Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis
Abstract
:1. Introduction
2. Synthesis of Mesoporous Zeolites
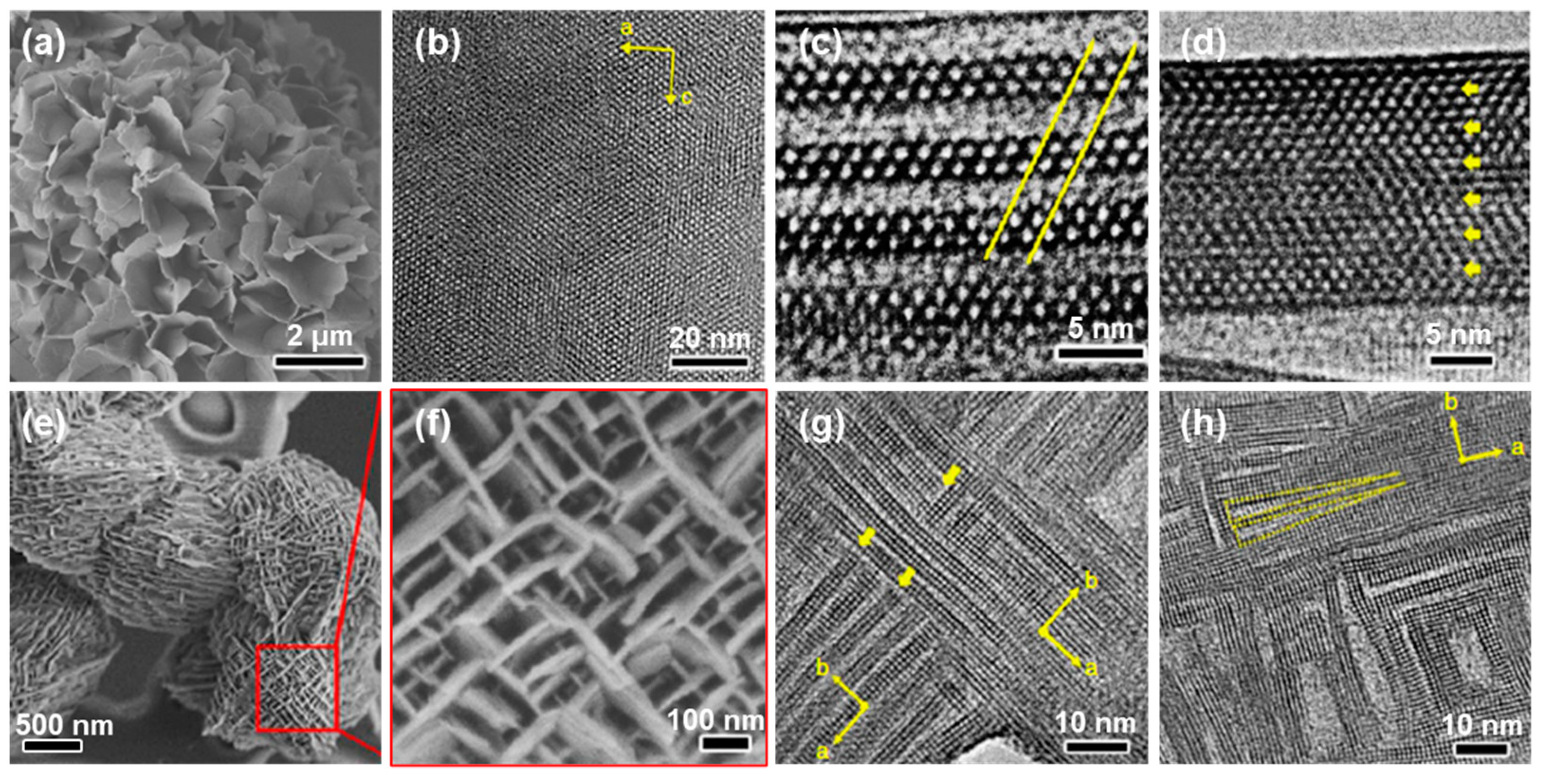
2.1. Templating Approach
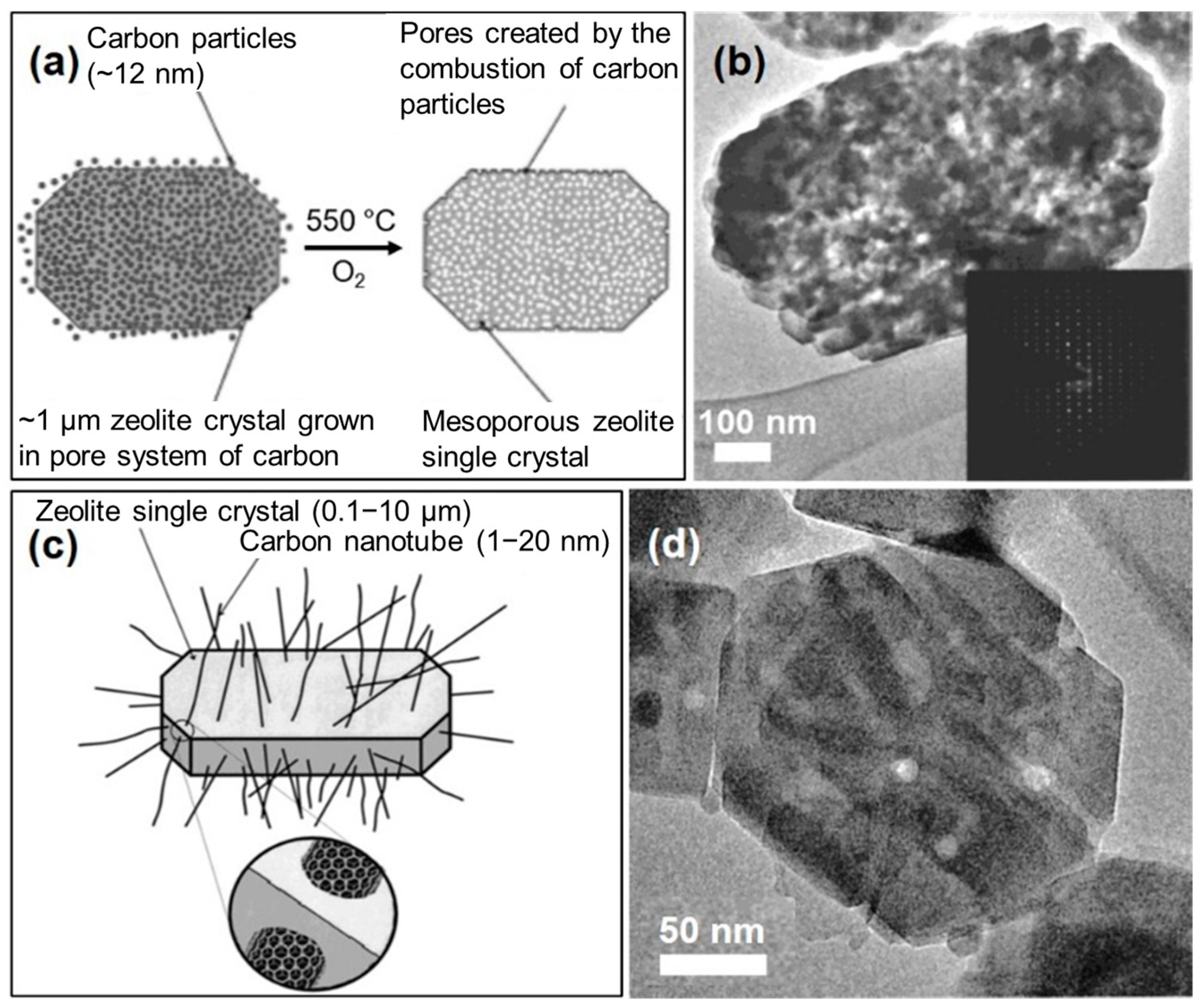
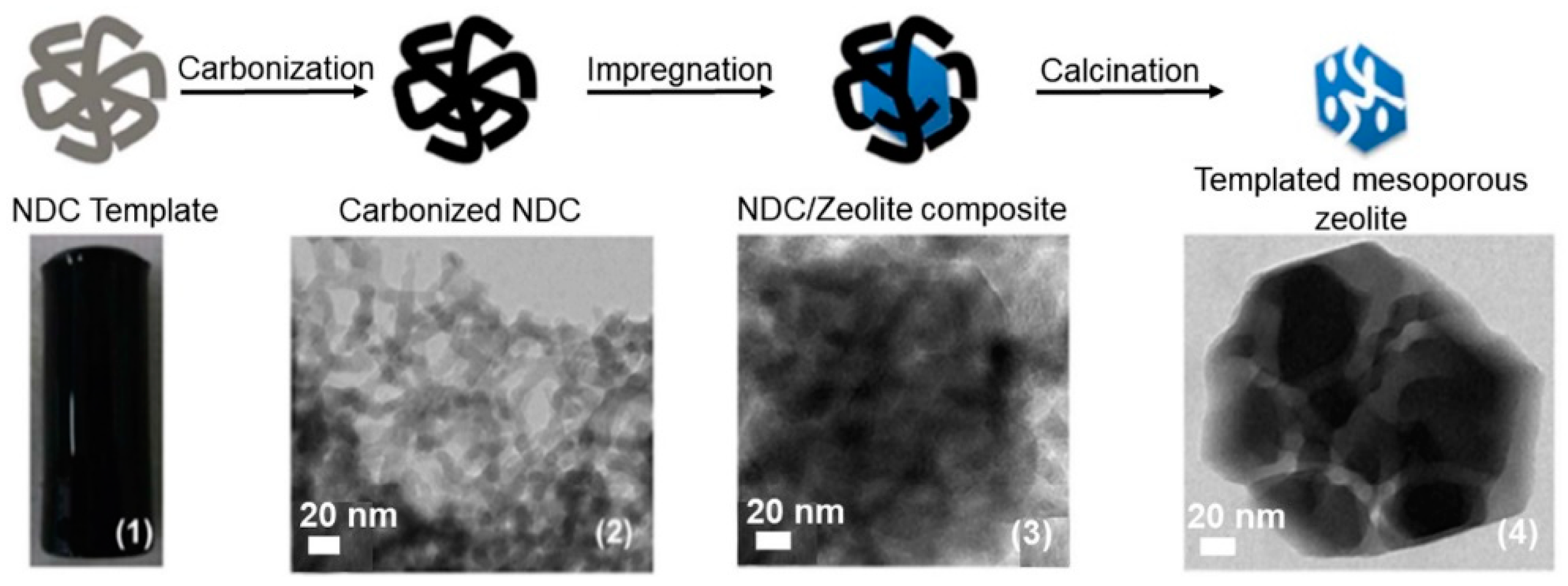
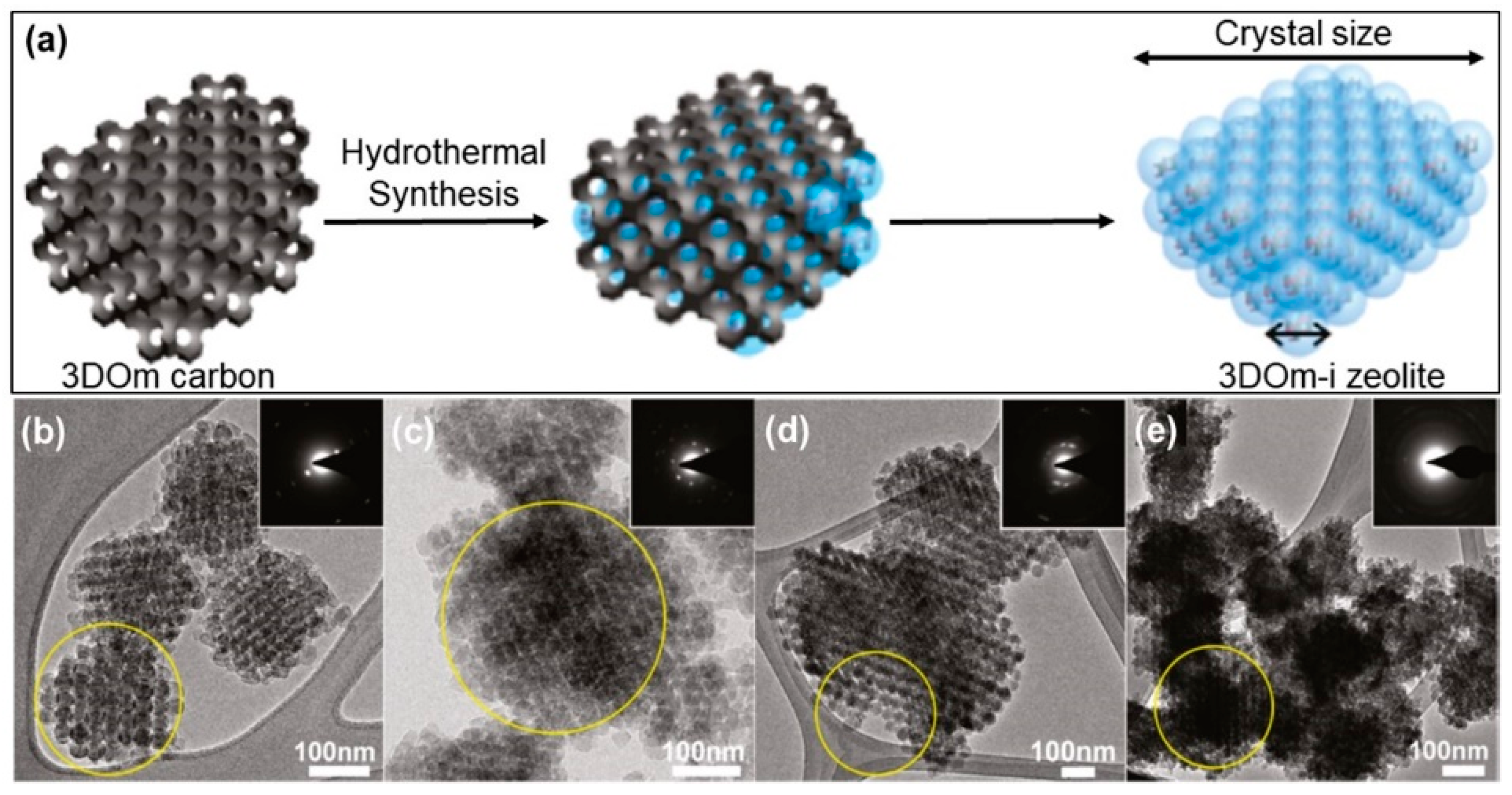
2.1.1. Hard-Templating Method
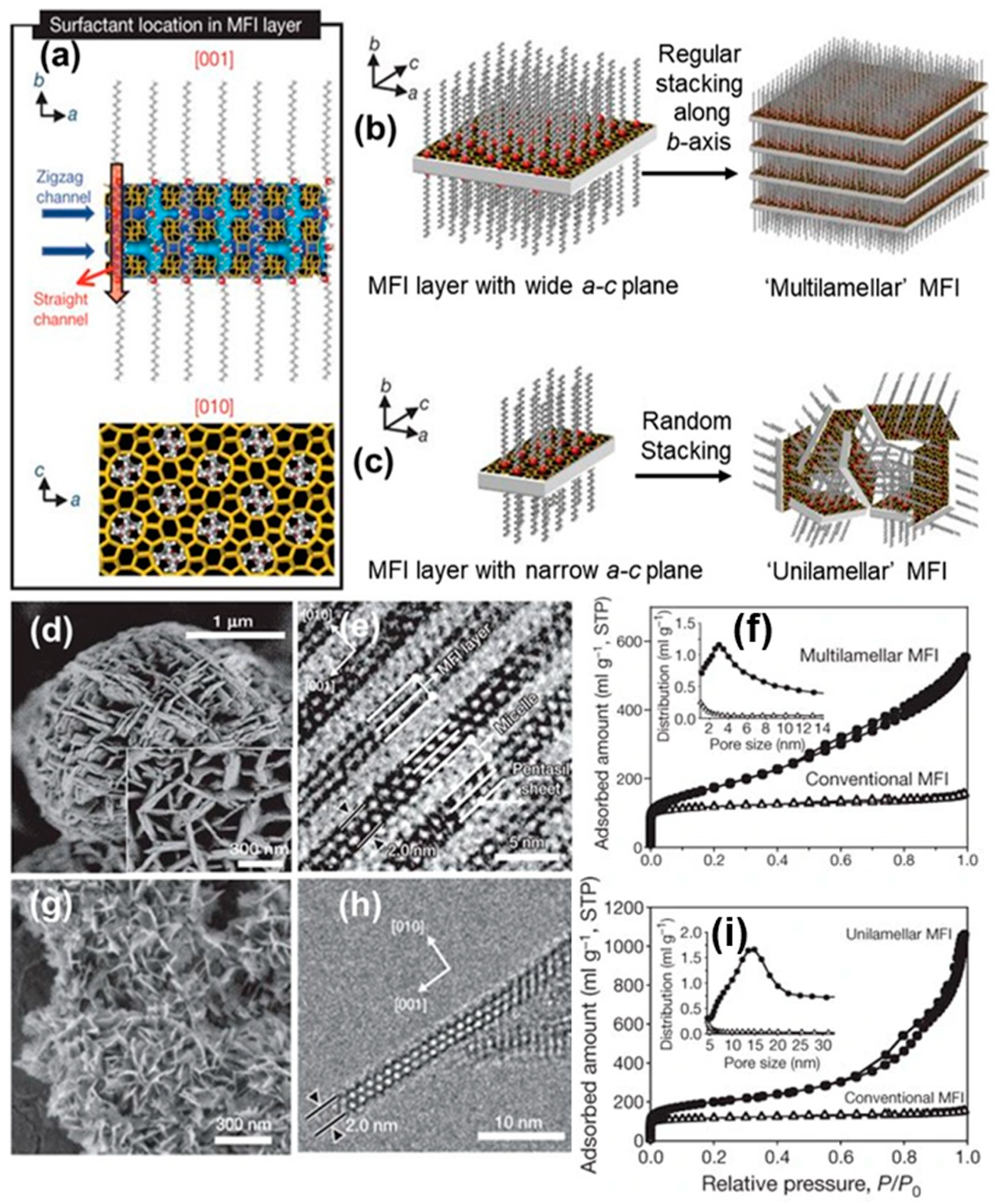
2.1.2. Soft-Templating Method
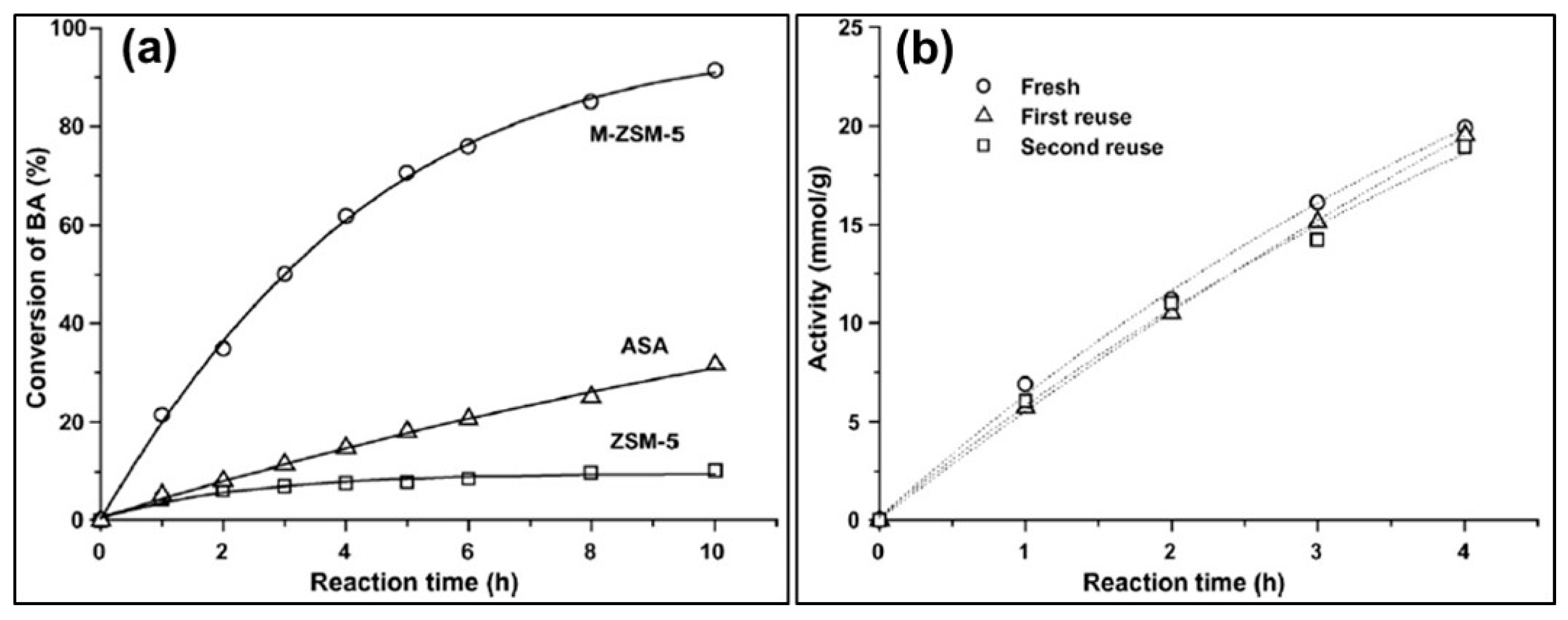
2.2. Post-Synthesis Treatment (Demetallation)
2.2.1. Dealumination
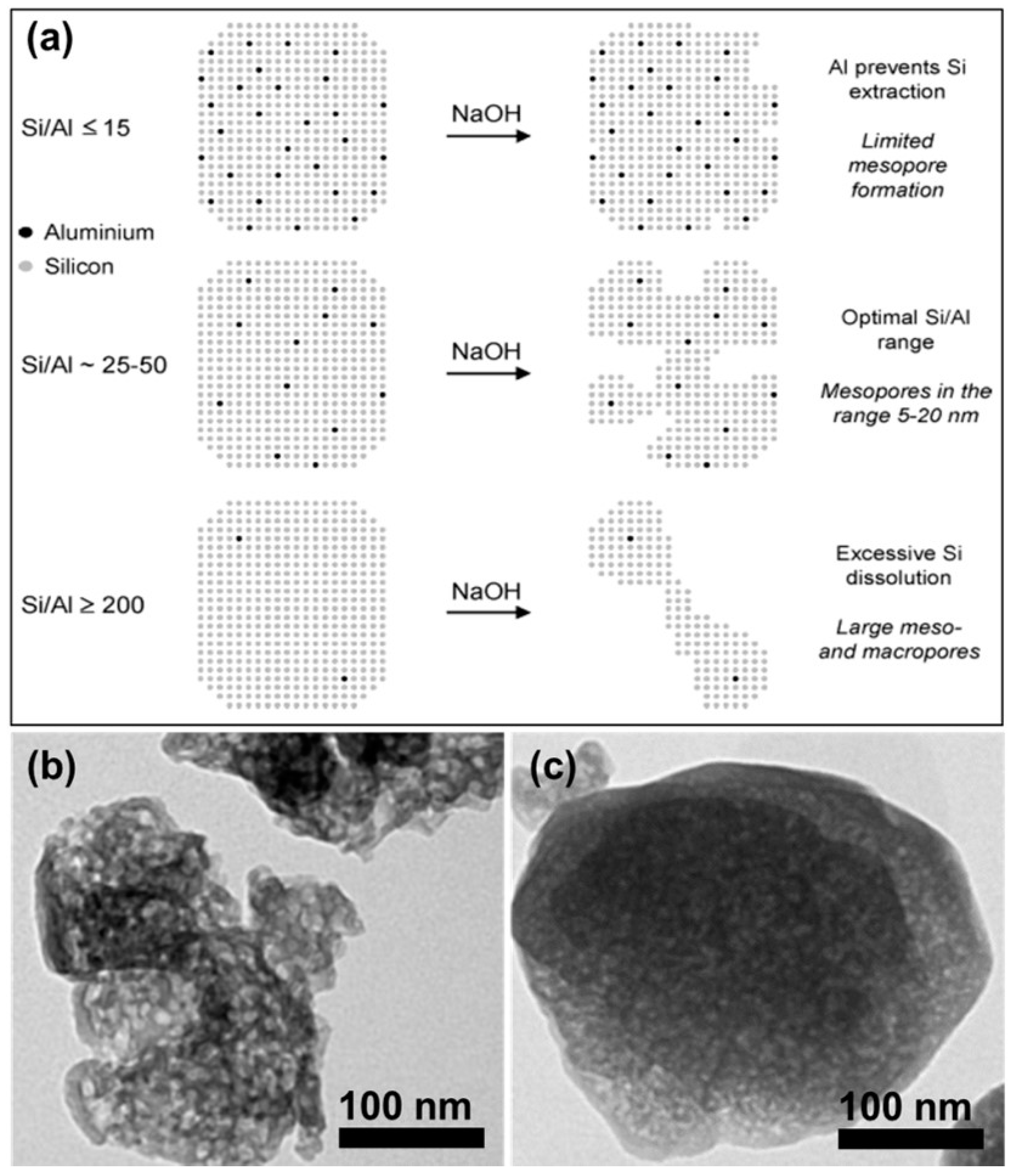
2.2.2. Desilication
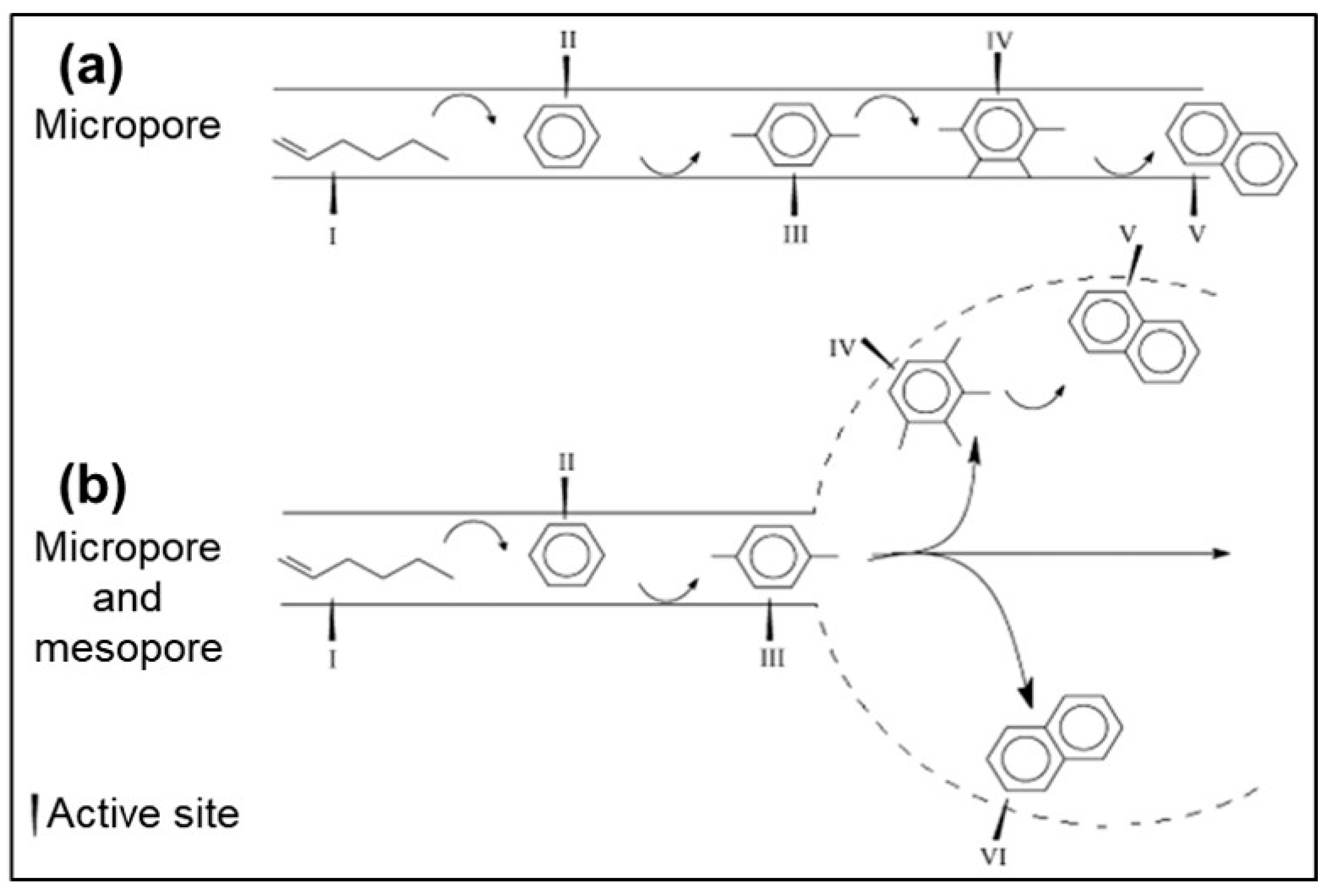
3. Application of Mesoporous Zeolites in Heterogeneous Catalysis
3.1. Catalytic Cracking
3.2. Isomerization
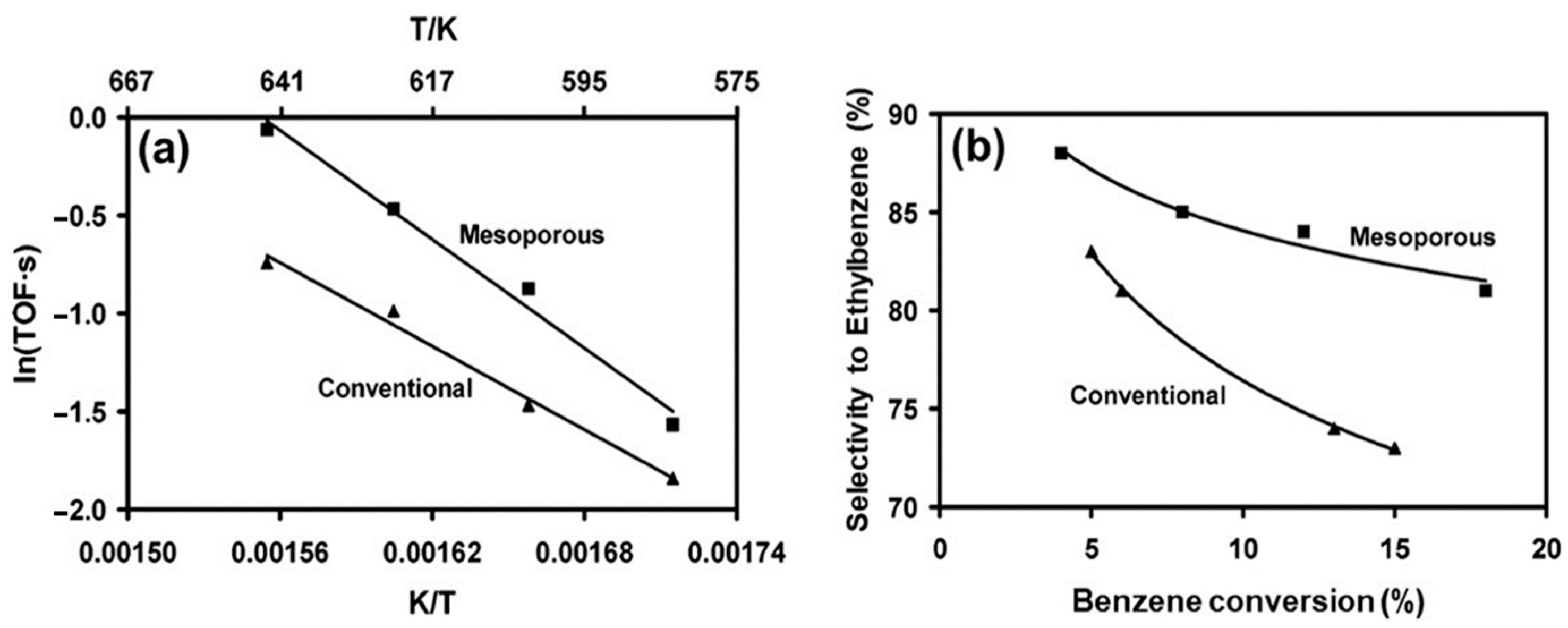
3.3. Alkylation and Acylation Reactions
3.4. Methanol-to-Hydrocarbon (MTH) Conversion
3.5. Fischer–Tropsch Synthesis (FTS)
3.6. Fine-Chemical Syntheses
3.6.1. Conversion of Glycerol
3.6.2. Cycloaddition of CO2 with Epoxides
3.7. Dehydrogenation of Light Alkanes
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Li, R.; Xue, T.; Bingre, R.; Gao, Y.; Louis, B.; Wang, Q. Microporous Zeolite@Vertically Aligned Mg–Al Layered Double Hydroxide Core@Shell Structures with Improved Hydrophobicity and Toluene Adsorption Capacity under Wet Conditions. ACS Appl. Mater. Interfaces 2018, 10, 34834–34839. [Google Scholar] [CrossRef]
- Park, B.-G.; Chung, K.-H. Catalytic properties of microporous zeolites in the catalytic cracking of m-diisopropylbenzene. Mol. Catal. 2018, 461, 80–85. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.-P.; Retoux, R.; Boullay, P.; Goupil, J.-M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O.; Khan, I.; Helal, A.; Al-Maythalony, B.A.; et al. Propene Adsorption-Chemisorption Behaviors on H-SAPO-34 Zeolite Catalysts at Different Temperatures. Catalysts 2019, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Xin, Y.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Zhang, Z. Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3. Catalysts 2019, 9, 882. [Google Scholar] [CrossRef] [Green Version]
- Bhadauria, J.; Singh, B.K.; Tomar, A.; Tomar, R. Synthesis and characterization of analogue of mordenite and its role as a catalyst for Friedel-Crafts acylation of anisole. J. Chem. Pharm. Res. 2011, 3, 245–257. [Google Scholar]
- Singh, B.K.; Lee, S.; Na, K. An overview on metal-related catalysts: Metal oxides, nanoporous metals and supported metal nanoparticles on metal organic frameworks and zeolites. Rare Metals 2020, 39, 751–766. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, Y.; Singh, B.K.; Na, K. Selective and rapid capture of Sr2+ with LTA zeolites: Effect of crystal sizes and mesoporosity. Appl. Surf. Sci. 2020, 506, 145029. [Google Scholar] [CrossRef]
- Millini, R.; Bellussi, G.; Smeets, S.; Xiaodong, Z.; Strohmaier, K. Zeolites in Catalysis: Properties and Applications. R. Soc. Chem. 2017, 28, 4. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Structure Commission of the International Zeolite Association (IZA-SC). Database of Zeolite Structures. 2013. Available online: http://www.iza-online.org/ (accessed on 15 November 2021).
- Na, K.; Somorjai, G.A. Hierarchically nanoporous zeolites and their heterogeneous catalysis: Current status and future perspectives. Catal. Lett. 2015, 145, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Bereciartua, P.J.; Cantín, Á.; Corma, A.; Jordá, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W.; Kortunov, P.; Ravikovitch, P.I.; et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.; Yip, A.C.K. Advances in the Green Synthesis of Microporous and Hierarchical Zeolites: A Short Review. Catalysts 2019, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Abdulridha, S.; Jiao, Y.; Xu, S.; Zhang, R.; Ren, Z.; Garforth, A.A.; Fan, X. A Comparative Study on Mesoporous Y Zeolites Prepared by Hard-Templating and Post-Synthetic Treatment Methods. Appl. Catal. A Gen. 2021, 612, 117986. [Google Scholar] [CrossRef]
- Losch, P.; Hoff, T.C.; Kolb, J.F.; Bernardon, C.; Tessonnier, J.-P.; Louis, B. Mesoporous ZSM-5 zeolites in acid catalysis: Top-down vs. Bottom-up approach. Catalysts 2017, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Nawaz, F.; Xiao, F.-S. Templating route for synthesizing mesoporous zeolites with improved catalytic properties. Nano Today 2009, 4, 292–301. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Major advances in the development of ordered mesoporous materials. Chem. Commun. 2020, 56, 7836–7848. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; de Jong, K.P.; Zečević, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Jing, Z.; Cao, F.; Sun, H.; Che, S. Surfactants with aromatic-group tail and single quaternary ammonium head for directing single-crystalline mesostructured zeolite nanosheets. Chem. Mater. 2014, 26, 4612–4619. [Google Scholar] [CrossRef]
- Jia, X.; Jo, C.; Yip, A.C. Synthesis Strategies for Hierarchical Zeolites. Heterogeneous Catalysts: Advanced Design. Charact. Appl. 2021, 1, 119–145. [Google Scholar]
- Jiao, Y.; Forster, L.; Xu, S.; Chen, H.; Han, J.; Liu, X.; Zhou, Y.; Liu, J.; Zhang, J.; Yu, J.; et al. Creation of Al-Enriched Mesoporous ZSM-5 Nanoboxes with High Catalytic Activity: Converting Tetrahedral Extra-Framework Al into Framework Sites by Post Treatment. Angew. Chem. Int. Ed. 2020, 59, 19478–19486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyra, K.; Tarach, K.A.; Góra-Marek, K. Towards a greater olefin share in polypropylene cracking–Amorphous mesoporous aluminosilicate competes with zeolites. Appl. Catal. B 2021, 297, 120408. [Google Scholar] [CrossRef]
- Sun, M.H.; Chen, L.H.; Yu, S.; Li, Y.; Zhou, X.G.; Hu, Z.Y.; Sun, Y.H.; Xu, Y.; Su, B.L. Micron-Sized Zeolite Beta Single Crystals Featuring Intracrystal Interconnected Ordered Macro-Meso-Microporosity Displaying Superior Catalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 19582–19591. [Google Scholar] [CrossRef]
- Meng, Q.; Doetschman, D.C.; Rizos, A.K.; Lee, M.H.; Schulte, J.T.; Spyros, A.; Kanyi, C.W. Adsorption of organophosphates into microporous and mesoporous NaX zeolites and subsequent chemistry. Environ. Sci. Technol. 2011, 45, 3000–3005. [Google Scholar] [CrossRef]
- Fattahi, N.; Triantafyllidis, K.; Luque, R.; Ramazani, A. Zeolite-Based Catalysts: A Valuable Approach toward Ester Bond Formation. Catalysts 2019, 9, 758. [Google Scholar] [CrossRef] [Green Version]
- Möller, K.; Bein, T. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef] [Green Version]
- Madsen, C.; Jacobsen, C.H. Nanosized zeolite crystals—Convenient control of crystal size distribution by confined space synthesis. Chem. Commun. 1999, 8, 673–674. [Google Scholar] [CrossRef]
- Jacobsen, C.J.; Madsen, C.; Houzvicka, J.; Schmidt, I.; Carlsson, A. Mesoporous zeolite single crystals. J. Am. Chem. Soc. 2000, 122, 7116–7117. [Google Scholar] [CrossRef]
- Schmidt, I.; Boisen, A.; Gustavsson, E.; Ståhl, K.; Pehrson, S.; Dahl, S.; Carlsson, A.; Jacobsen, C.J. Carbon nanotube templated growth of mesoporous zeolite single crystals. Chem. Mater. 2001, 13, 4416–4418. [Google Scholar] [CrossRef]
- Schmidt, F.; Paasch, S.; Brunner, E.; Kaskel, S. Carbon templated SAPO-34 with improved adsorption kinetics and catalytic performance in the MTO-reaction. Microporous Mesoporous Mater. 2012, 164, 214–221. [Google Scholar] [CrossRef]
- Yang, Z.X.; Xia, Y.D.; Mokaya, R. Zeolite ZSM-5 with unique supermicropores synthesized using mesoporous carbon as a template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. ZSM-5 monolith of uniform mesoporous channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Uniform mesopore-donated zeolite Y using carbon aerogel templating. J. Phys. Chem. B 2003, 107, 10974–10976. [Google Scholar] [CrossRef]
- White, R.J.; Fischer, A.; Goebel, C.; Thomas, A. A sustainable template for mesoporous zeolite synthesis. J. Am. Chem. Soc. 2014, 136, 2715–2718. [Google Scholar] [CrossRef]
- Abdulridha, S.; Jiang, J.; Xu, S.; Zhou, Z.; Liang, H.; Mao, B.; Zhou, Y.; Garforth, A.A.; Jiao, Y.; Fan, X. Cellulose nanocrystals (CNCs) as hard templates for preparing mesoporous zeolite Y assemblies with high catalytic activity. Green Chem. 2020, 22, 5115–5122. [Google Scholar] [CrossRef]
- Wei, X.; Smirniotis, P.G. Synthesis and characterization of mesoporous ZSM-12 by using carbon particles. Microporous Mesoporous Mater. 2006, 89, 170–178. [Google Scholar] [CrossRef]
- Sakthivel, A.; Huang, S.J.; Chen, W.H.; Lan, Z.H.; Chen, K.H.; Kim, T.W.; Ryoo, R.; Chiang, A.S.; Liu, S.B. Replication of mesoporous aluminosilicate molecular sieves (RMMs) with zeolite framework from mesoporous carbons (CMKs). Chem. Mater. 2004, 16, 3168–3175. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, H. An ordered mesoporous aluminosilicate with completely crystalline zeolite wall structure. J. Am. Chem. Soc. 2006, 128, 10636–10637. [Google Scholar] [CrossRef]
- Fan, W.; Snyder, M.A.; Kumar, S.; Lee, P.S.; Yoo, W.C.; McCormick, A.V.; Penn, R.L.; Stein, A.; Tsapatsis, M. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat. Mater. 2008, 7, 984. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporous-imprinted structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hua, Z.; Liu, Z.; Wu, W.; Zhu, Y.; Shi, J. Direct synthetic strategy of mesoporous ZSM-5 zeolites by using conventional block copolymer templates and the improved catalytic properties. ACS Catal. 2011, 1, 287–291. [Google Scholar] [CrossRef]
- Shen, X.; Mao, W.; Ma, Y.; Xu, D.; Wu, P.; Terasaki, O.; Han, L.; Che, S. A hierarchical MFI zeolite with a two-dimensional square mesostructure. Angew. Chem. Int. Ed. 2018, 57, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, X.; Gong, Z.; Han, L.; Sun, H.; Che, S. Single-Crystalline MFI Zeolite with Sheet-Like Mesopores Layered along the a-Axis. Chem. Eur. J. 2019, 25, 738–742. [Google Scholar] [CrossRef]
- Xiao, F.S.; Wang, L.; Yin, C.; Lin, K.; Di, Y.; Li, J.; Xu, R.; Su, D.S.; Schlögl, R.; Yokoi, T.; et al. Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers. Angew. Chem. Int. Ed. 2006, 45, 3090–3093. [Google Scholar] [CrossRef] [Green Version]
- Na, K.; Park, W.; Seo, Y.; Ryoo, R. Disordered assembly of MFI zeolite nanosheets with a large volume of intersheet mesopores. Chem. Mat. 2011, 23, 1273–1279. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI zeolite nanosheets of a single-unit-cell thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.F.; Ryoo, R. Directing zeolite structures into hierarchically nanoporous architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Ma, Y.; Jing, Z.; Han, L.; Singh, B.; Feng, J.; Shen, X.; Cao, F.; Oleynikov, P.; Sun, H.; et al. π–π interaction of aromatic groups in amphiphilic molecules directing for single-crystalline mesostructured zeolite nanosheets. Nat. Commun. 2014, 5, 4262. [Google Scholar] [CrossRef]
- Singh, B.K.; Xu, D.; Han, L.; Ding, J.; Wang, Y.; Che, S. Synthesis of single-crystalline mesoporous ZSM-5 with three-dimensional pores via the self-assembly of a designed triply branched cationic surfactant. Chem. Mater. 2014, 26, 7183–7188. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Ryoo, R. The synthesis of a hierarchically porous BEA zeolite via pseudomorphic crystallization. Chem. Commun. 2009, 20, 2845–2847. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Ryoo, R. Cyclic diquaternary ammoniums for nanocrystalline BEA, MTW and MFI zeolites with intercrystalline mesoporosity. J. Mater. Chem. 2009, 19, 6713–6719. [Google Scholar] [CrossRef]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Hu, Z.; Ren, J.; Zhang, S.; Wang, Z.; Yuan, Z.-Y. ZnO Nanoclusters Supported on Dealuminated Zeolite β as a Novel Catalyst for Direct Dehyrdogenation of Propane to Propylene. ChemCatChem 2019, 11, 868–877. [Google Scholar] [CrossRef]
- Guisnet, M.; Ribeiro, F.R. Deactivation and Regeneration of Solid Catalysts; Imperial College Press: London, UK, 2011; pp. 3–18. [Google Scholar] [CrossRef]
- Zukal, A.; Patzelová, V.; Lohse, U. Secondary porous structure of dealuminated Y zeolites. Zeolites 1986, 6, 133–136. [Google Scholar] [CrossRef]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- van Oers, C.J.; Stevens, W.J.J.; Bruijn, E.; Mertens, M.; Lebedev, O.I.; van Tendeloo, G.; Meynen, V.; Cool, P. Formation of a combined micro-and mesoporous material using zeolite Beta nanoparticles. Microporous Mesoporous Mater. 2009, 120, 29–34. [Google Scholar] [CrossRef]
- Sulikowski, B. The fractal dimension in molecular sieves: Synthetic faujasite and related solids. J. Phys. Chem. B 1993, 97, 1420–1425. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P. Comparison of dealumination of zeolites beta, mordenite and ZSM-5 by treatment with acid under microwave irradiation. Microporous Mesoporous Mater. 2011, 144, 162–170. [Google Scholar] [CrossRef]
- Groen, J.C.; Zhu, W.; Brouwer, S.; Huynink, S.J.; Kapteijn, F.; Moulijn, J.A.; Pérez-Ramírez, J. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication. J. Am. Chem. Soc. 2007, 129, 355–360. [Google Scholar] [CrossRef]
- Ogura, M.; Shinomiya, S.-Y.; Tateno, J.; Nara, Y.; Nomura, M.; Kikuchi, E.; Matsukata, M. Alkali-treatment technique—new method for modification of structural and acid-catalytic properties of ZSM-5 zeolites. Appl. Catal. A Gen. 2001, 219, 33–43. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Optimal aluminum-assisted mesoporosity development in MFI zeolites by desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Desilication mechanism revisited: Highly mesoporous all-silica zeolites enabled through pore-directing agents. Chem. Eur. J. 2011, 17, 1137–1147. [Google Scholar] [CrossRef]
- Abelló, S.; Bonilla, A.; Pérez-Ramírez, J. Mesoporous ZSM-5 zeolite catalysts prepared by desilication with organic hydroxides and comparison with NaOH leaching. Appl. Catal. A Gen. 2009, 364, 191–198. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite catalysts with tunable hierarchy factor by pore-growth moderators. Adv. Funct. Mat. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Wojciechowski, B.W.; Corma, A. Catalytic Cracking: Catalysts, Chemistry, and Kinetics; Marcel Dekker Inc.: New York, NY, USA, 1986. [Google Scholar]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef] [Green Version]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.S.; Park, J.W.; Seo, G. Catalytic cracking of n-octane over alkali-treated MFI zeolites. Appl. Catal. A Gen. 2005, 288, 149–157. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Suzuki, K.; Aoyagi, Y.; Katada, N.; Choi, M.; Ryoo, R.; Niwa, M. Acidity and catalytic activity of mesoporous ZSM-5 in comparison with zeolite ZSM-5, Al-MCM-41 and silica–alumina. Catal. Today 2008, 132, 38–45. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhang, Z.; Xie, S.; Zhu, X.; Xu, L. Aromatization and isomerization of 1-hexene over alkali-treated HZSM-5 zeolites: Improved reaction stability. Appl. Catal. A Gen. 2008, 338, 100–113. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Wang, Y.; Bai, Y.; Sun, W.; Li, R. Synthesis and catalytic performance of a bi-phase core-shell zeolite composite. J. Porous Mater. 2009, 16, 731. [Google Scholar] [CrossRef]
- Christensen, C.H.; Schmidt, I.; Christensen, C.H. Improved performance of mesoporous zeolite single crystals in catalytic cracking and isomerization of n-hexadecane. Catal. Commun. 2004, 5, 543–546. [Google Scholar] [CrossRef]
- Kustova, M.Y.; Hasselriis, P.; Christensen, C.H. Mesoporous MEL–type zeolite single crystal catalysts. Catal. Lett. 2004, 96, 205–211. [Google Scholar] [CrossRef]
- Lei, Q.; Zhao, T.; Li, F.; Zhang, L.; Wang, Y. Catalytic cracking of large molecules over hierarchical zeolites. Chem. Commun. 2006, 16, 1769–1771. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Kong, D.; Wang, Y.; Xie, Z. Synthesis and catalytic performances of mesoporous zeolites templated by polyvinyl butyral gel as the mesopore directing agent. J. Phys. Chem. C 2008, 112, 17257–17264. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Paniagua, M.; Melero, J.A.; Riisager, A. Efficient isomerization of glucose to fructose over zeolites in consecutive reactions in alcohol and aqueous media. J. Am. Chem. Soc. 2013, 135, 5246–5249. [Google Scholar] [CrossRef]
- De Ménorval, B.; Ayrault, P.; Gnep, N.S.; Guisnet, M. Mechanism of n-butene skeletal isomerization over HFER zeolites: A new proposal. J. Catal. 2005, 230, 38–51. [Google Scholar] [CrossRef]
- Moushey, D.L.; Smirniotis, P.G. n-Heptane hydroisomerization over mesoporous zeolites made by utilizing carbon particles as the template for mesoporosity. Catal. Lett. 2009, 129, 20–25. [Google Scholar] [CrossRef]
- Modhera, B.K.; Chakraborty, M.; Bajaj, H.C.; Parikh, P.A. Influences of mesoporosity generation in ZSM-5 and zeolite beta on catalytic performance during n-hexane isomerization. Catal. Lett. 2011, 141, 1182–1190. [Google Scholar] [CrossRef]
- Fedyna, M.; Śliwa, M.; Jaroszewska, K.; Trawczyński, J. Effect of zeolite amount on the properties of Pt/(AlSBA-15 + Beta zeolite) micro-mesoporous catalysts for the hydroisomerization of n-heptane. Fuel 2020, 280, 118607. [Google Scholar] [CrossRef]
- Verboekend, D.; Thomas, K.; Milina, M.; Mitchell, S.; Pérez-Ramírez, J.; Gilson, J.-P. Towards more efficient monodimensional zeolite catalysts: N-alkane hydro-isomerisation on hierarchical ZSM-22. Catal. Sci. Technol. 2011, 1, 1331–1335. [Google Scholar] [CrossRef]
- Martens, J.A.; Verboekend, D.; Thomas, K.; Vanbutsele, G.; Gilson, J.-P.; Pérez-Ramírez, J. Hydroisomerization of emerging renewable hydrocarbons using hierarchical Pt/H-ZSM-22 catalyst. ChemSusChem 2013, 6, 421–425. [Google Scholar] [CrossRef]
- Musselwhite, N.; Na, K.; Sabyrov, K.; Alayoglu, S.; Somorjai, G.A. Mesoporous Aluminosilicate Catalysts for the Selective Isomerization of n-Hexane: The Roles of Surface Acidity and Platinum Metal. J. Am. Chem. Soc. 2015, 137, 10231–10237. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Zhang, Z.; Ling, F.; Sun, W. Synthesis, characterization and catalytic properties of Y-β zeolite composites. Pet. Sci. 2011, 8, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Pellet, R.J.; Casey, D.G.; Huang, H.M.; Kessler, R.V.; Kuhlman, E.J.; Oyoung, C.L.; Sawicki, R.A.; Ugolini, J.R. Isomerization of n-butene to isobutene by ferrierite and modified ferrierite catalysts. J. Catal. 1995, 157, 423–435. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.C. Skeletal isomerization of unsaturated fatty acids: The role of mesopores in HBeta zeolites. Catal. Lett. 2007, 115, 114–121. [Google Scholar] [CrossRef]
- Chica, A.; Diaz, U.; Fornés, V.; Corma, A. Changing the hydroisomerization to hydrocracking ratio of long chain alkanes by varying the level of delamination in zeolitic (ITQ-6) materials. Catal. Today 2009, 147, 179–185. [Google Scholar] [CrossRef]
- Fernandez, C.; Stan, I.; Gilson, J.-P.; Thomas, K.; Vicente, A.; Bonilla, A.; Pérez-Ramírez, J. Hierarchical ZSM-5 zeolites in shape-selective xylene isomerization: Role of mesoporosity and acid site speciation. Chem. Eur. J. 2010, 16, 6224–6233. [Google Scholar] [CrossRef]
- Mokrzycki, Ł.; Sulikowski, B. Desilication of ZSM-12 and MCM-22 type zeolites and their performance in isomerization of α-pinene. Stud. Surf. Sci. Catal. 2008, 174, 1231–1234. [Google Scholar] [CrossRef]
- Mokrzycki, Ł.; Sulikowski, B.; Olejniczak, Z. Properties of Desilicated ZSM-5, ZSM-12, MCM-22 and ZSM-12/MCM-41 Derivatives in Isomerization of α-Pinene. Catal. Lett. 2009, 127, 296. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Use of solid catalysts in Friedel−Crafts acylation reactions. Chem. Rev. 2006, 106, 1077–1104. [Google Scholar] [CrossRef]
- Bejblová, M.; Procházková, D.; Čejka, J. Acylation reactions over zeolites and mesoporous catalysts. ChemSusChem 2009, 2, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C.H. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Schmidt, I.; Christensen, C.H. Catalytic benzene alkylation over mesoporous zeolite single crystals: Improving activity and selectivity with a new family of porous materials. J. Am. Chem. Soc. 2003, 125, 13370–13371. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Törnqvist, E.; Schmidt, I.; Topsøe, H.; Christensen, C.H. Mesoporous zeolite single crystal catalysts: Diffusion and catalysis in hierarchical zeolites. Catal. Today 2007, 128, 117–122. [Google Scholar] [CrossRef]
- Sun, Y.; Prins, R. Friedel-Crafts alkylations over hierarchical zeolite catalysts. Appl. Catal. A Gen. 2008, 336, 11–16. [Google Scholar] [CrossRef]
- Tu, X.; Matsumoto, M.; Maeda, T.; Sugi, Y.; Matsuzaki, T.; Hanaoka, T.; Kubota, Y.; Kim, J.-H. Shape-selective ethylation of biphenyl over a highly dealuminated H-mordenite. Micropor. Mat. 1995, 3, 593–595. [Google Scholar] [CrossRef]
- Li, X.; Prins, R.; van Bokhoven, J.A. Synthesis and characterization of mesoporous mordenite. J. Catal. 2009, 262, 257–265. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, X.; Hu, H.; Ma, B. Synthesis of 2,6-dimethylnaphthalene by methylation of 2-methylnaphthalene on mesoporous ZSM-5 by desilication. Catal. Commun. 2008, 10, 336–340. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Baek, S.B.; Meena, A.; Sultan, S.; Kwak, J.H.; Kim, K.S. Template free facile synthesis of mesoporous mordenite for bulky molecular catalytic reactions. J. Ind. Eng. Chem. 2018, 57, 363–369. [Google Scholar] [CrossRef]
- Fu, D.; Paioni, A.L.; Lian, C.; van der Heijden, O.; Baldus, M.; Weckhuysen, B.M. Elucidating Zeolite Channel Geometry–Reaction Intermediate Relationships for the Methanol-to-Hydrocarbon Process. Angew. Chem. Int. Ed. 2020, 59, 20024–20030. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, T.; Li, H.; Cui, L.; Li, Z. Insight into the Selection of the Post-Treatment Strategy for ZSM-5 Zeolites for the Improvement of Catalytic Stability in the Conversion of Methanol to Hydrocarbons. Ind. Eng. Chem. Res. 2020, 59, 11125–11138. [Google Scholar] [CrossRef]
- Stöcker, M. Methanol-to-hydrocarbons: Catalytic materials and their behavior. Microporous Mesoporous Mater. 1999, 29, 3–48. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Cooke, D.; Crivelli, P.; Pérez-Ramírez, J. Impact of Pore Connectivity on the Design of Long-Lived Zeolite Catalysts. Angew. Chem. Int. Ed. 2015, 54, 1591–1594. [Google Scholar] [CrossRef]
- Erichsen, M.W.; Svelle, S.; Olsbye, U. The influence of catalyst acid strength on the methanol to hydrocarbons (MTH) reaction. Catal. Today 2013, 215, 216–223. [Google Scholar] [CrossRef]
- Schulz, H. “Coking” of zeolites during methanol conversion: Basic reactions of the MTO-, MTP- and MTG processes. Catal. Today 2010, 154, 183–194. [Google Scholar] [CrossRef]
- Haw, J.F.; Song, W.; Marcus, D.M.; Nicholas, J.B. The Mechanism of Methanol to Hydrocarbon Catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, X.; Zhang, Y.; Tang, Y. Future of nano-/hierarchical zeolites in catalysis: Gaseous phase or liquid phase system. Catal. Sci. Technol. 2015, 5, 772–785. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Crivelli, P.; Cooke, D.; Pérez-Ramírez, J. Mesopore quality determines the lifetime of hierarchically structured zeolite catalysts. Nat. Commun. 2014, 5, 3922. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Sun, W.; Hou, Y.; Ge, B.; Hu, L.; Nie, J.; Qian, W.; Wei, F. Crystal-plane effects of MFI zeolite in catalytic conversion of methanol to hydrocarbons. J. Catal. 2018, 360, 89–96. [Google Scholar] [CrossRef]
- Bjørgen, M.; Kolboe, S. The conversion of methanol to hydrocarbons over dealuminated zeolite H-beta. Appl. Catal. A Gen. 2002, 225, 285–290. [Google Scholar] [CrossRef]
- Yarulina, I.; Kapteijn, F.; Gascon, J. The importance of heat effects in the methanol to hydrocarbons reaction over ZSM-5: On the role of mesoporosity on catalyst performance. Catal. Sci. Technol. 2016, 6, 5320–5325. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, M.; Ryoo, R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process. J. Catal. 2010, 269, 219–228. [Google Scholar] [CrossRef]
- Schmidt, F.; Hoffmann, C.; Giordanino, F.; Bordiga, S.; Simon, P.; Carrillo-Cabrera, W.; Kaskel, S. Coke location in microporous and hierarchical ZSM-5 and the impact on the MTH reaction. J. Catal. 2013, 307, 238–245. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, A.; Wu, X.; Hai, G.; Hu, J.; Li, T.; Li, G. Preparation of hierarchical mesoporous Zn/HZSM-5 catalyst and its application in MTG reaction. J. Nat. Gas Chem. 2011, 20, 237–242. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Dong, M.; Zhao, H.; Huang, W. Production of gasoline range hydrocarbons from methanol on hierarchical ZSM-5 and Zn/ZSM-5 catalyst prepared with soft second template. J. Energy Chem. 2015, 24, 490–496. [Google Scholar] [CrossRef]
- Mentzel, U.V.; Højholt, K.T.; Holm, M.S.; Fehrmann, R.; Beato, P. Conversion of methanol to hydrocarbons over conventional and mesoporous H-ZSM-5 and H-Ga-MFI: Major differences in deactivation behavior. Appl. Catal. A 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Mezari, B.; Pestman, R.; Wannapakdee, W.; Hensen, E.J.M. On the Role of Acidity in Bulk and Nanosheet [T]MFI (T = Al3+, Ga3+, Fe3+, B3+) Zeolites in the Methanol-to-Hydrocarbons Reaction. ChemCatChem 2017, 9, 3942–3954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodarzi, F.; Herrero, I.P.; Kalantzopoulos, G.N.; Svelle, S.; Lazzarini, A.; Beato, P.; Olsbye, U.; Kegnæs, S. Synthesis of mesoporous ZSM-5 zeolite encapsulated in an ultrathin protective shell of silicate-1 for MTH conversion. Microporous Mesoporous Mater. 2020, 292, 109730. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Guo, G.; Chen, X.; Yu, J. Synthesis of tri-level hierarchical SAPO-34 zeolite with intracrystalline micro-meso-macroporosity showing superior MTO performance. J. Mater. Chem. A 2015, 3, 19783–19789. [Google Scholar] [CrossRef]
- Liu, X.; Ren, S.; Zeng, G.; Liu, G.; Wu, P.; Wang, G.; Chen, X.; Liu, Z.; Sun, Y. Coke suppression in MTO over hierarchical SAPO-34 zeolites. RSC Adv. 2016, 6, 28787–28791. [Google Scholar] [CrossRef]
- Jin, W.; Wang, B.; Tuo, P.; Li, C.; Li, L.; Zhao, H.; Gao, X.; Shen, B. Selective Desilication, Mesopores Formation, and MTO Reaction Enhancement via Citric Acid Treatment of Zeolite SAPO-34. Ind. Eng. Chem. Res. 2018, 57, 4231–4236. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, S.; Yu, X.; Chen, X.; Wang, G.; Wu, X.; Yu, G.; Qiu, M.; Yang, C.; Sun, Y. Melting-assisted solvent-free synthesis of hierarchical SAPO-34 with enhanced methanol to olefins (MTO) performance. Catal. Sci. Technol. 2018, 8, 423–427. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Sartipi, S.; Sun, X.; Rodríguez-Mirasol, J.; Cordero, T.; Kapteijn, F.; Gascon, J. Carbon/H-ZSM-5 composites as supports for bi-functional Fischer–Tropsch synthesis catalysts. Catal. Sci. Technol. 2016, 6, 2633–2646. [Google Scholar] [CrossRef] [Green Version]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Dry, M.E. High quality diesel via the Fischer–Tropsch process—A review. J. Chem. Technol. Biotechnol. 2001, 77, 43–50. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Rynkowski, J.; Gurgul, J.; Dzwigaj, S. Cobalt Based Catalysts Supported on Two Kinds of Beta Zeolite for Application in Fischer-Tropsch Synthesis. Catalysts 2019, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.H. Fischer-Tropsch Synthesis: Comparison of Performances of Iron and Cobalt Catalysts. Ind. Eng. Chem. Res. 2007, 46, 8938–8945. [Google Scholar] [CrossRef]
- Carvalho, A.; Marinova, M.; Batalha, N.; Marcilio, N.R.; Khodakov, A.Y.; Ordomsky, V.V. Design of nanocomposites with cobalt encapsulated in the zeolite micropores for selective synthesis of isoparaffins in Fischer–Tropsch reaction. Catal. Sci. Technol. 2017, 7, 5019–5027. [Google Scholar] [CrossRef]
- Liu, R.-J.; Xu, Y.; Qiao, Y.; Li, Z.-H.; Ma, X.-B. Factors influencing the Fischer-Tropsch synthesis performance of iron-based catalyst: Iron oxide dispersion, distribution and reducibility. Fuel Process. Technol. 2015, 139, 25–32. [Google Scholar] [CrossRef]
- Kang, J.; Cheng, K.; Zhang, L.; Zhang, Q.; Ding, J.; Hua, W.; Lou, Y.; Zhai, Q.; Wang, Y. Mesoporous Zeolite-Supported Ruthenium Nanoparticles as Highly Selective Fischer-Tropsch Catalysts for the Production of C5-C11 Isoparaffins. Angew. Chem. Int. Ed. 2011, 50, 5200–5203. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yin, Q.; Guo, J.; Ru, B.; Zhu, L. Improved Fischer–Tropsch synthesis for gasoline over Ru, Ni promoted Co/HZSM-5 catalysts. Fuel 2013, 108, 597–603. [Google Scholar] [CrossRef]
- Plana-Pallejà, J.; Abelló, S.; Berrueco, C.; Montané, D. Effect of zeolite acidity and mesoporosity on the activity of Fischer-Tropsch Fe/ZSM-5 bifunctional catalysts. Appl. Catal. A Gen. 2016, 515, 126–135. [Google Scholar] [CrossRef]
- Xing, C.; Yang, G.; Wu, M.; Yang, R.; Tan, L.; Zhu, P.; Wei, Q.; Li, J.; Mao, J.; Yoneyama, Y.; et al. Hierarchical zeolite Y supported cobalt bifunctional catalyst for facilely tuning the product distribution of Fischer-Tropsch synthesis. Fuel 2015, 148, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Cheng, K.; Kang, J.; Gu, B.; Yu, X.; Zhang, Q.; Wang, Y. Impact of Hydrogenolysis on the Selectivity of the Fischer–Tropsch Synthesis: Diesel Fuel Production over Mesoporous Zeolite-Y-Supported Cobalt Nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 4553–4556. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Li, Z.; Wang, H.; Wu, J.; Huang, J.; Zhao, Y. The influence of hierarchical zeolite composition and pore structure on behavior of cobalt-based Fischer-Tropsch synthesis catalysts. J. Porous Mater. 2015, 22, 1097–1104. [Google Scholar] [CrossRef]
- Sartipi, S.; Parashar, K.; Makkee, M.; Gascon, J.; Kapteijn, F. Breaking the Fischer–Tropsch synthesis selectivity: Direct conversion of syngas to gasoline over hierarchical Co/H-ZSM-5 catalysts. Catal. Sci. Technol. 2012, 3, 572. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-C.; Lee, S.; Cho, K.; Na, K.; Lee, C.; Ryoo, R. Mesoporous MFI Zeolite Nanosponge Supporting Cobalt Nanoparticles as a Fischer-Tropsch Catalyst with High Yield of Branched Hydrocarbons in the Gasoline Range. ACS Catal. 2014, 4, 3919–3927. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Two-dimensional itq-2 zeolite for biomass transformation: Synthesis of alkyl 5-benzyl-2-furoates as intermediates for fine chemicals. ACS Sustain. Chem. Eng. 2016, 4, 6152–6159. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Heracleous, E.; Vasalos, I.A.; Lemonidou, A.A. Ru-based catalysts for glycerol hydrogenolysis—Effect of support and metal precursor. Appl. Catal. B Environ. 2009, 92, 90–99. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkaplin, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol conversion in the aqueous solution under hydrogen over Ru/C plus an ion-exchange resin and its reaction mechanism. J. Catal. 2006, 240, 213–221. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Selective Catalytic Transfer Hydrogenolysis of Glycerol to 2-Isopropoxy-Propan-1-Ol over Noble Metal Ion-Exchanged Mordenite Zeolite. Catalysts 2019, 9, 885. [Google Scholar] [CrossRef] [Green Version]
- Beerthuis, R.; Huang, L.; Shiju, N.R.; Rothenberg, G.; Shen, W.; Xu, H. Facile Synthesis of a Novel Hierarchical ZSM-5 Zeolite: A Stable Acid Catalyst for Dehydrating Glycerol to Acrolein. ChemCatChem 2018, 10, 211–221. [Google Scholar] [CrossRef]
- Possato, L.; Diniz, R.; Garetto, T.; Pulcinelli, S.; Santilli, C.; Martins, L. A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J. Catal. 2013, 300, 102–112. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; Grau-Atienza, A.; Serrano, E.; Romero, A.A.; Garcia-Martinez, J.; Luque, R. The role of mesoporosity and Si/Al ratio in the catalytic etherification of glycerol with benzyl alcohol using ZSM-5 zeolites. J. Mol. Catal. A Chem. 2015, 406, 40–45. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, F.; Xiao, G. Performance of hierarchical HZSM-5 zeolites prepared by NaOH treatments in the aromatization of glycerol. RSC Adv. 2015, 5, 63697–63704. [Google Scholar] [CrossRef]
- Gustavsson, L.; Haus, S.; Lundblad, M.; Lundström, A.; Ortiz, C.A.; Sathre, R.; Truong, N.L.; Wikberg, P.-E. Climate change effects of forestry and substitution of carbon-intensive materials and fossil fuels. Renew. Sustain. Energy Rev. 2017, 67, 612–624. [Google Scholar] [CrossRef]
- McKechnie, J.; Colombo, S.; Chen, J.; Mabee, W.; MacLean, H.L. Forest bioenergy or forest carbon? Assessing trade-offs in greenhouse gas mitigation with wood-based fuels. Environ. Sci. Technol. 2011, 45, 789–795. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Decarbonization of fossil fuels as a strategy to control global warming. Renew. Sustain. Energy Rev. 2011, 15, 1828–1834. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Najafabadi, A.T. CO2 chemical conversion to useful products: An engineering insight to the latest advances toward sustainability. Int. J. Energy Res. 2013, 37, 485–499. [Google Scholar] [CrossRef]
- Li, C.-G.; Xu, L.; Wu, P.; Wu, H.; He, M. Efficient cycloaddition of epoxides and carbon dioxide over novel organic–inorganic hybrid zeolite catalysts. ChemComm 2014, 50, 15764–15767. [Google Scholar] [CrossRef]
- Kim, D.; Na, K. Organic-inorganic multifunctional hybrid catalyst giving catalytic synergies in cooperative coupling between CO2 and propylene oxide to propylene carbonate. J. CO2 Util. 2018, 27, 129–136. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metal and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Ryoo, R.; Kim, J.; Jo, C.; Han, S.W.; Kim, J.-C.; Park, H.; Han, J.; Shin, H.S.; Shin, J.W. Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 2020, 585, 221–224. [Google Scholar] [CrossRef]
- Han, S.W.; Park, H.; Han, J.; Kim, J.-C.; Lee, J.; Jo, C.; Ryoo, R. PtZn intermetallic compound nanoparticles in mesoporous zeolite exhibiting high catalyst durability for propane dehydrogenation. ACS Catal. 2021, 11, 9233–9241. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. https://doi.org/10.3390/catal11121541
Singh BK, Kim Y, Kwon S, Na K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts. 2021; 11(12):1541. https://doi.org/10.3390/catal11121541
Chicago/Turabian StyleSingh, Bhupendra Kumar, Yongseok Kim, Seungdon Kwon, and Kyungsu Na. 2021. "Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis" Catalysts 11, no. 12: 1541. https://doi.org/10.3390/catal11121541
APA StyleSingh, B. K., Kim, Y., Kwon, S., & Na, K. (2021). Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts, 11(12), 1541. https://doi.org/10.3390/catal11121541







