Novel CNT Supported Molybdenum Catalyst for Detection of L-Cysteine in Its Natural Environment
Abstract
:1. Introduction
2. Results and Discussion
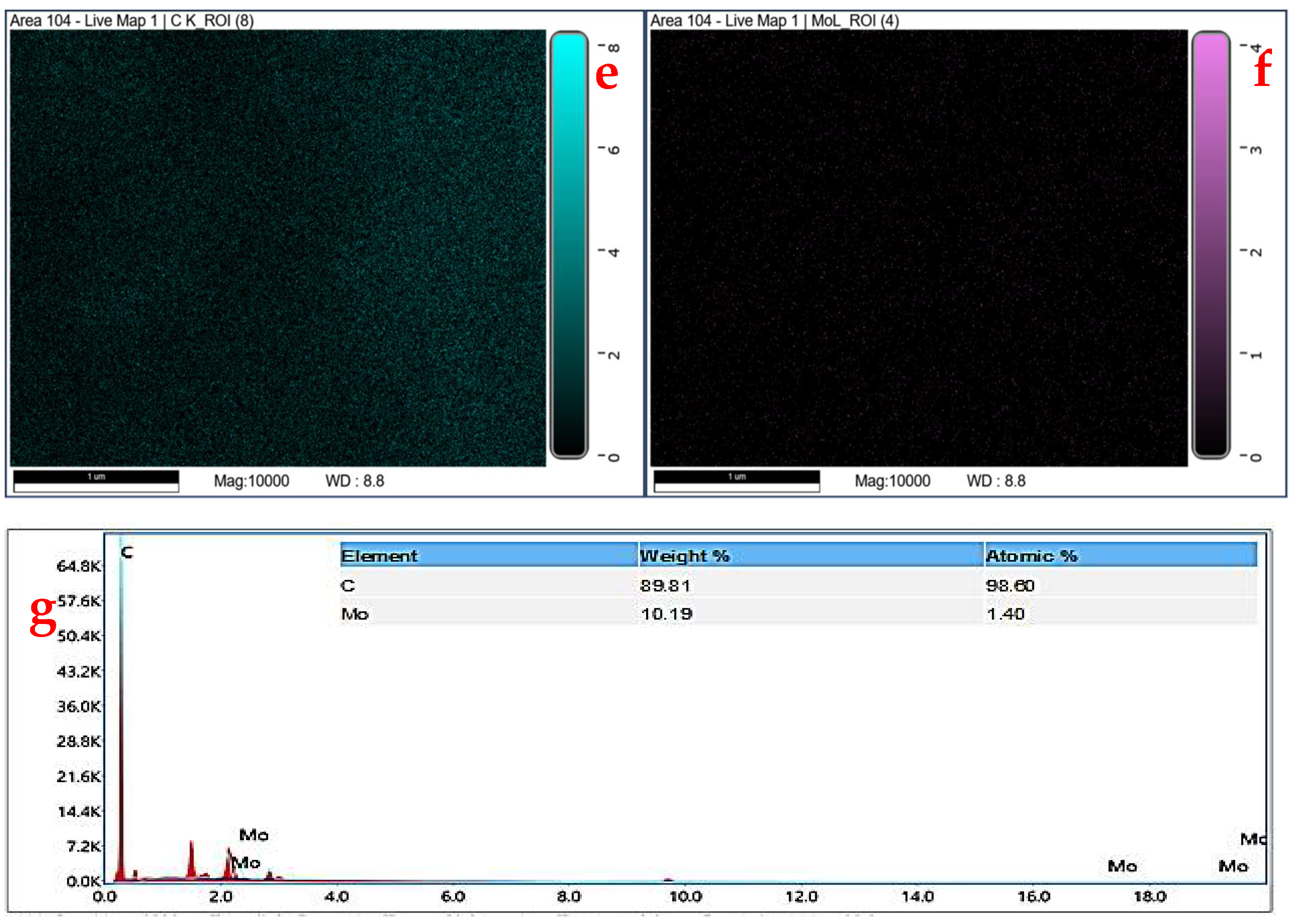
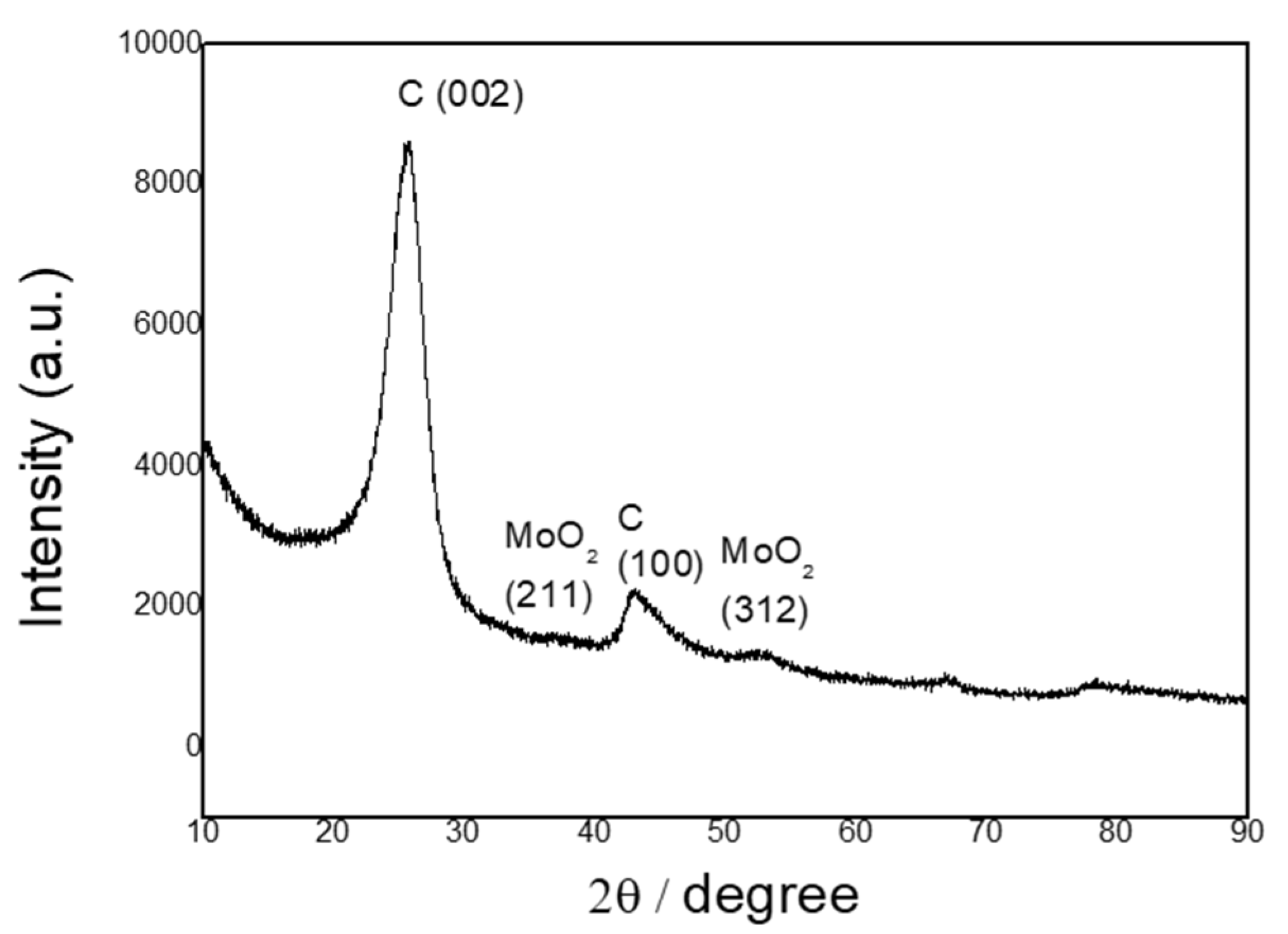
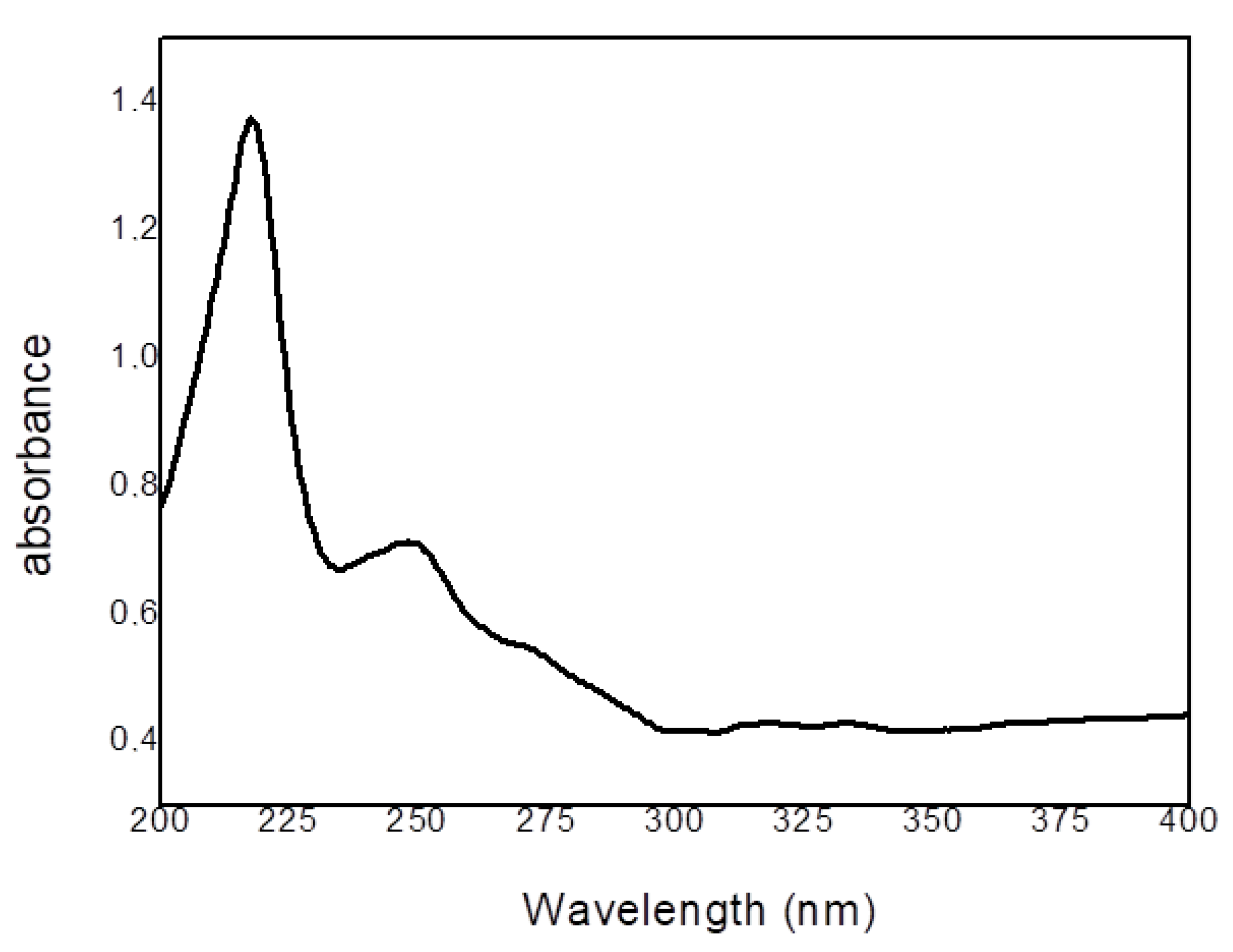
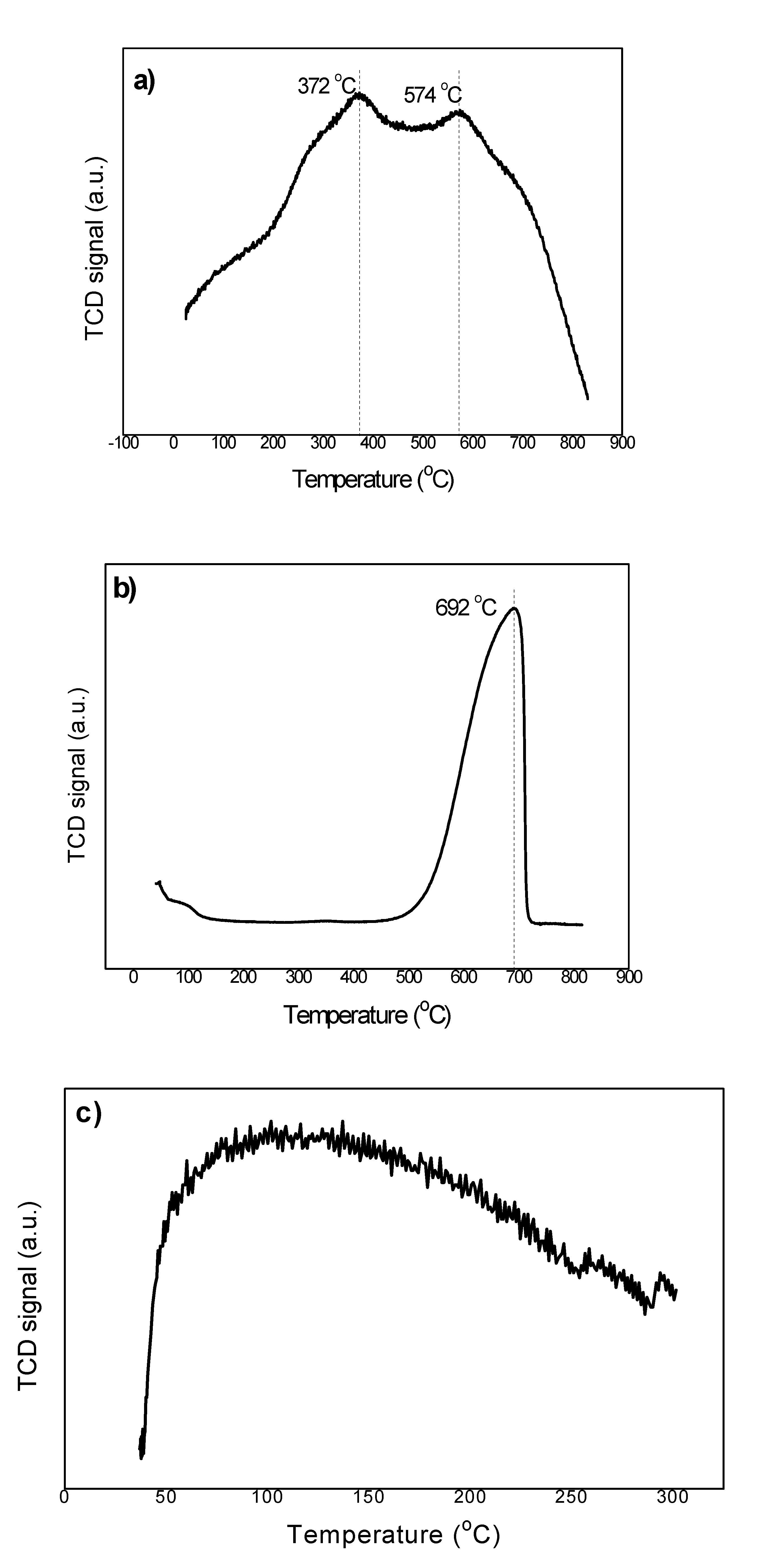
2.1. Physical Characterization
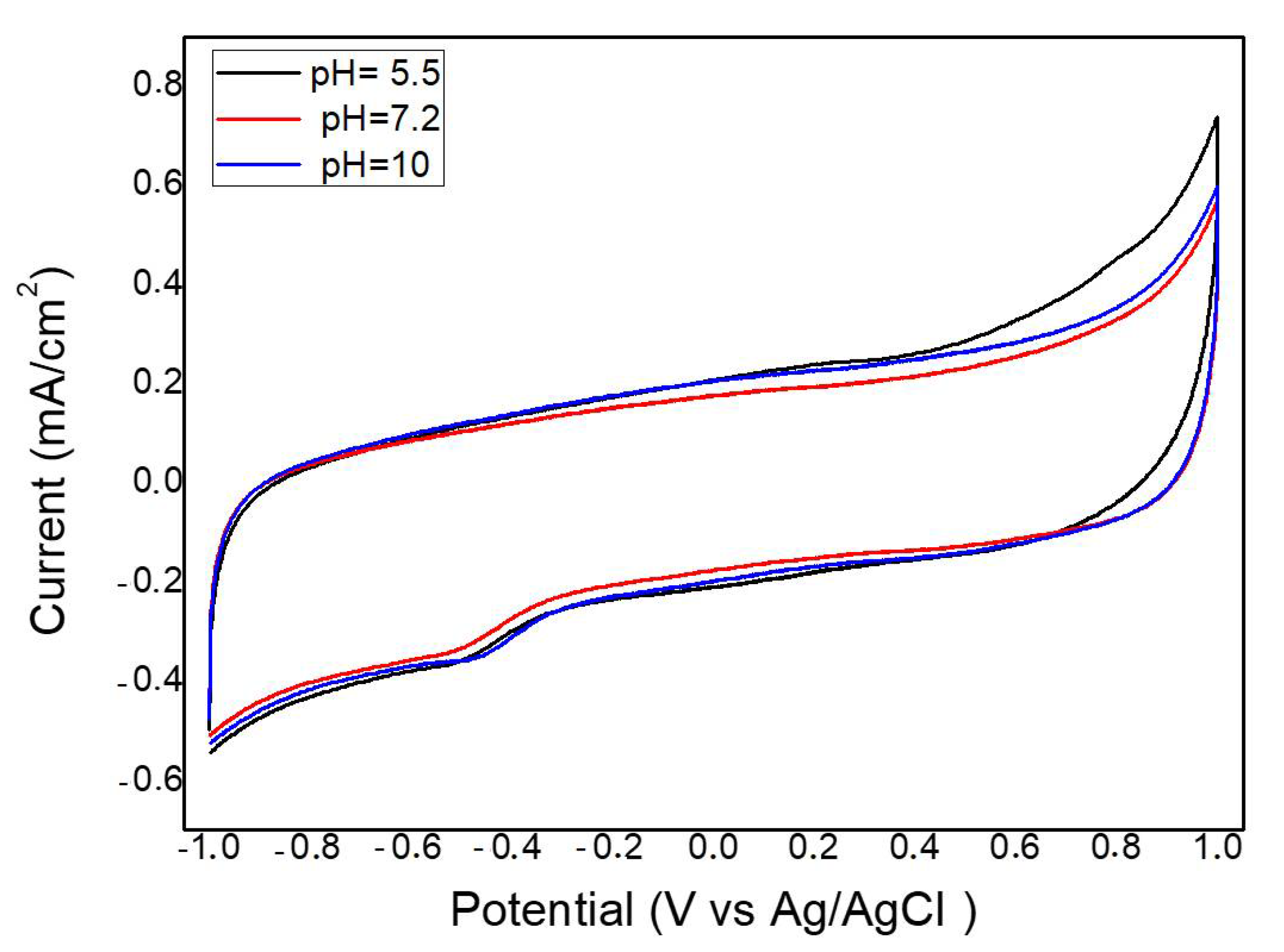
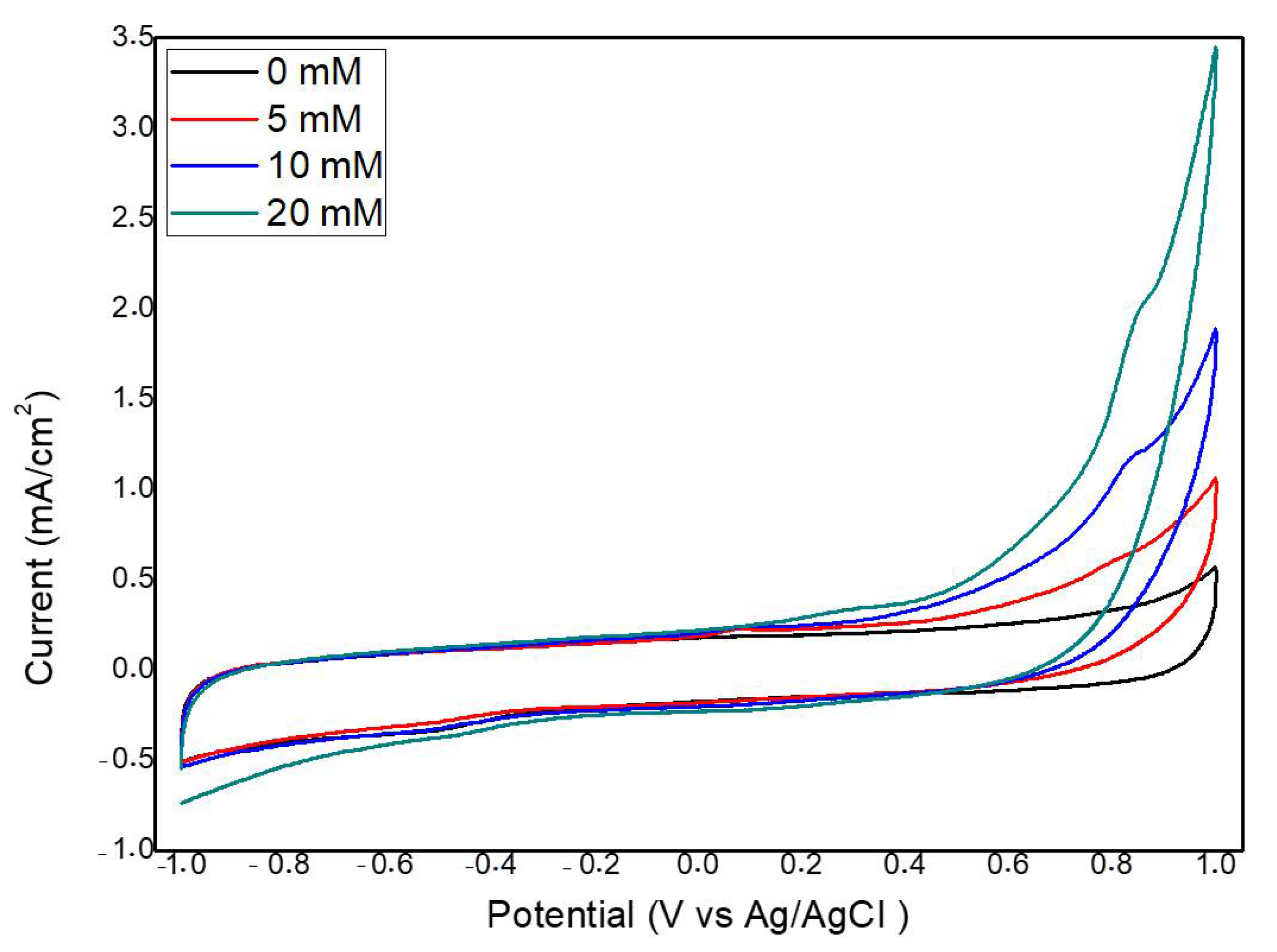
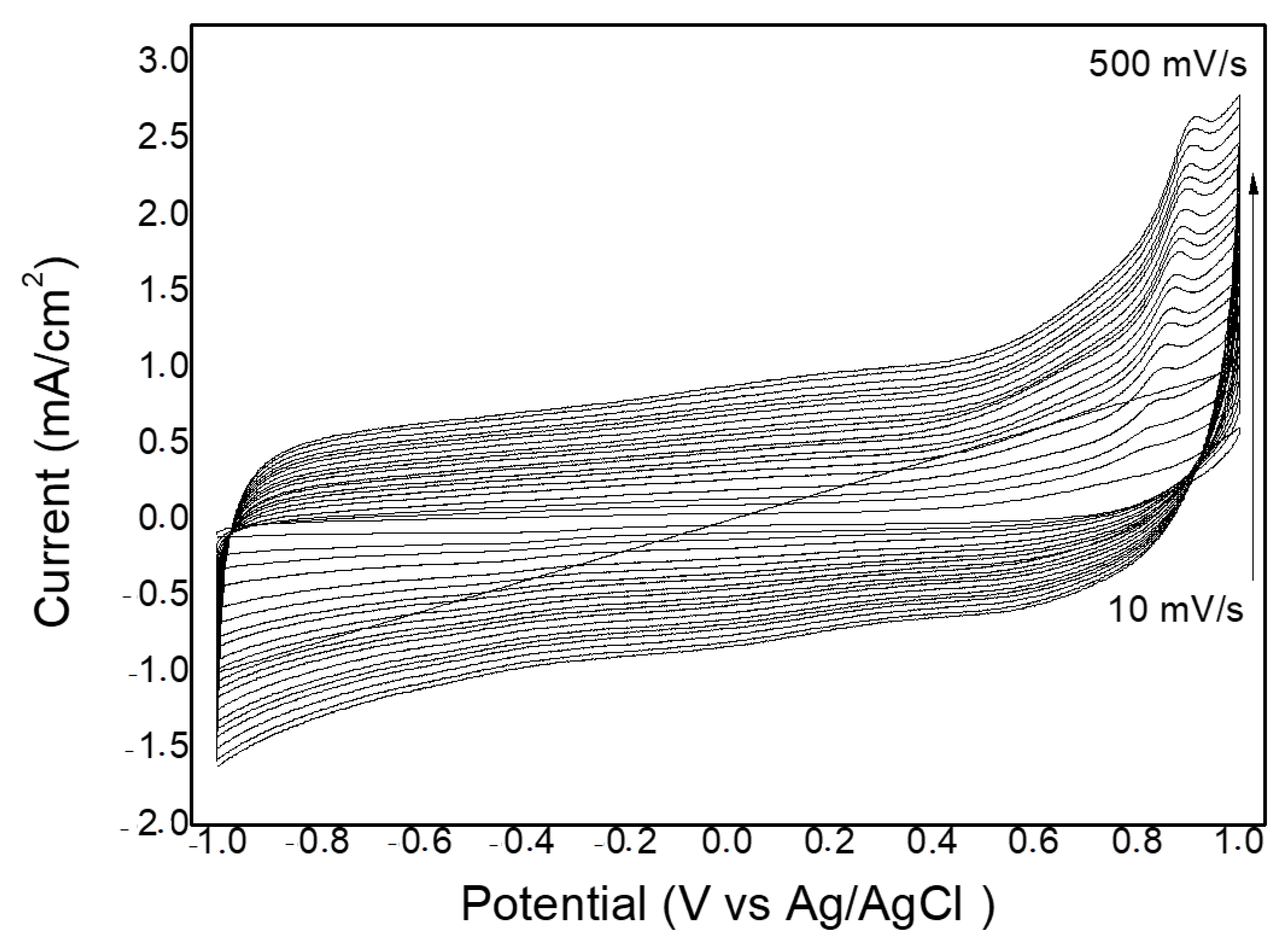
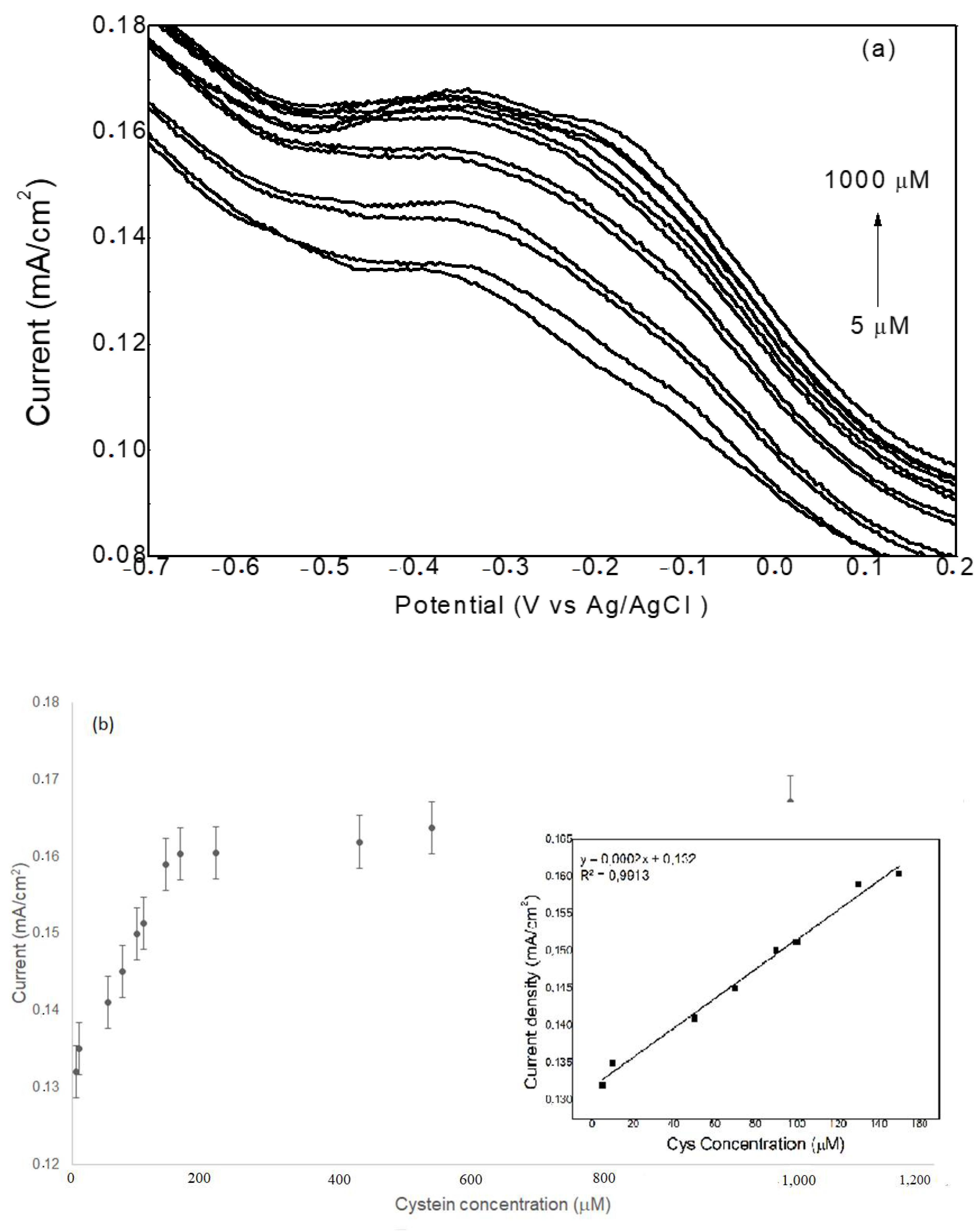
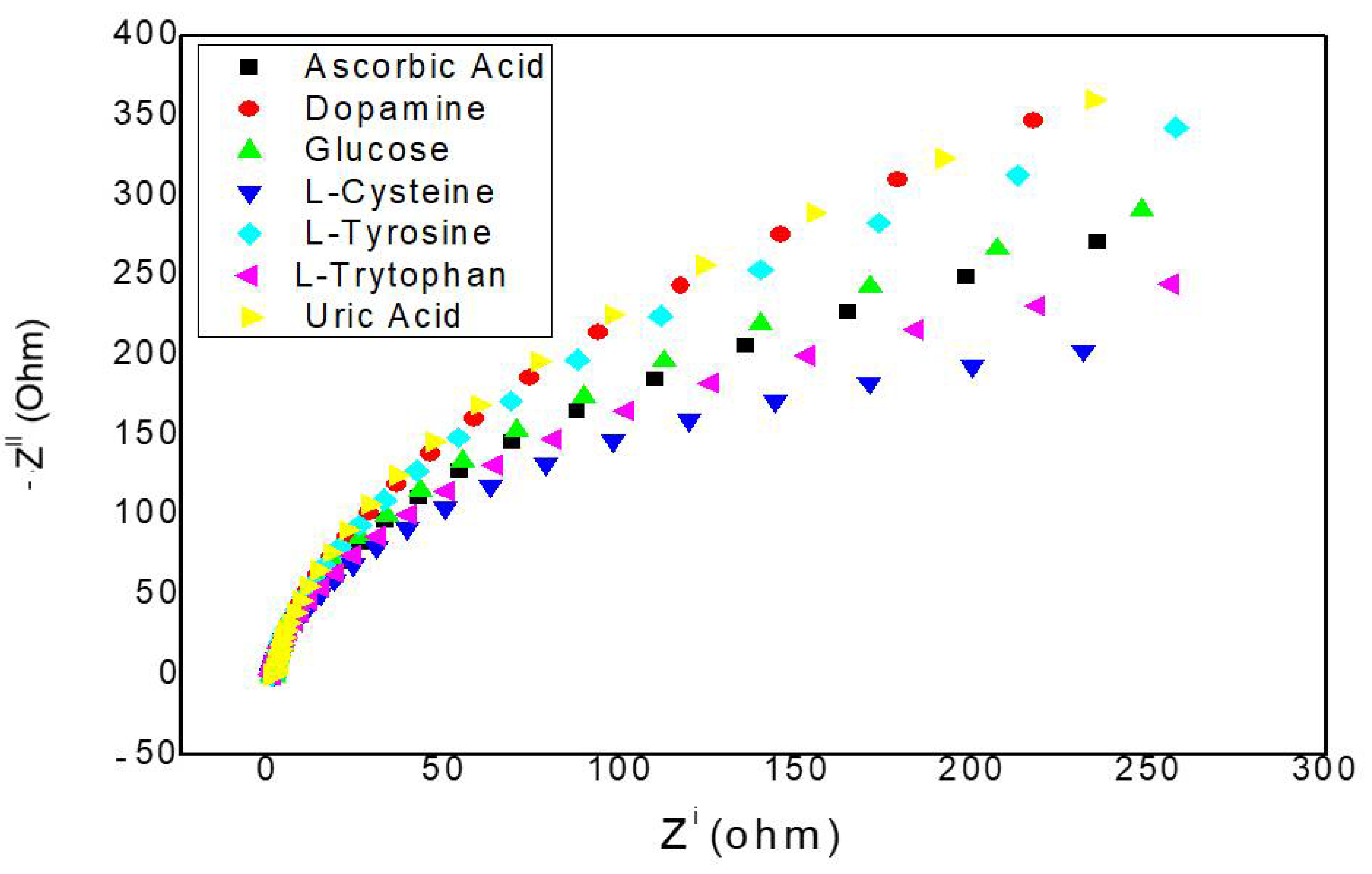
2.2. Electrochemical Measurements
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crawhall, J.C. Areview of the clinical presentation and laboratory findings in 2 uncommon herediatry disorders of sulfur aminoacid metabolism amd beta mercaplolactate cysteine disulfideureia and sulfite oxidase deficiency. Clin. Biochem. 1985, 18, 139–142. [Google Scholar] [CrossRef]
- Kos, J.; Lah, T.T. Cysteine proteinases and their endogenous inhibitors, Target proteins for prognosis, diagnosis and therapy in cancer (Review). Oncol. Rep. 1998, 5, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Derbyshire, J.F.; McPhie, P.; Jakoby, W.B. A review of the effects of manipulation of the cysteine residues of rat aryl sulfotransferase IV. Chem. Biol. Interact. 1998, 109, 107–116. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Role of the liver in regulation of body cysteine and taurine levels, A brief review. Neurochem. Res. 2004, 29, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Sarayama, Y.; Shimizu, H.; Yamada, Y.; Horikawa, T. Thiodiglycolic acid as a possible causative agent of fixed drug eruption provoked only after continuous administration of S-carboxymethyl-L-cysteine, case report and review of reported cases. Br. J. Dermatol. 2005, 153, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Cejpek, K. Biosynthesis of food constituents, Amino acids, 2. The alanine-valine-leucine, serine-cysteine-glycine, and aromatic and heterocyclic amino acids groups—A review. Czech J. Food Sci. 2006, 24, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Kargosha, K.; Ahmadi, S.H.; Zeeb, M.; Moeinossadat, S.R. Vapour phase Fourier transform infrared spectrometric determination of L-cysteine and L-cystine. Talanta 2008, 74, 753–759. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Velázquez-Gómez, M.; Lacorte, S.; Ramos, L. Exhaustive characterization of (semi-) volatile organic contaminants in car dust using comprehensive two-dimensional gas chromatography—Time-of-flight mass spectrometry. J. Hazard. Mater. 2021, 411, 125058. [Google Scholar] [CrossRef] [PubMed]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Heidari, M.; Ghaffarinejad, A. Electrochemical sensor for L-cysteine by using a cobalt(II)/aluminum(III) layered double hydroxide as a nanocatalyst. Microchim. Acta 2019, 186, 365. [Google Scholar] [CrossRef]
- Kurniawan, A.; Kurniawan, F.; Gunawan, F.; Chou, S.H.; Wang, M.J. Disposable electrochemical sensor based on copper-electrodeposited screen-printed gold electrode and its application in sensing L-Cysteine. Electrochim. Acta 2019, 293, 318–327. [Google Scholar] [CrossRef]
- Yao, J.; Liu, C.H.; Liu, L.; Chen, M.; Yang, M. An Electrochemical Sensor for Sensitive Determination of L-cysteine and Its Electrochemical Kinetics on AgNPs/GQDs/GCE Composite Modified Electrode. J. Electrochem. Soc. 2018, 165, B551–B558. [Google Scholar] [CrossRef]
- Pazalja, M.; Kahrovic, E.; Zahirovic, A.; Turkusic, E. Electrochemical Sensor for Determination of L-Cysteine Based on Carbon Electrodes Modified with Ru(III) Schiff Base Complex, Carbon Nanotubes and Nafion. Int. J. Electrochem. Sci. 2016, 11, 10939–10952. [Google Scholar] [CrossRef]
- Xiao, C.H.; Chen, J.H.; Liu, B.; Chu, X.C.; Wu, L.A.; Yao, S.Z. Sensitive and selective electrochemical sensing of L-cysteine based on a caterpillar-like manganese dioxide-carbon nanocomposite. Phys. Chem. Chem. Phys. 2011, 13, 1568–1574. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Y.; Lu, Z.W.; Du, H.J.; Ye, J.S. One pot synthesis of palladium-cobalt nanoparticles over carbon nanotubes as a sensitive non-enzymatic sensor for glucose and hydrogen peroxide detection. Sens. Actuators B Chem. 2017, 252, 1016–1025. [Google Scholar] [CrossRef]
- Hoa, L.T.; Hur, S.H. Non-Enzymatic Glucose Sensor Based on 3D Graphene Oxide Hydrogel Cross linked by Various Diamines. J. Nanosci. Nanotechnol. 2015, 15, 8697–8700. [Google Scholar] [CrossRef]
- Hoa, L.T.; Sun, K.G.; Hur, S.H. Highly sensitive non-enzymatic glucose sensor based on Pt nanoparticle decorated graphene oxide hydrogel. Sens. Actuators B Chem. 2015, 210, 618–623. [Google Scholar] [CrossRef]
- Qiu, H.W.; Xu, S.C.; Jiang, S.Z.; Li, Z.; Chen, P.X.; Gao, S.S.; Zhang, C.; Feng, D.J. A novel graphene-based tapered optical fiber sensor for glucose detection. Appl. Surf. Sci. 2015, 329, 390–395. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, W.; Zhang, X.; Peng, W. CuO nanoparticles on sulfur-doped graphene for nonenzymatic glucose sensing. Electrochim. Acta 2015, 156, 244–251. [Google Scholar] [CrossRef]
- Alal, O.; Caglar, A.; Kivrak, H.; Sahin, O. Dendrimer Templated Synthesis of Carbon Nanotube Supported PdAu Catalyst and its Application as Hydrogen Peroxide Sensor. Electroanalysis 2019, 31, 1646–1655. [Google Scholar] [CrossRef]
- Kivrak, H.D.; Aktas, N.; Caglar, A. Electrochemical production of Graphene Oxide and its application as a novel Hydrogen Peroxide sensor. Int. J. Nano Dimens. 2019, 10, 252–259. [Google Scholar]
- Kazici, H.C.; Salman, F.; Kivrak, H.D. Synthesis of Pd-Ni/C bimetallic materials and their application in non-enzymatic hydrogen peroxide detection. Mater. Sci. Pol. 2017, 35, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Y.; Li, Y.H.; Li, Y.C.; Li, S.X. A sensitive enzyme-free hydrogen peroxide sensor based on a chitosan-graphene quantum dot/silver nanocube nanocomposite modified electrode. Anal. Methods 2016, 8, 2448–2455. [Google Scholar] [CrossRef]
- Wang, X.; Deng, W.P.; Shen, L.; Yan, M.; Yu, J.H. A 3D electrochemical immunodevice based on an Au paper electrode and using Au nanoflowers for amplification. New J. Chem. 2016, 40, 2835–2842. [Google Scholar] [CrossRef]
- Kivrak, H.; Alal, O.; Atbas, D. Efficient and rapid microwave-assisted route to synthesize Pt-MnOx hydrogen peroxide sensor. Electrochim. Acta 2015, 176, 497–503. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M. A novel nonenzymatic hydrogen peroxide sensor based on the synthesized mesoporous carbon and silver nanoparticles nanohybrid. Sens. Actuators B Chem. 2014, 203, 919–925. [Google Scholar] [CrossRef]
- Ye, D.; Li, H.; Liang, G.; Luo, J.; Zhang, X.; Zhang, S.; Chen, H.; Kong, J. A three-dimensional hybrid of MnO2/graphene/carbon nanotubes based sensor for determination of hydrogen-peroxide in milk. Electrochim. Acta 2013, 109, 195–200. [Google Scholar] [CrossRef]
- Guzmán, C.; Orozco, G.; Verde, Y.; Jiménez, S.; Godínez, L.A.; Juaristi, E.; Bustos, E. Hydrogen peroxide sensor based on modified vitreous carbon with multiwall carbon nanotubes and composites of Pt nanoparticles-dopamine. Electrochim. Acta 2009, 54, 1728–1732. [Google Scholar] [CrossRef]
- Kivrak, H.; Selçuk, K.; Er, O.F.; Aktas, N. Nanostructured Electrochemical Cysteine Sensor Based on Carbon Nanotube Supported Ru, Pd, and Pt Catalysts. Mater. Chem. Phys. 2021, 267, 124689. [Google Scholar] [CrossRef]
- Kivrak, H.; Selcuk, K.; Er, O.F.; Aktas, N. Electrochemical Cysteine Sensor on Novel Ruthenium Based Ternary Catalyst. Int. J. Electrochem. Sci. 2021, 16, 210519. [Google Scholar] [CrossRef]
- Ramoraswi, N.O.; Ndungu, P.G. Photo-Catalytic Properties of TiO2 Supported on MWCNTs, SBA-15 and Silica-Coated MWCNTs Nanocomposites. Nanoscale Res. Lett. 2015, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.H.; Wang, N. Effect of Nitrogen Pressure on the Structure of Cr-N, Ta-N, Mo-N, and W-N Nanocrystals Synthesized by Arc Discharge. J. Nanomater. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Song, J.; Li, G.R.; Xiong, F.Y.; Gao, X.P. Synergistic effect of molybdenum nitride and carbon nanotubes on electrocatalysis for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 20580–20585. [Google Scholar] [CrossRef]
- Rondon, S.; Wilkinson, W.R.; Proctor, A.; Houalla, M.; Hercules, D.M. Characterization of Mo/C catalysts by XRD, XPS, and TOF SIMS. J. Phys. Chem. 1995, 99, 16709–16713. [Google Scholar] [CrossRef]
- Aritani, H.; Tanaka, T.; Funabiki, T.; Yoshida, S.; Eda, K.; Sotani, N.; Kudo, M.; Hasegawa, S. Study of the local structure of molybdenum-magnesium binary oxides by means of Mo L(3)-edge XANES and UV-vis spectroscopy. J. Phys. Chem. 1996, 100, 19495–19501. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Ressler, T.; Walter, A.; Tzolova-Muller, G.; Hess, C. Structure of molybdenum oxide supported on silica SBA-15 studied by Raman, UV-Vis and X-ray absorption spectroscopy. Appl. Catal. A Gen. 2011, 399, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.P.; Shen, Y. Influence of propyl alcohol addition during processing of molybdenum trioxide powders, Observation of novel dual-wavelength excitation photochromism. Mater. Sci. Semicond. 2015, 30, 271–274. [Google Scholar] [CrossRef]
- Allaedini, G.; Aminayi, P.; Tasirin, S.M. Methane decomposition for carbon nanotube production, Optimization of the reaction parameters using response surface methodology. Chem. Eng. Res. Des. 2016, 112, 163–174. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Eswaramoorthi, I.; Sundaramurthy, V.; Das, N.; Dalai, A.K.; Adjaye, J. Application of multi-walled carbon nanotubes as efficient support to NiMo hydrotreating catalyst. Appl. Catal. A Gen. 2008, 339, 187–195. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Chou, K.C. Study on oxidation mechanism and kinetics of MoO2 to MoO3 in air atmosphere. Int. J. Refract. Hard Met. 2016, 57, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Barrie, P.J. Analysis of temperature programmed desorption (TPD) data for the characterisation of catalysts containing a distribution of adsorption sites. Phys. Chem. Chem. Phys. 2008, 10, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, P.; Wang, K.; Xu, J.; Jiang, J. Catalytic hydrogenation and one step hydrogenation-esterification to remove acetic acid for bio-oil upgrading, model reaction study. Catal. Sci. Technol. 2016, 6, 7783–7792. [Google Scholar] [CrossRef]
- Castro e Silva, C.d.C.C.; Breitkreitz, M.C.; Santhiago, M.; Corrêa, C.C.; Kubota, L.T. Construction of a new functional platform by grafting poly (4-vinylpyridine) in multi-walled carbon nanotubes for complexing copper ions aiming the amperometric detection of l-cysteine. Electrochim. Acta 2012, 71, 150–158. [Google Scholar] [CrossRef]


| Sensor | Sensitivity | Linear Range | (LOD) | Ref. |
|---|---|---|---|---|
| CuFe2O4/rGO-Au | 4.73 μA/mM | 0.05–0.2 μM | 0.383 μM | [10] |
| copper-electrodeposited screen-printed gold electrode | 0.028 μA/mM | 400–1800 μM | 0.21 μM | [11] |
| AgNPs/GQDs/GCE | - | 0.1–200 μM | 0.01 μM | [12] |
| Ru(III) Schiff Base Complex | - | 411–4111 μM | 0.9 μM | [13] |
| MnO2-CNTs | - | 0.5–680 μM | [14] | |
| Ru/CNT | 305.8 μA/mM | 0–200 μM | 0.353 μM | [29] |
| Ru-Mo-Pd/CNT | 136 μA/mM | 5–200 μM | 0.1 μM | [30] |
| Sample | Added (mM) | Found (mM) | Recovery (%) |
|---|---|---|---|
| 1 | 1.5 | 1.5 | 98.0 |
| 2 | 3.0 | 2.9 | 97.3 |
| 3 | 6.0 | 5.9 | 98.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selçuk, K.; Kivrak, H.; Aktaş, N. Novel CNT Supported Molybdenum Catalyst for Detection of L-Cysteine in Its Natural Environment. Catalysts 2021, 11, 1561. https://doi.org/10.3390/catal11121561
Selçuk K, Kivrak H, Aktaş N. Novel CNT Supported Molybdenum Catalyst for Detection of L-Cysteine in Its Natural Environment. Catalysts. 2021; 11(12):1561. https://doi.org/10.3390/catal11121561
Chicago/Turabian StyleSelçuk, Kadir, Hilal Kivrak, and Nahit Aktaş. 2021. "Novel CNT Supported Molybdenum Catalyst for Detection of L-Cysteine in Its Natural Environment" Catalysts 11, no. 12: 1561. https://doi.org/10.3390/catal11121561
APA StyleSelçuk, K., Kivrak, H., & Aktaş, N. (2021). Novel CNT Supported Molybdenum Catalyst for Detection of L-Cysteine in Its Natural Environment. Catalysts, 11(12), 1561. https://doi.org/10.3390/catal11121561






