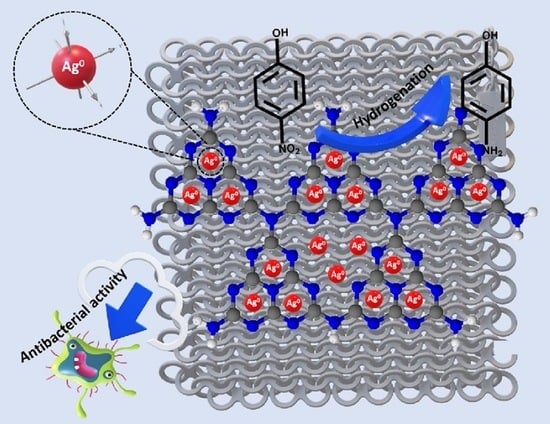
Self-Supporting g-C3N4 Nanosheets/Ag Nanoparticles Embedded onto Polyester Fabric as “Dip-Catalyst” for Synergic 4-Nitrophenol Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Graphitic Carbon Nitride (GCN)
2.2. Characterization of PES Based Materials
2.3. Antibacterial Activity and Antibacterial Adhesion
2.4. Catalytic Hydrogenation of 4-Nitrophenol over PES-GCNN/Ag0
2.5. Recyclability and Stability of Catalyst
2.6. Mechanism of 4-NP Reduction Reaction by PES-GCNN/Ag0
3. Experimental Section
3.1. Materials and Chemicals
3.2. Preparation of Graphitic Carbon Nitride Nanosheets (GCNN)
3.3. Preparation of Polyester Coated GCNN and GCNN/Ag NPs
3.4. Catalytic Hydrogenation of 4-Nitrophenol
3.5. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Melián, J.A.; Martín-Rodríguez, A.J.; Ortega-Méndez, A.; Araña, J.; Doña-Rodríguez, J.M.; Pérez-Peña, J. Degradation and detoxification of 4-nitrophenol by advanced oxidation technologies and bench-scale constructed wetlands. J. Environ. Manag. 2012, 105, 53–60. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.Y.; Liu, Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426–8430. [Google Scholar] [CrossRef]
- Lv, J.J.; Wang, A.J.; Ma, X.; Xiang, R.Y.; Chen, J.R.; Feng, J.J. One-pot synthesis of porous Pt-Au nanodendrites supported on reduced graphene oxide nanosheets toward catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 290–296. [Google Scholar] [CrossRef]
- Lu, S.; Yu, J.; Cheng, Y.; Wang, Q.; Barras, A.; Xu, W.; Szunerits, S.; Cornu, D.; Boukherroub, R. Preparation of silver nanoparticles/polydopamine functionalized polyacrylonitrile fiber paper and its catalytic activity for the reduction 4-nitrophenol. Appl. Surf. Sci. 2017, 411, 163–169. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Cai, Z.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: Their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]
- Layek, K.; Kantam, M.L.; Shirai, M.; Nishio-Hamane, D.; Sasaki, T.; Maheswaran, H. Gold nanoparticles stabilized on nanocrystalline magnesium oxide as an active catalyst for reduction of nitroarenes in aqueous medium at room temperature. Green Chem. 2012, 14, 3164–3174. [Google Scholar] [CrossRef]
- Chi, Y.; Tu, J.; Wang, M.; Li, X.; Zhao, Z. One-pot synthesis of ordered mesoporous silver nanoparticle/carbon composites for catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2014, 423, 54–59. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Y.; Feng, W.; Qi, J.; Zhang, G.; Zhang, F.; Fan, X. Robust and smart gold nanoparticles: One-step synthesis, tunable optical property, and switchable catalytic activity. J. Mater. Chem. 2011, 21, 6173–6178. [Google Scholar] [CrossRef]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold nanoparticles: Past, present, and future. Langmuir 2009, 25, 13840–13851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Sun, Y.; Mu, J.; Zhang, M.; Zhang, P.; Guo, Z.; Liang, P.; Wang, C.; Liu, Y. Tubular nanocomposite catalysts based on size-controlled and highly dispersed silver nanoparticles assembled on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 1387–1395. [Google Scholar] [CrossRef]
- Hsu, K.C.; Chen, D.H. Green synthesis and synergistic catalytic effect ofAg/reduced graphene oxide nanocomposite. Nanoscale Res. Lett. 2014, 9, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, J.R.; Lai, B.H.; Hsu, K.C.; Chen, D.H. One-pot green synthesis of silver/iron oxide composite nanoparticles for 4-nitrophenol reduction. J. Hazard. Mater. 2013, 248–249, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xiao, Z.; Zhai, S.; Zhai, B.; Zhang, F.; An, Q. Fabrication of highly-stable Ag/CA@GTA hydrogel beads and their catalytic application. RSC Adv. 2014, 4, 60460–60466. [Google Scholar] [CrossRef]
- Özkar, S.; Finke, R.G. Nanocluster formation and stabilization fundamental studies: Ranking commonly employed anionic stabilizers via the development, then application, of five comparative criteria. J. Am. Chem. Soc. 2002, 124, 5796–5810. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T. Photochemical green synthesis of calcium-alginate-stabilized ag and au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2010, 26, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Hortigüela, M.J.; Aranaz, I.; Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Chitosan gelation induced by the in situ formation of gold nanoparticles and its processing into macroporous scaffolds. Biomacromolecules 2011, 12, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chtchigrovsky, M.; Lin, Y.; Ouchaou, K.; Chaumontet, M.; Robitzer, M.; Quignard, F.; Taran, F. Dramatic effect of the gelling cation on the catalytic performances of alginate-supported palladium nanoparticles for the Suzuki-Miyaura reaction. Chem. Mater. 2012, 24, 1505–1510. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ashraf, M.; Hussain, T.; Rehman, A.; Malik, M.M.; Raza, Z.A.; Nawab, Y.; Zia, Q. In situ deposition of TiO2 nanoparticles on polyester fabric and study of its functional properties. Fibers Polym. 2015, 16, 1092–1097. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Montazer, M. Reduced graphene oxide/SnO2 nanocomposite on PET surface: Synthesis, characterization and application as an electro-conductive and ultraviolet blocking textile. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 507–513. [Google Scholar] [CrossRef]
- Mohammadi, M.; Karimi, L.; Mirjalili, M. Simultaneous synthesis of nano ZnO and surface modification of polyester fabric. Fibers Polym. 2016, 17, 1371–1377. [Google Scholar] [CrossRef]
- Guo, M.X.; Bian, S.W.; Shao, F.; Liu, S.; Peng, Y.H. Hydrothermal synthesis and electrochemical performance of MnO2/graphene/polyester composite electrode materials for flexible supercapacitors. Electrochim. Acta 2016, 209, 486–497. [Google Scholar] [CrossRef]
- Karimi, L.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.; Mirjalili, M. Optimizing the photocatalytic properties and the synergistic effects of graphene and nano titanium dioxide immobilized on cotton fabric. Appl. Surf. Sci. 2015, 332, 665–673. [Google Scholar] [CrossRef]
- Tian, M.; Tang, X.; Qu, L.; Zhu, S.; Guo, X.; Han, G. Robust ultraviolet blocking cotton fabric modified with chitosan/graphene nanocomposites. Mater. Lett. 2015, 145, 340–343. [Google Scholar] [CrossRef]
- Moazami, A.; Montazer, M.; Dolatabadi, M.K. Reduction of 4-nitrophenol to 4-aminophenol over sonoimmobilized silver/reduced graphene oxide nanocomposites on polyester fabric. Fibers Polym. 2017, 18, 2287–2297. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, M.; Wakida, T.; Saito, M.; Yamashiro, T.; Nishi, K.; Inoue, G.; Ishida, S. Ozone-gas treatment of cationic dyeable polyester and poly(butylene terephthalate) fibers. J. Appl. Polym. Sci. 2007, 104, 2423–2429. [Google Scholar] [CrossRef]
- Moazami, A.; Montazer, M.; Dolatabadi, M.K. Tunable functional properties on polyester fabric using simultaneous green reduction of graphene oxide and silver nitrate. Fibers Polym. 2016, 17, 1359–1370. [Google Scholar] [CrossRef]
- Majdoub, M.; Amedlous, A.; Anfar, Z.; Jada, A.; El Alem, N. Engineering of amine-based binding chemistry on functionalized graphene oxide/alginate hybrids for simultaneous and efficient removal of trace heavy metals: Towards drinking water. J. Colloid Interface Sci. 2021, 589, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Versatile graphene-promoting photocatalytic performance of semiconductors: Basic principles, synthesis, solar energy conversion, and environmental applications. Adv. Funct. Mater. 2013, 23, 4996–5008. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, J.; Li, X.; Hu, T.; Wang, J.; Sui, Y.; Wu, X.; Kong, L. Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J. Phys. Chem. Solids 2014, 75, 441–446. [Google Scholar] [CrossRef]
- Pan, C.; Xu, J.; Wang, Y.; Li, D.; Zhu, Y. Dramatic activity of C 3N 4/BiPO 4 photocatalyst with core/shell structure formed by self-assembly. Adv. Funct. Mater. 2012, 22, 1518–1524. [Google Scholar] [CrossRef]
- Lv, H.; Ji, G.; Yang, Z.; Liu, Y.; Zhang, X.; Liu, W.; Zhang, H. Enhancement photocatalytic activity of the graphite-like C3N4 coated hollow pencil-like ZnO. J. Colloid Interface Sci. 2015, 450, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Liu, W.J.; Jiang, H. Comparative investigation on photoreactivity and mechanism of biogenic and chemosythetic Ag/C3N4 composites under visible light irradiation. ACS Sustain. Chem. Eng. 2015, 3, 269–276. [Google Scholar] [CrossRef]
- Majdoub, M.; Amedlous, A.; Anfar, Z.; Moussaoui, O. MoS2 nanosheets/silver nanoparticles anchored onto textile fabric as “dip catalyst” for synergistic p-nitrophenol hydrogenation. Environ. Sci. Pollut. Res. 2021, 28, 64674–64686. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Yang, J. Separation and concentration of natural products by fast forced adsorption using well-dispersed velvet-like graphitic carbon nitride with response surface methodology optimisation. Talanta 2016, 154, 119–126. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Huang, Y.; Wang, X.; Hayat, T.; Li, J.; Xu, X.; Wang, X. Ultrathin g-C3N4 nanosheets coupled with amorphous Cu-doped FeOOH nanoclusters as 2D/0D heterogeneous catalysts for water remediation. Environ. Sci. Nano 2018, 5, 1179–1190. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, W.; Sun, Y.; Dong, F.; Zhou, Y.; Ho, W.K. Water-assisted production of honeycomb-like g-C3N4with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale 2015, 7, 2471–2479. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, X.; Ouyang, S.; Zhu, Y. Polyoxometalates covalently combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production. Catal. Sci. Technol. 2018, 8, 1686–1695. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2,5,8-Triamino-tri- s -triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon Nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid-State NMR, and Theoretical Studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Shang, J.K.; Jiang, Q.; Wang, Y.; Li, Y.X. Facile alkali-assisted synthesis of g-C3N4 materials and their high-performance catalytic application in solvent-free cycloaddition of CO2 to epoxides. RSC Adv. 2016, 6, 55382–55392. [Google Scholar] [CrossRef]
- Molina, J.; Zille, A.; Fernández, J.; Souto, A.P.; Bonastre, J.; Cases, F. Conducting fabrics of polyester coated with polypyrrole and doped with graphene oxide. Synth. Met. 2015, 204, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Berendjchi, A.; Khajavi, R.; Yousefi, A.A.; Yazdanshenas, M.E. Improved continuity of reduced graphene oxide on polyester fabric by use of polypyrrole to achieve a highly electro-conductive and flexible substrate. Appl. Surf. Sci. 2016, 363, 264–272. [Google Scholar] [CrossRef]
- Alongi, J.; Camino, G.; Malucelli, G. Heating rate effect on char yield from cotton, poly(ethylene terephthalate) and blend fabrics. Carbohydr. Polym. 2013, 92, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Hou, S.; Li, J.; Huang, X.; Wang, X.; Ma, L.; Shen, W.; Kang, F.; Huang, Z.-H. Silver Nanoparticles-Loaded Exfoliated Graphite and Its Anti-Bacterial Performance. Appl. Sci. 2017, 7, 852. [Google Scholar] [CrossRef] [Green Version]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Luo, Y.; Lu, W.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ag nanoparticles decorated polyaniline nanofibers: Synthesis, characterization, and applications toward catalytic reduction of 4-nitrophenol and electrochemical detection of H2O2 and glucose. Catal. Sci. Technol. 2012, 2, 800–806. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, Y.Y.; Pang, F.; Chen, G.Q.; Zhang, X. Ag nanoparticles supported on N-doped graphene hybrids for catalytic reduction of 4-nitrophenol. RSC Adv. 2014, 4, 43204–43211. [Google Scholar] [CrossRef]
- Qiao, X.Q.; Zhang, Z.W.; Tian, F.Y.; Hou, D.F.; Tian, Z.F.; Li, D.S.; Zhang, Q. Enhanced Catalytic Reduction of p-Nitrophenol on Ultrathin MoS2 Nanosheets Decorated with Noble Metal Nanoparticles. Cryst. Growth Des. 2017, 17, 3538–3547. [Google Scholar] [CrossRef]
- Wang, M.; Tian, D.; Tian, P.; Yuan, L. Synthesis of micron-SiO2 @nano-Ag particles and their catalytic performance in 4-nitrophenol reduction. Appl. Surf. Sci. 2013, 283, 389–395. [Google Scholar] [CrossRef]
- Du, X.; He, J.; Zhu, J.; Sun, L.; An, S. Ag-deposited silica-coated Fe3O4 magnetic nanoparticles catalyzed reduction of p-nitrophenol. Appl. Surf. Sci. 2012, 258, 2717–2723. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Synthesis and characterization of g-C3N4 nanosheets decorated Ag2S composites for investigation of catalytic reduction of 4-nitrophenol, antioxidant and antimicrobial activities. J. Mol. Struct. 2019, 1186, 423–433. [Google Scholar] [CrossRef]
- Baruah, B.; Gabriel, G.J.; Akbashev, M.J.; Booher, M.E. Facile synthesis of silver nanoparticles stabilized by cationic polynorbornenes and their catalytic activity in 4-nitrophenol reduction. Langmuir 2013, 29, 4225–4234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Amedlous, A.; Majdoub, M.; Amaterz, E.; Anfar, Z.; Benlhachemi, A. Synergistic Effect of g-C3N4 Nanosheets/Ag3PO4 Microcubes as Efficient n-p-Type Heterostructure based Photoanode for Photoelectrocatalytic Dye Degradation. J. Photochem. Photobiol. A Chem. 2021, 113127. [Google Scholar] [CrossRef]
- Lee, D.T.; Zhao, J.; Peterson, G.W.; Parsons, G.N. Catalytic “mOF-Cloth” Formed via Directed Supramolecular Assembly of UiO-66-NH2 Crystals on Atomic Layer Deposition-Coated Textiles for Rapid Degradation of Chemical Warfare Agent Simulants. Chem. Mater. 2017, 29, 4894–4903. [Google Scholar] [CrossRef]












| Sample Code | Stage 1: Ester Linkage Decomposition | Stage 2: C–C Cleavage (Oxidation) | ||
|---|---|---|---|---|
| Tdi–Tdf (°C) | Tdmax (°C) | Tdi–Tdf (°C) | Tdmax (°C) | |
| PES | 300.1–489.1 | 419.2 | 498.5–579.4 | 540.2 |
| PES-GCNN | 308.6–496.4 | 431.1 | 501.7–606.7 | 590.9 |
| PES-GCNN/Ag010 | 315.5–448.3 | 439.3 | 449.9–495.2 | 458.4 |
| Sample | m/m0(t = 0) | m/m0(t = 24h) |
|---|---|---|
| PES-GCNN | 1 | 1 |
| PES-GCNN/Ag0 10 | 1 | 0.99 |
| PES-Ag0 (reference) | 1 | 0.48 |
| Catalyst | Support | 4-NP (mM) | kapp (min−1) | Quantity of Catalyst Used (mg) | Reaction Time (min) | Conve-rsion % | Ref. |
|---|---|---|---|---|---|---|---|
| Ag NPs | None | 5 | 0.19 | 2.7 | 20 | 100 | [52] |
| RGO/Ag | Graphene oxide | 0.2 | 0.216 | 1 | 15 | 100 | [53] |
| RN–GO/Ag | N-doped graphene oxide | 0.2 | 0.444 | 1 | 6.6 | 100 | [53] |
| Ag/MoS2 | MoS2 | 0.1 | 0.29 | 1.5 | 12 | 94.5 | [54] |
| SiO2/Ag | SiO2 | 0.12 | 0.213 | 15 | 10 | 78 | [55] |
| Fe3O4@SiO2–Ag | Fe3O4@SiO2 | 5 | 0.83 | 3 | 4 | 96 | [56] |
| Ag/C | Carbon | 0.12 | 0.32 | 2 | 12 | 98 | [8] |
| Ag/PANI | Polyaniline | 5 | 1.28 | 2.7 | 3 | 100 | [52] |
| g-C3N4/Ag2S | Graphitic carbon nitride | 2.5 | 0.0311 | 0.0008 (10 mg/L) | 42 | 97 | [57] |
| PES-GCNN/Ag0 | PES-GCNN | 0.03 | 0.462 | 20 a | 5 | 90 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amedlous, A.; Majdoub, M.; Anfar, Z.; Amaterz, E. Self-Supporting g-C3N4 Nanosheets/Ag Nanoparticles Embedded onto Polyester Fabric as “Dip-Catalyst” for Synergic 4-Nitrophenol Hydrogenation. Catalysts 2021, 11, 1533. https://doi.org/10.3390/catal11121533
Amedlous A, Majdoub M, Anfar Z, Amaterz E. Self-Supporting g-C3N4 Nanosheets/Ag Nanoparticles Embedded onto Polyester Fabric as “Dip-Catalyst” for Synergic 4-Nitrophenol Hydrogenation. Catalysts. 2021; 11(12):1533. https://doi.org/10.3390/catal11121533
Chicago/Turabian StyleAmedlous, Abdallah, Mohammed Majdoub, Zakaria Anfar, and Elhassan Amaterz. 2021. "Self-Supporting g-C3N4 Nanosheets/Ag Nanoparticles Embedded onto Polyester Fabric as “Dip-Catalyst” for Synergic 4-Nitrophenol Hydrogenation" Catalysts 11, no. 12: 1533. https://doi.org/10.3390/catal11121533