Structural, Optical, and Catalytic Properties of MgCr2O4 Spinel-Type Nanostructures Synthesized by Sol–Gel Auto-Combustion Method
Abstract
:1. Introduction
2. Results and Discussion
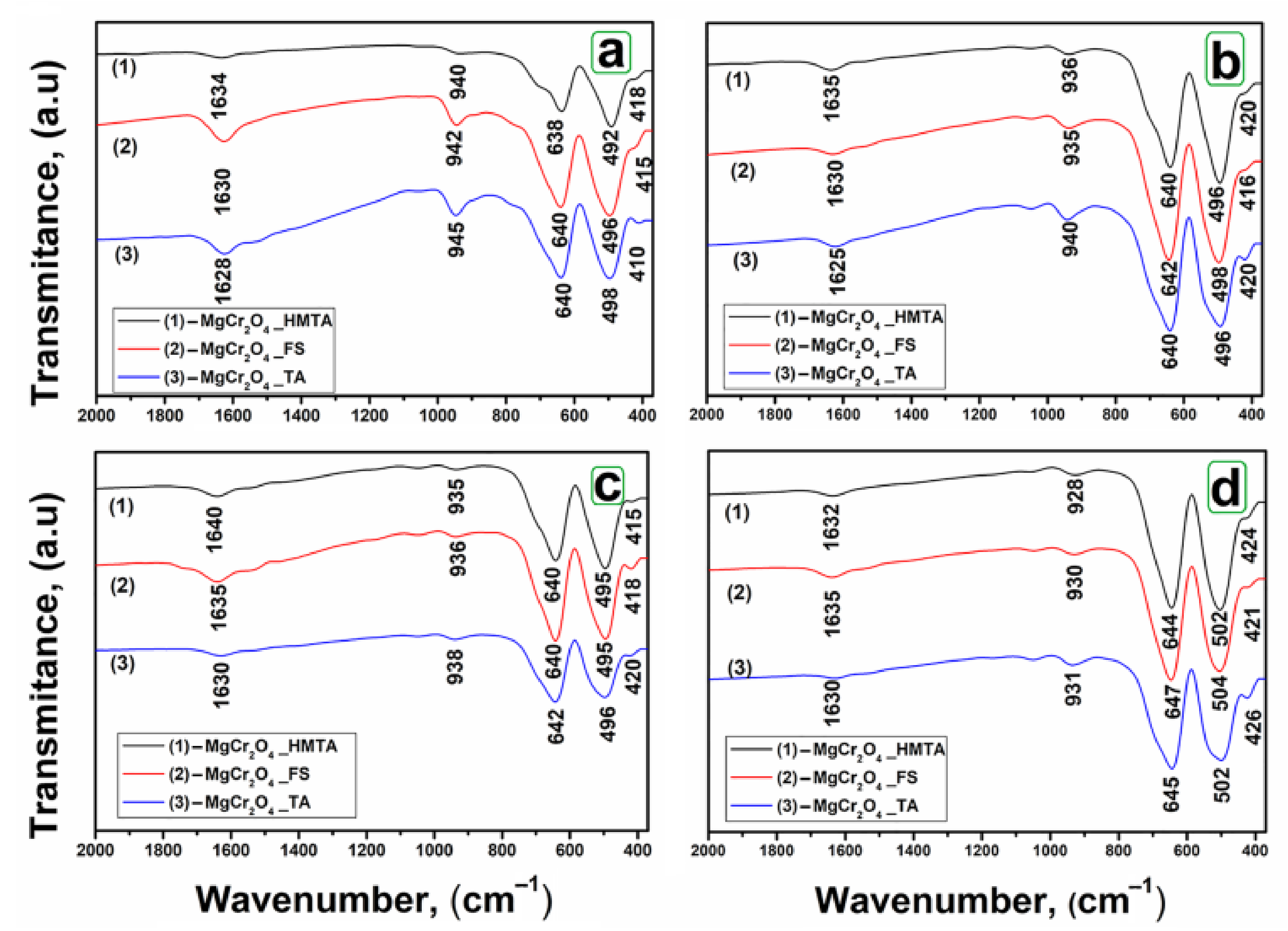
2.1. Infrared Spectroscopy
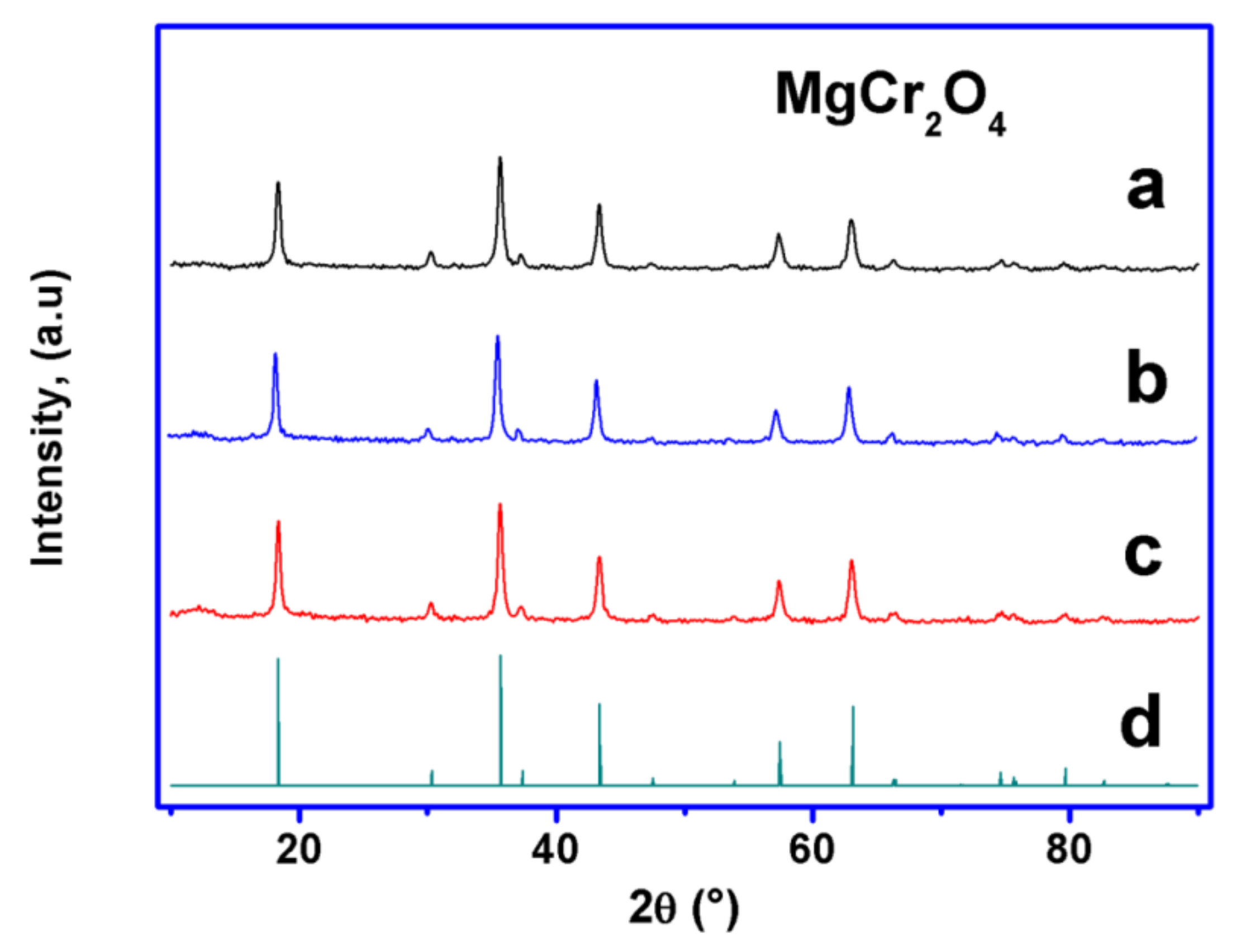
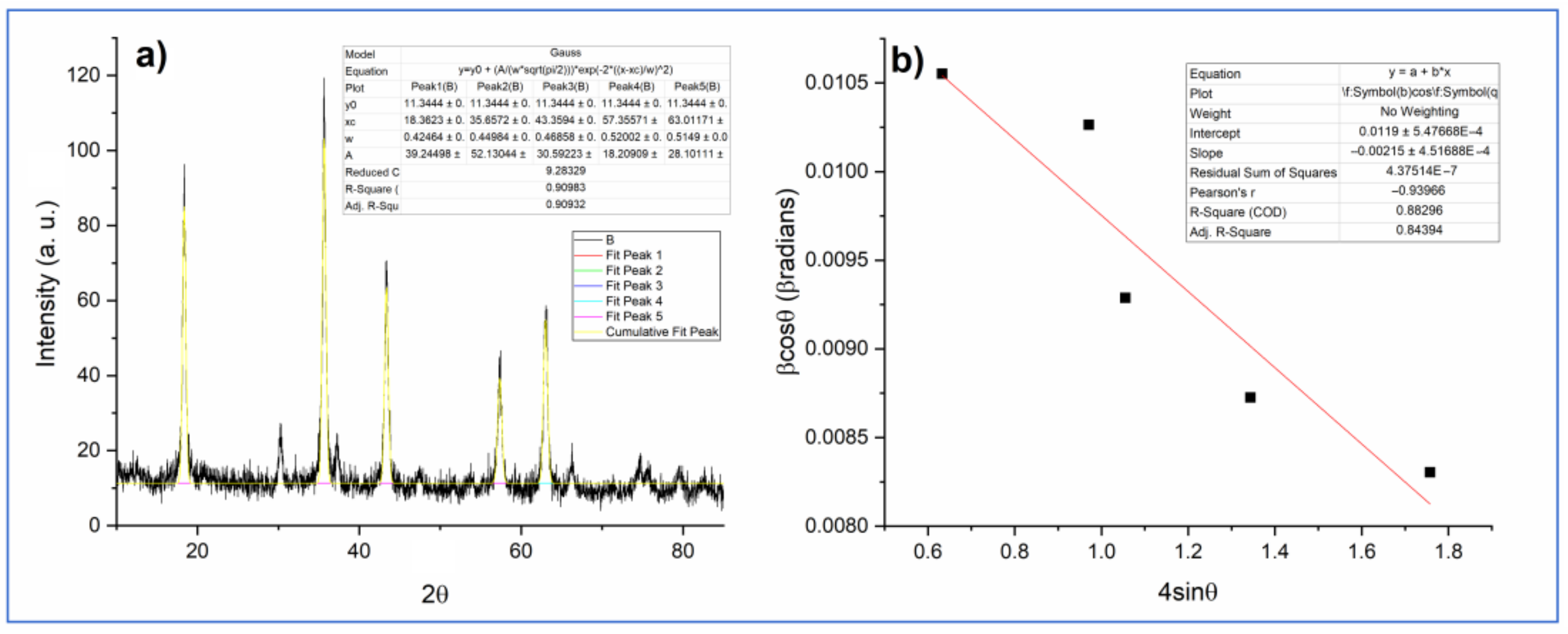
2.2. Phase Analysis
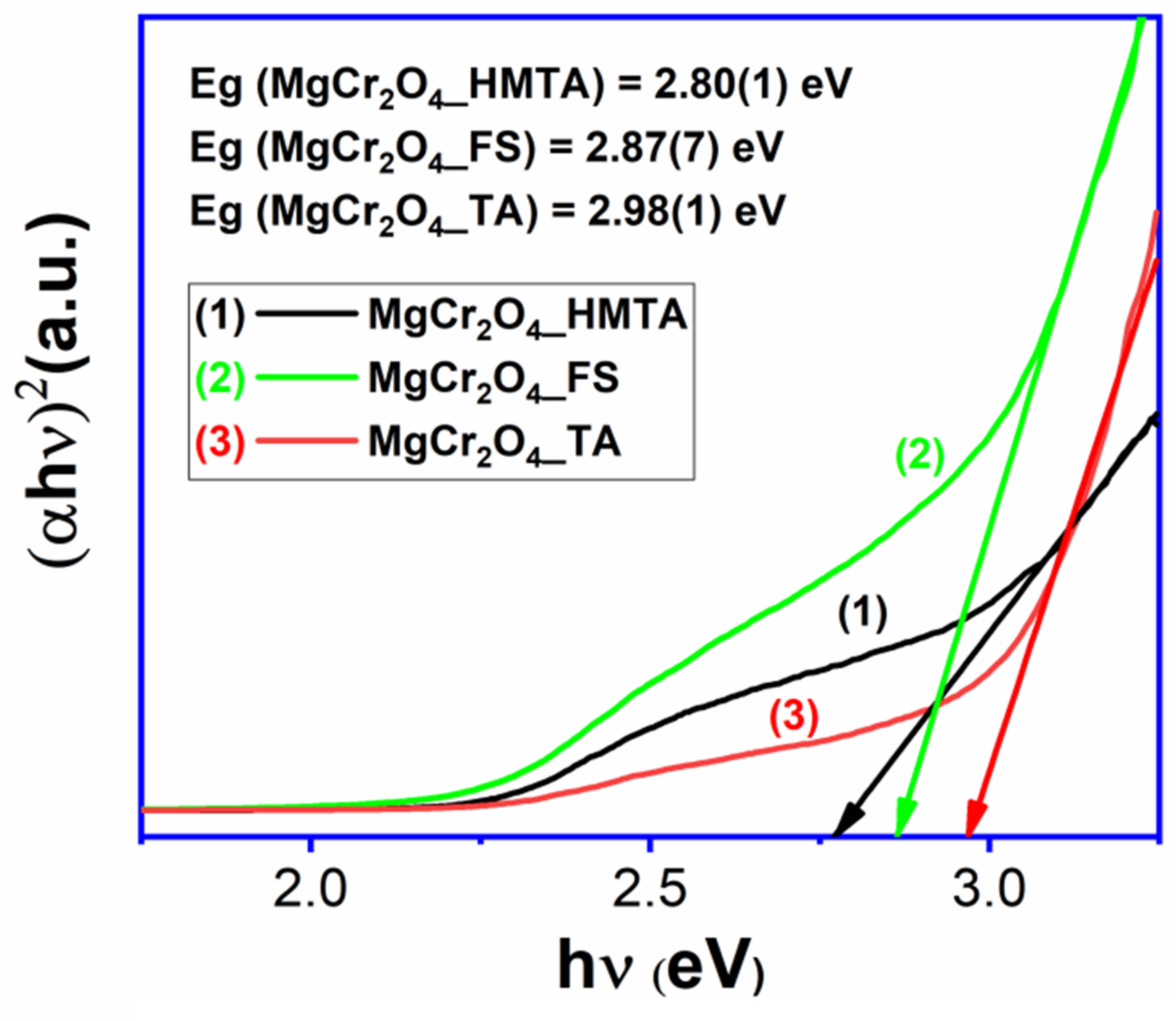
2.3. UV–VIS Analysis
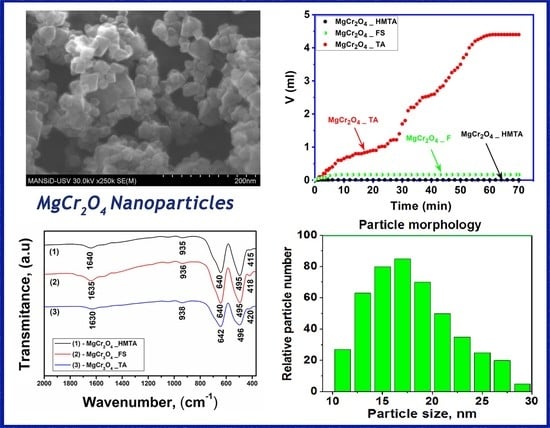
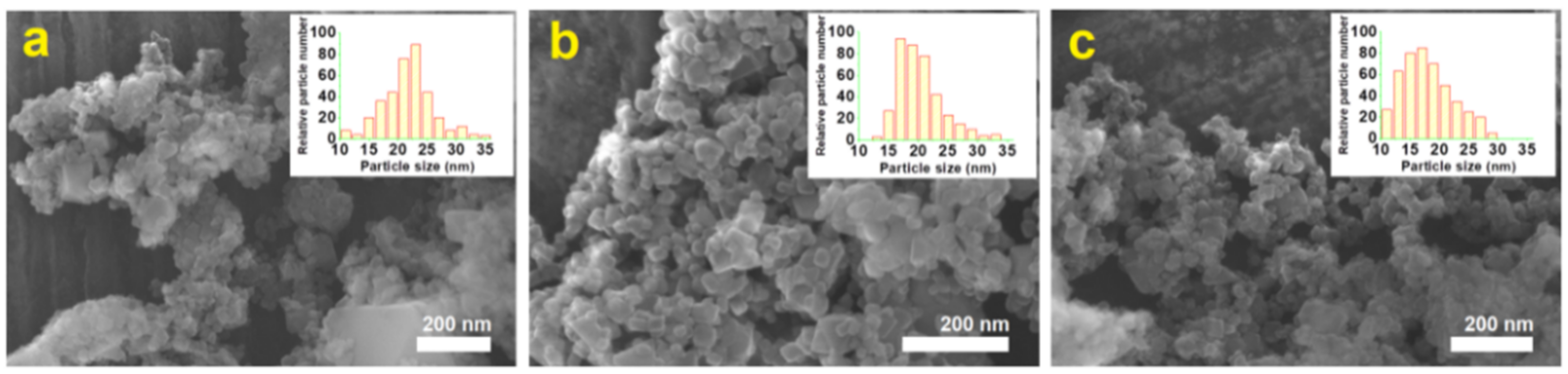
2.4. Morphological Analysis
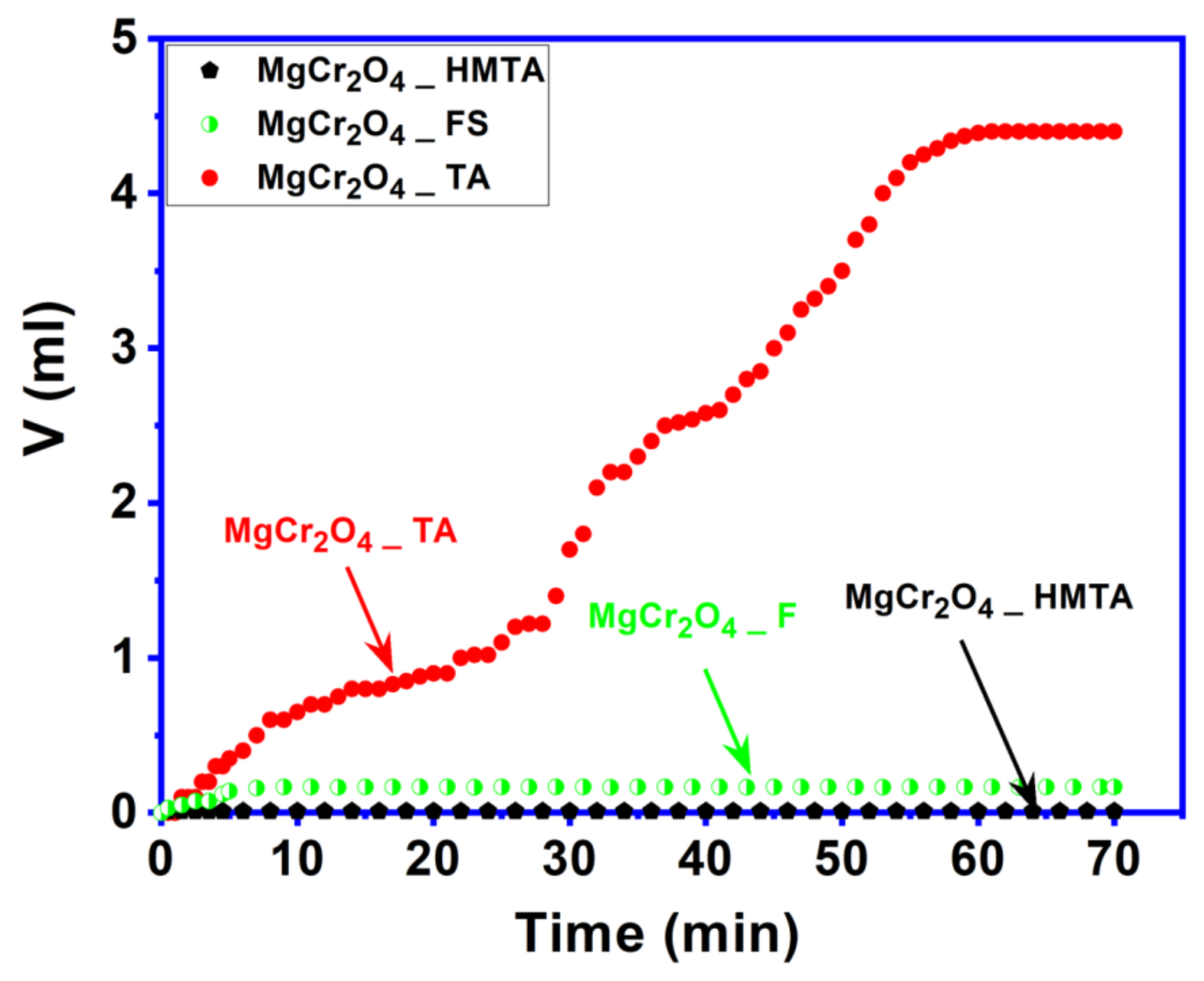
2.5. Catalytic Activity
3. Materials and Methods
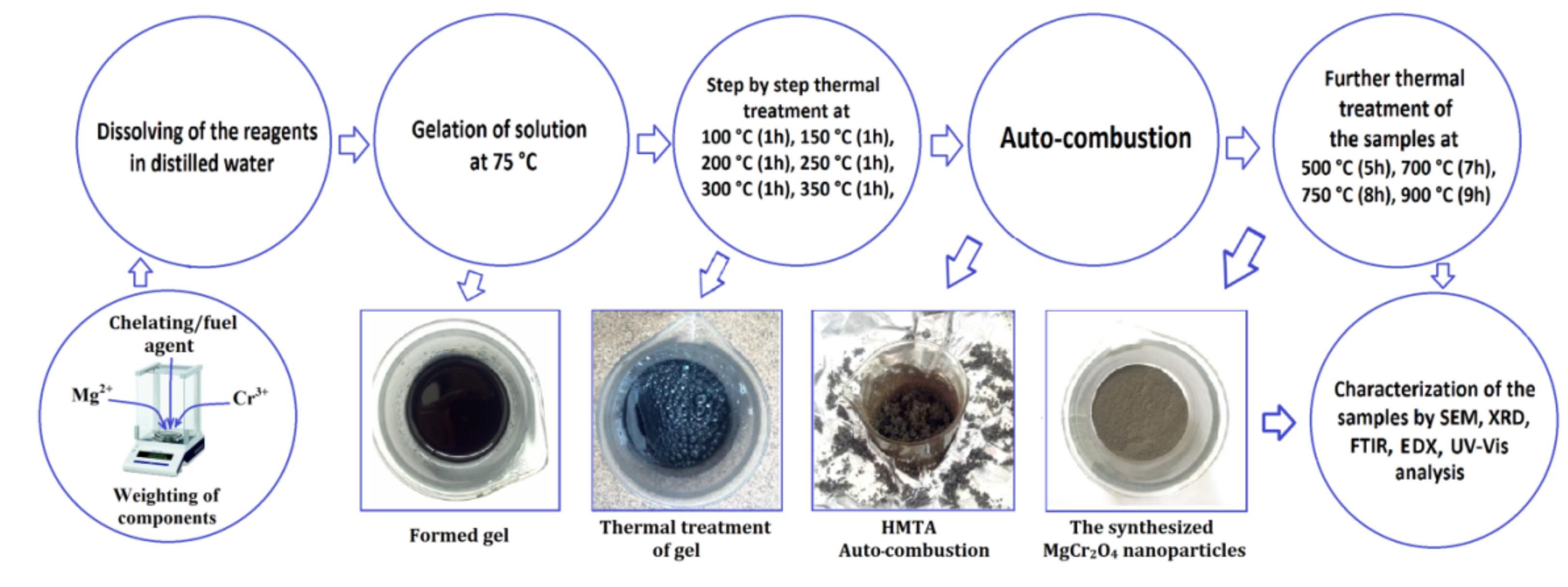
3.1. Synthesis of MgCr2O4 Nanoparticles
3.2. Nanoparticle Characterization
3.3. Study of the Catalytic Properties of MgCr2O4 Nanopowders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qing, Z.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar]
- Kinoshita, C.; Fukumoto, K.; Fukuda, K.; Garner, F.A.; Hollenberg, G.W. Why is magnesia spinel a radiation-resistant material? J. Nucl. Mater. 1995, 219, 143–151. [Google Scholar] [CrossRef]
- Rida, K.; Benabbas, A.; Bouremmad, F.; Pena, M.A.; Martinez-Arias, A. Influence of the synthesis method on structural properties and catalytic activity for oxidation of CO and C3H6 of pirochromite MgCr2O4. Appl. Catal. A Gen. 2010, 375, 101–106. [Google Scholar] [CrossRef]
- Tripathi, V.K.; Nagarajan, R. Rapid Synthesis of Mesoporous, Nano-Sized MgCr2O4 and Its Catalytic Properties. J. Am. Ceram. Soc. 2016, 99, 814–818. [Google Scholar] [CrossRef]
- Finocchio, E.; Busca, G.; Lorenzelli, V.; Willey, R.J. The Activation of Hydrocarbon CH Bonds over Transition Metal Oxide Catalysts: A FTIR Study of Hydrocarbon Catalytic Combustion over MgCr2O4. J. Catal. 1995, 151, 204–215. [Google Scholar] [CrossRef]
- Nitta, T.; Terada, Z.; Hayakawa, S. Humidity-Sensitive Electrical Conduction of MgCr2O4-TiO2 Porous Ceramics. J. Am. Ceram. Soc. 1980, 63, 295–300. [Google Scholar] [CrossRef]
- Schoonman, J.; Dekker, J.P.; Broers, J.W.; Kiwiet, N.J. Electrochemical vapor deposition of stabilized zirconia and interconnection materials for solid oxide fuel cells. Solid State Ion. 1991, 46, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Nitta, T.; Terada, J.; Fukushima, F. Multifunctional ceramic sensors: Humidity-gas sensor and temperature-humidity sensor. IEEE Trans. Electron. Devices. 1982, 29, 95–101. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Pingale, S.S.; Patil, S.F.; Vinod, M.P.; Pathak, G.; Vijayamohanan, K. Mechanism of humidity sensing of Ti-doped MgCr2O4 ceramics. Mater. Chem. Phys. 1996, 46, 72–76. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Peng, C.; Gao, L. Optical and photocatalytic properties of spinel ZnCr2O4 nanoparticles synthesized by a hydrothermal route. J. Am. Ceram. Soc. 2008, 91, 2388–2390. [Google Scholar] [CrossRef]
- Lü, H.; Ma, W.; Zhao, H.; Du, J.; Yu, X. Synthesis and characterization of MgCr2O4: Co2+ fabricated by a microwave method. Mater. Manuf. Process. 2011, 26, 1233–1235. [Google Scholar] [CrossRef]
- Marinković, Z.; Mančić, L.; Vulić, P.; Milošević, O. The influence of mechanical activation on the stoichiometry and defect structure of a sintered ZnO-Cr2O3 system. Mater. Sci. Forum Trans. Tech. Publ. 2004, 453, 423–428. [Google Scholar] [CrossRef]
- Messing, G.L.; Zhang, S.C.; Jayanthi, G.V. Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Dumitrescu, A.M.; Samoila, P.M.; Nica, V.; Doroftei, F.; Iordan, A.R.; Palamaru, M.N. Study of the chelating/fuel agents influence on NiFe2O4 samples with potential catalytic properties. Powder Technol. 2013, 243, 9–17. [Google Scholar] [CrossRef]
- Ansari, F.; Bazarganipour, M.; Salavati-Niasari, M. NiTiO3/NiFe2O4 nanocomposites: Simple sol–gel auto-combustion synthesis and characterization by utilizing onion extract as a novel fuel and green capping agent. Mater. Sci. Semicond. Process. 2016, 43, 34–40. [Google Scholar] [CrossRef]
- Ansari, F.; Sobhani, A.; Salavati-Niasari, M. Green synthesis of magnetic chitosan nanocomposites by a new sol–gel auto-combustion method. J. Magn. Magn. Mater. 2016, 410, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Slatineanu, T.; Diana, E.; Nica, V.; Oancea, V.; Caltun, O.F.; Iordan, A.R.; Palamaru, M.N. The influence of the chelating/combustion agents on the structure and magnetic properties of zinc ferrite. Cent. Eur. J. Chem. 2012, 10, 1799–1807. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhao, W.; Hu, R.; Chang, G.; Li, C.; Wang, L. Catalytic activity of spinel oxides MgCr2O4 and CoCr2O4 for methane combustion. Mater. Res. Bull. 2014, 57, 268–273. [Google Scholar] [CrossRef]
- Saberi, A.; Golestani-Fard, F.; Willert-Porada, M.; Negahdari, Z.; Liebscher, C.; Gossler, B. A novel approach to synthesis of nanosize MgAl2O4 spinel powder through sol–gel citrate technique and subsequent heat treatment. Ceram. Inter. 2009, 35, 933–937. [Google Scholar] [CrossRef]
- Habibi, N.; Wang, Y.; Arandiyan, H.; Rezaei, M. Low-temperature synthesis of mesoporous nanocrystalline magnesium aluminate (MgAl2O4) spinel with high surface area using a novel modified sol-gel method. Adv. Powder Technol. 2017, 28, 1249–1257. [Google Scholar] [CrossRef] [Green Version]
- Swietoslawski, W.; Starczewska, H. Influence of certain corrections on the results of measurements of the heat of combustion of organic substances. Bull. Int. Acad. Pol. Sci. Lett. Cl Med. 1928, 1928, 85–97. [Google Scholar]
- Clarke, T.; Stegeman, G. Heats of combustion of some mono-and disaccharides. J. Am. Ceram. Soc. 1939, 61, 1726–1730. [Google Scholar] [CrossRef]
- Westrum Jr, E.; Mansson, M.; Rapport, N. Enthalpies of formation of globular molecules. I. Adamantane and hexamethylenetetramine. J. Am. Chem. Soc. 1970, 92, 7296–7299. [Google Scholar] [CrossRef]
- Mao, L.; Cui, H.; Miao, C.; An, H.; Zhai, J.; Li, Q. Preparation of MgCr2O4 from waste tannery solution and effect of sulfate, chloride, and calcium on leachability of chromium. J. Mater. Cycles Waste Manag. 2016, 18, 573–581. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. Adv. Catal. 2014, 57, 99–318. [Google Scholar]
- Ehrenberg, H.; Knapp, M.; Baehtz, C.; Klemme, S. Tetragonal low-temperature phase of MgCr2O4. Powder Diffr. 2002, 17, 230–233. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Sol. State Sci. 2011, 13, 251–256. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ewais, E.M.; El-Amir, A.A.; Besisa, D.H.; Esmat, M.; El-Anadouli, B.E. Synthesis of nanocrystalline MgO/MgAl2O4 spinel powders from industrial wastes. J. Alloy. Comp. 2017, 691, 822–833. [Google Scholar] [CrossRef]
- Lushchik, A.; Feldbach, E.; Kotomin, E.A.; Kudryavtseva, I.; Kuzovkov, V.N.; Popov, A.I.; Seeman, V.; Shablonin, E. Distinctive features of diffusion-controlled radiation defect recombination in stoichiometric magnesium aluminate spinel single crystals and transparent polycrystalline ceramics. Sci. Rep. 2020, 10, 7810. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mykhailovych, V.; Kanak, A.; Cojocaru, Ş.; Chitoiu-Arsene, E.-D.; Palamaru, M.N.; Iordan, A.-R.; Korovyanko, O.; Diaconu, A.; Ciobanu, V.G.; Caruntu, G.; et al. Structural, Optical, and Catalytic Properties of MgCr2O4 Spinel-Type Nanostructures Synthesized by Sol–Gel Auto-Combustion Method. Catalysts 2021, 11, 1476. https://doi.org/10.3390/catal11121476
Mykhailovych V, Kanak A, Cojocaru Ş, Chitoiu-Arsene E-D, Palamaru MN, Iordan A-R, Korovyanko O, Diaconu A, Ciobanu VG, Caruntu G, et al. Structural, Optical, and Catalytic Properties of MgCr2O4 Spinel-Type Nanostructures Synthesized by Sol–Gel Auto-Combustion Method. Catalysts. 2021; 11(12):1476. https://doi.org/10.3390/catal11121476
Chicago/Turabian StyleMykhailovych, Vasyl, Andrii Kanak, Ştefana Cojocaru, Elena-Daniela Chitoiu-Arsene, Mircea Nicolae Palamaru, Alexandra-Raluca Iordan, Oleksandra Korovyanko, Andrei Diaconu, Viorela Gabriela Ciobanu, Gabriel Caruntu, and et al. 2021. "Structural, Optical, and Catalytic Properties of MgCr2O4 Spinel-Type Nanostructures Synthesized by Sol–Gel Auto-Combustion Method" Catalysts 11, no. 12: 1476. https://doi.org/10.3390/catal11121476