Molybdenum-Containing Metalloenzymes and Synthetic Catalysts for Conversion of Small Molecules
Abstract
1. Introduction
2. CO2 Electrocatalysts
2.1. Metalloenzymes with CO2 Reactivity
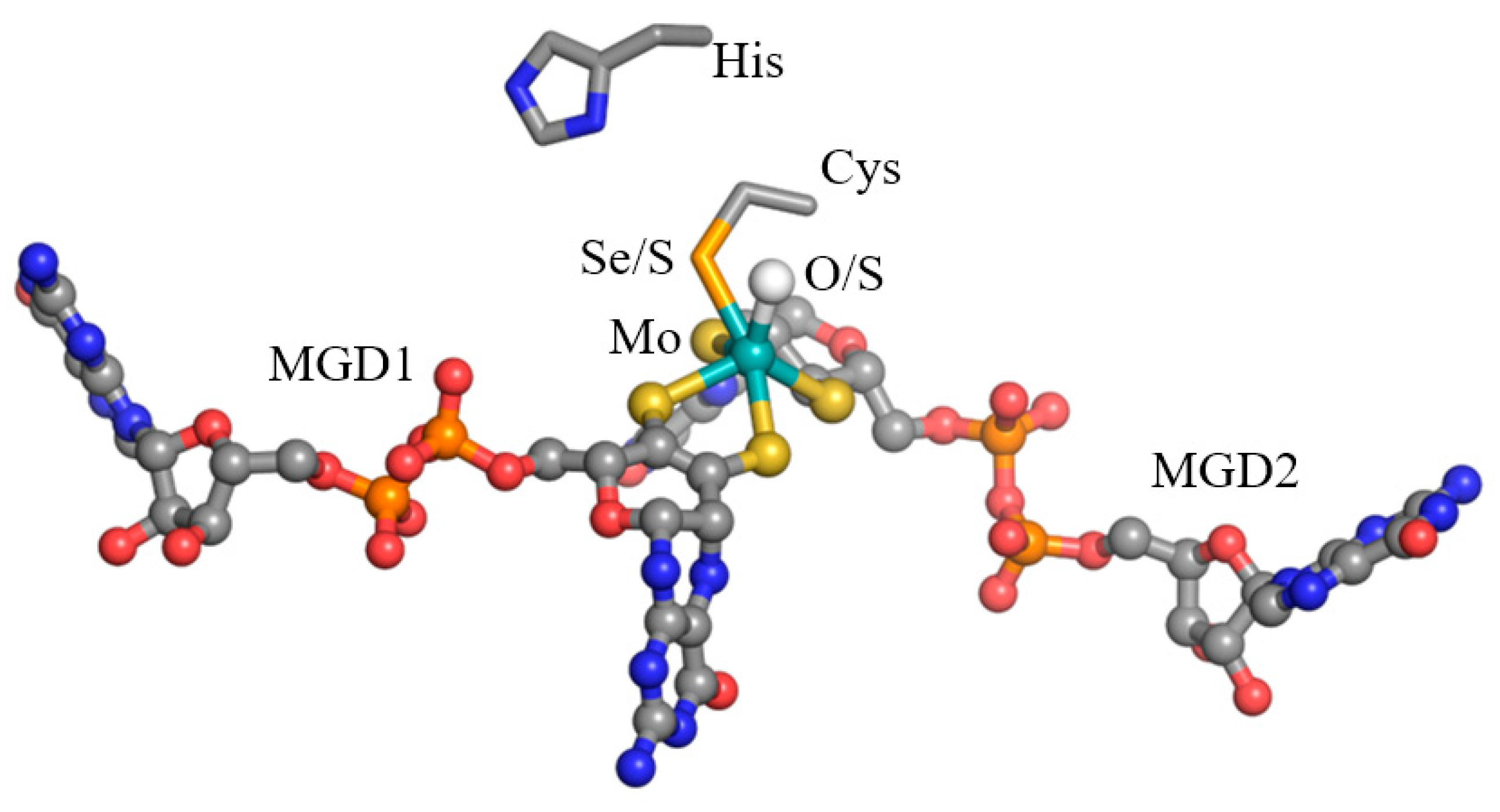
2.1.1. Formate Dehydrogenases
2.1.2. MoCu-Carbon Monoxide Dehydrogenases
2.2. Structural Analogs of CO2 Enzymes
2.2.1. FDH Analogs
2.2.2. MoCu-CODH Analogs
2.3. Synthetic Electrocatalysts for CO2 Reduction
2.3.1. Homogeneous Mo Complexes for CO2 Reduction
2.3.2. Heterogeneous Mo-Containing CO2 Reduction Electrocatalysts
2.4. FDH-Electrode Biohybrid
3. Nitrogen Fixation
3.1. Nitrogenase
3.2. Structural Analogs of Nitrogenase
3.3. Synthetic Electrocatalysts for N2 Reduction
3.3.1. Homogeneous Mo Complexes for N2 Reduction
3.3.2. Heterogeneous Mo-Containing N2-Reduction Electrocatalysts
3.4. Nitrogenase-Electrode Biohybrid
4. H2 Evolution
4.1. Homogeneous Mo Complexes for H2 Evolution
4.2. Heterogeneous Mo-Containing H2-Evolution Electrocatalyst
5. Heterogeneous Mo-Containing O2-Evolution Electrocatalysts
6. Conclusions
Funding
Conflicts of Interest
References
- van der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Lee, D.-K.; Sim, U. Photocatalytic and electrocatalytic approaches towards atmospheric nitrogen reduction to ammonia under ambient conditions. Nano Converg. 2019, 6, 15. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Rosca, V.; Duca, M.; de Groot, M.T.; Koper, M.T.M. Nitrogen Cycle Electrocatalysis. Chem. Rev. 2009, 109, 2209–2244. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Janani, G.; Choi, H.; Surendran, S.; Sim, U. Recent advances in rational design of efficient electrocatalyst for full water splitting across all pH conditions. MRS Bull. 2020, 45, 539–547. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef]
- Bullock, R.M.; Chen, J.G.; Gagliardi, L.; Chirik, P.J.; Farha, O.K.; Hendon, C.H.; Jones, C.W.; Keith, J.A.; Klosin, J.; Minteer, S.D.; et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science 2020, 369. [Google Scholar] [CrossRef]
- Eckenhoff, W.T.; Brennessel, W.W.; Eisenberg, R. Light-Driven Hydrogen Production from Aqueous Protons using Molybdenum Catalysts. Inorg. Chem. 2014, 53, 9860–9869. [Google Scholar] [CrossRef]
- Dos Santos, P.C.; Dean, D.R.; Hu, Y.; Ribbe, M.W. Formation and Insertion of the Nitrogenase Iron−Molybdenum Cofactor. Chem. Rev. 2004, 104, 1159–1174. [Google Scholar] [CrossRef]
- Dobbek, H. Structural aspects of mononuclear Mo/W-enzymes. Coord. Chem. Rev. 2011, 255, 1104–1116. [Google Scholar] [CrossRef]
- Cordas, C.M.; Moura, J.J.G. Molybdenum and tungsten enzymes redox properties—A brief overview. Coord. Chem. Rev. 2019, 394, 53–64. [Google Scholar] [CrossRef]
- Lin, Y.-W. Rational Design of Artificial Metalloproteins and Metalloenzymes with Metal Clusters. Molecules 2019, 24, 2743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, P.; Li, J.; Xiang, B. Two-Dimensional Material Molybdenum Disulfides as Electrocatalysts for Hydrogen Evolution. Catalysts 2017, 7, 285. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Axley, M.J.; Grahame, D.A. Kinetics for formate dehydrogenase of Escherichia coli formate-hydrogenlyase. J. Biol. Chem. 1991, 266, 13731–13736. [Google Scholar] [CrossRef]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef]
- Hille, R.; Hall, J.; Basu, P. The Mononuclear Molybdenum Enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef]
- Hartmann, T.; Leimkühler, S. The oxygen-tolerant and NAD+-dependent formate dehydrogenase from Rhodobacter capsulatus is able to catalyze the reduction of CO2 to formate. FEBS J. 2013, 280, 6083–6096. [Google Scholar] [CrossRef]
- Maia, L.B.; Fonseca, L.; Moura, I.; Moura, J.J.G. Reduction of Carbon Dioxide by a Molybdenum-Containing Formate Dehydrogenase: A Kinetic and Mechanistic Study. J. Am. Chem. Soc. 2016, 138, 8834–8846. [Google Scholar] [CrossRef] [PubMed]
- Boyington, J.C.; Gladyshev, V.N.; Khangulov, S.V.; Stadtman, T.C.; Sun, P.D. Crystal structure of formate dehydrogenase H: Catalysis involving Mo, molybdopterin, selenocysteine, and an Fe4S4 cluster. Science 1997, 275, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, H.C.A.; Romao, M.J. Formate-reduced E-coli formate dehydrogenase H: The reinterpretation of the crystal structure suggests a new reaction mechanism. J. Biol. Inorg. Chem. 2006, 11, 849–854. [Google Scholar] [CrossRef]
- Mota, C.S.; Rivas, M.G.; Brondino, C.D.; Moura, I.; Moura, J.J.G.; González, P.J.; Cerqueira, N.M.F.S.A. The mechanism of formate oxidation by metal-dependent formate dehydrogenases. J. Biol. Inorg. Chem. 2011, 16, 1255–1268. [Google Scholar] [CrossRef]
- Cerqueira, N.M.F.S.A.; Fernandes, P.A.; Gonzalez, P.J.; Moura, J.J.G.; Ramos, M.J. The Sulfur Shift: An Activation Mechanism for Periplasmic Nitrate Reductase and Formate Dehydrogenase. Inorg. Chem. 2013, 52, 10766–10772. [Google Scholar] [CrossRef] [PubMed]
- Schrapers, P.; Hartmann, T.; Kositzki, R.; Dau, H.; Reschke, S.; Schulzke, C.; Leimkuhler, S.; Haumann, M. Sulfido and Cysteine Ligation Changes at the Molybdenum Cofactor during Substrate Conversion by Formate Dehydrogenase (FDH) from Rhodobacter capsulatus. Inorg. Chem. 2015, 54, 3260–3271. [Google Scholar] [CrossRef]
- Duffus, B.R.; Schrapers, P.; Schuth, N.; Mebs, S.; Dau, H.; Leimkuhler, S.; Haumann, M. Anion Binding and Oxidative Modification at the Molybdenum Cofactor of Formate Dehydrogenase from Rhodobacter capsulatus Studied by X-ray Absorption Spectroscopy. Inorg. Chem. 2020, 59, 214–225. [Google Scholar] [CrossRef]
- Hille, R.; Dingwall, S.; Wilcoxen, J. The aerobic CO dehydrogenase from Oligotropha carboxidovorans. J. Biol. Inorg. Chem. 2015, 20, 243–251. [Google Scholar] [CrossRef]
- Dobbek, H.; Gremer, L.; Kiefersauer, R.; Huber, R.; Meyer, O. Catalysis at a dinuclear [CuSMo(=O)OH] cluster in a CO dehydrogenase resolved at 1.1-A resolution. Proc. Natl. Acad. Sci. USA 2002, 99, 15971–15976. [Google Scholar] [CrossRef]
- Gnida, M.; Ferner, R.; Gremer, L.; Meyer, O.; Meyer-Klaucke, W. A Novel Binuclear [CuSMo] Cluster at the Active Site of Carbon Monoxide Dehydrogenase: Characterization by X-ray Absorption Spectroscopy. Biochemistry 2003, 42, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Siegbahn, P.E.M.; Shestakov, A.F. Quantum chemical modeling of CO oxidation by the active site of molybdenum CO dehydrogenase. J. Comput. Chem. 2005, 26, 888–898. [Google Scholar] [CrossRef]
- Hofmann, M.; Kassube, J.K.; Graf, T. The mechanism of Mo-/Cu-dependent CO dehydrogenase. J. Biol. Inorg.Chem. 2005, 10, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hemann, C.F.; Hille, R. Kinetic and spectroscopic studies of the molybdenum-copper CO dehydrogenase from Oligotropha carboxidovorans. J. Biol. Chem. 2010, 285, 12571–12578. [Google Scholar] [CrossRef]
- Seo, J.; Kim, E. O-Atom Exchange between H2O and CO2 Mediated by a Bis(dithiolene)tungsten Complex. Inorg. Chem. 2012, 51, 7951–7953. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Holm, R.H. Bis(Dithiolene)molybdenum Analogues Relevant to the DMSO Reductase Enzyme Family: Synthesis, Structures, and Oxygen Atom Transfer Reactions and Kinetics. J. Am. Chem. Soc. 2001, 123, 1920–1930. [Google Scholar] [CrossRef]
- Sung, K.-M.; Holm, R.H. Oxo Transfer Reactions Mediated by Bis(dithiolene)tungsten Analogues of the Active Sites of Molybdoenzymes in the DMSO Reductase Family: Comparative Reactivity of Tungsten and Molybdenum. J. Am. Chem. Soc. 2001, 123, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Shearer, J.; Williard, P.G.; Kim, E. Reactivity of a biomimetic W(iv) bis-dithiolene complex with CO2 leading to formate production and structural rearrangement. Dalton Trans. 2019, 48, 17441–17444. [Google Scholar] [CrossRef] [PubMed]
- Fogeron, T.; Todorova, T.K.; Porcher, J.-P.; Gomez-Mingot, M.; Chamoreau, L.-M.; Mellot-Draznieks, C.; Li, Y.; Fontecave, M. A Bioinspired Nickel(bis-dithiolene) Complex as a Homogeneous Catalyst for Carbon Dioxide Electroreduction. ACS Catal. 2018, 8, 2030–2038. [Google Scholar] [CrossRef]
- Fogeron, T.; Retailleau, P.; Chamoreau, L.-M.; Li, Y.; Fontecave, M. Pyranopterin Related Dithiolene Molybdenum Complexes as Homogeneous Catalysts for CO2 Photoreduction. Angew. Chem. Int. 2018, 57, 17033–17037. [Google Scholar] [CrossRef] [PubMed]
- Mouchfiq, A.; Todorova, T.K.; Dey, S.; Fontecave, M.; Mougel, V. A bioinspired molybdenum–copper molecular catalyst for CO2 electroreduction. Chem. Sci. 2020, 11, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.H.; Cotton, F.A.; Extine, M.W.; Reichert, W.W. The molybdenum-molybdenum triple bond Insertion reactions of hexakis(alkoxy)dimolybdenum compounds with carbon dioxide and single-crystal x-ray structural characterization of bis(tert-butylcarbonato)tetrakis(tert-butoxy)dimolybdenum. J. Am. Chem. Soc. 1978, 100, 1727–1734. [Google Scholar] [CrossRef]
- Alvarez, R.; Carmona, E.; Cole-Hamilton, D.J.; Galindo, A.; Gutierrez-Puebla, E.; Monge, A.; Poveda, M.L.; Ruiz, C. Formation of acrylic acid derivatives from the reaction of carbon dioxide with ethylene complexes of molybdenum and tungsten. J. Am. Chem. Soc. 1985, 107, 5529–5531. [Google Scholar] [CrossRef]
- Chakraborty, S.; Blacque, O.; Berke, H. Ligand assisted carbon dioxide activation and hydrogenation using molybdenum and tungsten amides. Dalton Trans. 2015, 44, 6560–6570. [Google Scholar] [CrossRef]
- Man, M.L.; Zhou, Z.; Ng, S.M.; Lau, C.P. Synthesis, characterization and reactivity of heterobimetallic complexes (η5-C5R5)Ru(CO)(μ-dppm)M(CO)2(η5-C5H5) (R = H, CH3; M = Mo, W). Interconversion of hydrogen/carbon dioxide and formic acid by these complexes. Dalton Trans. 2003, 3727–3735. [Google Scholar] [CrossRef]
- Minato, M.; Zhou, D.-Y.; Sumiura, K.-I.; Oshima, Y.; Mine, S.; Ito, T.; Kakeya, M.; Hoshino, K.; Asaeda, T.; Nakada, T.; et al. Reactivity Patterns of O2, CO2, Carboxylic Acids, and Triflic Acid with Molybdenum Silyl Hydrido Complexes Bearing Polydentate Phosphinoalkyl–Silyl Ligands: Pronounced Effects of Silyl Ligands on Reactions. Organometallics 2012, 31, 4941–4949. [Google Scholar] [CrossRef]
- McWeeny, R.; Mason, R.; Towl, A.D.C. The geometries of and bonding in certain transition metal complexes. Discuss. Faraday Soc. 1969, 47, 20–26. [Google Scholar] [CrossRef]
- Haszeldine, R.N.; Parish, R.V.; Setchfield, J.H. Organosilicon chemistry: XI. The stereochemistry of Ir(H)Cl(SiR3)CO(PPh3)2, and the trans-influence of substituted silyl, germyl, and stannyl groups. J. Organomet. Chem. 1973, 57, 279–285. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Flores, M.; Groy, T.L.; Trovitch, R.J. A Highly Active Manganese Precatalyst for the Hydrosilylation of Ketones and Esters. J. Am. Chem. Soc. 2014, 136, 882–885. [Google Scholar] [CrossRef]
- Pal, R.; Groy, T.L.; Trovitch, R.J. Conversion of Carbon Dioxide to Methanol Using a C–H Activated Bis(imino)pyridine Molybdenum Hydroboration Catalyst. Inorg. Chem. 2015, 54, 7506–7515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hanna, B.S.; Dineen, A.; Williard, P.G.; Bernskoetter, W.H. Functionalization of Carbon Dioxide with Ethylene at Molybdenum Hydride Complexes. Organometallics 2013, 32, 3969–3979. [Google Scholar] [CrossRef]
- Fong, L.K.; Fox, J.R.; Cooper, N.J. Reactions of carbon dioxide with the electron-rich polyhydride complex [Mo(dmpe)2H4]. Organometallics 1987, 6, 223–231. [Google Scholar] [CrossRef]
- Hor, T.S.A.; Chee, S.-M. Substituted metal carbonyls: III. Chromium, molybdenum and tungsten tricarbonyl complexes containing bipyridyl and a unidentate diphosphine: Facile synthesis via trimethylamine N-oxide-induced decarbonylations. J. Organomet. Chem. 1987, 331, 23–28. [Google Scholar] [CrossRef]
- Clark, M.L.; Grice, K.A.; Moore, C.E.; Rheingold, A.L.; Kubiak, C.P. Electrocatalytic CO2 reduction by M(bpy-R)(CO)4 (M = Mo, W, R = H, tBu) complexes. Electrochemical, spectroscopic, and computational studies and comparison with group 7 catalysts. Chem. Sci. 2014, 5, 1894–1900. [Google Scholar] [CrossRef]
- Zhang, Y.; MacIntosh, A.D.; Wong, J.L.; Bielinski, E.A.; Williard, P.G.; Mercado, B.Q.; Hazari, N.; Bernskoetter, W.H. Iron catalyzed CO2 hydrogenation to formate enhanced by Lewis acid co-catalysts. Chem. Sci. 2015, 6, 4291–4299. [Google Scholar] [CrossRef]
- Zhang, Y.; Williard, P.G.; Bernskoetter, W.H. Synthesis and Characterization of Pincer-Molybdenum Precatalysts for CO2 Hydrogenation. Organometallics 2016, 35, 860–865. [Google Scholar] [CrossRef]
- Summers, D.P.; Leach, S.; Frese, K.W. The electrochemical reduction of aqueous carbon dioxide to methanol at molybdenum electrodes with low overpotentials. J. Electroanal. Chem. 1986, 205, 219–232. [Google Scholar] [CrossRef]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 2014, 5, 4470. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, P.; Asadi, M.; Liu, C.; Sharifi-Asl, S.; Sayahpour, B.; Behranginia, A.; Zapol, P.; Shahbazian-Yassar, R.; Curtiss, L.A.; Salehi-Khojin, A. Tailoring the Edge Structure of Molybdenum Disulfide toward Electrocatalytic Reduction of Carbon Dioxide. ACS Nano 2017, 11, 453–460. [Google Scholar] [CrossRef]
- DiMeglio, J.L.; Rosenthal, J. Selective Conversion of CO2 to CO with High Efficiency Using an Inexpensive Bismuth-Based Electrocatalyst. J. Am. Chem. Soc. 2013, 135, 8798–8801. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Q.; Kang, X.; Liu, H.; Qian, Q.; Zhang, Z.; Han, B. Molybdenum–Bismuth Bimetallic Chalcogenide Nanosheets for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2016, 55, 6771–6775. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Shi, G.; Yu, L.; Ba, X.; Zhang, X.; Zhou, J.; Yu, Y. Copper nanoparticle interspersed MoS2 nanoflowers with enhanced efficiency for CO2 electrochemical reduction to fuel. Dalton Trans. 2017, 46, 10569–10577. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Chen, S.; Yang, D.; Zhang, Q.; Song, L.; Xiao, H.; Zhang, Q.; Gu, L.; Wang, X. Edge-Exposed Molybdenum Disulfide with N-Doped Carbon Hybridization: A Hierarchical Hollow Electrocatalyst for Carbon Dioxide Reduction. Adv. Energy Mater. 2019, 9, 1900072. [Google Scholar] [CrossRef]
- Lv, K.; Suo, W.; Shao, M.; Zhu, Y.; Wang, X.; Feng, J.; Fang, M.; Zhu, Y. Nitrogen doped MoS2 and nitrogen doped carbon dots composite catalyst for electroreduction CO2 to CO with high Faradaic efficiency. Nano Energy 2019, 63, 103834. [Google Scholar] [CrossRef]
- Asadi, M.; Motevaselian, M.H.; Moradzadeh, A.; Majidi, L.; Esmaeilirad, M.; Sun, T.V.; Liu, C.; Bose, R.; Abbasi, P.; Zapol, P.; et al. Highly Efficient Solar-Driven Carbon Dioxide Reduction on Molybdenum Disulfide Catalyst Using Choline Chloride-Based Electrolyte. Adv. Energy Mater. 2019, 9, 1803536. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Liu, W.; Sun, Y.; Ju, Z.; Yao, T.; Wang, C.; Ju, H.; Zhu, J.; Wei, S.; et al. Carbon Dioxide Electroreduction into Syngas Boosted by a Partially Delocalized Charge in Molybdenum Sulfide Selenide Alloy Monolayers. Angew. Chem. Int. Ed. 2017, 56, 9121–9125. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, L.; Zhu, Q.; Wu, C.; Yang, D.; Chen, C.; Han, B. MoP Nanoparticles Supported on Indium-Doped Porous Carbon: Outstanding Catalysts for Highly Efficient CO2 Electroreduction. Angew. Chem. Int. Ed. 2018, 57, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, S.; Cordier, S.; Paofai, S.; Boukherroub, R.; Jain, S.L. Photoreduction of CO2 to methanol with hexanuclear molybdenum [Mo6Br14]2− cluster units under visible light irradiation. RSC Adv. 2014, 4, 10420–10423. [Google Scholar] [CrossRef]
- Kumar, P.; Mungse, H.P.; Cordier, S.; Boukherroub, R.; Khatri, O.P.; Jain, S.L. Hexamolybdenum clusters supported on graphene oxide: Visible-light induced photocatalytic reduction of carbon dioxide into methanol. Carbon 2015, 94, 91–100. [Google Scholar] [CrossRef]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen quenching of electronically excited hexanuclear molybdenum and tungsten halide clusters. J. Phys. Chem. A 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Cordier, S.; Kirakci, K.; Méry, D.; Perrin, C.; Astruc, D. Mo6X8i Nanocluster cores (X=Br, I): From inorganic solid state compounds to hybrids. Inorganica Chim. Acta 2006, 359, 1705–1709. [Google Scholar] [CrossRef]
- Srikanth, S.; Maesen, M.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour. Technol. 2014, 165, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.K.; Lee, S.H.; Yoon, S.; Jung, K.-D. Conversion of CO2 to formate in an electroenzymatic cell using Candida boidinii formate dehydrogenase. J. Mol. Catal. B Enzym. 2014, 102, 9–15. [Google Scholar] [CrossRef]
- Kim, S.-H.; Chung, G.-Y.; Kim, S.-H.; Vinothkumar, G.; Yoon, S.-H.; Jung, K.-D. Electrochemical NADH regeneration and electroenzymatic CO2 reduction on Cu nanorods/glassy carbon electrode prepared by cyclic deposition. Electrochim. Acta 2016, 210, 837–845. [Google Scholar] [CrossRef]
- Amao, Y.; Shuto, N. Formate dehydrogenase–viologen-immobilized electrode for CO2 conversion, for development of an artificial photosynthesis system. Res. Chem. Intermed. 2014, 40, 3267–3276. [Google Scholar] [CrossRef]
- Srikanth, S.; Alvarez-Gallego, Y.; Vanbroekhoven, K.; Pant, D. Enzymatic Electrosynthesis of Formic Acid through Carbon Dioxide Reduction in a Bioelectrochemical System: Effect of Immobilization and Carbonic Anhydrase Addition. ChemPhysChem 2017, 18, 3174–3181. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Ong, J.; Li, S.F.Y. Specific and sustainable bioelectro-reduction of carbon dioxide to formate on a novel enzymatic cathode. Chemosphere 2016, 162, 228–234. [Google Scholar] [CrossRef]
- Bassegoda, A.; Madden, C.; Wakerley, D.W.; Reisner, E.; Hirst, J. Reversible Interconversion of CO2 and Formate by a Molybdenum-Containing Formate Dehydrogenase. J. Am. Chem. Soc. 2014, 136, 15473–15476. [Google Scholar] [CrossRef]
- Yuan, M.; Sahin, S.; Cai, R.; Abdellaoui, S.; Hickey, D.P.; Minteer, S.D.; Milton, R.D. Creating a Low-Potential Redox Polymer for Efficient Electroenzymatic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 6582–6586. [Google Scholar] [CrossRef]
- Ferguson, S.J. Nitrogen cycle enzymology. Curr. Opin. Chem. Biol. 1998, 2, 182–193. [Google Scholar] [CrossRef]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The Natural History of Nitrogen Fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Shilov, A.E. Catalytic reduction of molecular nitrogen in solutions. Russ. Chem. Bull. 2003, 52, 2555–2562. [Google Scholar] [CrossRef]
- Haber, F.J.N. The production of ammonia from nitrogen and hydrogen. Naturwissenschaften 1922, 10, 1041. [Google Scholar] [CrossRef]
- Service, R.F. New recipe produces ammonia from air, water, and sunlight. Science 2014. [Google Scholar] [CrossRef]
- Eady, R.R. Structure−Function Relationships of Alternative Nitrogenases. Chem. Rev. 1996, 96, 3013–3030. [Google Scholar] [CrossRef]
- Garagounis, I.; Kyriakou, V.; Skodra, A.; Vasileiou, E.; Stoukides, M. Electrochemical Synthesis of Ammonia in Solid Electrolyte Cells. Front. Energy Res. 2014, 2. [Google Scholar] [CrossRef]
- Jia, H.-P.; Quadrelli, E.A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen. Chem. Soc. Rev. 2014, 43, 547–564. [Google Scholar] [CrossRef]
- MacKay, B.A.; Fryzuk, M.D. Dinitrogen Coordination Chemistry: On the Biomimetic Borderlands. Chem. Rev. 2004, 104, 385–402. [Google Scholar] [CrossRef]
- Deng, H.; Hoffmann, R. How N2 Might Be Activated by the FeMo-Cofactor in Nitrogenase. Angew. Chem. Int. Ed. 1993, 32, 1062–1065. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of Molybdenum Nitrogenase. Chem. Rev. 1996, 96, 2983–3012. [Google Scholar] [CrossRef]
- Howard, J.B.; Rees, D.C. Structural Basis of Biological Nitrogen Fixation. Chem. Rev. 1996, 96, 2965–2982. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef]
- Spatzal, T.; Aksoyoglu, M.; Zhang, L.; Andrade, S.L.A.; Schleicher, E.; Weber, S.; Rees, D.C.; Einsle, O. Evidence for Interstitial Carbon in Nitrogenase FeMo Cofactor. Science 2011, 334, 940. [Google Scholar] [CrossRef]
- Lancaster, K.M.; Roemelt, M.; Ettenhuber, P.; Hu, Y.; Ribbe, M.W.; Neese, F.; Bergmann, U.; DeBeer, S. X-ray Emission Spectroscopy Evidences a Central Carbon in the Nitrogenase Iron-Molybdenum Cofactor. Science 2011, 334, 974. [Google Scholar] [CrossRef] [PubMed]
- Wiig, J.A.; Hu, Y.; Lee, C.C.; Ribbe, M.W. Radical SAM-Dependent Carbon Insertion into the Nitrogenase M-Cluster. Science 2012, 337, 1672. [Google Scholar] [CrossRef]
- Wolff, T.E.; Berg, J.M.; Warrick, C.; Hodgson, K.O.; Holm, R.H.; Frankel, R.B. The molybdenum-iron-sulfur cluster complex [Mo2Fe6S9(SC2H5)8]3−. A synthetic approach to the molybdenum site in nitrogenase. J. Am. Chem. Soc. 1978, 100, 4630–4632. [Google Scholar] [CrossRef]
- Wolff, T.E.; Berg, J.M.; Hodgson, K.O.; Frankel, R.B.; Holm, R.H. Synthetic approaches to the molybdenum site in nitrogenase. Preparation and structural properties of the molybdenum-iron-sulfur “double-cubane” cluster complexes [Mo2Fe6S8(SC2H5)9]3− and [Mo2Fe6S9(SC2H5)8]3. J. Am. Chem. Soc. 1979, 101, 4140–4150. [Google Scholar] [CrossRef]
- Armstrong, W.H.; Holm, R.H. Synthesis and structure of a new type of molybdenum-iron-sulfur double-cubane cluster and evidence for formation of magnetically uncoupled S = 3/2 MoFe3S4 subclusters. J. Am. Chem. Soc. 1981, 103, 6246–6248. [Google Scholar] [CrossRef]
- Armstrong, W.H.; Mascharak, P.K.; Holm, R.H. Doubly bridged double cubanes containing MFe3S4 clusters (M = Mo, W). Synthesis, structure, and conversion to spin-quartet single clusters in solution. J. Am. Chem. Soc. 1982, 104, 4373–4383. [Google Scholar] [CrossRef]
- Cramer, S.P.; Gillum, W.O.; Hodgson, K.O.; Mortenson, L.E.; Stiefel, E.I.; Chisnell, J.R.; Brill, W.J.; Shah, V.K. The molybdenum site of nitrogenase. 2. A comparative study of molybdenum-iron proteins and the iron-molybdenum cofactor by x-ray absorption spectroscopy. J. Am. Chem. Soc. 1978, 100, 3814–3819. [Google Scholar] [CrossRef]
- Coucouvanis, D.; Demadis, K.D.; Kim, C.G.; Dunham, R.W.; Kampf, J.W. Single and double MoFe3S4 cubanes with molybdenum-coordinated polycarboxylate ligands. Syntheses and structural characterization of (Et4N)4{[MoFe3S4Cl4]2(μ-C2O4)} and (Et4N)3([MoFe3S4Cl4(C2O4)] clusters. J. Am. Chem. Soc. 1993, 115, 3344–3345. [Google Scholar] [CrossRef]
- Fomitchev, D.V.; McLauchlan, C.C.; Holm, R.H. Heterometal Cubane-Type MFe3S4 Clusters (M = Mo, V) Trigonally Symmetrized with Hydrotris(pyrazolyl)borate(1−) and Tris(pyrazolyl)methanesulfonate(1−) Capping Ligands. Inorg. Chem. 2002, 41, 958–966. [Google Scholar] [CrossRef]
- Bjornsson, R.; Neese, F.; Schrock, R.R.; Einsle, O.; DeBeer, S. The discovery of Mo(III) in FeMoco: Reuniting enzyme and model chemistry. J. Biol. Inorg. Chem. 2015, 20, 447–460. [Google Scholar] [CrossRef]
- Yang, J. Progress in Synthesizing Analogues of Nitrogenase Metalloclusters for Catalytic Reduction of Nitrogen to Ammonia. Catalysts 2019, 9, 939. [Google Scholar] [CrossRef]
- Tanifuji, K.; Ohki, Y. Metal–Sulfur Compounds in N2 Reduction and Nitrogenase-Related Chemistry. Chem. Rev. 2020, 120, 5194–5251. [Google Scholar] [CrossRef]
- Komori, K.; Oshita, H.; Mizobe, Y.; Hidai, M. Preparation and properties of molybdenum and tungsten dinitrogen complexes. J. Am. Chem. Soc. 1989, 111, 1939–1940. [Google Scholar] [CrossRef]
- Yandulov, D.V.; Schrock, R.R. Catalytic Reduction of Dinitrogen to Ammonia at a Single Molybdenum Center. Science 2003, 301, 76. [Google Scholar] [CrossRef]
- Yandulov, D.V.; Schrock, R.R. Reduction of Dinitrogen to Ammonia at a Well-Protected Reaction Site in a Molybdenum Triamidoamine Complex. J. Am. Chem. Soc. 2002, 124, 6252–6253. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Schrock, R.R. Catalytic reduction of dinitrogen under mild conditions. Chem. Commun. 2003, 19, 2389–2391. [Google Scholar] [CrossRef]
- Yandulov, D.V.; Schrock, R.R.; Rheingold, A.L.; Ceccarelli, C.; Davis, W.M. Synthesis and Reactions of Molybdenum Triamidoamine Complexes Containing Hexaisopropylterphenyl Substituents. Inorg. Chem. 2003, 42, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Ritleng, V.; Yandulov, D.V.; Weare, W.W.; Schrock, R.R.; Hock, A.S.; Davis, W.M. Molybdenum Triamidoamine Complexes that Contain Hexa-tert-butylterphenyl, Hexamethylterphenyl, or p-Bromohexaisopropylterphenyl Substituents. An Examination of Some Catalyst Variations for the Catalytic Reduction of Dinitrogen. J. Am. Chem. Soc. 2004, 126, 6150–6163. [Google Scholar] [CrossRef]
- Schrock, R.R. Catalytic Reduction of Dinitrogen to Ammonia at a Single Molybdenum Center. Acc. Chem. Res. 2005, 38, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Yandulov, D.V.; Schrock, R.R. Studies Relevant to Catalytic Reduction of Dinitrogen to Ammonia by Molybdenum Triamidoamine Complexes. Inorg. Chem. 2005, 44, 1103–1117. [Google Scholar] [CrossRef]
- Schrock, R.R. Reduction of dinitrogen. Proc. Natl. Acad. Sci. USA 2006, 103, 17087. [Google Scholar] [CrossRef]
- Schrock, R.R. Catalytic Reduction of Dinitrogen to Ammonia by Molybdenum: Theory versus Experiment. Angew. Chem. Int. Ed. 2008, 47, 5512–5522. [Google Scholar] [CrossRef]
- Tanaka, H.; Sasada, A.; Kouno, T.; Yuki, M.; Miyake, Y.; Nakanishi, H.; Nishibayashi, Y.; Yoshizawa, K. Molybdenum-Catalyzed Transformation of Molecular Dinitrogen into Silylamine: Experimental and DFT Study on the Remarkable Role of Ferrocenyldiphosphine Ligands. J. Am. Chem. Soc. 2011, 133, 3498–3506. [Google Scholar] [CrossRef]
- Arashiba, K.; Miyake, Y.; Nishibayashi, Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem. 2011, 3, 120–125. [Google Scholar] [CrossRef]
- Nishibayashi, Y. Molybdenum-catalyzed reduction of molecular dinitrogen under mild reaction conditions. Dalton Trans. 2012, 41, 7447–7453. [Google Scholar] [CrossRef]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Tanaka, H.; Kamaru, N.; Yoshizawa, K.; Nishibayashi, Y. Catalytic Formation of Ammonia from Molecular Dinitrogen by Use of Dinitrogen-Bridged Dimolybdenum–Dinitrogen Complexes Bearing PNP-Pincer Ligands: Remarkable Effect of Substituent at PNP-Pincer Ligand. J. Am. Chem. Soc. 2014, 136, 9719–9731. [Google Scholar] [CrossRef]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Tanaka, H.; Yoshizawa, K.; Nishibayashi, Y. Nitrogen fixation catalyzed by ferrocene-substituted dinitrogen-bridged dimolybdenum–dinitrogen complexes: Unique behavior of ferrocene moiety as redox active site. Chem. Sci. 2015, 6, 3940–3951. [Google Scholar] [CrossRef]
- Itabashi, T.; Mori, I.; Arashiba, K.; Eizawa, A.; Nakajima, K.; Nishibayashi, Y. Effect of substituents on molybdenum triiodide complexes bearing PNP-type pincer ligands toward catalytic nitrogen fixation. Dalton Trans. 2019, 48, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Arashiba, K.; Eizawa, A.; Tanaka, H.; Nakajima, K.; Yoshizawa, K.; Nishibayashi, Y. Catalytic Nitrogen Fixation via Direct Cleavage of Nitrogen–Nitrogen Triple Bond of Molecular Dinitrogen under Ambient Reaction Conditions. Bull. Chem. Soc. Jpn. 2017, 90, 1111–1118. [Google Scholar] [CrossRef]
- Eizawa, A.; Arashiba, K.; Egi, A.; Tanaka, H.; Nakajima, K.; Yoshizawa, K.; Nishibayashi, Y. Catalytic Reactivity of Molybdenum–Trihalide Complexes Bearing PCP-Type Pincer Ligands. Chem. Asian J. 2019, 14, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Ashida, Y.; Arashiba, K.; Nakajima, K.; Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 2019, 568, 536–540. [Google Scholar] [CrossRef]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.J.S. Light-driven dinitrogen reduction catalyzed by a CdS: Nitrogenase MoFe protein biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef]
- Nazemi, M.; Panikkanvalappil, S.R.; El-Sayed, M.A. Enhancing the rate of electrochemical nitrogen reduction reaction for ammonia synthesis under ambient conditions using hollow gold nanocages. Nano Energy 2018, 49, 316–323. [Google Scholar] [CrossRef]
- Manjunatha, R.; Schechter, A. Electrochemical synthesis of ammonia using ruthenium–platinum alloy at ambient pressure and low temperature. Electrochem. Commun. 2018, 90, 96–100. [Google Scholar] [CrossRef]
- Tao, H.; Choi, C.; Ding, L.-X.; Jiang, Z.; Han, Z.; Jia, M.; Fan, Q.; Gao, Y.; Wang, H.; Robertson, A.W.; et al. Nitrogen Fixation by Ru Single-Atom Electrocatalytic Reduction. Chem 2019, 5, 204–214. [Google Scholar] [CrossRef]
- Liu, H.-M.; Han, S.-H.; Zhao, Y.; Zhu, Y.-Y.; Tian, X.-L.; Zeng, J.-H.; Jiang, J.-X.; Xia, B.Y.; Chen, Y. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction. J. Mater. Chem. A 2018, 6, 3211–3217. [Google Scholar] [CrossRef]
- Huang, H.; Xia, L.; Shi, X.; Asiri, A.M.; Sun, X. Ag nanosheets for efficient electrocatalytic N2 fixation to NH3 under ambient conditions. Chem. Commun. 2018, 54, 11427–11430. [Google Scholar] [CrossRef]
- Oshikiri, T.; Ueno, K.; Misawa, H. Selective Dinitrogen Conversion to Ammonia Using Water and Visible Light through Plasmon-induced Charge Separation. Angew. Chem. Int. Ed. 2016, 55, 3942–3946. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, C.; Deng, K.; Wang, Z.; Xu, Y.; Li, X.; Xue, H.; Wang, L. One-pot synthesis of bi-metallic PdRu tripods as an efficient catalyst for electrocatalytic nitrogen reduction to ammonia. J. Mater. Chem. A 2019, 7, 801–805. [Google Scholar] [CrossRef]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The Challenge of Electrochemical Ammonia Synthesis: A New Perspective on the Role of Nitrogen Scaling Relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, T.; Wang, Z. Electrochemical reduction of aqueous nitrogen (N2) at a low overpotential on (110)-oriented Mo nanofilm. J. Mater. Chem. A 2017, 5, 18967–18971. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, L.; Ruther, R.E.; Hamers, R.J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 2013, 12, 836–841. [Google Scholar] [CrossRef]
- Kordali, V.; Kyriacou, G.; Lambrou, C. Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell. Chem. Commun. 2000, 1673–1674. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon-Nanotube-Based Electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ji, X.; Ren, X.; Cui, G.; Li, L.; Xie, F.; Wang, H.; Li, B.; Sun, X. MoO3 nanosheets for efficient electrocatalytic N2 fixation to NH3. J. Mater. Chem. A 2018, 6, 12974–12977. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Ren, X.; Luo, Y.; Shi, X.; Asiri, A.M.; Zheng, B.; Sun, X. Efficient Electrochemical N2 Reduction to NH3 on MoN Nanosheets Array under Ambient Conditions. ACS Sustain. Chem. Eng. 2018, 6, 9550–9554. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical Ammonia Synthesis via Nitrogen Reduction Reaction on a MoS2 Catalyst: Theoretical and Experimental Studies. Adv. Mat. 2018, 30, 1800191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, B.; Liang, Z.; Cui, H.; Tian, J. High-Performance Electrocatalytic Conversion of N2 to NH3 Using 1T-MoS2 Anchored on Ti3C2 MXene under Ambient Conditions. ACS Appl. Mater. Interfaces 2020, 12, 26060–26067. [Google Scholar] [CrossRef] [PubMed]
- Danyal, K.; Inglet, B.S.; Vincent, K.A.; Barney, B.M.; Hoffman, B.M.; Armstrong, F.A.; Dean, D.R.; Seefeldt, L.C. Uncoupling Nitrogenase: Catalytic Reduction of Hydrazine to Ammonia by a MoFe Protein in the Absence of Fe Protein-ATP. J. Am. Chem. Soc. 2010, 132, 13197–13199. [Google Scholar] [CrossRef]
- Milton, R.D.; Abdellaoui, S.; Khadka, N.; Dean, D.R.; Leech, D.; Seefeldt, L.C.; Minteer, S.D. Nitrogenase bioelectrocatalysis: Heterogeneous ammonia and hydrogen production by MoFe protein. Energy Environ. Sci. 2016, 9, 2550–2554. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yuan, M.; Cai, R.; Lim, K.; Minteer, S.D. Nitrogenase Bioelectrocatalysis: ATP-Independent Ammonia Production Using a Redox Polymer/MoFe Protein System. ACS Catal. 2020, 10, 6854–6861. [Google Scholar] [CrossRef]
- Badalyan, A.; Yang, Z.-Y.; Seefeldt, L.C. A Voltammetric Study of Nitrogenase Catalysis Using Electron Transfer Mediators. ACS Catal. 2019, 9, 1366–1372. [Google Scholar] [CrossRef]
- Jensen, B.B.; Burris, R.H. Nitrous oxide as a substrate and as a competitive inhibitor of nitrogenase. Biochemistry 1986, 25, 1083–1088. [Google Scholar] [CrossRef]
- Davis, L.C. Hydrazine as a substrate and inhibitor of Azotobacter vinelandii nitrogenase. Arch. Biochem. Biophys. 1980, 204, 270–276. [Google Scholar] [CrossRef]
- Vaughn, S.A.; Burgess, B.K. Nitrite, a new substrate for nitrogenase. Biochemistry 1989, 28, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent Development in Hydrogen Evolution Reaction Catalysts and Their Practical Implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Reibenspies, J.H.; Lai, C.-H.; Lee, W.-Z.; Darensbourg, M.Y. Analysis of an Organometallic Iron Site Model for the Heterodimetallic Unit of [NiFe]Hydrogenase. J. Am. Chem. Soc. 1997, 119, 7903–7904. [Google Scholar] [CrossRef]
- Donovan, E.S.; Felton, G.A.N. Electrochemical analysis of cyclopentadienylmetal carbonyl dimer complexes: Insight into the design of hydrogen-producing electrocatalysts. J. Organomet. Chem. 2012, 711, 25–34. [Google Scholar] [CrossRef]
- Hu, C.; Fan, W.Y. Molybdenum carbonyl complexes as HER electrocatalysts. Mol. Catal. 2019, 479, 110615. [Google Scholar] [CrossRef]
- DuBois, M.R.; VanDerveer, M.C.; DuBois, D.L.; Haltiwanger, R.C.; Miller, W.K. Characterization of reactions of hydrogen with coordinated sulfido ligands. J. Am. Chem. Soc. 1980, 102, 7456–7461. [Google Scholar] [CrossRef]
- Appel, A.M.; DuBois, D.L.; Rakowski DuBois, M. Molybdenum−Sulfur Dimers as Electrocatalysts for the Production of Hydrogen at Low Overpotentials. J. Am. Chem. Soc. 2005, 127, 12717–12726. [Google Scholar] [CrossRef]
- Karunadasa, H.I.; Montalvo, E.; Sun, Y.; Majda, M.; Long, J.R.; Chang, C.J. A Molecular MoS2 Edge Site Mimic for Catalytic Hydrogen Generation. Science 2012, 335, 698. [Google Scholar] [CrossRef] [PubMed]
- Karunadasa, H.I.; Chang, C.J.; Long, J.R. A molecular molybdenum-oxo catalyst for generating hydrogen from water. Nature 2010, 464, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Rajagopal, A.; Damm-Ruttensperger, M.; Schwarz, B.; Nägele, F.; Daccache, L.; Fantauzzi, D.; Jacob, T.; Streb, C. Understanding homogeneous hydrogen evolution reactivity and deactivation pathways of molecular molybdenum sulfide catalysts. Sustain. Energ. Fuels 2018, 2, 1020–1026. [Google Scholar] [CrossRef]
- Cao, J.-P.; Zhou, L.-L.; Fu, L.-Z.; Zhao, J.-X.; Lu, H.-X.; Zhan, S.-Z. A molybdenum–Schiff base complex, a new molecular electro-catalyst for generating hydrogen from acetic acid or water. Catal. Commun. 2014, 57, 1–4. [Google Scholar] [CrossRef]
- Cao, J.-P.; Zhou, L.-L.; Fu, L.-Z.; Zhan, S. A molecular molybdenum electrocatalyst for generating hydrogen from acetic acid or water. J. Power Sources 2014, 272, 169–175. [Google Scholar] [CrossRef]
- Seo, J.; Williard, P.G.; Kim, E. Deoxygenation of Mono-oxo Bis(dithiolene) Mo and W Complexes by Protonation. Inorg. Chem. 2013, 52, 8706–8712. [Google Scholar] [CrossRef]
- Porcher, J.-P.; Fogeron, T.; Gomez-Mingot, M.; Derat, E.; Chamoreau, L.-M.; Li, Y.; Fontecave, M. A Bioinspired Molybdenum Complex as a Catalyst for the Photo- and Electroreduction of Protons. Angew. Chem. Int. Ed. 2015, 54, 14090–14093. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100. [Google Scholar] [CrossRef]
- Seger, B.; Laursen, A.B.; Vesborg, P.C.K.; Pedersen, T.; Hansen, O.; Dahl, S.; Chorkendorff, I. Hydrogen Production Using a Molybdenum Sulfide Catalyst on a Titanium-Protected n+p-Silicon Photocathode. Angew. Chem. Int. Ed. 2012, 51, 9128–9131. [Google Scholar] [CrossRef]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Growth and Activation of an Amorphous Molybdenum Sulfide Hydrogen Evolving Catalyst. ACS Catal. 2013, 3, 2002–2011. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Bonde, J.; Zhang, J.; Ooi, B.-L.; Andersson, K.; Ulstrup, J.; Chorkendorff, I. Hydrogen Evolution on Supported Incomplete Cubane-type [Mo3S4]4+ Electrocatalysts. J. Phys. Chem. C 2008, 112, 17492–17498. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F.; Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nat. Chem. 2014, 6, 248–253. [Google Scholar] [CrossRef]
- Du, K.; Zheng, L.; Wang, T.; Zhuo, J.; Zhu, Z.; Shao, Y.; Li, M. Electrodeposited Mo3S13 Films from (NH4)2Mo3S13·2H2O for Electrocatalysis of Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 18675–18681. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xu, X.; Gao, B.; Ren, Z. Thiomolybdate [Mo3S13]2– Nanoclusters Anchored on Reduced Graphene Oxide-Carbon Nanotube Aerogels for Efficient Electrocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 8908–8917. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, W.; Ma, L.; Yu, M.; Ren, X.; He, M.; Polen, S.; Click, K.; Garrett, B.; Lu, J.; et al. Dimeric [Mo2S12]2− Cluster: A Molecular Analogue of MoS2 Edges for Superior Hydrogen-Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 15181–15185. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, M.; Hou, J.; Wang, F.; Cui, E.; Kong, C.; Min, S. Thiomolybdate [Mo3S13]2− nanocluster: A molecular mimic of MoS2 active sites for highly efficient photocatalytic hydrogen evolution. Chem. Commun. 2018, 54, 603–606. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Wang, R.; Wang, S.; Xi, X.-J.; Ma, L.-F.; Zang, S.-Q. Encapsulating [Mo3S13]2− clusters in cationic covalent organic frameworks: Enhancing stability and recyclability by converting a homogeneous photocatalyst to a heterogeneous photocatalyst. Chem. Commun. 2018, 54, 13563–13566. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Tran, T.V.; Orio, M.; Torelli, S.; Truong, Q.D.; Nayuki, K.; Sasaki, Y.; Chiam, S.Y.; Yi, R.; Honma, I.; et al. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide. Nat. Mat. 2016, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Feliz, M.; Puche, M.; Atienzar, P.; Concepción, P.; Cordier, S.; Molard, Y. In Situ Generation of Active Molybdenum Octahedral Clusters for Photocatalytic Hydrogen Production from Water. ChemSusChem 2016, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Puche, M.; García-Aboal, R.; Mikhaylov, M.A.; Sokolov, M.N.; Atienzar, P.; Feliz, M.J.N. Enhanced Photocatalytic Activity and Stability in Hydrogen Evolution of Mo6 Iodide Clusters Supported on Graphene Oxide. Nanomaterials 2020, 10, 1259. [Google Scholar] [CrossRef]
- Feliz, M.; Atienzar, P.; Amela-Cortés, M.; Dumait, N.; Lemoine, P.; Molard, Y.; Cordier, S. Supramolecular Anchoring of Octahedral Molybdenum Clusters onto Graphene and Their Synergies in Photocatalytic Water Reduction. Inorg. Chem. 2019, 58, 15443–15454. [Google Scholar] [CrossRef]
- Recatalá, D.; Llusar, R.; Gushchin, A.L.; Kozlova, E.A.; Laricheva, Y.A.; Abramov, P.A.; Sokolov, M.N.; Gómez, R.; Lana-Villarreal, T. Photogeneration of Hydrogen from Water by Hybrid Molybdenum Sulfide Clusters Immobilized on Titania. ChemSusChem 2015, 8, 148–157. [Google Scholar] [CrossRef]
- Tran, P.D.; Nguyen, M.; Pramana, S.S.; Bhattacharjee, A.; Chiam, S.Y.; Fize, J.; Field, M.J.; Artero, V.; Wong, L.H.; Loo, J.; et al. Copper molybdenum sulfide: A new efficient electrocatalyst for hydrogen production from water. Energy Environ. Sci. 2012, 5, 8912–8916. [Google Scholar] [CrossRef]
- Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef]
- Tang, H.; Dou, K.; Kaun, C.-C.; Kuang, Q.; Yang, S. MoSe2 nanosheets and their graphene hybrids: Synthesis, characterization and hydrogen evolution reaction studies. J. Mater. Chem. A 2014, 2, 360–364. [Google Scholar] [CrossRef]
- Chen, W.-F.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-Evolution Catalysts Based on Non-Noble Metal Nickel–Molybdenum Nitride Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 6131–6135. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F. Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 14433–14437. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple Phases of Molybdenum Carbide as Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 6407–6410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. In Situ CO2-Emission Assisted Synthesis of Molybdenum Carbonitride Nanomaterial as Hydrogen Evolution Electrocatalyst. J. Am. Chem. Soc. 2015, 137, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-J.; Gao, M.-R.; Liu, C.-H.; Li, S.-L.; Jiang, H.-L.; Lan, Y.-Q.; Han, M.; Yu, S.-H. Porous Molybdenum-Based Hybrid Catalysts for Highly Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 12928–12932. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, X.; Zhou, P.; Liang, X.; Wang, Z.; Liu, Y.; Wang, P.; Zheng, Z.; Dai, Y.; Li, Y.; et al. Molybdenum Nitride Electrocatalysts for Hydrogen Evolution More Efficient than Platinum/Carbon: Mo2N/CeO2@Nickel Foam. ACS Appl. Mater. Interfaces 2020, 12, 29153–29161. [Google Scholar] [CrossRef]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 Quantum Dots as Efficient Catalyst Materials for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Hartley, C.L.; DiRisio, R.J.; Screen, M.E.; Mayer, K.J.; McNamara, W.R. Iron Polypyridyl Complexes for Photocatalytic Hydrogen Generation. Inorg. Chem. 2016, 55, 8865–8870. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Li, J.; Yue, X.; Han, Y.; Shen, P.K.; Cui, Y. Porous MoO2 Nanosheets as Non-noble Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mat. 2016, 28, 3785–3790. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, N.; Fernandes, R.; Hanchate, S.; Miotello, A.; Kothari, D.C. Co-Mo-B Nanoparticles as a non-precious and efficient Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution. Electrochim. Acta 2017, 232, 64–71. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Liu, Y.; Shen, J.; Wang, H.; Yang, X.; Zhu, Y.; Li, C. Multimetallic Ni–Mo/Cu nanowires as nonprecious and efficient full water splitting catalyst. J. Mater. Chem. A 2017, 5, 4207–4214. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zheng, L.R.; Zhang, B.; Yang, H.G. Mo6+ activated multimetal oxygen-evolving catalysts. Chemi. Sci. 2017, 8, 3484–3488. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Lin, H.; Li, X.; Chan, H.C.; Yang, L.; Gao, Q. MoS2–Ni3S2 Heteronanorods as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Liu, C.-H.; Huang, W.; Wang, X.-L.; Dong, L.-Z.; Li, S.-L.; Lan, Y.-Q. Bimetallic Carbides-Based Nanocomposite as Superior Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 16977–16985. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Yue, X.; Du, H.; Shen, P.K. Mo- and Fe-Modified Ni(OH)2/NiOOH Nanosheets as Highly Active and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Catal. 2018, 8, 2359–2363. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Huang, H.; Wang, C.; Gao, L.; Ma, T. One-dimensional MoO2–Co2Mo3O8@C nanorods: A novel and highly efficient oxygen evolution reaction catalyst derived from metal–organic framework composites. Chem. Commun. 2018, 54, 2739–2742. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Adimi, S.; Guo, X.; Thomas, T.; Zhu, Y.; Guo, H.; Priyanga, G.S.; Yoo, P.; Wang, J.; Chen, J.; et al. A Surface-Oxide-Rich Activation Layer (SOAL) on Ni2Mo3N for a Rapid and Durable Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 18036–18041. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Y.-T.; Yao, R.-Q.; Wan, W.-B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.-Y.; Zheng, W.-T.; Jiang, Q. Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef]



























| Catalyst | FE | Current Density | CO2 Reduction Product (Major) | Ref. |
|---|---|---|---|---|
| Mo electrode | 50% | - | Methanol | [58] |
| layer-stacked MoS2 | ~98% | −65 mA/cm2 at −0.76 V vs. RHE | CO | [59] |
| 5% Nb-doped VA-MoS2 | 82% | −237 mA/cm2 at −0.8 V vs. RHE | CO | [60] |
| Ta-doped VA-MoS2 | - | −98~68 mA/cm2 at −0.8 V vs. RHE | CO | [60] |
| MoBiSx nanosheets | 71% | −12.1 mA/cm2 at −0.7 V vs. SHE | Methanol | [62] |
| MoS/Se monolayer | 45% | −43 mA/cm2 at −1.15 V vs. RHE | CO | [68] |
| Cu-doped MoS2 | 85% | −17 mA/cm2 at −1.7 V vs. SCE | CO | [64] |
| NCMSH | 93% | −34.31 mA/cm2 at −0.7 V vs. RHE | CO | [65] |
| MoS2 nanoflake (choline chloride) | 93% | −315 mA/cm2 at −0.8 V vs. RHE | CO | [67] |
| N-MoS2@NCDs | 90% | −36 mA/cm2 at −0.9 V vs. RHE | CO | [66] |
| MoP@In-PC | 97% | −43.8 mA/cm2 at −2.2 V vs. Ag/AgNO3 | Formic acid | [69] |
| Catalyst | FE | NH3 Formation Rate | Ref. |
|---|---|---|---|
| (110)-oriented Mo nanofilm | 0.72% | 3.09 × 10−11 mols−1cm−2 at −0.49 V vs. RHE | [138] |
| MoO3 nanosheets | 1.9% | 4.80 × 10−10 mols−1cm−2 at −0.5 V vs. RHE | [142] |
| MoN nanosheets | 1.15% | 3.01 × 10−10 mols−1cm−2 at −0.3 V vs. RHE | [143] |
| MoS2 | 1.17% | 8.08 × 10−11 mols−1cm−2 at −0.5 V vs. RHE | [144] |
| 1T-MoS2@Ti3C2 | 10.94% | 30.33 μg h−1mg−1cat. at −0.3 V vs. RHE | [145] |
| Catalyst | Onset Potential | Overpotential | Tafel Slope (mV/dec) | Exchanged Current Density (A/cm2) | Ref. |
|---|---|---|---|---|---|
| MoS2 nanoparticles | - | - | 55~60 | 1.3 × 10−7 | [170] |
| [Mo3S4]4+ cluster | −0.2 V vs. NHE | - | 120 | 2.2 × 10−7 | [175] |
| MoSx|Ti|n+p-Si photocathode | 0.33 V vs. RHE | - | 39 | - | [171] |
| MoS3–CV | - | 200 mV at −15 mA/cm2 | 40 | 1.3 × 10−7 | [172] |
| amorphous MoS3 | - | 200 mV at −4.8 mA/cm2 | 42 | - | [173] |
| MoS2+x film | - | 170 mV at −20 mA/cm2 | - | - | [174] |
| MoSe2 nanosheets | −0.15 V vs. RHE | 290 mV at −10 mA/cm2 | 101 | - | [189] |
| MoSe2|RGO | −0.05 V vs. RHE | 115 mV at −10 mA/cm2 | 69 | - | [189] |
| Cu2MoS4 | 135 mV (onset) | 95 | 4.0 × 10−5 | [187] | |
| MoS2/CoSe2 | −11 mV vs. RHE | 68 mV at −10 mA/cm2 | 36 | 7.3 × 10−5 | [188] |
| NiMoNx nanosheet | −78 mV vs. RHE | - | 35.9 | 2.4 × 10−4 | [190] |
| commercial MoB | 100 mV (onset) | 55 | 1.4 × 10−6 | [192] | |
| commercial Mo2C | 100 mV (onset) | 56 | 1.3 × 10−6 | [192] | |
| MoP/S | - | 90 mV at −10 mA/cm2 | 50 | 2.0 × 10−4 | [191] |
| MoP | - | 117 mV at −10 mA/cm2 | 50 | 5.0 × 10−5 | [191] |
| γ-MoC | −0.24 V vs. RHE (at 0.18 mA/cm2) | - | 121.6 | 3.2 × 10−6 | [193] |
| Mo carbonitride | −0.05 V vs. RHE | - | 46~51 | - | [194] |
| MoO2@PC-RGO | 0 V vs. RHE | 64 mV at −10 mA/cm2 | 41 | 4.8 × 10−4 | [195] |
| Mo2N/CeO2@NF-0.05 | - | 26 mV at −10 mA/cm2 | 37.8 | - | [196] |
| [Mo3S13]2− nanocluster | 180 mV at −10 mA/cm2 | 40 | [176] | ||
| Mo3S13 film | −130 mV vs. RHE | 200 mV at −10 mA/cm2 | 37 | [177] | |
| [Mo3S13]2− attached at (rGO-CNTs) aerogels | −110 mV vs. RHE | 179 mV at −10 mA/cm2 | 60.2 | [178] | |
| dimeric [Mo2S12]2− cluster | 161mV at −10 mA/cm2 | 39 | [179] |
| Catalyst | Current Density (mA/cm2) | Overpotential | Tafel Slope (mV/dec) | Ref. |
|---|---|---|---|---|
| MoS2 on NF | 20 | 310 mV | 105 | [198] |
| Mesoporous MoO2 nanosheets on NF | 10 | 260 mV | 54 | [199] |
| Co-Mo-B | 10 | 320 mV | 56 | [200] |
| Ni-Mo/Cu nanowire | 20 | 280 mV | 66 | [201] |
| FeCoMo nanocomposite | 10 | 277 mV | 27.74 | [202] |
| MoS2-Ni3S2 heteronanorods | 10 | 249 mV | 57 | [203] |
| bimetallic Co/Mo carbides | 10 | 260 mV | 50 | [204] |
| MoFe:Ni(OH)2/NiOOH nanosheet | 100 | 280 mV | 47 | [205] |
| MSQDs-AC | 10 | 370 mV | 39 | [197] |
| MoO2-Co2Mo3O8@C nanorods | 10 | 320 mV | 88 | [206] |
| Ni2Mo3N hetero-metal nitride | 10 | 270 mV | 59 | [207] |
| EO Co3Mo alloy nanoparticles | 164 | 350 mV | 82 | [208] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Lee, J.; Seo, J. Molybdenum-Containing Metalloenzymes and Synthetic Catalysts for Conversion of Small Molecules. Catalysts 2021, 11, 217. https://doi.org/10.3390/catal11020217
Kim D, Lee J, Seo J. Molybdenum-Containing Metalloenzymes and Synthetic Catalysts for Conversion of Small Molecules. Catalysts. 2021; 11(2):217. https://doi.org/10.3390/catal11020217
Chicago/Turabian StyleKim, Donghyeon, Jaeheon Lee, and Junhyeok Seo. 2021. "Molybdenum-Containing Metalloenzymes and Synthetic Catalysts for Conversion of Small Molecules" Catalysts 11, no. 2: 217. https://doi.org/10.3390/catal11020217
APA StyleKim, D., Lee, J., & Seo, J. (2021). Molybdenum-Containing Metalloenzymes and Synthetic Catalysts for Conversion of Small Molecules. Catalysts, 11(2), 217. https://doi.org/10.3390/catal11020217






