Metal(II) Coordination Polymers from Tetracarboxylate Linkers: Synthesis, Structures, and Catalytic Cyanosilylation of Benzaldehydes
Abstract
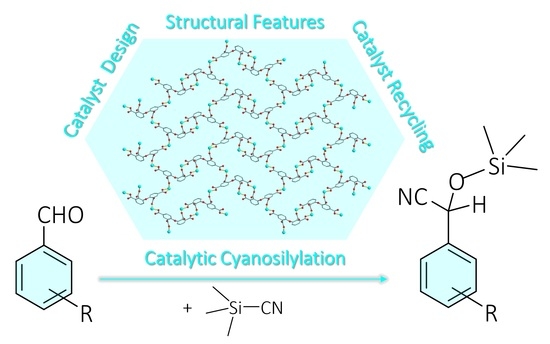
:1. Introduction
2. Results and Discussion
2.1. Hydrothermal Synthesis
2.2. Structure of [Cu2(µ4-dpa)(bipy)2(H2O)]n·6H2O (1)
2.3. Structure of [Mn2(µ6-dpa)(bipy)2]n (2)
2.4. Structure of [Zn2(µ4-dpa)(bipy)2(H2O)2]n·2H2O (3)
2.5. TGA & PXRD
2.6. Cyanosilylation of Benzaldehydes
3. Experimental
3.1. Chemicals & Equipment
3.2. Hydrothermal Synthesis & Analytical Data
3.3. Single-Crystal X-ray Diffraction
3.4. Catalytic Cyanosilylation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaskel, S. (Ed.) The Chemistry of Metal-Organic Frameworks, 2 Volume Set: Synthesis, Characterization, and Applications; John Wiley & Sons: Weinheim, Germany, 2016. [Google Scholar]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; RSC: Cambridge, UK, 2009. [Google Scholar]
- MacGillivray, L.R.; Lukehart, C.M. (Eds.) Metal-Organic Framework Materials; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2003, 2781–2804. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, I.; Kostakis, G.E. Recent Bio-Advances in Metal-Organic Frameworks. Molecules 2020, 25, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Avilés, A.; Muelas-Ramos, V.; Bedia, J.; Rodriguez, J.J.; Belver, C. Thermal post-treatments to enhance the water sta-bility of NH2-MIL-125(Ti). Catalysts 2020, 10, 603. [Google Scholar] [CrossRef]
- Yu, L.; Dong, X.L.; Gong, Q.H.; Acharya, S.R.; Lin, Y.H.; Wang, H.; Han, Y.; Thonhauser, T.; Li, J. Splitting mono- and di-branched alkane isomers by a robust aluminum-based metal-organic framework material with optimal pore dimensions. J. Am. Chem. Soc. 2020, 142, 6925–6929. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, J. Microporous Metal–Organic Frameworks for Adsorptive Separation of C5–C6 Alkane Isomers. Acc. Chem. Res. 2019, 52, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2011, 112, 1126–1162. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Karabach, Y.Y.; Guedes da Silva, M.F.C.; Kopylovich, M.N.; Gil-Hernández, B.; Sanchiz, J.; Kirillov, A.M.; Pombeiro, A.J.L. Self-assembled 3D heterometallic CuII/FeII coordination polymers with octahedral net skeletons: Structural features, molecular magnetism, thermal and oxidation catalytic properties. Inorg. Chem. 2010, 49, 11096–11105. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Jiang, H. Metal–Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wen, M.; Cai, Y.; Shi, Z.F.; Arol, A.S.; Kirillova, M.V.; Kirillov, A.M. Metal−organic architectures assembled from multifunctionalpolycarboxylates: Hydrothermal self-assembly, structures, andcatalytic activity in alkane oxidation. Inorg. Chem. 2019, 58, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Z.; Wen, M.; Cai, Y.; Shi, Z.F.; Nesterov, D.S.; Kirillova, M.V.; Kirillov, A.M. Cobalt(II) Coordination Polymers Assem-bled from UnexploredPyridine-Carboxylic Acids: Structural Diversity and CatalyticOxidation of Alcohols. Inorg. Chem. 2019, 58, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Huang, C.; Mi, L.W. Metal-organic frameworks as acid- and/or base-functionalized catalysts for tandem reac-tions. Dalton Trans. 2020, 49, 14723–14730. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.A.; Gupta, A.K.; De, D. Flexible Zn-MOF Exhibiting Selective CO2 Adsorption and Efficient Lewis Acidic Catalytic Activity. Cryst. Growth Des. 2019, 19, 2010–2018. [Google Scholar] [CrossRef]
- Liu, J.-J.; Lu, Y.; Lu, W. Metal-dependent photosensitivity of three isostructural 1D CPs based on the 1,1′-bis(3-carboxylatobenzyl)-4,4′-bipyridinium moiety. Dalton Trans. 2020, 49, 4044–4049. [Google Scholar] [CrossRef]
- Gu, J.Z.; Cui, Y.H.; Liang, X.X.; Wu, J.; Lv, D.Y.; Kirillov, A.M. Srtructurally distinct metal−organic and H-bonded networks derivedfrom 5-(6-carboxypyridin-3-yl)isophthalic acid: Coordination and template effect of 4,4′-bipyridine. Cryst. Grwoth Des. 2016, 16, 4658–4670. [Google Scholar] [CrossRef]
- Han, S.-D.; Chen, Y.-Q.; Zhao, J.-P.; Liu, S.-J.; Miao, X.-H.; Hu, T.-L.; Bu, X.-H. Solvent-induced structural diversities from discrete cup-shaped Co8 clusters to Co8 cluster-based chains accompanied by in situ ligand conversion. Cryst. Eng. Comm. 2014, 16, 753–756. [Google Scholar] [CrossRef]
- Zou, X.Z.; Wu, J.; Gu, J.Z.; Zhao, N.; Feng, A.S.; Li, Y. Syntheses of two nickel(II) coordination compounds based on a rigid linear tricarboxylic acid. Chin. J. Inorg. Chem. 2019, 35, 1705–1711. [Google Scholar]
- Gu, J.; Gao, Z.; Tang, Y. pH and Auxiliary Ligand Influence on the Structural Variations of 5(2′-Carboxylphenyl) Nicotate Coordination Polymers. Cryst. Growth Des. 2012, 12, 3312–3323. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Gu, J.Z.; Qiu, W.D.; Feng, A.S. Temperature-dependent syntheses of two manganese(II) coordination com-pounds based on an ether-bridged tetracarboxylic acid. Chin. J. Struct. Chem. 2020, 39, 727–736. [Google Scholar]
- Hou, Y.L.; Peng, Y.L.; Diao, Y.X.; Liu, J.; Chen, L.Z.; Li, D. Side chain induced self-assembly and selective catalytic oxidation activity of copper(I)-coper(II)-N4 complexes. Cryst. Growth Des. 2020, 20, 1237–1241. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wan, S.M.; Kirillova, M.V.; Kirillov, A.M. H-bonded and metal(II)-organic architectures assembled from an unex-plored aromatic tricarboxylic acid: Structural variety and functional properties. Dalton Trans. 2020, 49, 7197–7209. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.J.H. Cyanohydrins in Nature and the Laboratory: Biology, Preparations, and Synthetic Applications. Chem. Rev. 1999, 99, 3649–3682. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M.; Holms, I.P. Chemically catalyzed asymmetric cyanohydrin syntheses. Angew. Chem. Int. Ed. 2004, 43, 2752–2778. [Google Scholar] [CrossRef]
- Lacour, M.-A.; Rahier, N.J.; Taillefer, M. Mild and Efficient Trimethylsilylcyanation of Ketones Catalysed by PNP Chloride. Chem. Eur. J. 2011, 17, 12276–12279. [Google Scholar] [CrossRef]
- Mowry, D.T. The preparation of nitriles. Chem. Rev. 1948, 42, 189–283. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Rúbio, G.M.D.M.; Da Silva, M.F.C.G.; Pombeiro, A.J.L. Zinc(II) and Copper(II) Metal-Organic Frameworks Constructed from a Terphenyl-4,4′′-dicarboxylic Acid Derivative: Synthesis, Structure, and Catalytic Application in the Cyanosilylation of Aldehydes. Eur. J. Inorg. Chem. 2016, 2016, 5557–5567. [Google Scholar] [CrossRef]
- Xi, Y.M.; Ma, Z.Z.; Wang, L.N.; Li, M.; Li, Z.J. Three-dimensional Ni(II)-MOF containing anasymmetric pyridyl-carboxylate ligand: Catalytic cyanosilylation of aldehydes and inhibits human promyelocytic leukemia cancer cells. J. Clust. Sci. 2019, 30, 1455–1464. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Liang, X.-X.; Cui, Y.-H.; Wu, J.; Shi, Z.-F.; Kirillov, A.M. Introducing 2-(2-carboxyphenoxy)terephthalic acid as a new versatile building block for design of diverse coordination polymers: Synthesis, structural features, luminescence sensing, and magnetism. CrystEngComm 2017, 19, 2570–2588. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Karabach, Y.Y.; Kirillova, M.V.; Haukka, M.; Pombeiro, A.J.L. Topologically Unique 2D Heterometallic CuII/Mg Coordination Polymer: Synthesis, Structural Features, and Catalytic Use in Alkane Hydrocarboxylation. Cryst. Growth Des. 2012, 12, 1069–1074. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Gu, J.; Kirillova, M.V.; Kirillov, A.M. Introducing a flexible tetracarboxylic acid linker into functional coordination polymers: Synthesis, structural traits, and photocatalytic dye degradation. New J. Chem. 2020, 44, 16082–16091. [Google Scholar] [CrossRef]
- Yu, Y. Auxiliary ligands induced two new Zn(II) compounds with the structural variations from 2D layer to 3D framework: Syntheses, structures and photoluminescent properties. J. Mol. Struct. 2017, 1149, 761–765. [Google Scholar] [CrossRef]
- Wang, L.-N.; Zhang, Y.-H.; Jiang, S.; Liu, Z.-Z. Three coordination polymers based on 3-(3′,5′-dicarboxylphenoxy)phthalic acid and auxiliary N-donor ligands: Syntheses, structures, and highly selective sensing for nitro explosives and Fe3+ ions. CrystEngComm 2019, 21, 4557–4567. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Liang, X.-X.; Cai, Y.; Wu, J.; Shi, Z.-F.; Kirillov, A.M. Hydrothermal assembly, structures, topologies, luminescence, and magnetism of a novel series of coordination polymers driven by a trifunctional nicotinic acid building block. Dalton Trans. 2017, 46, 10908–10925. [Google Scholar] [CrossRef]
- Jaros, S.W.; Da Silva, M.F.C.G.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-Triaza-7-phosphaadamantane Coordination Polymers Driven by Substituted Glutarate and Malonate Building Blocks: Self-Assembly Synthesis, Structural Features, and Antimicrobial Properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xu, M.-C.; Zhang, L.-J.; Yao, R.-X.; Zhang, X.-M. Solvent-free heterogeneous catalysis for cyanosilylation in a dynamic cobalt-MOF. Dalton Trans. 2015, 44, 12711–12716. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Van de Sluis, P.; Spek, A.L. Platon Program. Acta Cryst. 1990, 46, 194–201. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Llabrés i Xamena, F.X.; Gascon, J. (Eds.) Metal-Organic Frameworks as Heterogeneous Catalysts; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- García, H.; Navalón, S. (Eds.) Metal-Organic Frameworks: Applications in Separations and Catalysis; John Wiley & Sons: Weinheim, Germany, 2018. [Google Scholar]






| Entry | Catalyst | Time, h | Catalyst Loading, mol% | Solvent | Yield b, % |
|---|---|---|---|---|---|
| 1 | blank | 12 | - | CH2Cl2 | 3 |
| 2 | H3dpna | 12 | 3.0 | CH2Cl2 | 6 |
| 3 | ZnCl2 | 12 | 3.0 | CH2Cl2 | 8 |
| 4 | 1 | 12 | 3.0 | CH2Cl2 | 37 |
| 5 | 2 | 12 | 3.0 | CH2Cl2 | 27 |
| 6 | 3 | 12 | 3.0 | CH2Cl2 | 92 |
| 7 | 3 | 1 | 3.0 | CH2Cl2 | 40 |
| 8 | 3 | 2 | 3.0 | CH2Cl2 | 62 |
| 9 | 3 | 4 | 3.0 | CH2Cl2 | 73 |
| 10 | 3 | 6 | 3.0 | CH2Cl2 | 80 |
| 11 | 3 | 8 | 3.0 | CH2Cl2 | 84 |
| 12 | 3 | 10 | 3.0 | CH2Cl2 | 88 |
| 13 | 3 | 12 | 2.0 | CH2Cl2 | 73 |
| 14 | 3 | 12 | 4.0 | CH2Cl2 | 93 |
| 15 | 3 | 12 | 3.0 | CHCl3 | 82 |
| 16 | 3 | 12 | 3.0 | CH3CN | 76 |
| 17 | 3 | 12 | 3.0 | THF | 68 |
| 18 | 3 | 12 | 3.0 | CH3OH | 80 |

| Entry | Substrate (R-C6H4CHO) | Yield b, % |
|---|---|---|
| 1 | R = H | 61 |
| 2 | R = 2-NO2 | 82 |
| 3 | R = 3-NO2 | 87 |
| 4 | R = 4-NO2 | 92 |
| 5 | R = 4-Cl | 62 |
| 6 | R = 4-OH | 56 |
| 7 | R = 4-CH3 | 51 |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Chemical formula | C36H36Cu2N4O16 | C36H22Mn2N4O9 | C36H30Zn2N4O13 |
| Molecular weight | 907.78 | 764.45 | 857.38 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/c | P21/n |
| a/Å | 10.3607 (5) | 10.5312 (3) | 7.9352 (2) |
| b/Å | 35.8638 (11) | 21.2195 (5) | 13.1264 (4) |
| c/Å | 11.3823 (5) | 15.1835 (4) | 34.5048 (10) |
| α/(°) | 90 | 90 | 90 |
| β/(°) | 116.385 (6) | 107.751 (3) | 94.158 (3) |
| γ/(°) | 90 | 90 | 90 |
| V/Å | 3788.8 (3) | 3231.47 (17) | 3584.58 (18) |
| Z | 4 | 4 | 4 |
| F (000) | 1624 | 1552 | 1752 |
| Crystal size/mm | 0.25 × 0.23 × 0.21 | 0.18 × 0.16 × 0.15 | 0.26 × 0.24 × 0.20 |
| θ range for data collection | 4.508–69.993 | 3.699–66.992 | 3.604–70.060 |
| Limiting indices | −12 ≤ h ≤ 12, −43 ≤ k ≤ 30, −13 ≤ l ≤ 13 | −12 ≤ h ≤ 11, −25 ≤ k≤ 25, −18 ≤ l ≤ 17 | −8 ≤ h ≤ 9, −1 ≤ 15, −41 ≤ l ≤ 40 |
| Reflections collected/unique (Rint) | 19,512/7079 (0.0494) | 20,432/5753 (0.0993) | 13,030/6658 (0.0337) |
| Dc/(Mg·cm−3) | 1.402 | 1.571 | 1.589 |
| μ/mm−1 | 1.892 | 6.917 | 2.285 |
| Data/restraints/parameters | 7079/0/469 | 5753/0/460 | 6658/0/496 |
| Goodness-of-fit on F2 | 1.047 | 1.074 | 1.017 |
| Final R indices [(I ≥ 2σ(I))] R1, wR2 | 0.0537, 0.1444 | 0.0707, 0.1680 | 0.0515, 0.1169 |
| R indices (all data) R1, wR2 | 0.0710, 0.1546 | 0.0901, 0.1800 | 0.0619, 0.1238 |
| Largest diff. peak and hole/(e·Å−3) | 1.195 and −0.648 | 0.859 and −1.089 | 0.857 and −1.096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liang, C.; Zou, X.; Gu, J.; Kirillova, M.V.; Kirillov, A.M. Metal(II) Coordination Polymers from Tetracarboxylate Linkers: Synthesis, Structures, and Catalytic Cyanosilylation of Benzaldehydes. Catalysts 2021, 11, 204. https://doi.org/10.3390/catal11020204
Li Y, Liang C, Zou X, Gu J, Kirillova MV, Kirillov AM. Metal(II) Coordination Polymers from Tetracarboxylate Linkers: Synthesis, Structures, and Catalytic Cyanosilylation of Benzaldehydes. Catalysts. 2021; 11(2):204. https://doi.org/10.3390/catal11020204
Chicago/Turabian StyleLi, Yu, Chumin Liang, Xunzhong Zou, Jinzhong Gu, Marina V. Kirillova, and Alexander M. Kirillov. 2021. "Metal(II) Coordination Polymers from Tetracarboxylate Linkers: Synthesis, Structures, and Catalytic Cyanosilylation of Benzaldehydes" Catalysts 11, no. 2: 204. https://doi.org/10.3390/catal11020204
APA StyleLi, Y., Liang, C., Zou, X., Gu, J., Kirillova, M. V., & Kirillov, A. M. (2021). Metal(II) Coordination Polymers from Tetracarboxylate Linkers: Synthesis, Structures, and Catalytic Cyanosilylation of Benzaldehydes. Catalysts, 11(2), 204. https://doi.org/10.3390/catal11020204