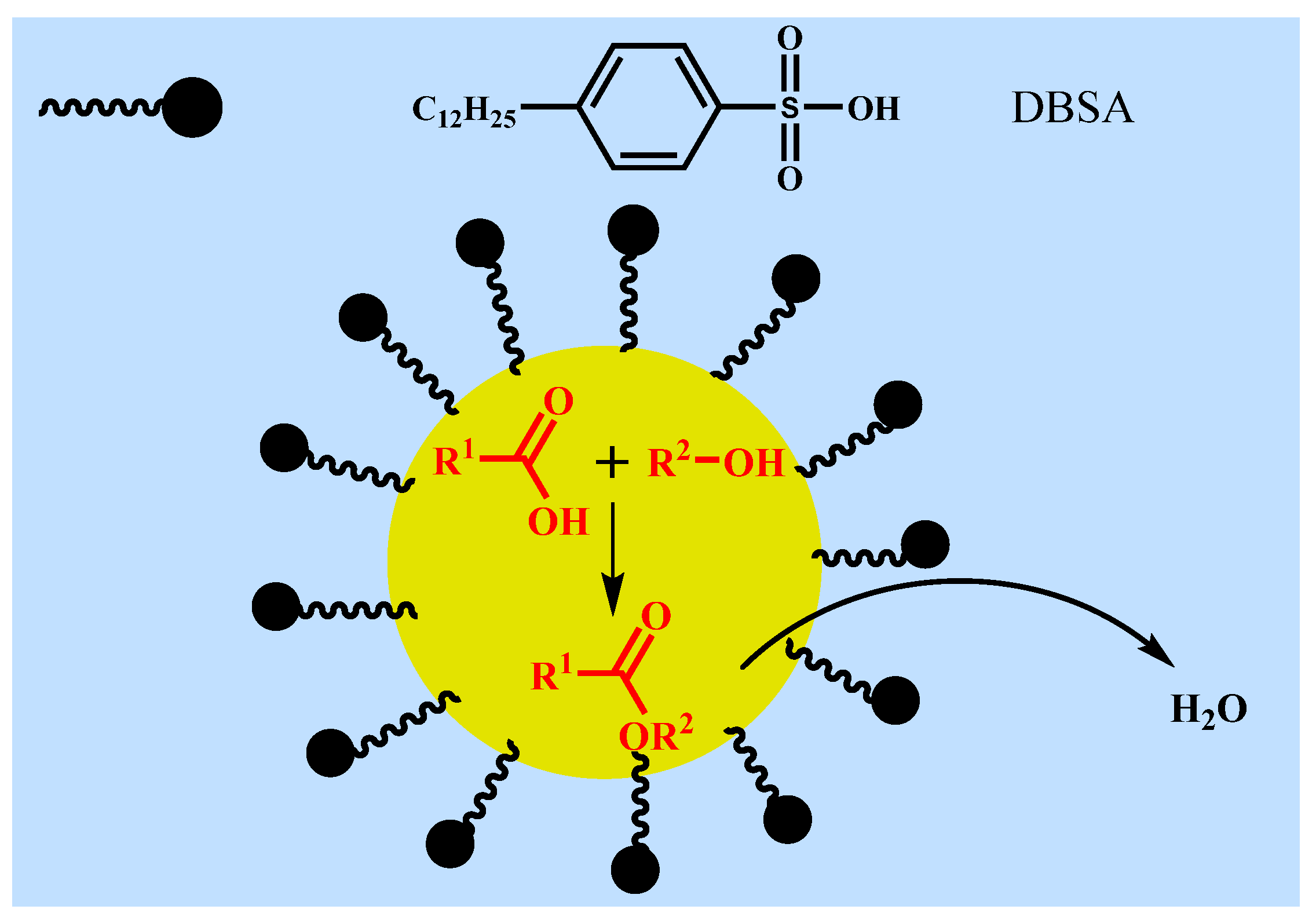
In fact, small amount of water will be produced and exist in the reaction mixture during esterification at such mild temperature (40 °C). The above experimental results showed that the catalytic effect of DBSA in water was significantly better than that of traditional acid catalysts (e.g., PTSA), and the inhibitory effect of water to DBSA decreases with increasing temperature, which may be related to the formation of O/W-type emulsion of DBSA in water. In order to further verify the mechanism of DBSA-catalyzed esterification, the effect of salt solution on esterification was studied (Figure 7). According to Figure 7a, when there was no water, the reaction system was homogeneous and transparent liquid. When 100% water was added (Figure 7b), the reaction system formed a white turbid emulsion, which was consistent with the speculation of Manabe et al. [16]. However, when the same amount of salt solution (saturated CaCl2 solution) was added (Figure 7c), the reaction system showed obvious stratification phenomenon, which indicated that inorganic salt (e.g., CaCl2) played a demulsifying effect [19]. Thus, DBSA indeed exhibited as a surfactant-combined acid catalyst, and the water generated by esterification will be removed from the reaction system due to its internal hydrophobicity (Figure 8). Through formation of emulsion of DBSA, esterification can take place smoothly at such mild temperature (40 °C) without removing the side-product water by special treatments (e.g., azeotropic distillation). During DBSA-catalyzed esterification, auto-isolation of water is the most important driving force of esterification [20].
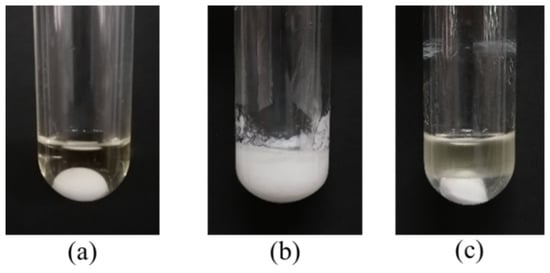
Figure 7.
Phenomenon of DBSA-catalyzed esterification of oleic acid with cetyl alcohol. (a): no water added, (b): 100% water added, (c): saturated CaCl2 solution added. Reaction conditions: a reaction temperature of 60 °C, DBSA 10 mol% DBSA, a molar ratio of 1.3:1 oleic acid to alcohol, a reaction time of 4 h and 100 wt% water of cetyl alcohol.
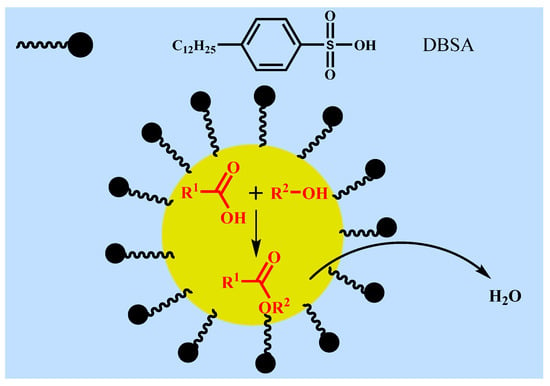
Figure 8.
Proposed mechanism of the DBSA-catalyzed esterification.
2.6. Comparison of Different Acyl Donors in DBSA-Catalyzed Reactions
Under optimum conditions (40 °C, 4 h, 10 mol% DBSA, and a molar ratio of 1.3:1 fatty acid to alcohol), the effect of DBSA on the esterification or transesterification of cetyl alcohol with different acyl donors (fatty acid or fatty acid glycerides) was also investigated (Table 1). The results showed that there were no significant differences in the catalytic activity of DBSA for the esterification of oleic acid from three different sources (84.4% purity prepared from high-oleic sunflower oil, 80% purity purchased commercially, 99% purity from Sigma-Aldrich, St. Louis, MO, USA) with cetyl alcohol, and the conversion rate was 93.3%, 93.7% and 94.0%, respectively. However, under the same conditions (40 °C), DBSA-catalyzed transesterification of high-oleic sunflower oil (fatty acid glycerides) with cetyl alcohol led to a low conversion rate 11.4%. In most cases, the reaction rate of acid-catalyzed esterification is faster than that of transesterification (reaction between cetyl alcohol and sunflower oil). Based on the above results, it was concluded that DBSA-catalyzed esterification for wax ester production was more favorable at a low reaction temperature (40 °C). Furthermore, at an increased reaction temperature (80 °C), the conversion rate of transesterification increased from 11.4% to 78.0% (Table 1). In general, DBSA-catalyzed esterification can take place at a low reaction temperature (40 °C) under solvent-free conditions.

Table 1.
DBSA-catalyzed reactions of cetyl alcohol with different acyl donors.
To identify the water tolerance of this low-temperature esterification, DBSA-catalyzed esterification was performed in a closed test tube and an open test tube, respectively (Table 2). The results showed that there were no significant differences in the conversion rates of the open test tube reaction and closed test tube reaction (92.4% vs. 93.7%), which indeed proved the inhibitory effect of water on DBSA-catalyzed esterification for rapid wax ester production.

Table 2.
Effect of different condition for esterification.
2.7. Analysis and Separation of Cetyl Oleate Wax Ester
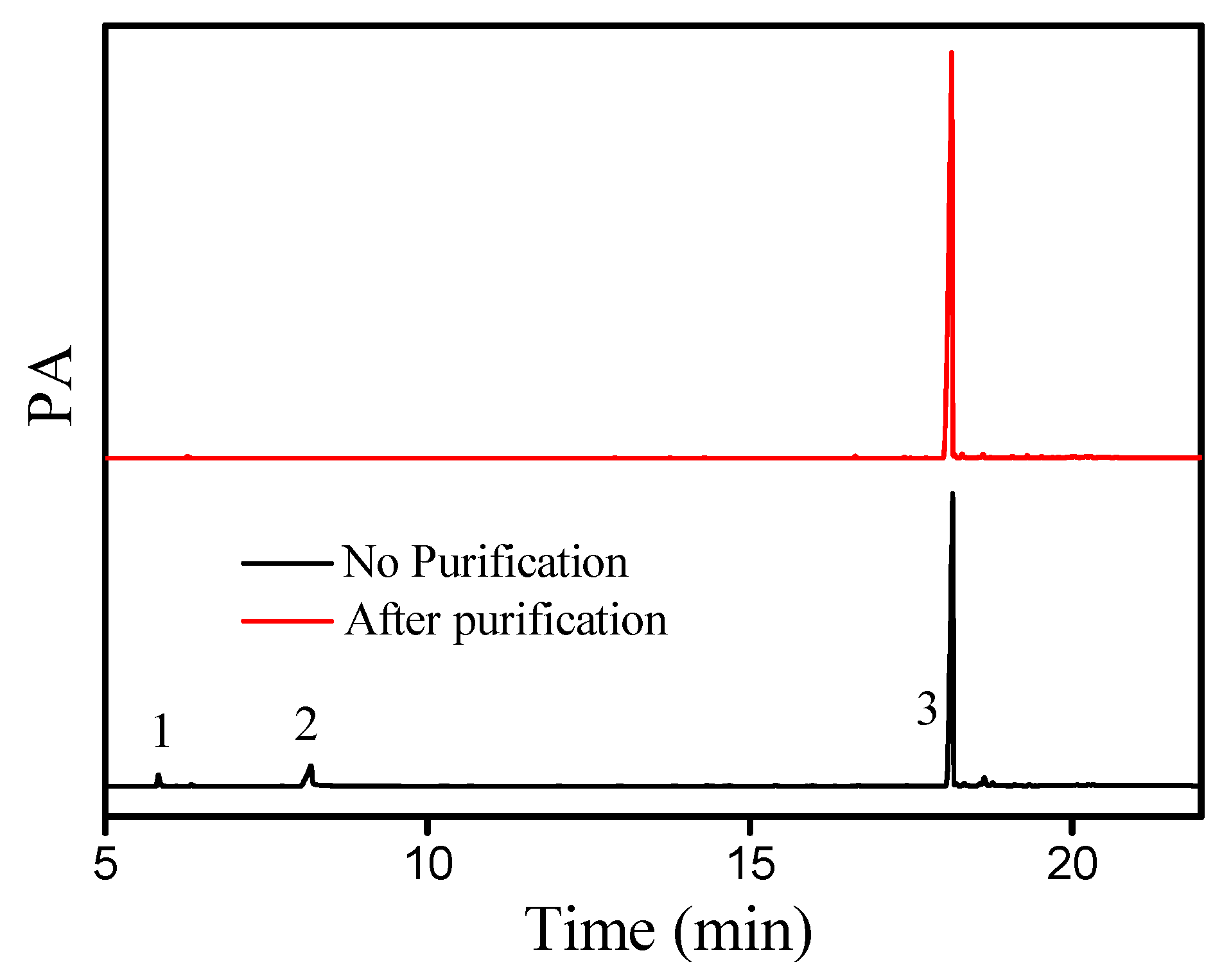
The gas chromatogram (GC) of the liquid wax ester (cetyl oleate) synthesized under optimal conditions is shown in Figure 9. In the presence of DBSA, no side products were observed after the esterification of oleic acid and cetyl alcohol. After purification by silica gel column chromatography (Figure 9), the cetyl oleate could be obtained as a light-yellow liquid, with a yield of 92.1% and a purity of 99%. Additionally, the chemical structure of pure cetyl oleate was identified by NMR (for more details, see Section 3.6).
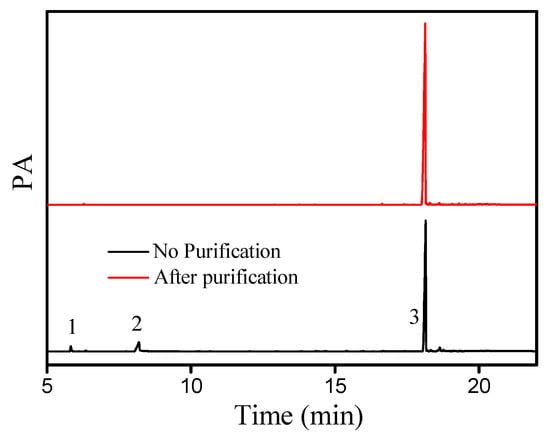
Figure 9.
Gas chromatogram of cetyl oleate after esterification. (1) Cetyl alcohol, (2) oleic acid, (3) and cetyl oleate.
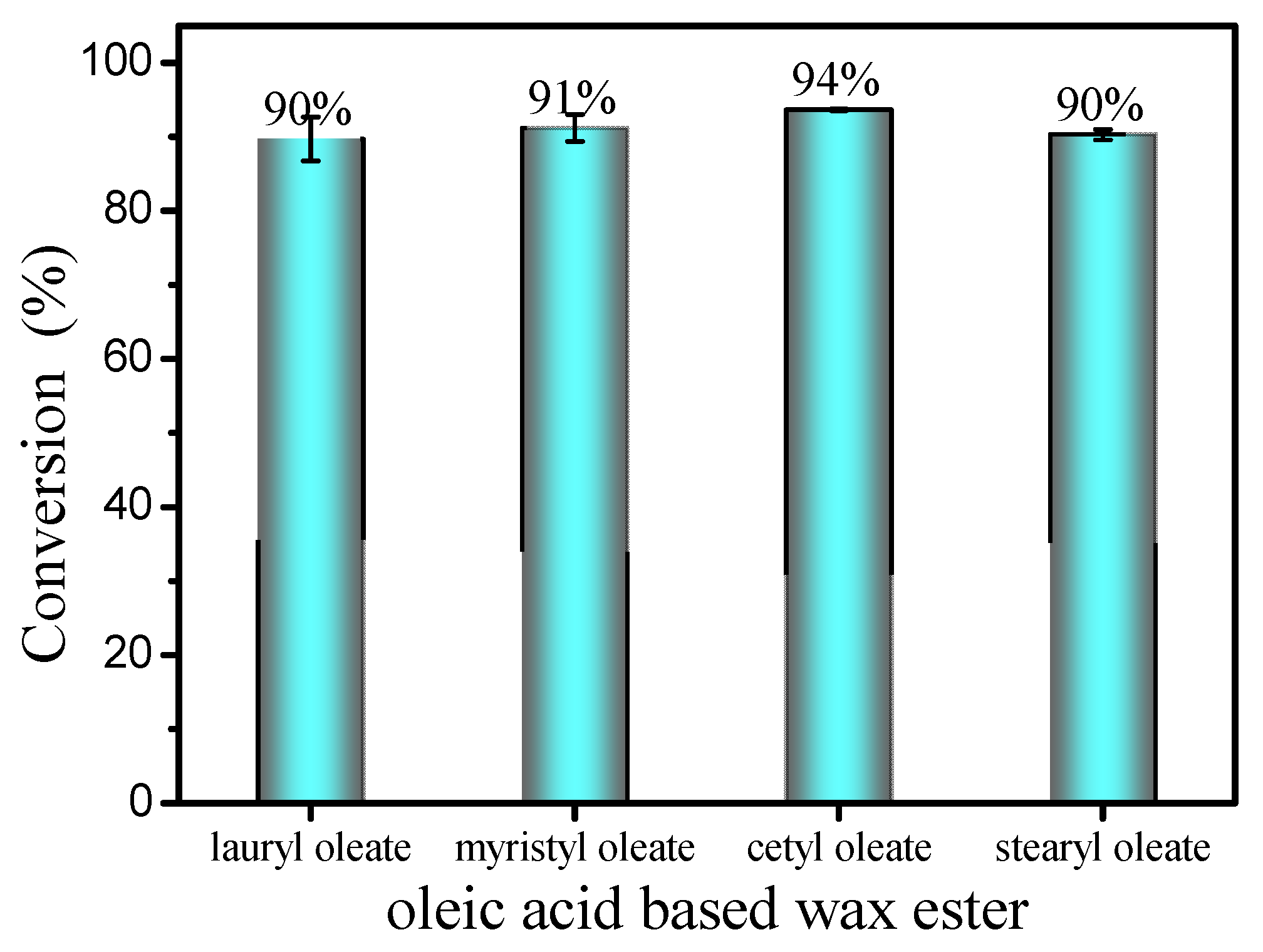
2.8. Synthesis of Different Oleic Acid-Based Wax Esters
The substrate scope for wax esters production was investigated under optimum reaction conditions (Figure 10). Esterification of oleic acid (99% purity) and various long-chain fatty alcohols (C12–C18) was conducted. Interestingly, esterification shows excellent conversion rates (90.0–94.0%) for various long-chain fatty alcohols, including lauryl alcohol, myristyl alcohol and stearyl alcohol. After purification by silica gel column chromatography, various liquid wax esters, including lauryl oleate, myristyl oleate and stearyloleate, were obtained with a purity of up to 99%.
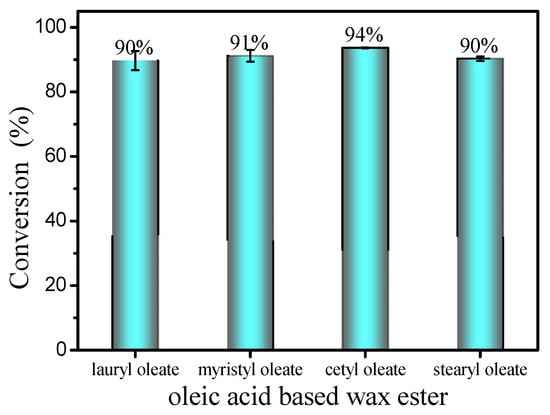
Figure 10.
Esterification of oleic acid with different long-chain alcohols. Reaction conditions: a reaction temperature of 40 °C, 10 mol% DBSA, a molar ratio of 1.3:1 fatty acids to alcohol and a reaction time of 4 h.
3. Materials and Methods
3.1. Materials
Cetyl alcohol (99%) and oleic acid (80%) were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). 4-dodecylbenzene sulfonic acid (DBSA, 95%) and oleic acid (99%) were obtained from Sigma Co. Ltd. (Shanghai, China). Zinc chloride (ZnCl2, 99%) and p-toluenesulfonic acid (PTSA, 98%) were purchased from Macklin Biochemical Co. Ltd. (Shanghai, China). Ferric Chloride (FeCl3, 98%) was obtained from Alfa Aesar Chemical Co. Ltd. (Tianjin, China). Methanesulfonic acid (MSA), sodium chloride (NaCl) and calcium chloride (CaCl2) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). Lauryl alcohol (99%), myristyl alcohol (98%) and stearyl alcohol (98%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). High-oleic sunflower oil was purchased from a local supermarket. N-hexane was of HPLC grade, and other chemicals were of analytical reagent grade.
3.2. Production of Fatty Acids
Sodium hydroxide (20 g) and ethanol (200 mL) were added into a 1 L three-neck flask, and stirred at 80 °C until sodium hydroxide dissolved. Then, 100 g of high-oleic sunflower seed oil was added into the flask and reacted for 3 h. After the reaction, a trace amount of unreacted sunflower seed oil was washed off by petroleum ether, and the soap was obtained after centrifugation. Then, the soap was stirred with sulfuric acid solution (3 mol/L) until two phases were formed. The upper layer was reserved, washed and dried to obtain the desired fatty acids.
3.3. Synthesis of Wax Esters
An amount of 1.5 mmol of fatty acid (0.42 g), 1.0 mmol of cetyl alcohol (0.24 g) and the catalyst (0.1 mmol, 10 mol% of cetyl alcohol) were added into the reaction tube. Then, the mixture was heated with stirring in an aluminum heating block at 40 °C for 4 h. After the reaction, the mixture was quenched with saturated NaHCO3(aq.). The product was extracted with n-hexane, washed with brine, dried over Na2SO4, concentrated, and then analyzed by gas chromatography (GC).
3.4. Determination of Wax Esters
A GC-7890B gas chromatography (Agilent) equipped with a DB-1ht capillary column (28 m × 250 μm × 0.1 μm) and a flame ionizing detector (FID) were used analyze the wax esters product. The column temperature was programmed at 100 °C, kept for 0 min, increased to 180 °C at a rate of 10 °C·min−1, maintained for 2 min; then increased to 230 °C at 10 °C·min−1; finally, elevated to 330 °C at 20 °C·min−1, and kept for 2 min. The injector and detector temperature were set at 350 and 360 °C, respectively.
The content of the product was quantified with hexadecane as the internal standard. The residual of cetyl alcohol is calculated by the equation: , where is the mass of component or internal standard, is area for component or internal standard and is the response factor for component or internal standard ( and ). The conversion rate of cetyl alcohol was calculated by the equation as follows.
where is the total mass of cetyl alcohol.
3.5. Purification of Wax Esters Product
As a typical example, the reaction mixture resulting from the esterification of oleic acid with cetyl alcohol contains a mixture of cetyl oleate and residual substrates. The purification of cetyl oleate was carried out by column chromatography using silica gel (200–300 mesh), eluting with hexane/diethylether mixtures (80:20, v/v). Finally, pure cetyl oleate was obtained as a liquid.
3.6. Characterization of Purified Wax Esters
The isolated products (liquid wax esters) of the esterification of oleic acid with various long-chain fatty alcohols were identified by NMR (see 1H NMR and 13C NMR patterns of products in Supplementary Materials).
Analytical data of oleic acid-based wax esters by NMR are listed as follows:
Lauryl Oleate: Light yellow liquid. Yield: 90%. 1H NMR (500 MHz, CDCl3): δ = 0.86 (br t, 6 H), 1.26 (br d, 38 H), 1.58–1.62 (br m, 4 H), 2.00 (br t, 4 H), 2.28 (br t, 2 H), 4.05 (tr, J = 10 Hz, 2 H), 5.33–5.35(br m, 2 H). 13C NMR (125 MHz, CDCl3): δ = 14.24, 22.83, 25.16, 26.09, 27.31, 27.36, 28.81, 29.26, 29.28, 29.32, 29.40, 29.47, 29.49, 29.67, 29.72, 29.78, 29.79, 29.84, 29.91, 32.06, 34.55, 64.54, 129.89, 130.13, 174.10.
Myristyl Oleate: Light yellow liquid. Yield: 89%. 1H NMR (500 MHz, CDCl3): δ = 0.86 (br t, 6 H), 1.26 (br d, 42 H), 1.58–1.62 (br m, 4 H), 2.00 (br t, 4 H), 2.28 (br t, 2 H), 4.05 (tr, J = 10 Hz, 2 H), 5.33–5.35(br m, 2 H). 13C NMR (125 MHz, CDCl3): δ = 14.25, 22.83, 25.17, 26.09, 27.31, 27.37, 28.81, 29.26, 29.29, 29.32, 29.41, 29.47, 29.51, 29.68, 29.73, 29.82, 29.84, 29.92, 32.05, 32.07 34.55, 64.54, 129.89, 130.13, 174.11.
Cetyl Oleate: Light yellow liquid. Yield: 92%. 1H NMR (500 MHz, CDCl3): δ = 0.86 (br t, 6 H), 1.26 (br d, 46 H), 1.62 (br t, 4 H), 2.00 (br t, 4 H), 2.28 (br t, 2 H), 4.05 (tr, J = 10 Hz, 2 H), 5.33–5.35(br m, 2 H). 13C NMR (125 MHz, CDCl3): δ = 14.25, 22.84, 25.17, 26.09, 27.32, 27.37, 28.82, 29.26, 29.29, 29.32, 29.41, 29.47, 29.51, 29.68, 29.73, 29.81, 29.84, 29.92, 32.08, 34.55, 64.54, 129.89, 130.13, 174.10.
Stearyl Oleate: Light yellow liquid. Yield: 91%. 1H NMR (500 MHz, CDCl3): δ = 0.86 (br t, 6 H), 1.26 (br d, 50 H), 1.62 (br t, 4 H), 2.00 (br t, 4 H), 2.28 (br t, 2 H), 4.05 (tr, J = 10 Hz, 2 H), 5.33–5.35(br m, 2 H). 13C NMR (125 MHz, CDCl3): δ = 14.25, 22.84, 25.17, 26.10, 27.32, 27.37, 28.82, 29.26, 29.29, 29.33, 29.41, 29.48, 29.52, 29.68, 29.74, 29.82, 29.85, 29.92, 32.06, 32.08, 34.55, 64.54, 129.89, 130.13, 174.09.
3.7. Data Analysis
All the experiments were replicated three times, and the results are expressed as the mean ± standard deviation (SD). The Design Expert 10.0 software was used in the analyses of data. The analysis of variance (ANOVA) was used to determine the optimal reaction conditions, and the significance of each influential factor was p < 0.05.
4. Conclusions
In this work, 4-dodecylbenzenesulfonic acid (DBSA) has been successfully used as an acid catalyst to synthesize oleic acid-based wax esters (e.g., cetyl oleate) through esterification of long-chain fatty alcohols (C12–C18) with oleic acid (prepared from high-oleic sunflower oil). It was found that the conversion rate increased significantly with the increasing hydrophobicity of the acid catalysts. Additionally, it was found that the inhibitory effect of water on DBSA-catalyzed esterification was weaker than that of PTSA, and the inhibitory effect also decreased rapidly with increasing temperature. At a reaction temperature of 40 °C, DBSA-catalyzed esterification could be applied in the production of various oleic acid-based wax esters with excellent yields under solvent-free conditions. DBSA-catalyzed esterification provides a mild and effective method for preparing liquid wax esters as jojoba oil analogues.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11121471/s1, 1H and 13C NMR patterns of products.
Author Contributions
Conceptualization, W.L. and G.Y.; funding acquisition, W.L.; investigation, Z.L.; supervision, W.L.; writing—original draft, Z.L.; writing—review and editing, W.L. and G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Postdoctoral Science Foundation Funded Project (2018T110730), the Scientific and Technological Key Project in Henan Province (212102110317) and the Henan University of Technology Excellent Young Teachers Project (2014003).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food. Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, E.C.G.; Papadaki, A.; Mallouchos, A.; Mandala, I.; Sousa, H.; Freire, D.M.G.; Koutinas, A.A. Enzymatic synthesis of bio-based wax esters from palm and soybean fatty acids using crude lipases produced on agricultural residues. Ind. Crop. Prod. 2019, 139, 1–6. [Google Scholar] [CrossRef]
- Li, J.J. Quantitative Analysis of Cosmetics Waxes by Using Supercritical Fluid Extraction (SFE)/Supercritical Fluid Chromatography (SFC) And Multivariate Data Analysis. Chemometr. Intell. Lab. 1999, 45, 385–395. [Google Scholar] [CrossRef]
- Ungcharoenwiwat, P.; H-Kittikun, A. Synthesis of Wax Esters from Crude Fish Fat by Lipase of Burkholderiasp. EQ3 and Commercial Lipases. J. Am. Oil. Chem. Soc. 2013, 90, 59–67. [Google Scholar] [CrossRef]
- Khalkar, S.; Bhowmick, D.; Pratap, A. Effect of Wax Esters as Friction modifiers in petroleum base stock. J. Oleo. Sci. 2012, 61, 723–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bi, Y.C.; Yu, M.; Zhou, H.Y. Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa Lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J. Mol. Catal. B-Enzym. 2016, 133, 1–5. [Google Scholar] [CrossRef]
- Deng, L.; Wang, X.J.; Nie, K.L.; Wang, F.; Liu, J.F.; Wang, P.; Tan, T.W. Synthesis of Wax Esters by Lipase-catalyzed Esterification with Immobilized Lipase from Candida sp. 99–125. Chinese. J. Chem. Eng. 2011, 19, 978–982. [Google Scholar]
- Keng, P.S.; Basri, M.; Zakaria, M.R.S.; Rahman, M.B.A.; Ariff, A.B.; Rahman, R.N.Z.A.; Salleh, A.B. Newly synthesized palm esters for cosmetics industry. Ind. Crop. Prod. 2009, 29, 37–44. [Google Scholar] [CrossRef]
- Al-Arafi, N.; Salimon, J. Production of Oleic Acid Based Wax Ester Using Acidic Homogeneous Catalysts. E-J. Chem. 2012, 9, 99–106. [Google Scholar] [CrossRef]
- Aracil, J.; Martinez, M.; Sánchez, N.; Corma, A. Formation of jojoba oil analog by esterification of oleic acid using zeolite as catalyst. Zeolites 1992, 12, 233–236. [Google Scholar] [CrossRef]
- Ieda, N.; Mantri, K.; Miyata, Y.; Ozaki, A.; Komura, K.; Sugi, Y. Esterification of Long-Chain Acids and Alcohols Catalyzed by Ferric Chloride Hexahydrate. Ind. Eng. Chem. Res. 2008, 47, 8631–8638. [Google Scholar] [CrossRef]
- Aissa, I.; Sellami, M.; Kamoun, A.; Gargouri, Y.; Miled, N. Optimization of Immobilized Lipase-Catalyzed Synthesis of Wax Esters by Response Surface Methodology. Curr. Chem. Biol. 2012, 6, 77–85. [Google Scholar]
- Alegría, A.; Cuellar, J. Esterification of oleic acid for biodiesel production catalyzed by 4-dodecylbenzenesulfonic acid. Appl. Catal. B-Environ. 2015, 179, 530–541. [Google Scholar] [CrossRef]
- Manabe, K.; Sun, X.M.; Kobayashi, S. Dehydration reactions in water. Surfactant-type Brønsted acid-catalyzed direct esterification of carboxylic acids with alcohols in an emulsion system. J. Am. Chem. Soc. 2001, 123, 10101–10102. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, X.Z.; Eli, W. Solvent-free esterification catalyzed by surfactant-combined catalysts at room temperature. New. J. Chem. 2007, 31, 348–351. [Google Scholar]
- Manabe, K.; Iimura, S.; Sun, X.M.; Kobayashi, S. Dehydration Reactions in Water. Brønsted Acid−Surfactant-Combined Catalyst for Ester, Ether, Thioether, and Dithioacetal Formation in Water. J. Am. Chem. Soc. 2002, 124, 11971–11978. [Google Scholar] [CrossRef] [PubMed]
- Aafaqi, R.; Mohamed, A.R.; Bhatia, S. Kinetics of esterification of palmitic acid with isopropanol using p-toluenesulfonic acid and zinc ethanoate supported over silica gel as catalysts. J. Chem. Technol. Biot. 2004, 79, 1127–1134. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; Santos, R.T.P.; Tapanes, N.C.O.; Ramos, A.L.D.; Antunes, O.A.C. Acid-Catalyzed Homogeneous Esterification Reaction for Biodiesel Production from Palm Fatty Acids. Catal. Lett. 2008, 122, 20–25. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, B.; Yang, G.L.; Bi, Y.L.; Chen, F.S. Rapid Salt-Assisted Microwave Demulsification of Oil-Rich Emulsion Obtained by Aqueous Enzymatic Extraction of Peanut Seeds. Eur. J. Lipid Sci. Technol. 2020, 122, 1900120. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, B.; Wang, X.; Chen, J.; Yang, G.L. Solvent-free synthesis of phytosterol linoleic acid esters at low temperature. RSC Adv. 2021, 11, 10738–10746. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).