Effects of Thermally Oxidized Vegetable Oil on Growth Performance and Carcass Characteristics, Gut Morphology, Nutrients Utilization, Serum Cholesterol and Meat Fatty Acid Profile in Broilers
Abstract
:1. Introduction
2. Results
2.1. Growth Performance
2.2. Slaughter Body Weight and Carcass Characteristics
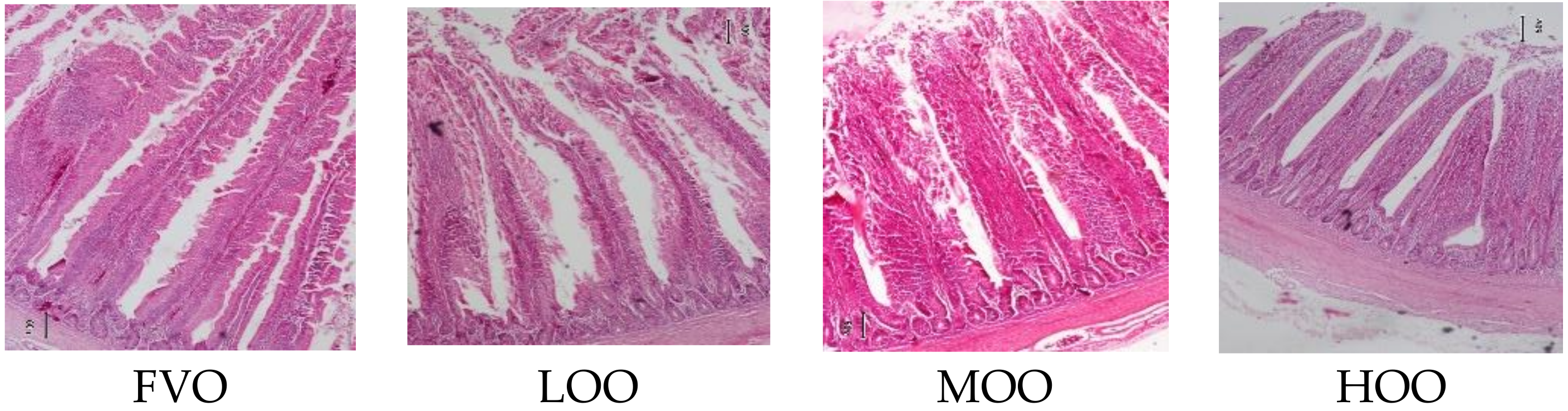
2.3. Gut Morphology
2.4. Nutrients Utilization
2.5. Serum Cholesterol
2.6. Meat Fatty Acid Profile
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Birds, Diets and Management
5.2. Traits Measured
5.2.1. Growth Performance and Carcass Characteristics
5.2.2. Tissue Collection and Morphometric Examination
5.2.3. Measurements of Ileal Digestibility of Crude Protein and Fat
5.2.4. Blood Sample Collection and Serum Cholesterol Analysis
5.2.5. Meat Fatty Acid Profile Analysis
5.3. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.M.M.; Mohammed, K.A.; Shakak, M.A.S. Effects of frying oils used in broiler rations on performance. J. Curr. Res. Sci. 2013, 1, 310–315. [Google Scholar]
- Van Ruth, S.; Rozijn, M.; Koot, A.; Garcia, R.P.; Van der Kamp, H.; Codony, R. Authentication of feeding fats: Classification of animal fats, fish oils and recycled cooking oils. Anim. Feed Sci. Technol. 2010, 155, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, A.; Akiba, Y.; Toyomizu, M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R690–R698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baião, N.C.; Lara, L. Oil and fat in broiler nutrition. Rev. Bras. Cienc. Avic. 2005, 7, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Turchini, G.; Francis, D.; Senadheera, S.; Thanuthong, T.; De Silva, S. Fish oil replacement with different vegetable oils in Murray cod: Evidence of an “omega-3 sparing effect” by other dietary fatty acids. Aquaculture 2011, 315, 250–259. [Google Scholar] [CrossRef]
- Liu, P.; Kerr, B.; Chen, C.; Weber, T.; Johnston, L.J.; Shurson, G.C. Methods to create thermally oxidized lipids and comparison of analytical procedures to characterize peroxidation. J. Anim. Sci. 2014, 92, 2950–2959. [Google Scholar] [CrossRef]
- Ghasemi-Sadabadi, M.; Veldkamp, T.; van Krimpen, M.; Ebrahimnezhad, Y.; Ghalehkandi, J.G.; Salehi, A.; Didehvar, M.; Khodaei, M.; Mehdizadeh, A. Determining tolerance of Japanese quail to different dietary fat peroxidation values by supplementation with Rosemary and Aloe Vera on performance and meat quality. Anim. Feed. Sci. Technol. 2020, 267, 114574. [Google Scholar] [CrossRef]
- Ahsan-ul-Haq, H.A.; Rasool, S.; Shah, T.H.; Anjum, I. Effect of sodium bentonite as aflatoxin binder in broiler feeds containing fungal infected grains. Pak. J. Agric. Sci. 2000, 37, 163–165. [Google Scholar]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids. 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Tan, L.; Rong, D.; Yang, Y.; Zhang, B. Effect of Oxidized Soybean Oils on Oxidative Status and Intestinal Barrier Function in Broiler Chickens. Rev. Bras. Cienc. Avic. 2018, 20, 333–342. [Google Scholar] [CrossRef]
- Liang, F.; Jiang, S.; Mo, Y.; Zhou, G.; Yang, L. Consumption of oxidized soybean oil increased intestinal oxidative stress and affected intestinal immune variables in yellow-feathered broilers. Asian-Australas J. Anim. Sci. 2015, 28, 1194. [Google Scholar] [CrossRef] [Green Version]
- Qaisrani, S.; Rizwan, M.; Yaseen, G.; Bibi, F.; Sarfraz, M.; Khan, N.; Naveed, S.; Pasha, T. Effects of dietary oxidized oil on growth performance, meat quality and biochemical indices in poultry—A review. Ann Anim. Sci. 2021, 21, 29–46. [Google Scholar] [CrossRef]
- McGill, J.; Firman, J.D. Effect of high peroxide value fats on performance of broilers in normal and immune challenged states. Int. J. Poult. Sci. 2021, 10, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Mazur-Kuśnirek, M.; Antoszkiewicz, Z.; Lipiński, K.; Kaliniewicz, J.; Czurgiel, S. The effect of polyphenols and Vitamin E on the antioxidant status and meat quality of broiler chickens fed low-quality oil. Arch. Anim. Breed. 2019, 62, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishawy, A.T.; Omar, A.E.; Gomaa, A.M. Growth performance and immunity of broilers fed rancid oil diets that supplemented with pomegranate peel extract and sage oil. Jpn. J. Vet. Res. 2016, 64, 31–38. [Google Scholar]
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Mirza, I.; Azim, A. Effect of fresh versus oxidized soybean oil on growth performance, organ weights and meat quality of broiler chicks. Pak. Vet. J. 2004, 24, 173–178. [Google Scholar]
- Ehr, I.; Kerr, B.; Persia, M. Effects of peroxidized corn oil on performance, AMEn, and abdominal fat pad weight in broiler chicks. Poult. Sci. 2015, 94, 1629–1634. [Google Scholar] [CrossRef]
- Lewis-McCrea, L.; Lall, S. Effects of moderately oxidized dietary lipid and the role of vitamin E on the development of skeletal abnormalities in juvenile Atlantic halibut (Hippoglossus hippoglossus). Aquac. Nutr. 2007, 262, 142–155. [Google Scholar] [CrossRef]
- Dong, X.L.; Lei, W.; Zhu, X.; Han, D.; Yang, Y.X.; Xie, S. Effects of dietary oxidized fish oil on growth performance and skin color of Chinese longsnout catfish (Leiocassis longirostris Günther). Aquac. Nutr. 2011, 17, e861–e868. [Google Scholar] [CrossRef]
- Tres, A.; Bou, R.; Guardiola, F.; Nuchi, C.D.; Magrinyà, N.; Codony, R. Use of recovered frying oils in chicken and rabbit feeds: Effect on the fatty acid and tocol composition and on the oxidation levels of meat, liver and plasma. Animal 2013, 7, 505. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Kerr, B.; Weber, T.; Chen, C.; Johnston, L.J.; Shurson, G.C. Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. Anim. Sci. J. 2014, 92, 2971–2979. [Google Scholar] [CrossRef]
- Tavárez, M.; Boler, D.; Bess, K.; Zhao, J.; Yan, F.; Dilger, A.; McKeith, F.; Killefer, J. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 2011, 90, 922–930. [Google Scholar] [CrossRef]
- Tan, L.; Rong, D.; Yang, Y.; Zhang, B. The Effect of Oxidized Fish Oils on Growth Performance, Oxidative Status, and Intestinal Barrier Function in Broiler Chickens. J. Appl. Poult. Res. 2018, 28, 31–41. [Google Scholar] [CrossRef]
- Lindblom, S.; Gabler, N.; Kerr, B. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in growing pigs. J. Anim. Sci. 2018, 96, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Zdunczyk, Z.; Jankowski, J.; Koncicki, A. Growth performance and physiological state of turkeys fed diets with higher content of lipid oxidation products, selenium, vitamin E and vitamin A. Worlds Poult. Sci. J. 2002, 58, 357–364. [Google Scholar] [CrossRef]
- Eder, K. The effect of an oxidized dietary oil on plasma cholesterol and thyroid hormone concentrations in miniature pigs fed on a hyperlipidaemic diet. J. Anim. Physiol. An. N. 1999, 82, 271–281. [Google Scholar] [CrossRef]
- Kumagai, T.; Matsukawa, N.; Kaneko, Y.; Kusumi, Y.; Mitsumata, M.; Uchida, K. A lipid peroxidation-derived inflammatory mediator: Identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J. Biol. Chem. 2004, 279, 48389–48396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.; Wang, J.; Qi, X.; Ji, F.; Liu, M.; Wu, S.; Zhang, H.; Qi, G. Effects of dietary oxidized oil on laying performance, lipid metabolism, and apolipoprotein gene expression in laying hens. Poult. Sci. 2011, 90, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.; Atwell, C.; Kitchell, M.; Shermer, W.; Ivey, F. Feeding of oxidized fats to broilers and swine: Effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Anim. Feed Sci. Technol. 1996, 62, 1–13. [Google Scholar] [CrossRef]
- Ahmed, I.; Qaisrani, S.N.; Azam, F.; Pasha, T.N.; Bibi, F.; Naveed, S.; Murtaza, S. Interactive Effects of threonine levels and protein source on growth performance and carcass traits, gut morphology, ileal digestibility of protein and amino acids, and immunity in broilers. Poult. Sci. 2020, 99, 280–289. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.P. Effect of High Peroxide Value Fats on Performance of Broilers in Normal and Immune Challenged States; University of Missouri-Columbia: Columbia, MO, USA, 2009; Available online: http://edt.missouri.edu/Summer2009/Thesis/McGillJ-072709-T21/ (accessed on 13 September 2021).
- Lu, T.; Harper, A.; Zhao, J.; Dalloul, R. Effects of a dietary antioxidant blend and vitamin E on growth performance, oxidative status, and meat quality in broiler chickens fed a diet high in oxidants. Poult. Sci. 2014, 93, 1649–1657. [Google Scholar] [CrossRef]
- Lankenau, E.J. The Role of the Epithelial Cell in the Initiating Events of ADP-Ribosylating Enterotoxin-Induced Toxicity and Adjuvanticity; Tulane University: New Orleans, LA, USA, 2003. [Google Scholar]
- Da Rocha, C.; Maiorka, A.; de Paula Valle, F.; Schramm, V.G.; Angeli, A.; da Silva, A.F. The effect of soybean oil quality and vitamin E supplementation on turkey diet nutrition. J. Appl. Poult. Res. 2012, 21, 318–324. [Google Scholar] [CrossRef]
- Papadia, C.; Kelly, P.; Caini, S.; Corazza, G.R.; Shawa, T.; Franzè, A.; Forbes, A.; Di Sabatino, A. Plasma citrulline as a quantitative biomarker of HIV-associated villous atrophy in a tropical enteropathy population. Clin. Nutr. 2010, 29, 795–800. [Google Scholar] [CrossRef]
- Dibner, J.; Richards, J. The digestive system: Challenges and opportunities. J. Appl. Poult. Res. 2004, 13, 86–93. [Google Scholar] [CrossRef]
- Luo, B.; Chen, D.W.; Tian, G.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; et al. Effects of Dietary Aged Maize with Oxidized Fish Oil on Growth Performance, Antioxidant Capacity and Intestinal Health in Weaned Piglets. Animals 2019, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Overholt, M.; Dilger, A.; Boler, D.; Kerr, B. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in finishing pigs. J. Anim. Sci. 2018, 96, 2789–2803. [Google Scholar] [CrossRef] [PubMed]
- Açikgöz, Z.; Bayraktar, Ö.; Altan, O.; Akhisaroglu, S.; Kırkpınar, F.; Altun, Z. The effects of moderately oxidised dietary oil with or without vitamin E supplementation on performance, nutrient digestibility, some blood traits, lipid peroxidation and antioxidant defence of male broilers. J. Sci. Food Agri. 2011, 91, 1277–1282. [Google Scholar] [CrossRef]
- Kerr, B.; Lindblom, S.; Overholt, M. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, gut integrity, and oxidative stress in nursery pigs. J. Anim. Sci. 2020, 98, 1–11. [Google Scholar] [CrossRef]
- Wiseman, J.; Salvador, F. The Influence of Free Fatty Acid Content and Degree of Saturation on the Apparent Metabolizable Energy Value of Fats Fed to Broilers. Poult. Sci. 1991, 70, 573–582. [Google Scholar] [CrossRef]
- DeRouchey, J.; Hancock, J.; Hines, R.; Maloney, C.; Lee, D.; Cao, H.; Dean, D.; Park, J. Effects of rancidity and free fatty acids in choice white grease on growth performance and nutrient digestibility in weanling pigs. J. Anim. Sci. 2004, 82, 2937–2944. [Google Scholar] [CrossRef] [Green Version]
- Márquez-Ruiz, G.; Camino, M.; Dobarganes, M. Digestibility of fatty acid monomers, dimers and polymers in the rat. J. Am. Oil Chem. Soc. 1992, 69, 930–934. [Google Scholar] [CrossRef]
- González-Muñoz, M.J.; Bastida, S.; Muniz, F. Short-Term in Vivo Digestibility of Triglyceride Polymers, Dimers, and Monomers of Thermoxidized Palm Olein Used in Deep-Frying. J. Agric. Food Chem. 1998, 46, 5188–5193. [Google Scholar] [CrossRef]
- Hornick, J.-L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef]
- Bayraktar, Ö.; Altan, O.; Açikgöz, Z.; Baysal, Ş.; Seremet, C. Effects of oxidised oil and vitamin E on performance and some blood traits of heat-stressed male broilers. S. Afr. J. Anim. Sci. 2011, 41, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Hochgraf, E.; Cogan, U.; Mokady, S. Dietary oxidized linoleic acid enhances liver cholesterol biosynthesis and secretion in rats. J. Nutr. Biochem. 2000, 11, 176–180. [Google Scholar] [CrossRef]
- Abdulla, N.R.; Loh, T.C.; Akit, H.; Sazili, A.Q.; Foo, H.L.; Kareem, K.Y.; Mohamad, R.; Abdul Rahim, R. Effects of dietary oil sources, calcium and phosphorus levels on growth performance, carcass characteristics and bone quality of broiler chickens. J. Appl. Anim. Res. 2017, 45, 423–429. [Google Scholar] [CrossRef]
- Dorra, T.M.; Hamady, G.A.A.; Abdel-Moneim, M.A.A. The Use of Recovered Frying oil in Broiler Chicken Diets: Effect on Performance, Meat Quality and Blood Parameters. Res. J. Anim. Vet. Fish Sci. 2014, 2, 11–15. [Google Scholar]
- Crespo, N.; Esteve-Garcia, E. Dietary fatty acid profile modifies abdominal fat deposition in broiler chickens. Poult. Sci. 2001, 80, 71–78. [Google Scholar] [CrossRef] [PubMed]
- American Oil Chemists’ Society. AOCS Official Methods and Recommended Practices of the AOCS, 6th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2007. [Google Scholar]
- Aviagen. Broiler Ross Nutrition Specifications. 2019. Available online: http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf (accessed on 2 December 2019).
- Gopinger, E.; Xavier, E.G.; Elias, M.C.; Catalan, A.A.; Castro, M.L.; Nunes, A.P.; Roll, V.F. The effect of different dietary levels of canola meal on growth performance, nutrient digestibility, and gut morphology of broiler chickens. Poult. Sci. 2014, 93, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Asiedu, A.; Baidoot, S.; Nyachoti, C.; Marquardt, R. Response of early-weaned pigs to spray-dried porcine or animal plasma-based diets supplemented with egg-yolk antibodies against enterotoxigenic Escherichia coli. J. Anim. Sci. 2002, 80, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Gas Chromatography and Lipids; Oily Press: Bridgwater Somerset, UK, 1989. [Google Scholar]
- Qian, M. Gas chromatography. In Food Analysis Laboratory Manual, 3rd ed.; Kluwer Academics: New York, NY, USA, 2003; pp. 230–278. [Google Scholar]
| Effects | FI 1 | BWG 2 | FCR 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (Days) | 0–21 | 22–35 | 0–35 | 0–21 | 22–35 | 0–35 | 0–21 | 22–35 | 0–35 |
| Treatments ** | |||||||||
| FVO | 57.5 a | 135.5 a | 91.4 a | 46.7 a | 81.8 a | 62.7 a | 1.23 b | 1.66 b | 1.46 c |
| LOO | 58.2 a | 135.4 a | 89.1 a | 45.2 a | 78.5 ab | 59.5 a | 1.29 b | 1.72 ab | 1.50 b |
| MOO | 53.3 b | 126.5 b | 84.6 b | 39.3 b | 71.0 c | 54.0 b | 1.36 a | 1.78 a | 1.56 a |
| HOO | 53.7 b | 131.6 ab | 82.9 b | 39.7 b | 75.2 bc | 52.9 b | 1.35 a | 1.75 ab | 1.57 a |
| Pooled SE | 0.85 | 2.28 | 1.12 | 0.61 | 1.62 | 0.88 | 0.01 | 0.02 | 0.01 |
| p-value | 0.001 | 0.041 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.040 | 0.001 |
| Treatments ** | Body Weight (g) | Slaughter Weight (g) | Dressing (%)1 | Giblets (%) 1 | Breast Part (%) 1 | Leg Quarter (%) 1 |
|---|---|---|---|---|---|---|
| FVO | 2195 a | 1454 a | 66.2 | 4.46 | 27.3 | 19.2 |
| LOO | 2083 ab | 1375 b | 66.0 | 4.47 | 26.4 | 19.3 |
| MOO | 1890 bc | 1240 c | 65.6 | 4.56 | 25.7 | 19.8 |
| HOO | 1851 c | 1210 c | 65.4 | 4.34 | 26.5 | 19.6 |
| Pooled SE | 47.1 | 31.4 | 0.97 | 0.47 | 0.68 | 0.42 |
| p-value | 0.001 | 0.001 | 0.70 | 0.87 | 0.48 | 0.73 |
| Treatments ** | VH 1 | CD 2 | VCR 3 |
|---|---|---|---|
| FVO | 1450 a | 162 c | 8.95 a |
| LOO | 1427 a | 174 c | 8.20 b |
| MOO | 1096 b | 189 b | 5.79 c |
| HOO | 1037 b | 203 a | 5.11 d |
| Pooled SE | 27.0 | 5.00 | 0.16 |
| p-value | 0.001 | 0.001 | 0.001 |
| Treatments ** | CP (%) | EE (%) | Serum Cholesterol (mg/dL) |
|---|---|---|---|
| FVO | 68.5 a | 69.4 a | 82.1 b |
| LOO | 67.4 a | 66.5 b | 84.0 b |
| MOO | 63.1 b | 65.2 bc | 88.1 b |
| HOO | 60.5 c | 61.6 d | 106.0 a |
| Pooled SE | 1.20 | 0.05 | 5.32 |
| p-value | 0.041 | 0.032 | 0.023 |
| Treatments ** | Fatty Acids Profile (% of Total Fatty Acids) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:4 | C22:6 | SFA 1 | MUFA 2 | PUFA 3 | |
| FVO | 0.43 b | 15.2 d | 2.20 | 8.97 c | 28.5 | 41.1 a | 0.80 a | 3.73 a | 0.41 a | 24.6 b | 30.7 b | 46.0 a |
| LOO | 0.45 b | 15.7 c | 2.46 | 9.33 b | 28.5 | 39.2 b | 0.76 ab | 3.39 ab | 0.37 a | 25.5 b | 31.0 ab | 43.7 b |
| MOO | 0.48 ab | 17.5 b | 2.60 | 9.67 a | 28.6 | 36.5 c | 0.75 b | 3.00 b | 0.31 b | 27.7 ab | 31.1 ab | 40.6 c |
| HOO | 0.50 a | 19.3 a | 2.65 | 9.90 a | 28.7 | 31.2 d | 0.70 c | 2.86 b | 0.28 b | 29.7 a | 31.2 a | 35.0 d |
| Pooled SE | 0.02 | 0.17 | 0.11 | 0.12 | 0.32 | 0.23 | 0.01 | 0.10 | 0.02 | 0.19 | 0.04 | 0.05 |
| p-value | 0.04 | 0.001 | 0.16 | 0.008 | 0.82 | 0.001 | 0.03 | 0.005 | 0.001 | 0.001 | 0.03 | 0.001 |
| Ingredients (%) | Starter (Day 0–21) | Grower (Day 22–35) | ||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| Corn | 48.8 | 48.8 | 57.0 | 57.0 |
| Soybean meal (44%) | 35.4 | 35.4 | 30.6 | 30.6 |
| Wheat bran | 3.00 | 3.00 | 2.00 | 2.00 |
| Fresh vegetable (sunflower) oil * | 5.00 | - | 5.00 | - |
| Oxidized vegetable (sunflower) oil 1 | - | 5.00 | - | 5.00 |
| Canola meal | 4.00 | 4.00 | 2.00 | 2.00 |
| DCP 2 | 3.00 | 3.00 | 2.50 | 2.50 |
| Mineral premix 4 | 0.25 | 0.25 | 0.20 | 0.20 |
| Vitamin premix 3 | 0.20 | 0.20 | 0.20 | 0.20 |
| L-Lysine | 0.05 | 0.05 | 0.15 | 0.15 |
| DL-Methionine | 0.30 | 0.30 | 0.30 | 0.30 |
| Total | 100 | 100 | 100 | 100 |
| Calculated composition (%) | ||||
| ME (kcal/kg) | 2988 | 2988 | 3100 | 3100 |
| DM | 88.9 | 88.9 | 88.6 | 88.6 |
| CP | 23.0 | 23.0 | 20.6 | 20.6 |
| EE | 2.85 | 2.85 | 2.94 | 2.94 |
| CF | 4.32 | 4.32 | 3.84 | 3.84 |
| Lysine-HCL | 1.26 | 1.26 | 1.10 | 1.10 |
| Methionine | 0.50 | 0.50 | 0.45 | 0.45 |
| Met + Cys | 0.91 | 0.91 | 0.84 | 0.84 |
| Arginine | 1.34 | 1.34 | 1.19 | 1.19 |
| Threonine | 0.83 | 0.83 | 0.73 | 0.73 |
| Valine | 0.93 | 0.93 | 0.84 | 0.84 |
| Analyzed composition (%) | ||||
| ME (kcal/kg) | 2980 | 2978 | 3090 | 3087 |
| DM | 88.7 | 88.6 | 88.5 | 88.2 |
| CP | 22.8 | 22.8 | 20.5 | 20.5 |
| EE | 2.84 | 2.83 | 2.93 | 2.93 |
| CF | 4.30 | 4.31 | 3.83 | 3.83 |
| Lysine-HCL | 1.24 | 1.24 | 1.09 | 1.08 |
| Methionine | 0.49 | 0.49 | 0.44 | 0.44 |
| Met + Cys | 0.90 | 0.90 | 0.82 | 0.82 |
| Arginine | 1.33 | 1.32 | 1.17 | 1.17 |
| Threonine | 0.82 | 0.81 | 0.72 | 0.71 |
| Valine | 0.91 | 0.91 | 0.83 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, G.; Sarfraz, M.A.; Naveed, S.; Ahmad, F.; Bibi, F.; Irshad, I.; Asif, M.; Pasha, T.N.; Qaisrani, S.N. Effects of Thermally Oxidized Vegetable Oil on Growth Performance and Carcass Characteristics, Gut Morphology, Nutrients Utilization, Serum Cholesterol and Meat Fatty Acid Profile in Broilers. Catalysts 2021, 11, 1528. https://doi.org/10.3390/catal11121528
Yaseen G, Sarfraz MA, Naveed S, Ahmad F, Bibi F, Irshad I, Asif M, Pasha TN, Qaisrani SN. Effects of Thermally Oxidized Vegetable Oil on Growth Performance and Carcass Characteristics, Gut Morphology, Nutrients Utilization, Serum Cholesterol and Meat Fatty Acid Profile in Broilers. Catalysts. 2021; 11(12):1528. https://doi.org/10.3390/catal11121528
Chicago/Turabian StyleYaseen, Ghulam, Muhammad A. Sarfraz, Saima Naveed, Farooq Ahmad, Fehmeada Bibi, Irfan Irshad, Muhammad Asif, Talat N. Pasha, and Shafqat N. Qaisrani. 2021. "Effects of Thermally Oxidized Vegetable Oil on Growth Performance and Carcass Characteristics, Gut Morphology, Nutrients Utilization, Serum Cholesterol and Meat Fatty Acid Profile in Broilers" Catalysts 11, no. 12: 1528. https://doi.org/10.3390/catal11121528
APA StyleYaseen, G., Sarfraz, M. A., Naveed, S., Ahmad, F., Bibi, F., Irshad, I., Asif, M., Pasha, T. N., & Qaisrani, S. N. (2021). Effects of Thermally Oxidized Vegetable Oil on Growth Performance and Carcass Characteristics, Gut Morphology, Nutrients Utilization, Serum Cholesterol and Meat Fatty Acid Profile in Broilers. Catalysts, 11(12), 1528. https://doi.org/10.3390/catal11121528






