Removal of Organic Dyes from Water and Wastewater Using Magnetic Ferrite-Based Titanium Oxide and Zinc Oxide Nanocomposites: A Review
Abstract
1. Introduction
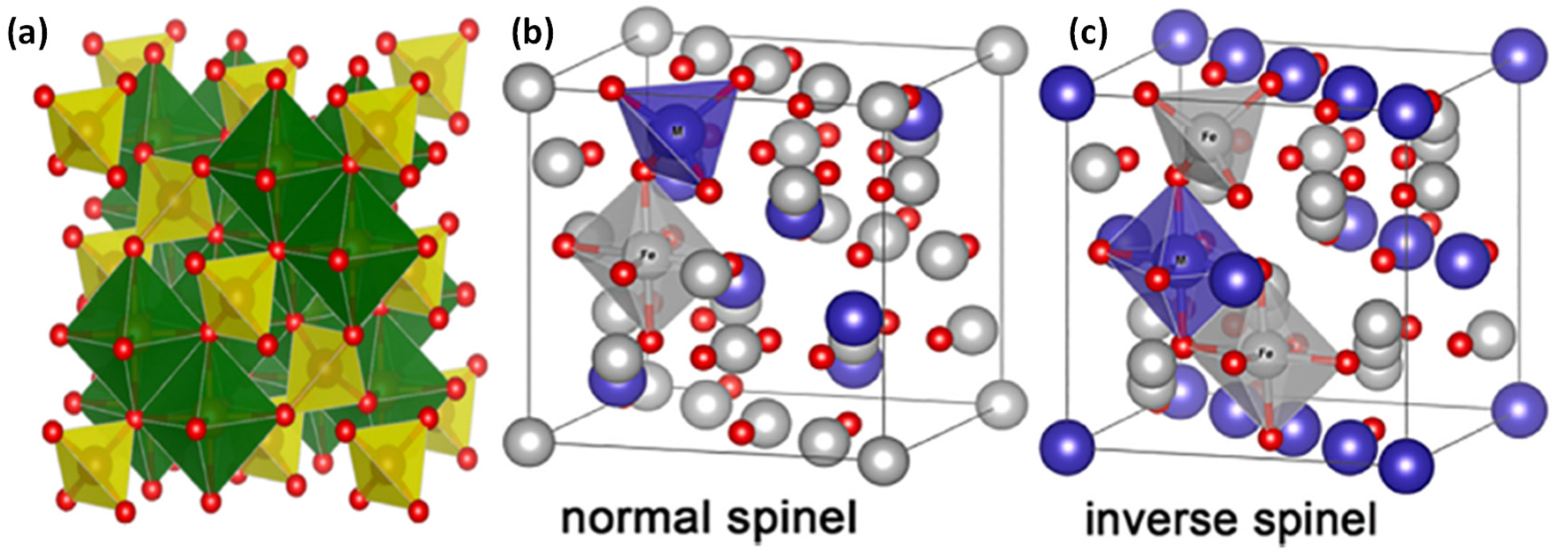
2. Magnetic Ferrites Nanoparticles
2.1. Methods of Synthesis of Magnetic Spinel Ferrites
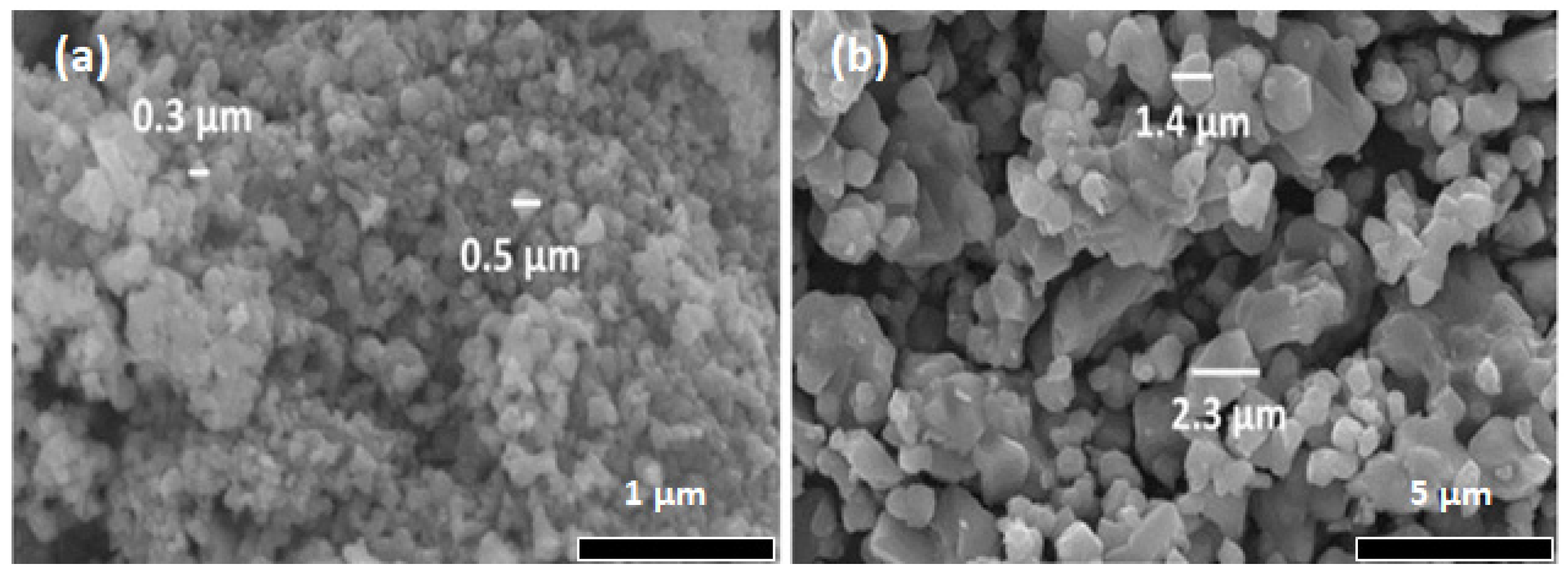
2.2. Characterization Methods
3. Photocatalytic Application of Magnetic Ferrites and Their Nanocomposites
3.1. Nickel Ferrite and Nanocomposites
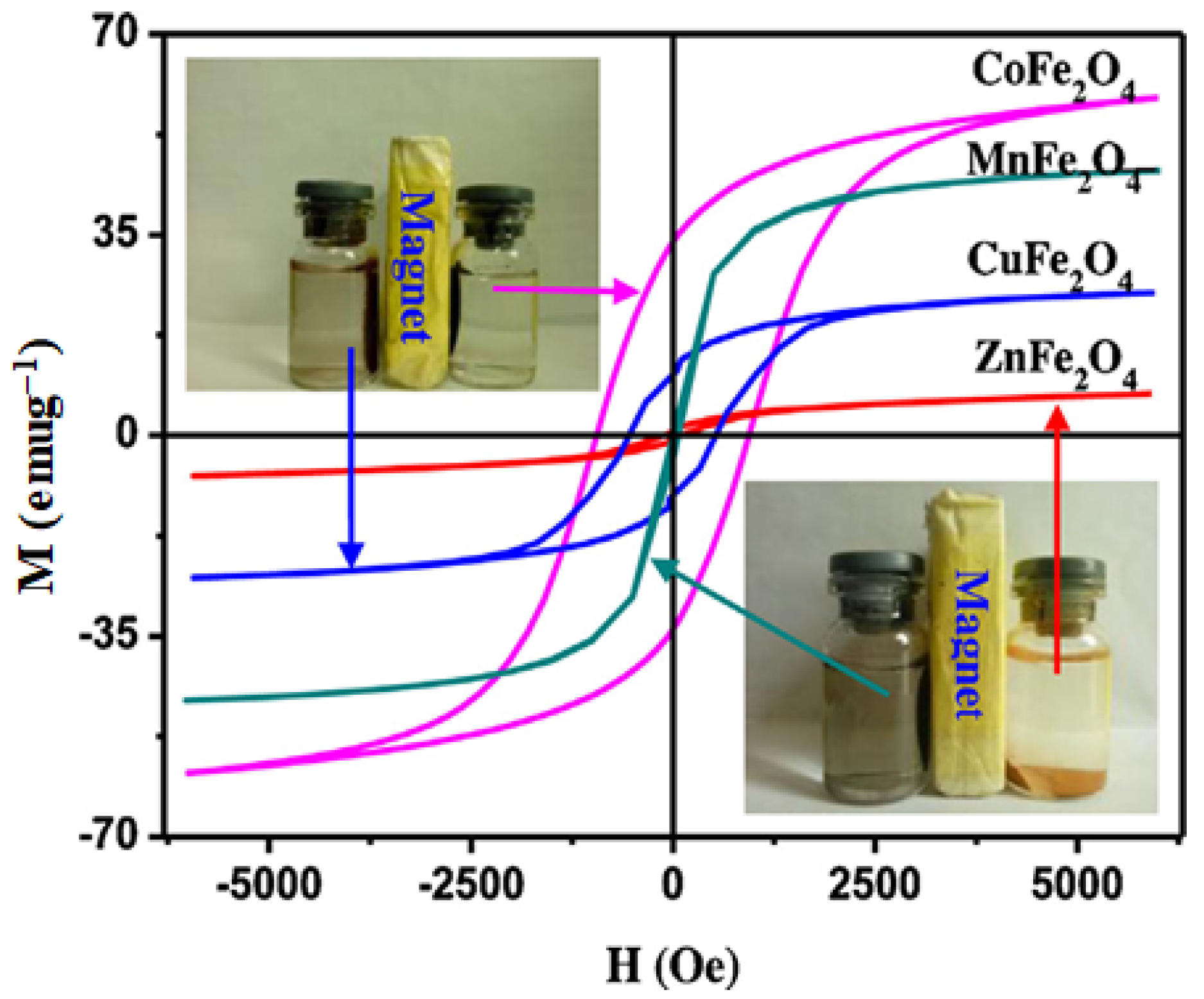
3.2. Zinc Ferrite and Nanocomposites
3.3. Cobalt Ferrite and Nanocomposites
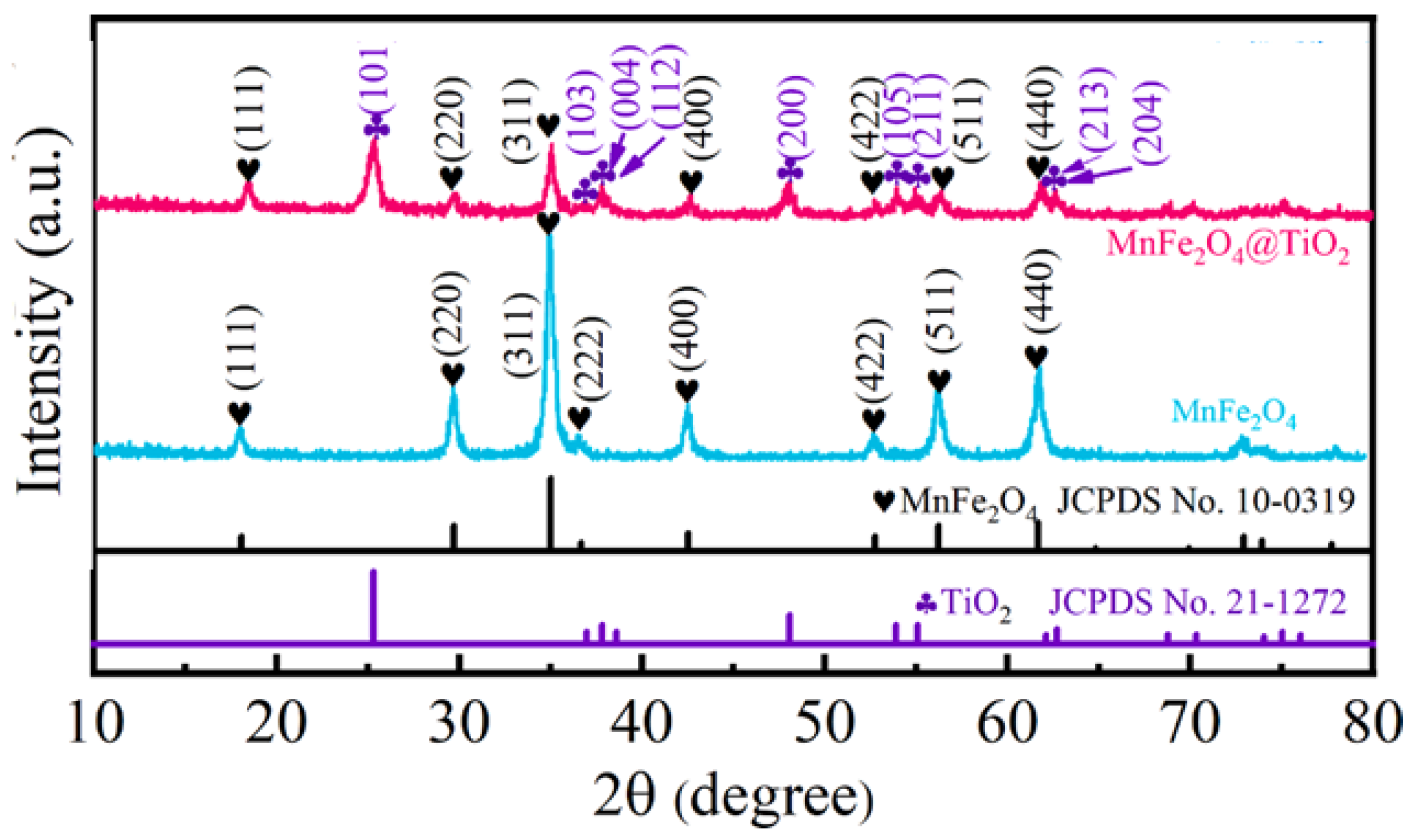
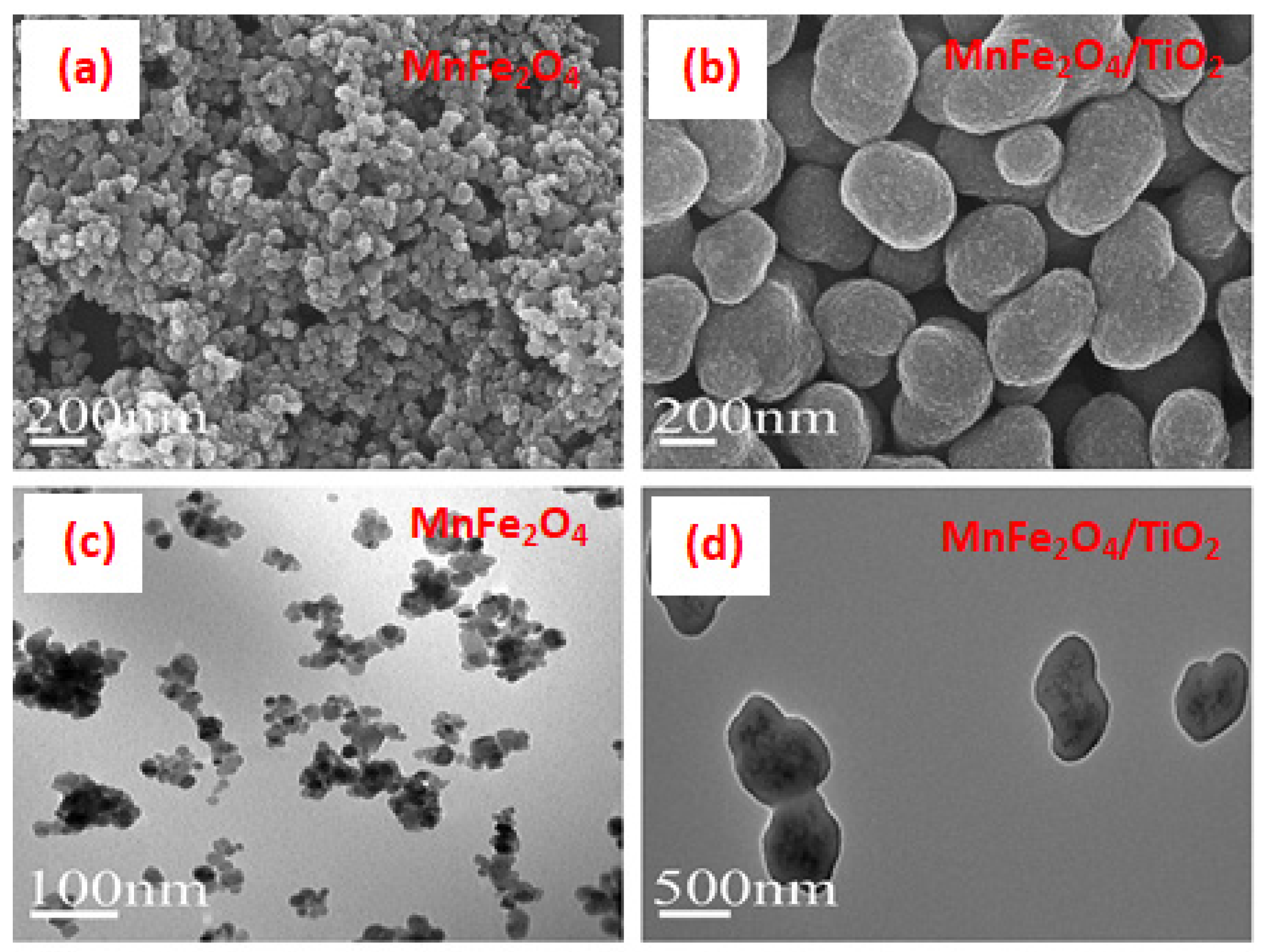
3.4. Manganese Ferrite and Nanocomposites
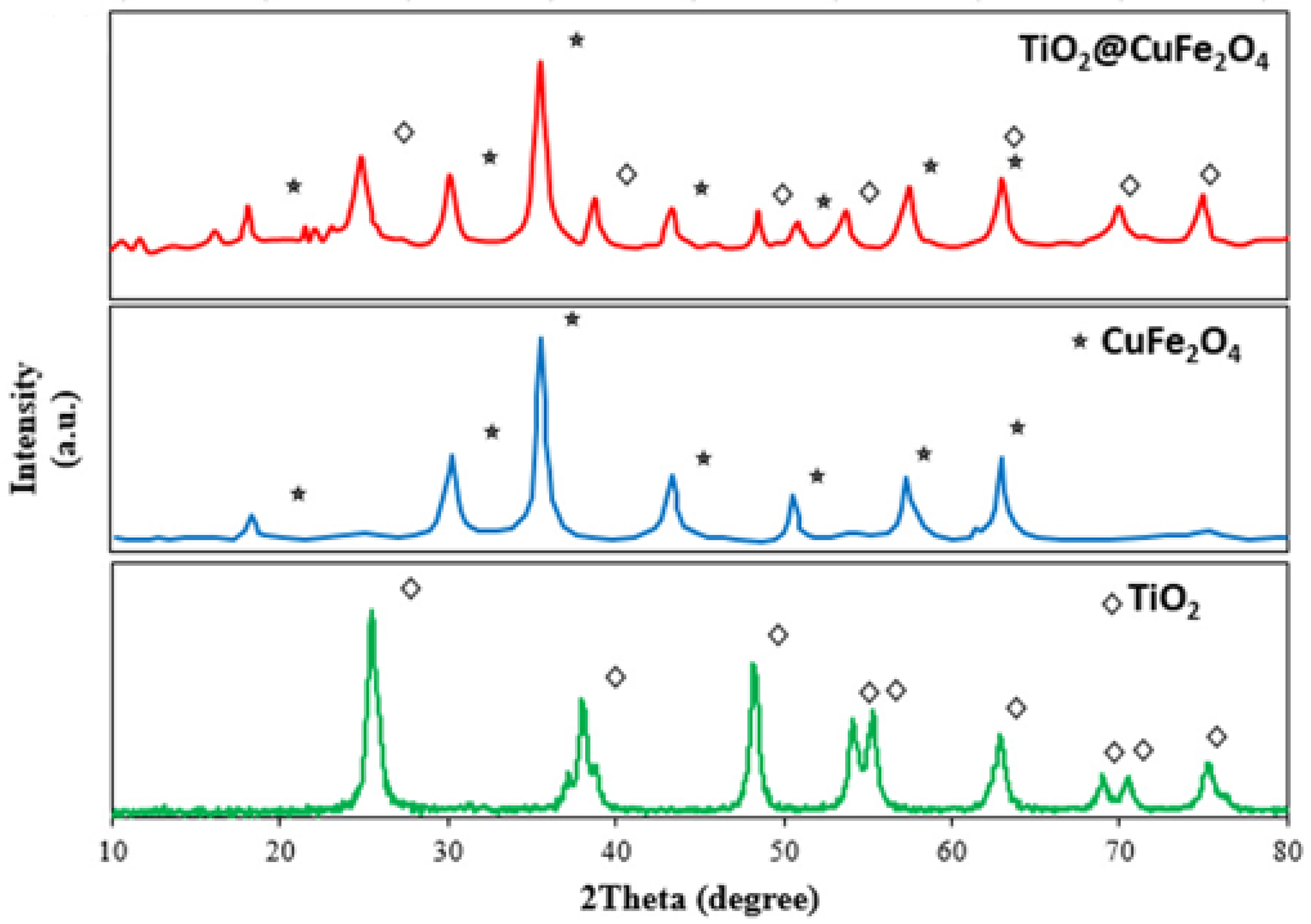
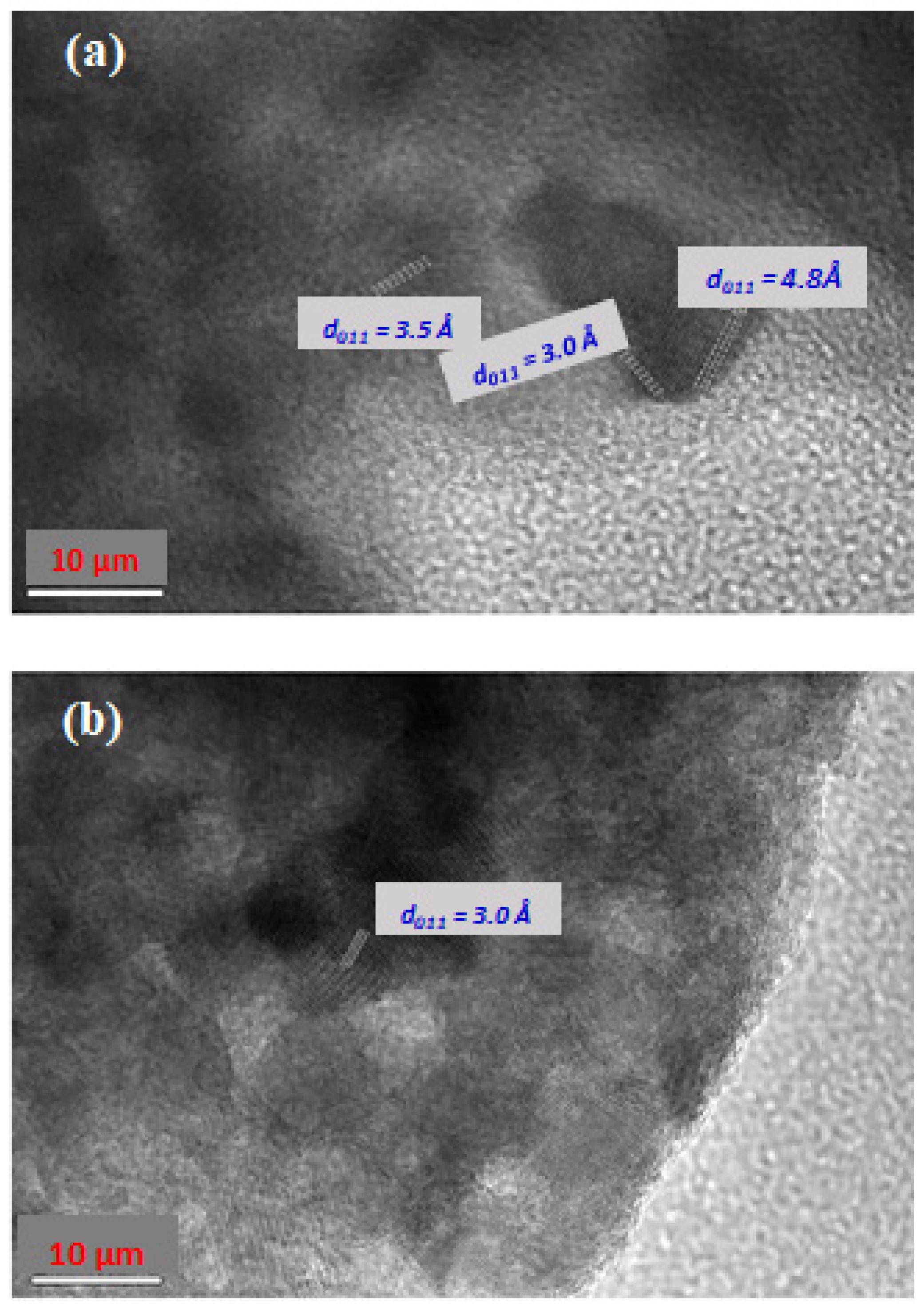
3.5. Copper Ferrite and Nanocomposites
3.6. Mixed-Metal Ferrites and Nanocomposites
4. Factors Affecting the Photocatalytic Activity of Magnetic Nanocomposites
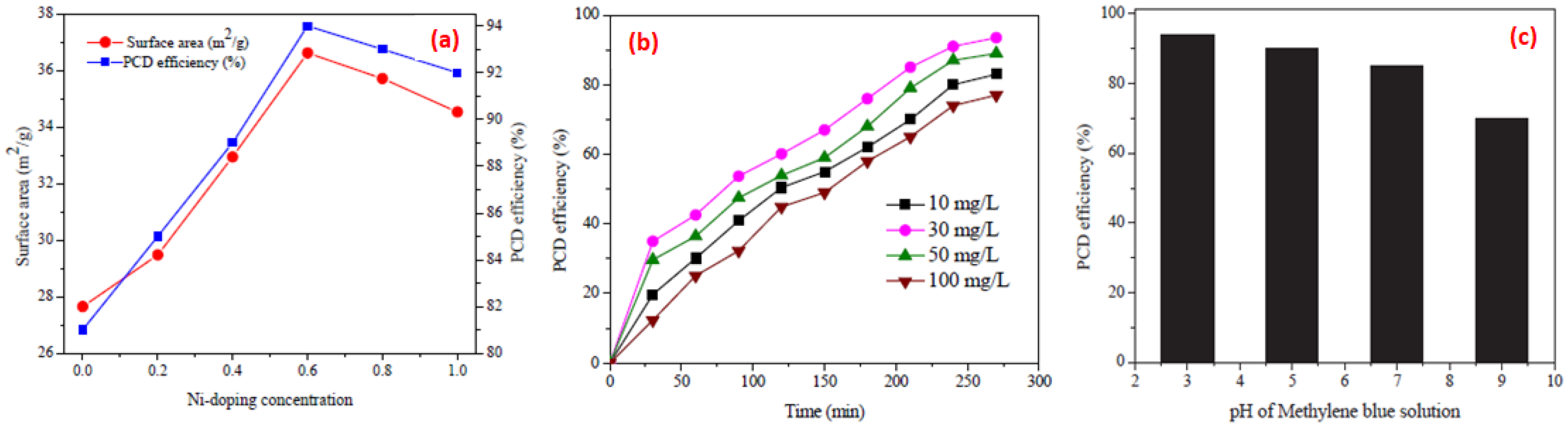
4.1. Catalyst Surface Area
4.2. Effect of Catalyst Amount
4.3. Effect of pH
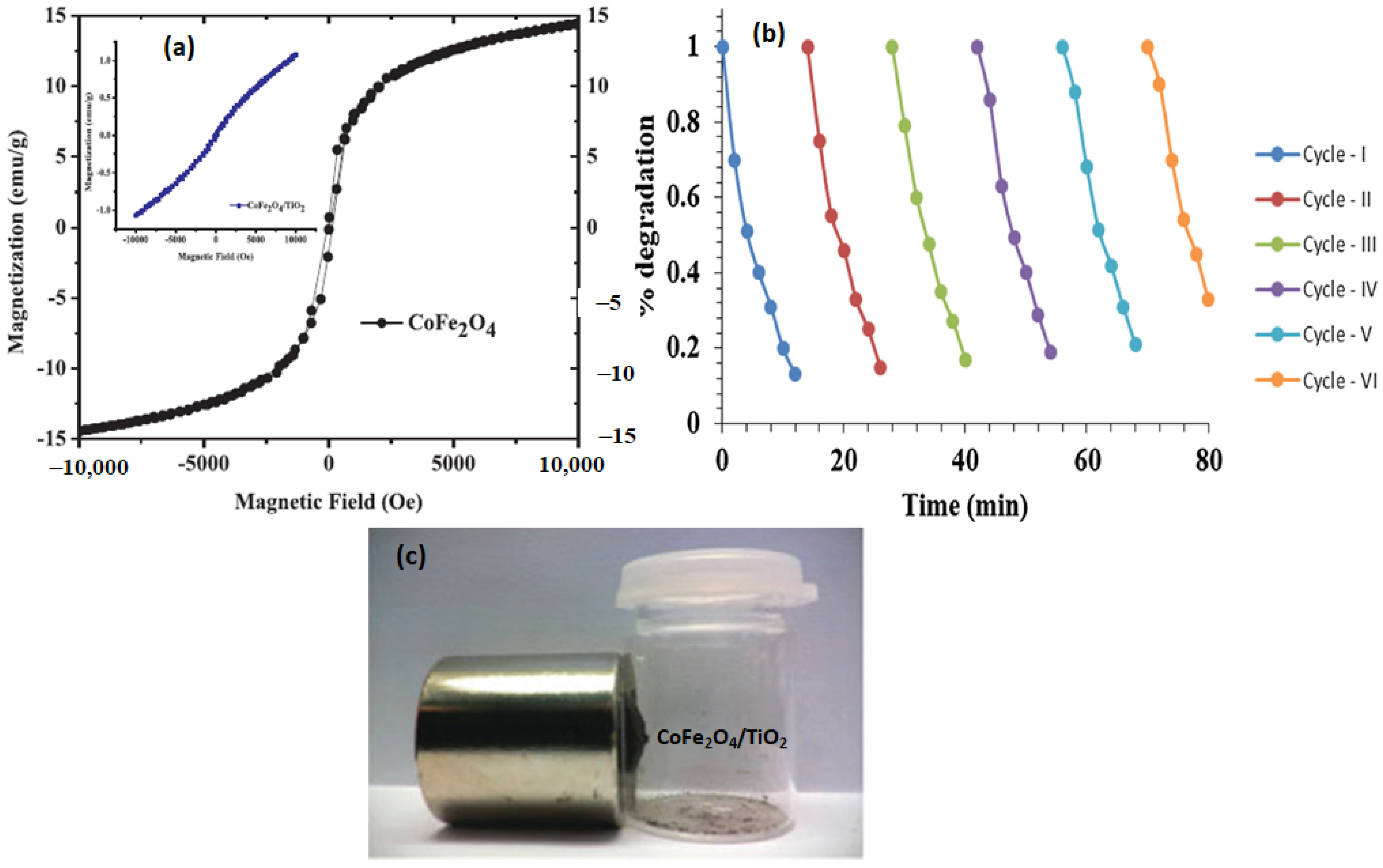
5. Reusability of the Magnetic Nanocatalyst
6. The Overlooked Social Dimension
7. Conclusions and Recommendation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB113 | Acid Blue 113 |
| CR | Congo Red |
| 4-CP | 4-Chlorophenol |
| DSC | Differential Scanning Calorimetry |
| EB | Evans Blue |
| Ms | Saturation Magnetization |
| MNPs | Magnetic Nanoparticles |
| MB | Methylene Blue |
| MO | Methylene Orange |
| MG | Malachite Green |
| RB5 | Reactive Blue 5 |
| SEM | Scanning Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TEM | Transmission Electron Microscopy |
| TC | Curie Temperature |
| UV | Ultraviolet |
| VSM | Vibrating Sample Magnetometer |
| XRD | X-ray Diffraction |
References
- Liu, Y.; Sun, L.; Wu, J.; Fang, T.; Cai, R.; Wei, A. Preparation and photocatalytic activity of ZnO/Fe2O3 nanotube composites. Mater. Sci. Eng. B 2015, 194, 9–13. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Cahino, A.M.; Loureiro, R.G.; Dantas, J.; Madeira, V.S.; Fernandes, P.C.R. Characterization and evaluation of ZnO/CuO catalyst in the degradation of methylene blue using solar radiation. Ceram. Int. 2019, 45, 13628–13636. [Google Scholar] [CrossRef]
- de Oliveira, P.L.; Lima, N.S.; de Melo Costa, A.C.F.; Cavalcanti, E.B.; de Sousa Conrado, L. Obtaining TiO2: CoFe2O4 nanocatalyst by Pechini method for diuron degradation and mineralization. Ceram. Int. 2020, 46, 9421–9435. [Google Scholar] [CrossRef]
- Wilson, A.; Mishra, S.R.; Gupta, R.; Ghosh, K. Preparation and photocatalytic properties of hybrid core–shell reusable CoFe2O4–ZnO nanospheres. J. Magn. Magn. Mater. 2012, 324, 2597–2601. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Mohammadi, M.R.; Ghorbani, M. On the assessment of photocatalytic activity and charge carrier mechanism of TiO2@SnO2 core-shell nanoparticles for water decontamination. J. Photochem. Photobiol. A Chem. 2017, 338, 171–177. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadizadeh, M.R. Simultaneous improvement of photocatalytic and superhydrophilicity properties of nano TiO2 thin films. Chem. Eng. Res. Des. 2012, 90, 1473–1479. [Google Scholar] [CrossRef]
- Ahmed, M.A.; El-Katori, E.E.; Gharni, Z.H. Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol–gel method. J. Alloys Compd. 2013, 553, 19–29. [Google Scholar] [CrossRef]
- Harraz, F.A.; Mohamed, R.M.; Rashad, M.M.; Wang, Y.C.; Sigmund, W. Magnetic nanocomposite based on titania–silica/cobalt ferrite for photocatalytic degradation of methylene blue dye. Ceram. Int. 2014, 40, 375–384. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W. Determination and toxicity evaluation of the generated products in sulfamethoxazole degradation by UV/CoFe2O4/TiO2. J. Hazard. Mater. 2016, 314, 197–203. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. Process. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Sundararajan, R.; Srinivasan, V. Catalytic decomposition of nitrous oxide on CuxCo3−xO4 spinels. Appl. Catal. 1991, 73, 165–171. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Silva, M.R.; Kiminami, R.H.; Costa, A.C.F.; Daramola, M.O. Catalytic performance of NiFe2O4 and Ni0.3Zn0.7Fe2O4 magnetic nanoparticles during biodiesel production. Arab. J. Chem. 2020, 13, 4462–4476. [Google Scholar] [CrossRef]
- Hajalilou, A.; Mazlan, S.A. A review on preparation techniques for synthesis of nanocrystalline soft magnetic ferrites and investigation on the effects of microstructure features on magnetic properties. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–15. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Fu, Y.Q.; Li, R.R.; Yao, J.; Jiang, S.T. Novel multifunctional NiFe2O4/ZnO hybrids for dye removal by adsorption, photocatalysis and magnetic separation. Appl. Surf. Sci. 2016, 369, 1–10. [Google Scholar] [CrossRef]
- Maynez-Navarro, O.D.; Mendez-Rojas, M.A.; Flores-Cervantes, D.X.; Kuri, U.S.; Sanchez-Salas, J.L. Recyclable and Photocatalytic Properties of ZnFe2O4/ZnO for Wastewater Treatment and Disinfection. ChemistrySelect 2020, 5, 15167–15174. [Google Scholar] [CrossRef]
- Rahmayeni, R.A.; Stiadi, Y.; Jamarun, N.; Emriadi, A.S. Photocatalytic Performance of ZnO-ZnFe2O4 Magnetic Nanocomposites on Degradation of Congo Red Dye Under Solar Light Irradiation. J. Mater. Environ. Sci. 2017, 8, 1634–1643. [Google Scholar]
- Reddy, D.H.K.; Yun, Y.S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Wang, G.; Jiang, P.; Zhang, J.; Wu, L.; Li, K. Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances. Chem. Eng. J. 2013, 219, 295–302. [Google Scholar] [CrossRef]
- Andjelković, L.; Šuljagić, M.; Lakić, M.; Jeremić, D.; Vulić, P.; Nikolić, A.S. A study of the structural and morphological properties of Ni–ferrite, Zn–ferrite and Ni–Zn–ferrites functionalized with starch. Ceram. Int. 2018, 44, 14163–14168. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Yuliantika, D.; Taufiq, A.; Hidayat, A.; Hidayat, N.; Soontaranon, S. Exploring structural properties of cobalt ferrite nanoparticles from natural sand. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 515, p. 012047. [Google Scholar]
- Narang, S.B.; Pubby, K. Nickel spinel ferrites: A review. J. Magn. Magn. Mater. 2021, 519, 167163. [Google Scholar] [CrossRef]
- Hazra, S.; Ghosh, N.N. Preparation of nanoferrites and their applications. J. Nanosci. Nanotechnol. 2014, 14, 1983–2000. [Google Scholar] [CrossRef]
- Amiri, G.R.; Yousefi, M.H.; Abolhassani, M.R.; Manouchehri, S.; Keshavarz, M.H.; Fatahian, S. Magnetic properties and microwave absorption in Ni–Zn and Mn–Zn ferrite nanoparticles synthesized by low-temperature solid-state reaction. J. Magn. Magn. Mater. 2011, 323, 730–734. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, A.; Kashyap, S.C. Micro structural, dielectric and magnetic behavior of spin deposited nanocrystalline nickel-zinc ferrite thin films for microwave applications. J. Magn. Magn. Mater. 2007, 308, 137–142. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Min, F.; Zhu, J.; Zhang, M. Microwave-assisted synthesis and magnetic properties of Ni1−xZnxFe2O4 ferrite powder. J. Magn. Magn. Mater. 2014, 354, 295–298. [Google Scholar] [CrossRef]
- Shafi, K.; Koltypin, Y.; Gedanken, A.; Prozorov, R.; Balogh, J.; Lendvai, J.; Felner, I. Sonochemical preparation of nano- sized amorphous NiFe2O4 particles. J. Mater. Chem. Phys. 1997, 101, 6409–6414. [Google Scholar]
- Chen, D.H.; He, X.R. Synthesis of nickel ferrite nanoparticles by sol–gel method. Mater. Res. Bull. 2001, 36, 1369–1377. [Google Scholar] [CrossRef]
- Zahi, S.; Hashim, M.; Daud, A.R. Synthesis, magnetic properties and microstructure of Ni–Zn ferrite by sol–gel technique. J. Magn. Magn. Mater. 2007, 308, 177–182. [Google Scholar] [CrossRef]
- Atif, M.; Nadeem, M.; Grössinger, R.; Turtelli, R.S. Studies on the magnetic, magneto strictive and electrical properties of sol–gel synthesized Zn doped nickel ferrite. J. Alloys Compd. 2011, 509, 5720–5724. [Google Scholar] [CrossRef]
- Kumar, S.J.; Prameela, P.; Rao, K.S.; Kiran, J.N.; Rao, K.H. Structural and Magnetic Properties of Copper-Substituted Nickel–Zinc Nanoparticles Prepared by Sol-Gel Method. J. Supercond. Nov. Magn. 2020, 33, 693–705. [Google Scholar] [CrossRef]
- Yang, J.M.; Tsuo, W.J.; Yen, F.S. Preparation of ultrafine nickel ferrite powders using mixed Ni and Fe tartrates. J. Solid State Chem. 1999, 145, 50–57. [Google Scholar] [CrossRef]
- Maaz, K.; Karim, S.; Mumtaz, A.; Hasanain, S.K.; Liu, J.; Duan, J.L. Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J. Magn. Magn. Mater. 2009, 321, 1838–1842. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.B.; Cornejo, D.R.; Costa, A.C.F.M. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Diniz, V.C.S.; Silva, M.R.; Kiminami, R.H.G.A.; Costa, A.C.F.M. Síntese e caracterização do ferroespinélio Ni0.7Zn0.3Fe2O4: Avaliação de desempenho na esterificação metílica e etílica. Cerâmica 2017, 63, 223–232. [Google Scholar] [CrossRef][Green Version]
- Abbas, M.K.; Khan, M.A.; Mushtaq, F.; Warsi, M.F.; Sher, M.; Shakir, I.; AlyAboud, M.F. Impact of Dy on structural, dielectric and magnetic properties of Li-Tb-nanoferrites synthesized by micro-emulsion method. Ceram. Int. 2017, 43, 5524–5533. [Google Scholar] [CrossRef]
- Rozman, M.; Drofenik, M. Hydrothermal synthesis of manganese zinc ferrites. J. Am. Ceram. Soc. 1995, 78, 2449–2455. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Xing, Y.; Song, S.; Yu, S.; Shi, W.; Guo, X.; Yang, J.; Lei, Y.; Cao, F. Studies on the magnetism of cobalt ferrite nanocrystals synthesized by hydrothermal method. J. Solid State Chem. 2008, 181, 245–252. [Google Scholar] [CrossRef]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Su, L.; Ma, Y.; Ren, C.; Guo, Q.; Chen, X. CuFe2O4 magnetic nanoparticles: A simple and efficient catalyst for the reduction of nitrophenol. Chem. Eng. J. 2013, 221, 16–24. [Google Scholar] [CrossRef]
- Chen, C.; Zuo, W.Q.; Yang, J.C.E.; Cui, H.J.; Fu, M.L. Yolk–shell structured CoFe2O4 microspheres as novel catalysts for peroxymonosulfate activation for efficient degradation of butyl paraben. RSC Adv. 2016, 6, 10136. [Google Scholar] [CrossRef]
- George, M.; John, A.M.; Nair, S.S.; Joy, P.A.; Anantharaman, M.R. Finite size effects on the structural and magnetic properties of sol–gel synthesized NiFe2O4 powders. J. Magn. Magn. Mater. 2006, 302, 190–195. [Google Scholar] [CrossRef]
- El-Okr, M.M.; Salem, M.A.; Salim, M.S.; El-Okr, R.M.; Ashoush, M.; Talaat, H.M. Synthesis of cobalt ferrite nano-particles and their magnetic characterization. J. Magn. Magn. Mater. 2011, 323, 920–926. [Google Scholar] [CrossRef]
- Konecny, A.P.; Covarrubias, J.; Wang, H. Magnetic Nanoparticle Design and Application in Magnetic Hyperthermia. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Bossmann, S.H., Wang, H., Eds.; RSC: London, UK, 2017. [Google Scholar]
- Ali, A.; Hira Zafar, M.Z.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar] [CrossRef]
- Gopalan Sibi, M.; Verma, D.; Kim, J. Magnetic core–shell nanocatalysts: Promising versatile catalysts for organic and photocatalytic reactions. Catal. Rev. 2020, 62, 163–311. [Google Scholar] [CrossRef]
- Bhagwat, V.R.; Humbe, A.V.; More, S.D.; Jadhav, K.M. Sol-gel auto combustion synthesis and characterizations of cobalt ferrite nanoparticles: Different fuels approach. Mater. Sci. Eng. B 2019, 248, 114388. [Google Scholar] [CrossRef]
- Venturini, J.; Zampiva, R.Y.S.; Arcaro, S.; Bergmann, C.P. Sol-gel synthesis of substoichiometric cobalt ferrite (CoFe2O4) spinels: Influence of additives on their stoichiometry and magnetic properties. Ceram. Int. 2018, 44, 12381–12388. [Google Scholar] [CrossRef]
- Motavallian, P.; Abasht, B.; Abdollah-Pour, H. Zr doping dependence of structural and magnetic properties of cobalt ferrite synthesized by sol–gel based Pechini method. J. Magn. Magn. Mater. 2018, 451, 577–586. [Google Scholar] [CrossRef]
- Hossain, M.S.; Alam, M.B.; Shahjahan, M.; Begum, M.H.A.; Hossain, M.M.; Islam, S.; Khatun, N.; Hossain, M.; Alam, M.S.; Al-Mamun, M. Synthesis, structural investigation, dielectric and magnetic properties of Zn2+-doped cobalt ferrite by the sol–gel technique. J. Adv. Dielectr. 2018, 8, 1850030. [Google Scholar] [CrossRef]
- Ansari, F.; Sobhani, A.; Salavati-Niasari, M. Simple sol-gel synthesis and characterization of new CoTiO3/CoFe2O4 nanocomposite by using liquid glucose, maltose and starch as fuel, capping and reducing agents. J. Colloid Interface Sci. 2018, 514, 723–732. [Google Scholar] [CrossRef]
- Raut, A.V.; Barkule, R.S.; Shengule, D.R.; Jadhav, K.M. Synthesis, structural investigation and magnetic properties of Zn2+ substituted cobalt ferrite nanoparticles prepared by the sol–gel auto-combustion technique. J. Magn. Magn. Mater. 2014, 358, 87–92. [Google Scholar] [CrossRef]
- Sajjia, M.; Oubaha, M.; Hasanuzzaman, M.; Olabi, A.G. Developments of cobalt ferrite nanoparticles prepared by the sol–gel process. Ceram. Int. 2014, 40, 1147–1154. [Google Scholar] [CrossRef]
- Jain, S.R.; Adiga, K.C.; Verneker, V.P. A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust. Flame 1981, 40, 71–79. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Costa, A.C. Transesterification reaction for biodiesel production from soybean oil using Ni0.5Zn0.5Fe2O4 nanomagnetic catalyst: Kinetic study. Int. J. Energy Res. 2020, 44, 6674–6684. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.B.; Pontes, J.R.; Freitas, N.L.; Fernandes, P.C.; Costa, A.C.F. Biodiesel production on bench scale from different sources of waste oils by using NiZn magnetic heterogeneous nanocatalyst. Int. J. Energy Res. 2021, 45, 10924–10945. [Google Scholar] [CrossRef]
- Costa, A.C.F.M.; Morelli, M.R.; Kiminami, R.H.G.A. Combustion synthesis: Effect of urea on the reaction and characteristics of Ni-Zn ferrite powders. J. Mater. Synth. Process. 2002, 9, 347–352. [Google Scholar] [CrossRef]
- Hwang, C.C.; Tsai, J.S.; Huang, T.H.; Peng, C.H.; Chen, S.Y. Combustion synthesis of Ni–Zn ferrite powder—influence of oxygen balance value. J. Solid State Chem. 2005, 178, 382–389. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Al-Lohedan, H.A.; Ramalingam, R.J. Studies on Opuntia dilenii haw mediated multifunctional ZnFe2O4 nanoparticles: Optical, magnetic and catalytic applications. Mater. Chem. Phys. 2017, 194, 153–164. [Google Scholar] [CrossRef]
- Segadães, A.M.; Morelli, M.R.; Kiminami, R.H.G.A. Combustion synthesis of aluminium titanate. J. Eur. Ceram. Soc. 1998, 8, 771–781. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Kiminami, A.G.H.R.; Silva, M.R.; Costa, A.C.F.M. Síntese do ferroespinélio ZnFe2O4 e avaliação do seu desempenho em reações de esterificação e transesterificação via rota metílica. Rev. Eletrônica Mater. Process. 2015, 10, 137–143. [Google Scholar]
- Salunkhe, A.B.; Khot, V.M.; Phadatare, M.R.; Pawar, S.H. Combustion synthesis of cobalt ferrite nanoparticles—Influence of fuel to oxidizer ratio. J. Alloys Compd. 2012, 514, 91–96. [Google Scholar] [CrossRef]
- Khorrami, S.A.; Manuchehri, Q.S. Magnetic properties of cobalt ferrite synthesized by hydrothermal and co-precipitation methods: A comparative study. J. Appl. Chem. Res. 2013, 7, 15–23. [Google Scholar]
- Ren, Y.; Lin, L.; Ma, J.; Yang, J.; Feng, J.; Fan, Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B Environ. 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Soto-Arreola, A.; Huerta-Flores, A.M.; Mora-Hernández, J.M.; Torres-Martínez, L.M. Comparative study of the photocatalytic activity for hydrogen evolution of MFe2O4 (M = Cu, Ni) prepared by three different methods. J. Photochem. Photobiol. A Chem. 2018, 357, 20–29. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Kumar, N.; Karthigeyan, A.; Neppolian, B. Visible light photocatalytic activities of ZnFe2O4/ZnO nanoparticles for the degradation of organic pollutants. Mater. Chem. Phys. 2016, 181, 106–115. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Ferreira, L.F.; Silva, V.C. Magnetic materials for photocatalytic applications—A review. J. Sol-Gel Sci. Technol. 2020, 96, 1–14. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A. A review on self-modification of zirconium dioxide nanocatalysts with enhanced visible-light-driven photodegradation of organic pollutants. J. Hazard. Mater. 2022, 423, 126996. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Vo, D.V.N.; Cornejo-Ponce, L. Recent advancements of spinel ferrite based binary nanocomposite photocatalysts in wastewater treatment. Chemosphere 2021, 274, 129734. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Evans, D.G.; Duan, X. Stoichiometric synthesis of pure MFe2O4 (M = Mg, Co, and Ni) spinel ferrites from tailored layered double hydroxide (hydrotalcite-like) precursors. Chem. Mater. 2004, 16, 1597–1602. [Google Scholar] [CrossRef]
- Tiwari, R.; De, M.; Tewari, H.S.; Ghoshal, S.K. Structural and magnetic properties of tailored NiFe2O4 nanostructures synthesized using auto-combustion method. Results Phys. 2020, 16, 102916. [Google Scholar] [CrossRef]
- Vepulanont, K.; Sa-Nguanprang, S.; Buapoon, S.; Bunluesak, T.; Suebsom, P.; Chaisong, K.; Udomsri, N.; Karnchana, N.; Laokae, D.; Chanadee, T. Nickel ferrite ceramics: Combustion synthesis, sintering, characterization, and magnetic and electrical properties. J. Asian Ceram. Soc. 2021, 9, 639–651. [Google Scholar] [CrossRef]
- Adeleke, J.T.; Theivasanthi, T.; Thiruppathi, M.; Swaminathan, M.; Akomolafe, T.; Alabi, A.B. Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl. Surf. Sci. 2018, 455, 195–200. [Google Scholar] [CrossRef]
- Hou, X.; Feng, J.; Liu, X.; Ren, Y.; Fan, Z.; Wei, T.; Meng, J.; Zhang, M. Synthesis of 3D porous ferromagnetic NiFe2O4 and using as novel adsorbent to treat wastewater. J. Colloid Interface Sci. 2011, 362, 477–485. [Google Scholar] [CrossRef]
- Verma, K.C.; Singh, V.P.; Ram, M.; Shah, J.; Kotnala, R.K. Structural, microstructural and magnetic properties of NiFe2O4, CoFe2O4 and MnFe2O4 nanoferrite thin films. J. Magn. Magn. Mater. 2011, 323, 3271–3275. [Google Scholar] [CrossRef]
- Xiong, P.; Fu, Y.; Wang, L.; Wang, X. Multi-walled carbon nanotubes supported nickel ferrite: A magnetically recyclable photocatalyst with high photocatalytic activity on degradation of phenols. Chem. Eng. J. 2012, 195, 149–157. [Google Scholar] [CrossRef]
- Peng, T.; Zhang, X.; Lv, H.; Zan, L. Preparation of NiFe2O4 nanoparticles and its visible-light-driven photoactivity for hydrogen production. Catal. Commun. 2012, 28, 116–119. [Google Scholar] [CrossRef]
- Ren, A.; Liu, C.; Hong, Y.; Shi, W.; Lin, S.; Li, P. Enhanced visible-light-driven photocatalytic activity for antibiotic degradation using magnetic NiFe2O4/Bi2O3 heterostructures. Chem. Eng. J. 2014, 258, 301–308. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Kanhe, N.S.; Bhoraskar, S.V.; Bhat, S.K.; Bulakhe, R.N.; Shim, J.J.; Mathe, V.L. Micro-structural analysis of NiFe2O4 nanoparticles synthesized by thermal plasma route and its suitability for BSA adsorption. J. Mater. Sci. Mater. Med. 2015, 26, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, I.; Eftekhar, M. Characterization and evaluation catalytic efficiency of NiFe2O4 nano spinel in removal of reactive dye from aqueous solution. Powder Technol. 2013, 250, 147–153. [Google Scholar] [CrossRef]
- Hung, D.Q.; Thanh, N.K. Preparation of NiFe2O4-TiO2 nanoparticles and study of their photocatalytic activity. VNU J. Sci. Math.-Phys. 2011, 27, 204–211. [Google Scholar]
- Shao, R.; Sun, L.; Tang, L.; Chen, Z. Preparation and characterization of magnetic core–shell ZnFe2O4@ZnO nanoparticles and their application for the photodegradation of methylene blue. Chem. Eng. J. 2013, 217, 185–191. [Google Scholar] [CrossRef]
- Fan, G.; Gu, Z.; Yang, L.; Li, F. Nanocrystalline zinc ferrite photocatalysts formed using the colloid mill and hydrothermal technique. Chem. Eng. J. 2009, 155, 534–541. [Google Scholar] [CrossRef]
- Cheng, P.; Li, W.; Zhou, T.; Jin, Y.; Gu, M. Physical and photocatalytic properties of zinc ferrite doped titania under visible light irradiationJ. Photochem. Photobiol. A Chem. 2004, 168, 97–101. [Google Scholar] [CrossRef]
- Su, M.; He, C.; Sharma, V.K.; Abou Asi, M.; Xia, D.; Li, X.Z.; Deng, H.; Xiong, Y. Mesoporous zinc ferrite: Synthesis, characterization, and photocatalytic activity with H2O2/visible light. J. Hazard. Mater. 2012, 211, 95–103. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Zinc ferrite nanoparticle as a magnetic catalyst: Synthesis and dye degradation. Mater. Res. Bull. 2013, 48, 4255–4260. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, H.; Li, Q. Visible-light-driven photocatalytic properties of ZnO/ZnFe2O4 core/shell nanocable arrays. Appl. Catal. B Environ. 2014, 160, 408–414. [Google Scholar] [CrossRef]
- Yuan, Z.H. Synthesis, characterization and photocatalyticactivity of ZnFe2O4/TiO2 nanocomposite. J. Mater. Chem. 2001, 11, 1265–1268. [Google Scholar] [CrossRef]
- Hou, X.; Feng, J.; Xu, X.; Zhang, M. Synthesis and characterizations of spinel MnFe2O4 nanorod by seed–hydrothermal route. J. Alloys Compd. 2010, 491, 258–263. [Google Scholar] [CrossRef]
- Patil, S.B.; Naik, H.B.; Nagaraju, G.; Viswanath, R.; Rashmi, S.K. Sugarcane juice mediated eco-friendly synthesis of visible light active zinc ferrite nanoparticles: Application to degradation of mixed dyes and antibacterial activities. Mater. Chem. Phys. 2018, 212, 351–362. [Google Scholar] [CrossRef]
- Sripriya, R.C.; Vigneaswari, B.; Raj, V.A. Comparative Studies of Magneto-Optical and Photocatalytic Properties of Magnetically Recyclable Spinel ZnFe2O4 Nanostructures by Combustion Methods. Int. J. Nanosci. 2019, 18, 1850020. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Zheng, J.; Song, X.; Liu, X.; Chen, W.; Li, Y.; Guo, J. Synthesis of hexagonal CoFe2O4/ZnO nanoparticles and their electromagnetic properties. Mater. Lett. 2012, 73, 143–146. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S.; Ashokkumar, M. CoFe2O4/TiO2 nanocatalysts for the photocatalytic degradation of Reactive Red 120 in aqueous solutions in the presence and absence of electron acceptors. Chem. Eng. J. 2013, 220, 302–310. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Molla-Abbasi, P.; Haji-Aghajani, Z. Photo-catalyst CoFe2O4–TiO2: Application in photo-degradation of organic dyes and magnetic nanocomposite preparation. J. Mater. Sci. Mater. Electron. 2016, 27, 4879–4886. [Google Scholar] [CrossRef]
- Krishna, S.; Sathishkumar, P.; Pugazhenthiran, N.; Guesh, K.; Mangalaraja, R.V.; Kumaran, S.; Gracia-Pinilla, M.A.; Anandan, S. Heterogeneous sonocatalytic activation of peroxomonosulphate in the presence of CoFe2O4/TiO2 nanocatalysts for the degradation of Acid Blue 113 in an aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104024. [Google Scholar]
- Li, C.J.; Wang, J.N.; Wang, B.; Gong, J.R.; Lin, Z. A novel magnetically separable TiO2/CoFe2O4 nanofiber with high photocatalytic activity under UV–vis light. Mater. Res. Bull. 2012, 47, 333–337. [Google Scholar] [CrossRef]
- Chandel, N.; Sharma, K.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Singh, P. Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes. Arab. J. Chem. 2020, 13, 4324–4340. [Google Scholar] [CrossRef]
- Arief, S.; Jamarun, N.; Stiadi, Y. Magnetically separable ZnO-MnFe2O4 nanocomposites synthesized in organic-free media for dye degradation under natural sunlight. Orient. J. Chem. 2017, 33, 2758. [Google Scholar]
- Akhlaghi, N.; Najafpour-Darzi, G. Manganese ferrite (MnFe2O4) Nanoparticles: From synthesis to application—A review. J. Ind. Eng. Chem. 2021, 103, 292–304. [Google Scholar] [CrossRef]
- Deraz, N.M.; Shaban, S. Optimization of catalytic, surface and magnetic properties of nanocrystalline manganese ferrite. J. Anal. Appl. Pyrol. 2009, 86, 173–179. [Google Scholar] [CrossRef]
- Chang, L.; Pu, Y.; Jing, P.; Cui, Y.; Zhang, G.; Xu, S.; Cao, B.; Guo, J.; Chen, F.; Qiao, C. Magnetic core-shell MnFe2O4@TiO2 nanoparticles decorated on reduced graphene oxide as a novel adsorbent for the removal of ciprofloxacin and Cu(II) from water. Appl. Surf. Sci. 2021, 541, 148400. [Google Scholar] [CrossRef]
- Zamani, A.; Seyed Sadjadi, M.; Mahjoub, A.R.; Yousefi, M.; Farhadyar, N. Synthesis and characterization ZnFe2O4@MnO and MnFe2O4@ZnO magnetic nanocomposites: Investigation of photocatalytic activity for the degradation of Congo Red under visible light irradiation. Int. J. Nano Dimens. 2020, 11, 58–73. [Google Scholar]
- Silambarasu, A.; Manikandan, A.; Balakrishnan, K.; Jaganathan, S.K.; Manikandan, E.; Aanand, J.S. Comparative study of structural, morphological, magneto-optical and photo-catalytic properties of magnetically reusable spinel MnFe2O4 nano-catalysts. J. Nanosci. Nanotechnol. 2018, 18, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Selvamani, T.; Prasad, G.G.; Asiri, A.M.; Wu, J.J. Magnetic and catalytic properties of inverse spinel CuFe2O4 nanoparticles. J. Magn. Magn. Mater. 2017, 432, 437–443. [Google Scholar] [CrossRef]
- You-Yi, Y.; Heng-Qiang, Z. Reduced graphene oxide coupled magnetic CuFe2O4-TiO2 nanoparticles with enhanced photocatalytic activity for methylene blue degradation. Struct Chem. 2016, 35, 472–480. [Google Scholar]
- Shevale, V.B.; Dhodamani, A.G.; Delekar, S.D. Catalytic reclamation of silver present in photographic waste using magnetically separable TiO2@CuFe2O4 nanocomposites and thereof its use in antibacterial activity. ACS Omega 2020, 5, 1098–1108. [Google Scholar] [CrossRef]
- Janani, B.; Syed, A.; Thomas, A.M.; Al-Rashed, S.; Elgorban, A.M.; Raju, L.L.; Khan, S.S. A simple approach for the synthesis of bi-functional pn type ZnO@CuFe2O4 heterojunction nanocomposite for photocatalytic and antimicrobial application. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 130, 114664. [Google Scholar] [CrossRef]
- Zhang, C.F.; Zhong, X.C.; Yu, H.Y.; Liu, Z.W.; Zeng, D.C. Effects of cobalt doping on the microstructure and magnetic properties of Mn–Zn ferrites prepared by the co-precipitation method. Phys. B Condens. Matter. 2009, 404, 2327–2331. [Google Scholar] [CrossRef]
- Manikandan, A.; Durka, M.; Antony, S.A. Magnetically recyclable spinel MnxZn1–xFe2O4 (0.0 ≤ x ≤ 0.5) nano-photocatalysts. Adv. Sci. Eng. Med. 2015, 7, 33–46. [Google Scholar] [CrossRef]
- Shaikh, P.A.; Kambale, R.C.; Rao, A.V.; Kolekar, Y.D. Structural, magnetic and electrical properties of Co–Ni–Mn ferrites synthesized by co-precipitation method. J. Alloys Compd. 2010, 492, 590–596. [Google Scholar] [CrossRef]
- Ciocarlan, R.G.; Seftel, E.M.; Mertens, M.; Pui, A.; Mazaj, M.; Tusar, N.N.; Cool, P. Novel magnetic nanocomposites containing quaternary ferrites systems Co0.5Zn0.25M0.25Fe2O4 (M = Ni, Cu, Mn, Mg) and TiO2-anatase phase as photocatalysts for wastewater remediation under solar light irradiation. Mater. Sci. Eng. B 2018, 230, 1–7. [Google Scholar] [CrossRef]
- Jesudoss, S.K.; Vijaya, J.J.; Kennedy, L.J.; Rajan, P.I.; Al-Lohedan, H.A.; Ramalingam, R.J.; Kaviyarasu, K.; Bououdina, M. Studies on the efficient dual performance of Mn1–xNixFe2O4 spinel nanoparticles in photodegradation and antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 165, 121–132. [Google Scholar] [CrossRef]
- Naik, M.M.; Naik, H.B.; Nagaraju, G.; Vinuth, M.; Vinu, K.; Viswanath, R. Green synthesis of zinc doped cobalt ferrite nanoparticles: Structural, optical, photocatalytic and antibacterial studies. Nano-Struct. Nano-Objects. 2019, 19, 100322. [Google Scholar] [CrossRef]
- Baig, M.M.; Pervaiz, E.; Afzal, M.J. Catalytic activity and kinetic studies of Core@ Shell nanostructure NiFe2O4@TiO2 for photocatalytic degradation of methyl orange dye. J. Chem. Soc. Pak. 2020, 42, 531. [Google Scholar]
- Baldrian, P.; Merhautová, V.; Gabriel, J.; Nerud, F.; Stopka, P.; Hrubý, M.; Beneš, M.J. Decolorization of synthetic dyes by hydrogen peroxide with heterogeneous catalysis by mixed iron oxides. Appl. Catal. B Environ. 2006, 66, 258–264. [Google Scholar] [CrossRef]
- Neris, A.M.; Schreiner, W.H.; Salvador, C.; Silva, U.C.; Chesman, C.; Longo, E.; Santos, I.M.G. Photocatalytic evaluation of the magnetic core@ shell system (Co, Mn) Fe2O4@TiO2 obtained by the modified Pechini method. Mater. Sci. Eng. B 2018, 229, 218–226. [Google Scholar] [CrossRef]
- Cao, X.; Gu, L.; Lan, X.; Zhao, C.; Yao, D.; Sheng, W. Spinel ZnFe2O4 nanoplates embedded with Ag clusters: Preparation, characterization, and photocatalytic application. Mater. Chem. Phys. 2007, 106, 175–180. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Sun, Y.Q.; Gao, D.Z.; Xu, Y.Y. Quasi-cube ZnFe2O4 nanocrystals: Hydrothermal synthesis and photocatalytic activity with TiO2 (Degussa P25) as nanocomposite. Mater. Res. Bull. 2010, 45, 755–760. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Chen, F. Preparation and characterization of magnetic TiO2/ZnFe2O4 photocatalysts by a sol–gel method. Res. Chem. Intermed. 2008, 34, 375–380. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Zhao, Q.; Wang, L. A general, one-step and template-free synthesis of sphere-like zinc ferrite nanostructures with enhanced photocatalytic activity for dye degradation. J. Colloid Interface Sci. 2011, 358, 102–108. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wang, S.; Wu, Q.; Liu, C. Study on nanomagnets supported TiO2 photocatalysts prepared by a sol–gel process in reverse microemulsion combining with solvent-thermal technique. J. Hazard. Mater. 2009, 169, 1045–1053. [Google Scholar] [CrossRef]
- Fu, Y.P.; Chang, W.K.; Wang, H.C.; Liu, C.W.; Lin, C.H. Synthesis and characterization of anatase TiO2 nanolayer coating on Ni–Cu–Zn ferrite powders for magnetic photocatalyst. J. Mater. Res. 2010, 25, 134–140. [Google Scholar] [CrossRef]
- Dom, R.; Subasri, R.; Radha, K.; Borse, P.H. Synthesis of solar active nanocrystalline ferrite, MFe2O4 (M: Ca, Zn, Mg) photocatalyst by microwave irradiation. Solid State Commun. 2011, 151, 470–473. [Google Scholar] [CrossRef]
- Chen, C.H.; Liang, Y.H.; Zhang, W.D. ZnFe2O4/MWCNTs composite with enhanced photocatalytic activity under visible-light irradiation. J. Alloys Compd. 2010, 501, 168–172. [Google Scholar] [CrossRef]
- Rana, S.; Srivastava, R.S.; Sorensson, M.M.; Misra, R.D.K. Synthesis and characterization of nanoparticles with magnetic core and photocatalytic shell: Anatase TiO2–NiFe2O4 system. Mater. Sci. Eng. B 2005, 119, 144–151. [Google Scholar] [CrossRef]
- Cheng, P.; Deng, C.; Gu, M.; Shangguan, W. Visible-light responsive zinc ferrite doped titania photocatalyst for methyl orange degradation. J. Mater. Sci. 2007, 42, 9239–9244. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Hankare, P.P.; Patil, R.P.; Sasikala, R. Effect of sintering on photocatalytic degradation of methyl orange using zinc ferrite. Mater. Lett. 2011, 65, 371–373. [Google Scholar] [CrossRef]
- Xu, S.; Feng, D.; Shangguan, W. Preparations and photocatalytic properties of visible-light-active zinc ferrite-doped TiO2 photocatalyst. J. Phys. Chem. C 2009, 113, 2463–2467. [Google Scholar] [CrossRef]
- Arifin, M.N.; Karim, K.M.R.; Abdullah, H.; Khan, M.R. Synthesis of titania doped copper ferrite photocatalyst and its photoactivity towards methylene blue degradation under visible light irradiation. Bull. Chem. React. Eng. Catal. 2019, 14, 219–227. [Google Scholar] [CrossRef]
- Zouhier, M.; Tanji, K.; Navio, J.A.; Hidalgo, M.C.; Jaramillo-Páez, C.; Kherbeche, A. Preparation of ZnFe2O4/ZnO composite: Effect of operational parameters for photocatalytic degradation of dyes under UV and visible illumination. J. Photochem. Photobiol. A Chem. 2020, 390, 112305. [Google Scholar] [CrossRef]
- To Loan, N.T.; Hien Lan, N.T.; Thuy Hang, N.T.; Quang Hai, N.; Tu Anh, D.T.; Thi Hau, V.; Van Tan, L.; Van Tran, T. CoFe2O4 nanomaterials: Effect of annealing temperature on characterization, magnetic, photocatalytic, and photo-fenton properties. Processes 2019, 7, 885. [Google Scholar] [CrossRef]
- Mir, S.H.; Jennings, B.D.; Akinoglu, G.E.; Selkirk, A.; Gatensby, R.; Mokarian-Tabari, P. Enhanced Dye Degradation through Multi-Particle Confinement in a Porous Silicon Substrate: A Highly Efficient, Low Band Gap Photocatalyst. Adv. Opt. Mater. 2021, 9, 2002238. [Google Scholar] [CrossRef]
- Sciortino, F.; Sanchez-Ballester, N.M.; Mir, S.H.; Rydzek, G. Functional Elastomeric Copolymer Membranes Designed by Nanoarchitectonics Approach for Methylene Blue Removal. J. Inorg. Organomet. Polym. 2021, 31, 1967–1977. [Google Scholar] [CrossRef]
- Velmurugan, R.; Swaminathan, M. An efficient nanostructured ZnO for dye sensitized degradation of Reactive Red 120 dye under solar light. Sol. Energy Mater Sol. Cells. 2011, 95, 942–950. [Google Scholar] [CrossRef]
- Selvam, K.; Balachandran, S.; Velmurugan, R.; Swaminathan, M. Mesoporous nitrogen doped nano titania—A green photocatalyst for the effective reductive cleavage of azoxybenzenes to amines or 2-phenyl indazoles in methanol. Appl. Catal. A Gen. 2012, 413, 213–222. [Google Scholar] [CrossRef]
- Mathubala, G.; Manikandan, A.; Antony, S.A.; Ramar, P. Photocatalytic degradation of methylene blue dye and magneto-optical studies of magnetically recyclable spinel NixMn1−xFe2O4 (x = 0.0–1.0) nanoparticles. J. Mol. Struct. 2016, 1113, 79–87. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.; Mansur, H.S. Advanced Functional Nanostructures based on Magnetic Iron Oxide Nanomaterials for Water Remediation: A Review. Water Res. 2020, 190, 116693. [Google Scholar] [CrossRef]
- Padmapriya, G.; Manikandan, A.; Krishnasamy, V.; Jaganathan, S.K.; Antony, S.A. Spinel NixZn1−xFe2O4 (0.0 ≤ x ≤ 1.0) nano-photocatalysts: Synthesis, characterization and photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2016, 1119, 39–47. [Google Scholar] [CrossRef]
- Jia, Z.; Ren, D.; Liang, Y.; Zhu, R. A new strategy for the preparation of porous zinc ferrite nanorods with subsequently light-driven photocatalytic activity. Mater. Lett. 2011, 65, 3116–3119. [Google Scholar] [CrossRef]
- Velmurugan, R.; Krishnakumar, B.; Subash, B.; Swaminathan, M. Preparation and characterization of carbon nanoparticles loaded TiO2 and its catalytic activity driven by natural sunlight. Sol. Energy Mater Sol. Cells 2013, 108, 205–212. [Google Scholar] [CrossRef]
- Mandal, S.S.; Bhattacharyya, A.J. Electrochemical sensing and photocatalysis using Ag–TiO2 microwires. J. Chem. Sci. 2012, 124, 969–978. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater. Sci. Eng. Int. J. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Mirkhani, V.; Tangestaninejad, S.; Moghadam, M.; Habibi, M.H.; Rostami-Vartooni, A. Photocatalytic degradation of azo dyes catalyzed by Ag doped TiO2 photocatalyst. J. Iran. Chem. Soc. 2009, 6, 578–587. [Google Scholar] [CrossRef]
- Suwarnkar, M.B.; Dhabbe, R.S.; Kadam, A.N.; Garadkar, K.M. Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceram. Int. 2014, 40, 5489–5496. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, F.; Wang, M.; Ding, J.; Sun, S.; Bao, J.; Gao, C. Facile synthesis, structure and visible light photocatalytic activity of recyclable ZnFe2O4/TiO2. Appl. Surf. Sci. 2014, 319, 83–89. [Google Scholar] [CrossRef]
- Gupta, V.K.; Eren, T.; Atar, N.; Yola, M.L.; Parlak, C.; Karimi-Maleh, H. CoFe2O4@TiO2 decorated reduced graphene oxide nanocomposite for photocatalytic degradation of chlorpyrifos. J. Mol. Liq. 2015, 208, 122–129. [Google Scholar] [CrossRef]
- Haw, C.; Chiu, W.; Rahman, S.A.; Khiew, P.; Radiman, S.; Shukor, R.A.; Hamid, M.A.A.; Ghazali, N. The design of new magnetic-photocatalyst nanocomposites (CoFe2O4–TiO2) as smart nanomaterials for recyclable-photocatalysis applications. New J. Chem. 2016, 40, 1124–1136. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. A potential natural solar light active photocatalyst using magnetic ZnFe2O4@TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution. J. Clean. Prod. 2020, 268, 122023. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R.A. Fabrication of highly visible-light-responsive ZnFe2O4/TiO2 heterostructures for the enhanced photocatalytic degradation of organic dyes. RSC Adv. 2016, 6, 103428–103437. [Google Scholar] [CrossRef]
- Xu, S.H.; Feng, D.L.; Li, D.X.; Shangguan, W.F. Preparation of magnetic photocatalyst TiO2 supported on NiFe2O4 and effect of magnetic carrier on photocatalytic activity. Chin. J. Chem. 2008, 26, 842–846. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R.A. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef]
- Saad, D.; Byrne, D.; Drechsel, P. Social perspectives on the effective management of wastewater. In Physico-Chemical Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; InTech: London, UK, 2017; pp. 253–267. [Google Scholar]
- Wichelns, D.; Drechsel, P.; Qadir, M. Wastewater: Economic Asset in an Urbanizing World. In Wastewater; Drechsel, P., Qadir, M., Wichelns, D., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 3–14. [Google Scholar]
- Friedler, E.; Lahav, O.; Jizhaki, H.; Lahav, T. Study of urban population attitudes towards various wastewater reuse options: Israel as a case study. J. Environ. Manag. 2006, 81, 360–370. [Google Scholar] [CrossRef]
- Gebrezgabher, S.; Rao, K.; Hanjra, M.A.; Hernández-Sancho, F. Business models and economic approaches for recovering energy from wastewater and fecal sludge. In Wastewater; Springer: Dordrecht, The Netherlands, 2015; pp. 217–245. [Google Scholar]








| Synthesis Method | Sol-Gel | Co-Precipitation | Hydrothermal | Combustion |
|---|---|---|---|---|
| Crystallite size (nm) | 37.3 | 33.0 | 15.0 | 38.0 |
| Ms (emu·g−1) | 58.9 | 60.9 | 56.9 | 59.0 |
| Magnetic Nanoparticles (MNPs) | Organic Pollutants | Dye (mg/L) | Photocatalyst (mg/L) | Irradiation Time (min) | Irradiation Source | Degradation (%) | References |
|---|---|---|---|---|---|---|---|
| NiFe2O4/TiO2 | Methyl Orange | UV–Vis | 90 | [120] | |||
| CoFe2O4 | Bromophenol Blue | 50 | 25 | 24 | n/A | 2 | [121] a |
| CuFe2O4 | Bromophenol blue | 50 | 25 | 24 | n/A | 48 | [121] |
| FeFe2O4 | Bromophenol blue | 50 | 25 | 24 | n/A | 99 | [121] |
| MnFe2O4 | Bromophenol blue | 50 | 25 | 24 | n/A | 0 | [121] |
| CoFe2O4 | Chicago Sky Blue | 50 | 25 | 24 | n/A | 93 | [121] |
| CuFe2O4 | Chicago Sky Blue | 50 | 25 | 24 | n/A | 95 | [121] |
| FeFe2O4 | Chicago Sky Blue | 50 | 25 | 24 | n/A | 98 | [121] |
| MnFe2O4 | Chicago Sky Blue | 50 | 25 | 24 | n/A | 91 | [121] |
| CoFe2O4 | Cu Phthalocyanine | 50 | 25 | 24 | n/A | 70 | [121] |
| CuFe2O4 | Cu Phthalocyanine | 50 | 25 | 24 | n/A | 75 | [121] |
| FeFe2O4 | Cu Phthalocyanine | 50 | 25 | 24 | n/A | 92 | [121] |
| MnFe2O4 | Cu Phthalocyanine | 50 | 25 | 24 | n/A | 19 | [121] |
| CoFe2O4 | Eosin Yellowish | 50 | 25 | 24 | n/A | 25 | [121] |
| CuFe2O4 | Eosin Yellowish | 50 | 25 | 24 | n/A | 53 | [121] |
| FeFe2O4 | Eosin Yellowish | 50 | 25 | 24 | n/A | 85 | [121] |
| MnFe2O4 | Eosin Yellowish | 50 | 25 | 24 | n/A | 12 | [121] |
| CoFe2O4 | Evans Blue | 50 | 25 | 24 | n/A | 73 | [121] |
| CuFe2O4 | Evans Blue | 50 | 25 | 24 | n/A | 92 | [121] |
| FeFe2O4 | Evans Blue | 50 | 25 | 24 | n/A | 99 | [121] |
| MnFe2O4 | Evans Blue | 50 | 25 | 24 | n/A | 8 | [121] |
| CoFe2O4 | Naphthol Blue Black | 50 | 25 | 24 | n/A | 68 | [121] |
| CuFe2O4 | Naphthol Blue Black | 50 | 25 | 24 | n/A | 95 | [121] |
| FeFe2O4 | Naphthol Blue Black | 50 | 25 | 24 | n/A | 93 | [121] |
| MnFe2O4 | Naphthol Blue Black | 50 | 25 | 24 | n/A | 75 | [121] |
| CoFe2O4 | Phenol Red | 50 | 25 | 24 | n/A | 85 | [121] |
| CuFe2O4 | Phenol Red | 50 | 25 | 24 | n/A | 86 | [121] |
| FeFe2O4 | Phenol Red | 50 | 25 | 24 | n/A | 81 | [121] |
| MnFe2O4 | Phenol Red | 50 | 25 | 24 | n/A | 63 | [121] |
| CoFe2O4 | Poly B-411 | 50 | 25 | 24 | n/A | 0 | [121] |
| CuFe2O4 | Poly B-411 | 50 | 25 | 24 | n/A | 7 | [121] |
| FeFe2O4 | Poly B-411 | 50 | 25 | 24 | n/A | 38 | [121] |
| MnFe2O4 | Poly B-411 | 50 | 25 | 24 | n/A | 0 | [121] |
| CoFe2O4 | Reactive Orange 16 | 50 | 25 | 24 | n/A | 21 | [121] |
| CuFe2O4 | Reactive Orange 16 | 50 | 25 | 24 | n/A | 86 | [121] |
| FeFe2O4 | Reactive Orange 16 | 50 | 25 | 24 | n/A | 77 | [121] |
| MnFe2O4 | Reactive Orange 16 | 50 | 25 | 24 | n/A | 6 | [121] |
| CuFe2O4 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 81 | [115] |
| CuFe2O4-TiO2 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 84 | [115] |
| Cu0.9Mn0.1Fe2O4/TiO2 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 88 | [115] |
| Cu0.8Mn0.2Fe2O4/TiO2 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 92 | [115] |
| Cu0.7Mn0.3Fe2O4/TiO2 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 94 | [115] |
| Cu0.6Mn0.4Fe2O4/TiO2 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 96 | [115] |
| Cu0.5Mn0.5Fe2O4/TiO2 | 4-chlorophenol | 200 | 30 | 30 | UV–Vis | 98 | [115] |
| (Co,Mn)Fe2O4@TiO2 | Azo dye | 10 | 10 | 960 | UV | 76 | [122] |
| ZnFe2O4 | Rhodamine B | 10 | 20 | 150 | 200–700 nm | 60 | [123] |
| TiO2/ZnFe2O4 | Rhodamine B | 9.6 | 10 | 150 | λ = 254 nm | 99.7 | [124] |
| TiO2/ZnFe2O4 (1:1) | Rhodamine B | n/A | n/A | 150 | UV | 47 | [125] |
| TiO2/ZnFe2O4 (2:1) | Rhodamine B | n/A | n/A | 150 | UV | 58 | [125] |
| TiO2/ZnFe2O4 (3:1) | Rhodamine B | n/A | n/A | 150 | UV | 87 | [125] |
| TiO2/ZnFe2O4 (4:1) | Rhodamine B | n/A | n/A | 150 | UV | 95 | [125] |
| ZnFe2O4 | Rhodamine B | 20 | 80 | 300 | UV–Vis | 38.4 | [126] |
| ZnFe2O4 nanospheres | Rhodamine B | 20 | 80 | 300 | UV–Vis | 100 | [126] |
| ZnFe2O4 | Rhodamine B | 20 | 500 | 360 | UV-light | 45 | [86] |
| ZnFe2O4 b | Rhodamine B | 20 | 500 | 360 | UV-light | 88 | [86] |
| ZnFe2O4 c | Rhodamine B | 20 | 500 | 360 | UV-light | 75 | [86] |
| ZnFe2O4 d | Rhodamine B | 20 | 500 | 360 | UV-light | 60 | [86] |
| ZnFe2O4 b | Rhodamine B | 20 | 500 | 360 | Dark | 0 | [86] |
| TiO2/CoFe2O4 (10%) | Methylene Blue | 5 | 0.5 | 60 | UV | 56 | [127] |
| TiO2/CoFe2O4 (20%) | Methylene Blue | 5 | 0.5 | 60 | UV | 60 | [127] |
| TiO2/CoFe2O4 (30%) | Methylene Blue | 5 | 0.5 | 60 | UV | 57 | [127] |
| TiO2/Ni-Cu-Zn ferrite | Methylene Blue | 20 | 13 | 120 | UV | 82 | [128] |
| TiO2/Ni-Cu-Zn ferrite | Methylene Blue | 20 | 20 | 120 | UV | 98 | [128] |
| TiO2/Ni-Cu-Zn ferrite | Methylene Blue | 20 | 26 | 120 | UV | 99 | [128] |
| TiO2/Ni-Cu-Zn ferrite | Methylene Blue | 20 | 33 | 120 | UV | 95 | [128] |
| ZnFe2O4 | Methylene Blue | 10 | 6 | 180 | UV | 28 | [129] |
| MnFe2O4 e | Methylene Blue | 7 | 300 | 1200 | Visible light | 15.2 | [92] |
| MnFe2O4 f | Methylene Blue | 7 | 300 | 1200 | Visible light | 67.2 | [92] |
| ZnFe2O4 | Methylene Blue | 10 | 100 | 360 | UV–Vis | 8 | [130] |
| ZnFe2O4 + H2O2 | Methylene Blue | 10 | 100 | 360 | UV–Vis | 52 | [130] |
| ZnFe2O4 + H2O2 | Methylene Blue | 10 | 100 | 360 | Dark | 45 | [130] |
| TiO2(57%)/CoFe2O4 (37%) | Methyl Orange | 6 | n/A | 250 | UV | 0 | [114] |
| TiO2(62%)/CoFe2O4 (30%) | Methyl Orange | 6 | n/A | 250 | UV | 25 | [114] |
| CoFe2O4/ZnO | Methyl Orange | 50 | 30 | 300 | UV | 93.9 | [114] |
| TiO2/ZnFe2O4 | Methyl Orange | 8 | 80 | 420 | UV | 80 | [131] |
| TiO2/ZnFe2O4 g | Methyl Orange | 10 | 50 | 180 | UV–Vis | 5 | [87] |
| TiO2/ZnFe2O4 h | Methyl Orange | 10 | 50 | 180 | UV–Vis | 13 | [87] |
| TiO2/ZnFe2O4 i | Methyl Orange | 10 | 50 | 180 | UV–Vis | 27 | [87] |
| TiO2/ZnFe2O4 (0.15%) | Methyl Orange | 25 | 5 | 240 | UV–Vis | 65 | [132] |
| TiO2/ZnFe2O4 (0.30%) | Methyl Orange | 25 | 5 | 240 | UV–Vis | 75 | [132] |
| TiO2/ZnFe2O4 (1.5%) | Methyl Orange | 25 | 5 | 240 | UV–Vis | 84 | [132] |
| TiO2/ZnFe2O4 (3.0%) | Methyl Orange | 25 | 5 | 240 | UV–Vis | 73 | [132] |
| TiO2/ZnFe2O4 (6.05%) | Methyl Orange | 25 | 5 | 240 | UV–Vis | 55 | [132] |
| ZnFe2O4 | Methyl Orange | 25 | 5 | 240 | UV–Vis | 4 | [132] |
| ZnFe2O4 | Methyl Orange | 10 | 4 | 60 | UV-light | 75 | [133] |
| ZnFe2O4 | Methyl Orange | 10 | 100 | 240 | UV–Vis | 5 | [134] |
| TiO2/ZnFe2O4 | Methyl Orange | 10 | 100 | 240 | UV–Vis | 40 | [134] |
| TiO2/ZnFe2O4 (1.5%) | Methyl Orange | 10 | 100 | 240 | UV–Vis | 12 | [134] |
| TiO2/ZnFe2O4 (3.0%) | Methyl Orange | 10 | 100 | 240 | UV–Vis | 34 | [134] |
| TiO2/ZnFe2O4 (4.5%) | Methyl Orange | 10 | 100 | 240 | UV–Vis | 24 | [134] |
| TiO2/ZnFe2O4 (6.0%) | Methyl Orange | 10 | 100 | 240 | UV–Vis | 18 | [134] |
| CuFe2O4-TiO2 | Methylene Blue | 50 | 1000 | 180 | UV–Vis | 83.7 | [135] |
| ZnFe2O4/ZnO | Methylene Blue | 20 | 1000 | 360 | UV | 90 | [136] |
| ZnFe2O4/ZnO | Remazol Brilliant Blue | 20 | 1000 | 360 | UV | 100 | [136] |
| CoFe2O4 + H2O2 | Rhodamine B | 10 | 100 | 270 | UV-Vis | 90.6 | [137] |
| CuFe2O4-TiO2 | Methylene Blue | 12 | 100 | 150 | UV-Vis | 47 | [111] |
| Magnetic Nanocomposites | Organic Pollutants | Dye (mg/L) | Photocatalyst (g/L) | Irradiation Time (min) | pH | Cycles of Reusability of MNPs | Degradation Efficiency (%) | Irradiation Source | References |
|---|---|---|---|---|---|---|---|---|---|
| CoFe2O4/TiO2-SiO2 | Methylene blue dye | 0.3 | 0.33 | 30 | 6 | 6 | 93.2 | UV | [10] |
| NiFe2O4 | Reactive blue 5 | 0.05 | 0.03 | 10 | 1–11 | 4 | 85.0 | n/A | [83] |
| NiFe2O4/ZnO | Congo red (CR) | 0.02 | 0.05 | 40 | 5.5 | 5 | 97.0 | UV–Vis | [16] |
| CoFe2O4/ZnO | Methylene blue dye | 5 | 0.025 | 60 | n/A | 3 | - | UV–Vis | [5] |
| CoFe2O4/TiO2 | Methylene blue dye | n/A | n/A | 360 | n/A | 5 | 93.8 | UV | [153] |
| ZnFe2O4/TiO2/Cu | Naproxen | 0.03 | 0.1 | 120 | 4–9 | 5 | 72.3 | Sunlight | [154] |
| ZnFe2O4/TiO2 | Rhodamine B | 10 | 1 | 30 | 3–11 | 5 | >99 | UV–Vis | [155] |
| CoFe2O4/TiO2/rGO | Chlorpyrifos | 5 | 0.4 | 60 | 5.8 | 8 | - | UV | [152] |
| ZnFe2O4/TiO2 | Methylene blue dye | 20 | 0.05 | 360 | 5.6 | 5 | 93.2 | UV–Vis | [151] |
| ZnFe2O4/ZnO | Methylene blue dye | n/A | 0.1 | 120 | n/A | 3 | - | UV | [17] |
| NiFe2O4/TiO2 | Methyl Orange | 10 | 1 | 300 | 9.5–10 | 3 | - | Visible light | [156] |
| Cu0.5Mn0.5Fe2O4/TiO2 | 4-chlorophenol | 30 | 0.2 | 300 | 8 | 5 | 98.0 | UV | [115] |
| ZnO/MnFe2O4 | Methylene blue dye | n/A | n/A | 300 | 3–9 | 6 | 86.0 | UV–Vis | [113] |
| CuFe2O4/TiO2 | Methylene blue dye | 30 | 1 | 120 | 2.2 | 3 | 81.2 | UV–Vis | [135] |
| ZnFe2O4/ZnO | Methylene blue dye | 10 | 0.1 | 200 | 9–10 | 3 | 79.0 | UV | [85] |
| MnFe2O4/TiO2-rGO | Ciprofloxacin | 30 | 0.06 | 300 | 1–6 | 6 | 75.0 | UV–Vis | [106] |
| ZnFe2O4/TiO2 | Bisphenol A | 10 | 1 | 300 | 9.5–10 | 5 | >90 | UV–Vis | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapossa, A.B.; Mhike, W.; Adalima, J.L.; Tichapondwa, S. Removal of Organic Dyes from Water and Wastewater Using Magnetic Ferrite-Based Titanium Oxide and Zinc Oxide Nanocomposites: A Review. Catalysts 2021, 11, 1543. https://doi.org/10.3390/catal11121543
Mapossa AB, Mhike W, Adalima JL, Tichapondwa S. Removal of Organic Dyes from Water and Wastewater Using Magnetic Ferrite-Based Titanium Oxide and Zinc Oxide Nanocomposites: A Review. Catalysts. 2021; 11(12):1543. https://doi.org/10.3390/catal11121543
Chicago/Turabian StyleMapossa, António B., Washington Mhike, José L. Adalima, and Shepherd Tichapondwa. 2021. "Removal of Organic Dyes from Water and Wastewater Using Magnetic Ferrite-Based Titanium Oxide and Zinc Oxide Nanocomposites: A Review" Catalysts 11, no. 12: 1543. https://doi.org/10.3390/catal11121543
APA StyleMapossa, A. B., Mhike, W., Adalima, J. L., & Tichapondwa, S. (2021). Removal of Organic Dyes from Water and Wastewater Using Magnetic Ferrite-Based Titanium Oxide and Zinc Oxide Nanocomposites: A Review. Catalysts, 11(12), 1543. https://doi.org/10.3390/catal11121543






