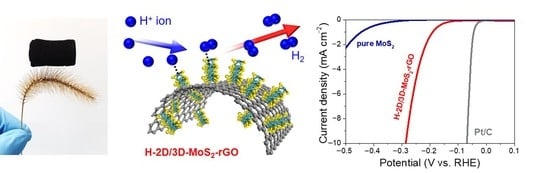
Hierarchically Ordinated Two-Dimensional MoS2 Nanosheets on Three-Dimensional Reduced Graphene Oxide Aerogels as Highly Active and Stable Catalysts for Hydrogen Evolution Reaction
Abstract
:1. Introduction
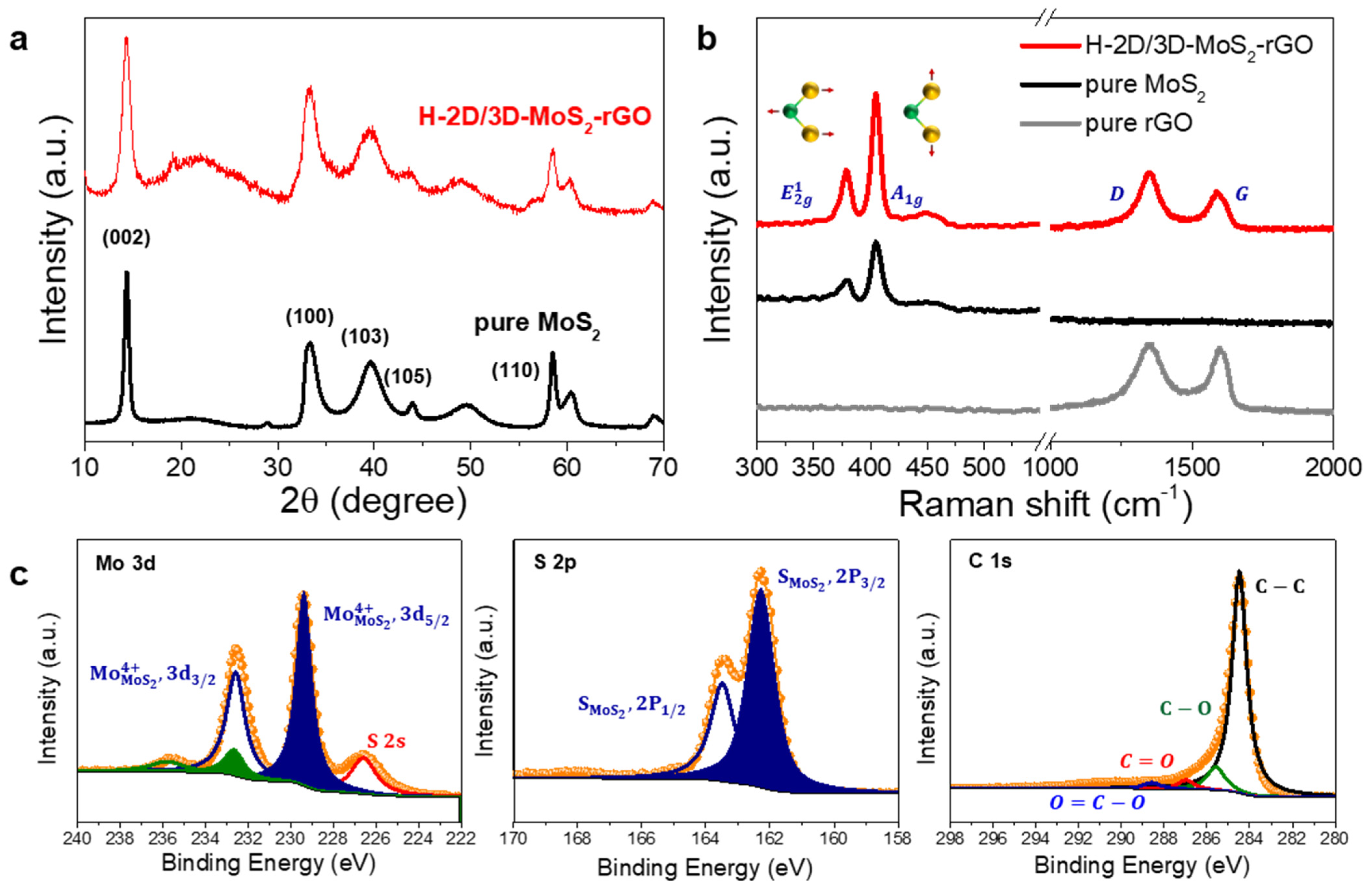
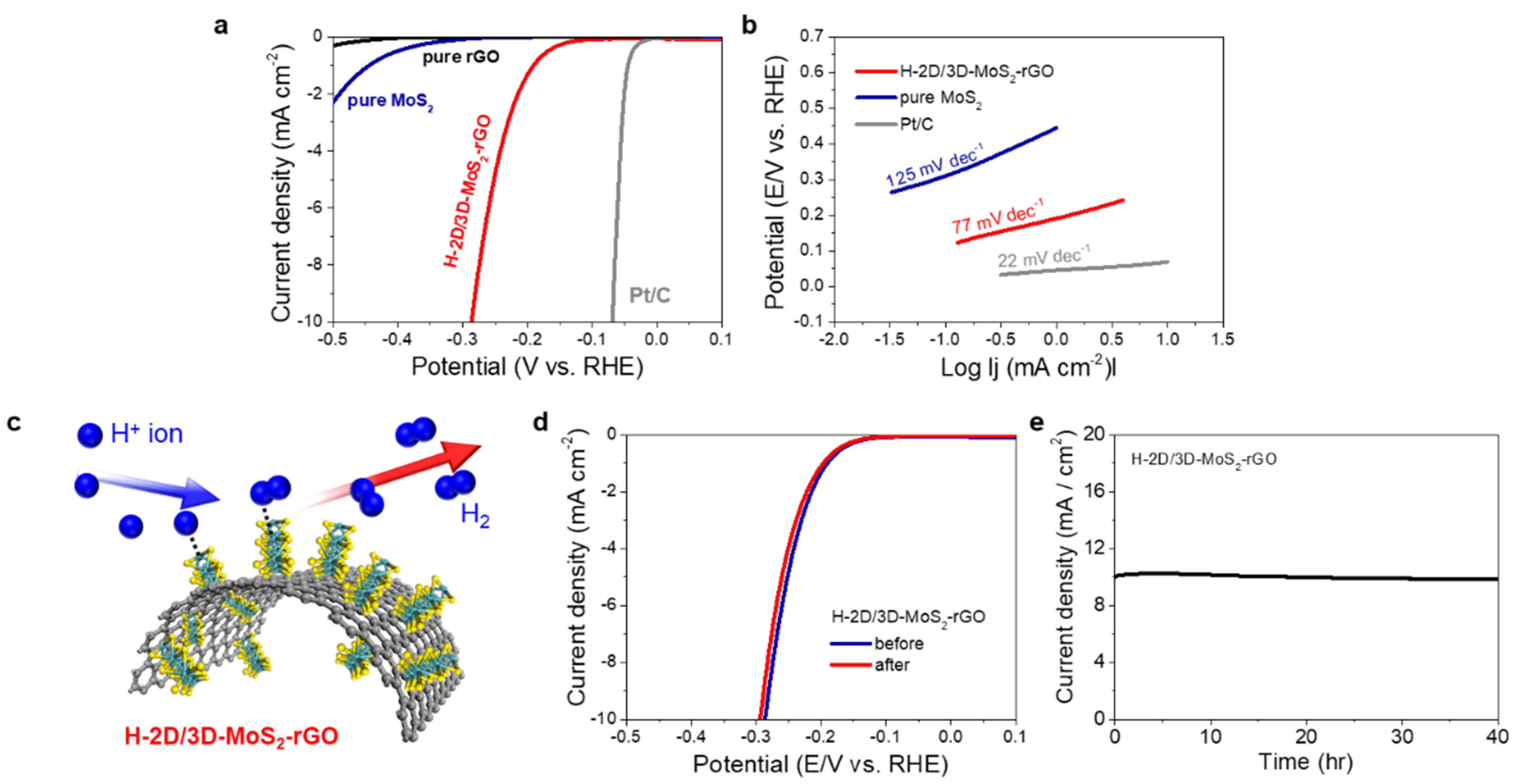
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of H-2D/3D-MoS2-rGO
3.2. Characterization and Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turner, J.A. A realizable renewable energy future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlapbach, L. Hydrogen-fuelled vehicles. Nature 2009, 406, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Ko, A.R.; Han, S.B.; Kim, H.S.; Park, K.W. Synthesis of octahedral Pt–Pd alloy nanoparticles for improved catalytic activity and stability in methanol electrooxidation. Phys. Chem. Chem. Phys. 2011, 13, 5569–5572. [Google Scholar] [CrossRef]
- Lee, Y.W.; Ko, A.R.; Kim, D.Y.; Han, S.B.; Park, K.W. Octahedral Pt-Pd alloy catalysts with enhanced oxygen reduction activity and stability in proton exchange membrane fuel cells. RSC Adv. 2012, 2, 1119–1125. [Google Scholar] [CrossRef]
- Jiang, Z.; Ren, J.; Li, Y.; Zhang, X.; Zhang, P.; Huang, J.; Du, C.; Chen, J. Low-cost high-performance hydrogen evolution electrocatalysts based on Pt-CoP polyhedra with low Pt loading in both alkaline and neutral media. Dalton Trans. 2019, 48, 8920–8930. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nøskov, J.K.; Seh, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [Green Version]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2017, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merki, D.; Hu, X.L. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kwak, D.H.; Han, S.B.; Hwang, E.T.; Kim, M.C.; Lee, J.Y.; Lee, Y.W.; Park, K.W. Synthesis of hollow carbon nanostructures as a non-precious catalyst for oxygen reduction reaction. Electrochim. Acta 2016, 191, 805–812. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnaes, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides-efficient and viable materials for electro- and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Growth and activation of an amorphous molybdenum sulfide hydrogen evolving catalyst. ACS Catal. 2013, 3, 2002–2011. [Google Scholar] [CrossRef] [Green Version]
- Park, K.W.; Lee, Y.W.; Oh, J.K.; Kim, D.Y.; Han, S.B.; Ko, A.R.; Kim, S.J.; Kim, H.S. TiO2-based nanowire supported catalysts for methanol electrooxidation in direct methanol fuel cells. J. Ind. Eng. Chem. 2011, 17, 696–699. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Jo, S.H.; Lee, Y.W.; Hong, J.; Sohn, J.I. Simple and facile fabrication of anion-vacancy-induced MoO3-X catalysts for enhanced hydrogen evolution activity. Catalysts 2020, 10, 1180. [Google Scholar] [CrossRef]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.B.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef]
- Ko, A.R.; Kim, J.Y.; Oh, J.K.; Lee, Y.W.; Han, S.B.; Park, K.W. Synergy effect of nanostructure electrodes supported by tungsten carbide and oxide for methanol electrooxidation. Phys. Chem. Chem. Phys. 2010, 12, 15181–15183. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2 nanosheet. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, Y.W.; Han, S.B.; Ko, A.R.; Kim, D.Y.; Kim, H.S.; Kim, S.J.; Roh, B.W.; Hwang, I.C.; Park, K.W. 3-Dimensional TiO2 nanostructure supports and their improved electrochemical properties in methanol electrooxidation. Catal. Sci. Technol. 2011, 1, 394–396. [Google Scholar] [CrossRef]
- Yu, C.; Xu, F.; Luo, L.; Abbo, H.S.; Titinchi, S.J.J.; Shen, P.K.; Tsiakaras, P.; Yin, S. Bimetallic Ni–Co phosphide nanosheets self-supported on nickel foam as high-performance electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 317, 191–198. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, L.; Luo, L.; Lu, J.; Yin, S.; Shen, P.K.; Tsiakaras, P. N-doped porous molybdenum carbide nanobelts as efficient catalysts for hydrogen evolution reaction. Appl. Catal. B-Environ. 2018, 224, 533–540. [Google Scholar] [CrossRef]
- Jing, S.; Lu, J.; Yu, G.; Yin, S.; Luo, L.; Zhang, Z.; Ma, Y.; Chen, W.; Shen, P.K. Carbon-encapsulated WOx hybrids as efficient catalysts for hydrogen evolution. Adv. Mater. 2018, 30, 1705979. [Google Scholar] [CrossRef]
- Wang, D.; Lu, J.; Luo, L.; Jing, S.; Abbo, H.S.; Titinchi, S.J.J.; Chen, Z.; Tsiakaras, P.; Yin, S. Enhanced hydrogen evolution activity over microwave-assisted functionalized 3D structured graphene anchoring FeP nanoparticles. Electrochim. Acta 2019, 317, 242–249. [Google Scholar] [CrossRef]
- Xu, F.; Lu, J.; Luo, L.; Yu, C.; Tang, Z.; Abbo, H.S.; Titinchi, S.J.J.; Zhu, J.; Shen, P.K.; Yin, S. Cu2S-Cu3P nanowire arrays self-supported on copper foam as boosting electrocatalysts for hydrogen evolution. Energy Technol. 2019, 7, 1800993. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Chung, D.Y.; Park, S.K.; Chung, Y.H.; Yu, S.H.; Sung, Y.E. Edge-exposed MoS2 nano-assembled structures as efficient electrocatalysts for hydrogen evolution reaction. Nanoscale 2014, 6, 2131–2136. [Google Scholar] [CrossRef]
- Kong, D.S.; Wang, H.T.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lu, Z.; Prinz, F.B.; Hsu, P.C.; Bradshaw, D.; Kong, D.; Wang, H.; Cui, Y.; Cha, J.J.; Zheng, G.; et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and it’s application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar]
- Yang, Y.; Fei, H.L.; Ruan, G.D.; Xiang, C.S.; Tour, J.M. Edge-oriented MoS2 nanoporous films as flexible electrodes for hydrogen evolution reactions and supercapacitor devices. Adv. Mater. 2014, 26, 8163–8168. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, H.Q.; Yuan, S.G.; Qian, H. Synthesis and characterization of vertically standing MoS2 nanosheets. Sci. Rep. 2016, 6, 21171. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, Y.; Shi, J.; Zhang, Y.; Liu, Z. Morphological engineering of CVD-grown transition metal dichalcogenides for efficient electrochemical hydrogen evolution. Adv. Mater. 2016, 28, 6207–6212. [Google Scholar]
- Park, T.; Bae, C.; Lee, H.; Leem, M.; Kim, H.; Ahn, W.; Kim, J.; Lee, E.; Shin, H.; Kim, H. Non-equilibrium fractal growth of MoS2 for electrocatalytic hydrogen evolution. CrystalEngComm 2019, 21, 478–486. [Google Scholar] [CrossRef]
- Qiu, X.; Huang, Y.; Nie, Z.; Ma, B.; Tan, Y.; Wu, Z.; Zhang, N.; Xie, X. Support interactions dictated active edge sites over MoS2–carbon composites for hydrogen evolution. Nanoscale 2020, 12, 1109–1117. [Google Scholar] [CrossRef]
- Shi, J.; Ma, D.; Han, G.; Zhang, Y.; Ji, Q.; Gao, T.; Sun, J.; Song, X.; Li, C.; Zhang, Y.; et al. Controllable growth and transfer of monolayer MoS2 on Au foils and its potential application in hydrogen evolution reaction. ACS Nano 2014, 8, 10196–10204. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, J.; Yan, K.; Wang, H.; Wang, Z.; Yang, S. Space-confined growth of MoS2 nanosheets within graphite: The layered hybrid of MoS2 and graphene as an active catalyst for hydrogen evolution reaction. Chem. Mater. 2014, 26, 2344–2353. [Google Scholar] [CrossRef]
- Zhang, B.; Jiu, J.; Wang, J.; Ruan, Y.; Ji, X.; Xu, K.; Chen, C.; Wan, H.; Miao, L.; Jiang, J. Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution. Nano Energy 2017, 37, 74–80. [Google Scholar] [CrossRef]
- Li, Y.; Majewski, M.B.; Islam, S.M.; Hao, S.; Murthy, A.A.; DiStefano, J.G.; Hanson, E.D.; Xu, Y.; Wolverton, C.; Kanatzidis, M.G.; et al. Morphological engineering of winged Au@MoS2 heterostructures for electrocatalytic hydrogen evolution. Nano Lett. 2018, 18, 7104–7110. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Luo, Y.; Mahmood, A.; Liu, B.; Cheng, H.M. Engineering two-dimensional materials and their heterostructures as high-performance electrocatalysts. Electrochem. Energy Rev. 2019, 2, 373–394. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Zhang, Y.; Yang, B.; Qi, Q.; Sun, M.; Zi, F.; Leung, M.K.H.; Huang, B. Interface modulation of MoS2/metal oxide heterostructures for efficient hydrogen evolution electrocatalysis. Small 2020, 16, 2002212. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube−graphene hybrid support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Seifi, M.; Rozati, S.M. MoS2 coating on different carbonaceous materials: Comparison of electrochemical properties and hydrogen evolution reaction performance. J. Electroanal. Chem. 2019, 847, 113198. [Google Scholar] [CrossRef]
- Liao, L.; Zhu, J.; Bian, X.; Zhu, L.; Scanlon, M.D.; Girault, H.H.; Liu, B. MoS2 formed on mesoporous graphene as a highly active catalyst for eydrogen evolution. Adv. Funct. Mater. 2013, 23, 5326–5333. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chen, M.; Li, X.; Zhang, X.; Lin, L.; Liu, W.; Liu, Y. A facile layer-by-layer fabrication of three dimensional MoS2-rGO-CNTs with high performance for hydrogen evolution reaction. Electrochim. Acta 2019, 300, 235–241. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Ahmad, H.; Fan, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Compos. Pt. B-Eng. 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Smith, A.T.; Lachance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.-B.; Qi, X.; Chen, B.; Bao, S.Y.; Yin, Z.; Wu, X.-J.; Luo, Z.; Wei, J.; Zhang, H.-L.; Zhang, H. MoS2 nanoflower-decorated reduced graphene oxide paper for high-performance hydrogen evolution reaction. Nanoscale 2014, 6, 5624–5629. [Google Scholar] [CrossRef]
- Kamila, S.; Mohanty, B.; Samantara, A.K.; Guha, P.; Ghosh, A.; Jena, B.; Satyam, P.V.; Mishira, B.K.; Jana, B.K. Highly active 2D layered MoS2-rGO hybrids for energy conversion and storage applications. Sci. Rep. 2017, 7, 8378–8390. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Jung, J.M.; Ko, T.Y.; Kim, S.; Kim, S.I.; Nah, J.; Ryu, S.; Nam, K.T.; Lee, M.H. Catalytic synergy effect of MoS2/reduced graphene oxide hybrids for a highly efficient hydrogen evolution reaction. RSC Adv. 2017, 7, 5480–5487. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Zhong, L.; Zhang, B.; Wang, H.F.; Zhang, Q. 3D Mesoporous van der Waals Heterostructures for Trifunctional Energy Electrocatalysis. Adv. Mater. 2018, 30, 1705110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Fan, X.; Wang, S.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W. (0D3D) MoS2 on porous graphene as catalysts for enhanced electrochemical hydrogen evolution. Carbon 2017, 121, 163–169. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, B.; Wen, Z.; Cui, S.; Guo, X.; He, Z.; Chen, J. A 3D hybrid of layered MoS2/nitrogen-doped graphene nanosheet aerogels: An effective catalyst for hydrogen evolution in microbial electrolysis cells. J. Mater. Chem. A 2014, 2, 13795. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xiao, H.; Zhou, B.; Huang, F.; Zhou, S.; Xiao, W.; Wang, D. Hierarchical MoS2–rGO nanosheets with high MoS2 loading with enhanced electro-catalytic performance. Appl. Surf. Sci. 2015, 358, 152–158. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, Y.-W.; Lee, S.W.; Ha, J.S.; Lee, S.-S.; Son, J.G. Ice-templated self-assembly of VOPO4-graphene nanocomposites for vertically porous 3D supercapacitor electrodes. Sci. Rep. 2015, 5, 13696. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphite oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved Hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Materials | Overpotential | Tafel Slope | Reference |
|---|---|---|---|
| (mV vs. RHE) | (mV dec−1) | ||
| MoS2Ag/rGO | 290 | 102 | [53] |
| 3D MoS2/N-GAs | 261 | 230 | [58] |
| hierarchical MoS2–rGO nanosheets | 250 | 98 | [59] |
| 3D MoS2/rGO | >300 | 92 | [56] |
| G@MoS2 | 302 | 112 | [57] |
| H-2D/3D-MoS2-rGO | 286 | 77 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lee, S.; Kim, M.-C.; Park, Y.; Jang, A.-R.; Ahn, W.; Sohn, J.I.; Park, J.B.; Hong, J.; Lee, Y.-W. Hierarchically Ordinated Two-Dimensional MoS2 Nanosheets on Three-Dimensional Reduced Graphene Oxide Aerogels as Highly Active and Stable Catalysts for Hydrogen Evolution Reaction. Catalysts 2021, 11, 182. https://doi.org/10.3390/catal11020182
Choi H, Lee S, Kim M-C, Park Y, Jang A-R, Ahn W, Sohn JI, Park JB, Hong J, Lee Y-W. Hierarchically Ordinated Two-Dimensional MoS2 Nanosheets on Three-Dimensional Reduced Graphene Oxide Aerogels as Highly Active and Stable Catalysts for Hydrogen Evolution Reaction. Catalysts. 2021; 11(2):182. https://doi.org/10.3390/catal11020182
Chicago/Turabian StyleChoi, Hyeonggeun, Suok Lee, Min-Cheol Kim, Yeonsu Park, A-Rang Jang, Wook Ahn, Jung Inn Sohn, Jong Bae Park, John Hong, and Young-Woo Lee. 2021. "Hierarchically Ordinated Two-Dimensional MoS2 Nanosheets on Three-Dimensional Reduced Graphene Oxide Aerogels as Highly Active and Stable Catalysts for Hydrogen Evolution Reaction" Catalysts 11, no. 2: 182. https://doi.org/10.3390/catal11020182
APA StyleChoi, H., Lee, S., Kim, M.-C., Park, Y., Jang, A.-R., Ahn, W., Sohn, J. I., Park, J. B., Hong, J., & Lee, Y.-W. (2021). Hierarchically Ordinated Two-Dimensional MoS2 Nanosheets on Three-Dimensional Reduced Graphene Oxide Aerogels as Highly Active and Stable Catalysts for Hydrogen Evolution Reaction. Catalysts, 11(2), 182. https://doi.org/10.3390/catal11020182