Abstract
One of the major aspects and advantages of solar energy conversion is the photocatalytic hydrogen generation using semiconductor materials for an eco-friendly technology. Designing a low-cost efficient material to overcome limited light absorption as well as rapid recombination of photogenerated charge carriers is essential to achieve considerable hydrogen generation. In recent years, sulfide based semiconductors have attracted scientific research interest due to their excellent solar response and narrow band gap. The present review focuses on the recent approaches in the development of hierarchical ternary sulfide based photocatalysts with a special focus on ZnIn2S4. We also observe how the electronic structure of ZnIn2S4 is beneficial for water splitting and the various strategies involved for improving the material efficiency for photocatalytic hydrogen generation. The review places emphasis on the latest advancement/new insights on ZnIn2S4 being used as an efficient material for hydrogen generation through photocatalytic water splitting. Recent progress on essential aspects which govern light absorption, charge separation and transport are also discussed in detail.
1. Introduction
Depletion of fossil fuels and deterioration of energy supply demands a better solution for sustainable energy, driving extensive research on the use of Hydrogen (H2) as an alternate energy source. The conversion of sunlight and water, two major resources of energy on earth, into H2 has been advocated as an ideal goal in providing a clean and green energy system without compromising environmental safety. The utilization of solar energy to dissociate water into H2 and oxygen (O2) through photocatalysis assisted by a semiconductor photocatalyst is gaining momentum among various ways of H2 production. With the pioneering work from Honda and Fujishima on the splitting of water using single-crystal TiO2 [1,2], many revolutionary ideas have contributed towards developing an efficient photocatalyst for the effective utilization of solar energy and high H2 yield in absence of any carbonaceous by-products [3,4,5,6]. The mechanism of photo splitting of water involves absorption of radiation with an energy greater than the band-gap of the photocatalyst. It mainly consists of two half-reactions: oxidation of water to form O2 and the reduction of protons to form H2 [7,8,9,10,11,12,13,14,15]
H2O + h+vb → 2H+ + ½ O2 [Photooxidation of water]
2 H+ + 2 e−cb → H2 [Photoreduction of protons]
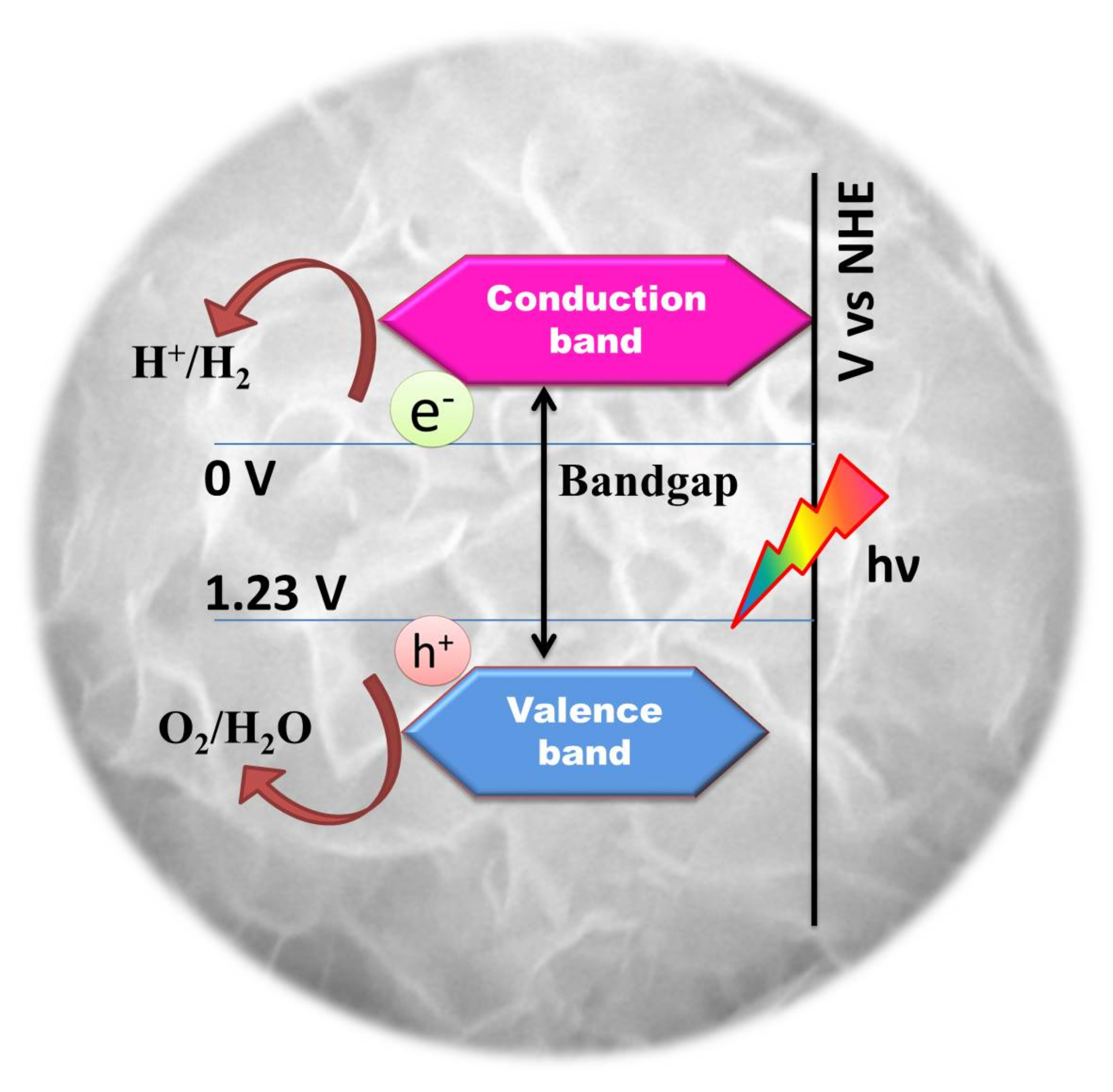
These redox reactions can be initiated only when the positions of the conduction band (CB) is more negative than H2 evolution potential (0V vs Normal Hydrogen Electrode (NHE)) and the valence band (VB) is more positive than the water oxidation potential (+1.23 V Vs NHE) [16]. For photo-assisted water splitting, oxide semiconductors continue to hold a reputation due to their ease of availability, low cost and photostability. Various oxide semiconductors, especially oxides consisting of metal cations (Ga3+, In3+, Ge4+, Sn4+, Sb5+) with d10 configuration and metal cations (Ti4+, Zr4+, Nb5+, Ta5+, W6+) with d0 configuration have been reported for good photocatalytic activity when assisted by co-catalyst like RuO2 [17,18,19,20,21,22,23]. Perovskite structured metal oxides like SrTiO3 and KTaO3 can split water without an external bias in powdered form and show enhanced results when combined with NiO or Rh cocatalyst [24,25]. However, since their valence band comprises of deep 2p oxygen orbital, their O2p levels lie at a potential of 3 eV making the effective band-gap (Eg) to fall in the UV region (4% of the available spectra). For the efficient utilization of the solar spectrum, the band-gap of a photocatalyst should be in the visible region having Eg < 3 eV. This cultivates the need of material with a narrow band-gap having suitable valence band positions. In this regard, metal sulfides are known for their shallow valence band which consists of S3p levels at more negative potential when compared to O2p levels in metal oxides thereby narrowing the bandgap energy [26]. The suitable band edge positions in CdS with narrow band-gap energy ~2.4 eV makes it one of the most studied materials for solar water splitting application. Regardless of the benefits, CdS experiences photocorrosion that greatly eradicates its practical application. Sulfide ions present on the surface of CdS get easily oxidized by photogenerated holes to form solid sulfur which is irreversible [27]. Nevertheless, the development of a photocatalytic corrosion-resistant, visible light-absorbing material with enhanced photoactivity is the doorway to effectuate the cycle of converting sunlight to hydrogen. Hence in view of the prospective applications, ternary semiconductor chalcogenides with hierarchical structures are emerging with greater scope in photocatalysis [28]. Numerous investigations have claimed that the ternary metal sulfides (ZnIn2S4, CdIn2S4, CaIn2S4, MgIn2S4, etc) with AB2X4 (where A = Zn, Cd, Mg, Ca, B = Ga, In and C = S) structure could be a new class of potential visible-light active photocatalysts due to their appreciable chemical stability and optical band gaps. These ternary sulphide compounds with AB2X4 structure have been explored for photo-assisted decomposition of water as listed in Table 1 and Table 2. Among the ternary metal sulfides, ZnIn2S4 is the only member of the family with a layered structure with potential applications in charge storage, opto-electronics and photoconduction [29]. In the hexagonal layered structure of ZnIn2S4, the atoms are arranged in layers along the c-axis. Zn atoms are tetrahedrally bonded to the S atoms (Zn-S4) while the In atoms have two different environments, a tetrahedral arrangement with four S atoms (In-S4) and an octahedral arrangement with 6 S atoms (In-S6). This layered arrangement of atoms is responsible for the improved photocatalytic performance of ZnIn2S4 [30,31,32]. It has also been shown that the ZnIn2S4 structure initially shows a resistivity drop during the exposure to light creating electron/hole pairs. The doubly negative charged surface traps the holes leading to an enhanced free-electron transport to the surface [33]. It is also known that a photocatalyst with a p-block metal ion having d10 configuration exhibits good photocatalytic performance during water decomposition [18]. Hence, the presence of In3+ metal ions with d10 configuration in ZnIn2S4 could be promising for good photocatalytic activity for water decomposition. This review with detailed information on photocatalytic activity of various ternary semiconductor sulfide chalcogenides with hierarchical structures will give a bird’s eye view on their performance with a special emphasis on ZnIn2S4.

Table 1.
Photocatalytic activity of ZnIn2S4-based material in H2 generation with respective parameters.

Table 2.
Other ternary metal sulfides and composites for photocatalytic H2 generation with respective parameters.
2. Crystalline Structures of Sulfide Based Photocatalysts
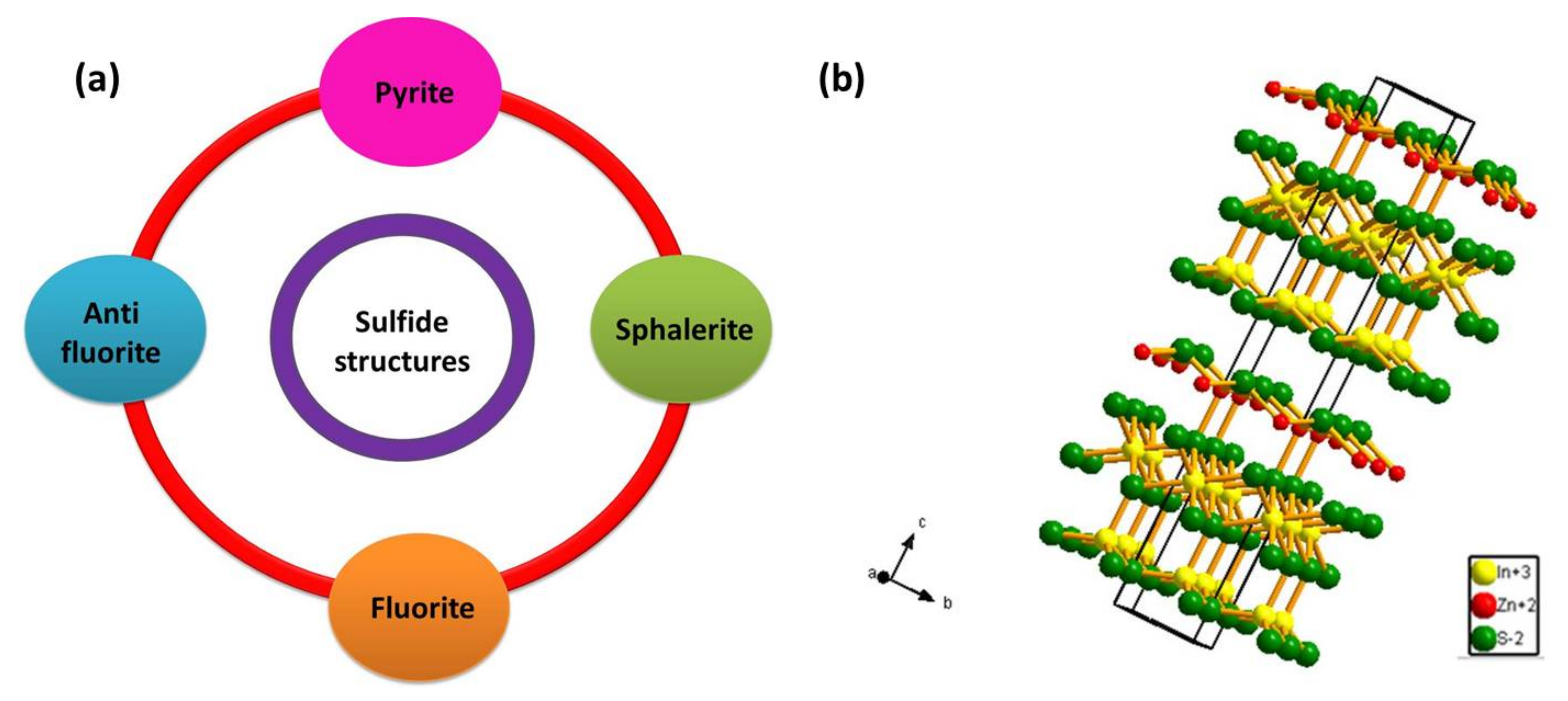
When a metal or semi-metal cation is combined with a sulfur anion, it forms a metal sulfide compound with a stoichiometry of MxSy (MS, M2S, M3S4, MS2). Bi-metal sulfides are formed in a similar approach with a stoichiometry of A1-xBxSy, where x and y are integers [57]. The occupation of metal and sulfur atoms in metal sulfide are arrangements of a close-packed system with four notable structures (Figure 1a). In the first case, we have a symmetrical sodium chloride (NaCl) structure type with each ion occupying an octahedron position with six nearest neighbors. It is called the pyrite structure when the crystal comprises two sulfide ions in each octahedron position [57,58,59]. Then we have the sphalerite structure where the metal ions are bounded by six oppositely charged ions positioned tetrahedrally [60]. The next important structure type is called the fluorite, in which every metal cation is surrounded by eight anions and every anion, in turn, is surrounded by four cations. If this symmetry is reversed with every metal cation surrounded by four anions and every anion, in turn, is surrounded by eight cations, then it is called the anti-fluorite structure [57,61].
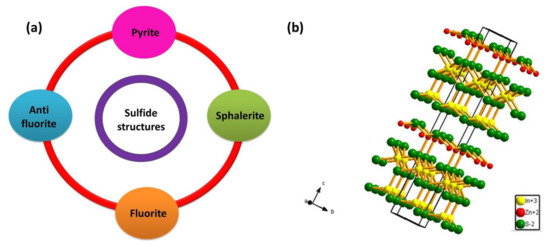
Figure 1.
(a) Classification of sulfide compounds based on their structure. (b) The layered hexagonal structure of ZnIn2S4.
Another metal sulfide category has a cubic spinel structure possessing a stoichiometry of AB2S4, where A site is occupied by divalent metal ion (such as Mg, Zn, Fe, Cu etc) arranged tetragonally, while B site is occupied by trivalent metal ions (such as In, Cr, Ti, Co) arranged octahedrally [57]. The oxidation state adopted by sulfur is usually 2- and hence to maintain the valence symmetry in the AB2S4 structure, the divalent A-site is occupied by cation with 2+ oxidation state and trivalent B-site is occupied by cation with 3+ oxidation state with the structure possibility of A2+B3+S42− [57]. These structures are called ternary metal chalcogenides. From among the family of AB2S4 semiconductors, ZnIn2S4 is the only member with a layered structure (see Figure 1b) [62]. It is a potentially visible-light-responsive photocatalyst reported with three different polymorphs, including, cubic, hexagonal and rhombohedral phase [63]. ZnIn2S4 displays less toxicity when compared to metal sulfides such as CdS and Sb2S3 while exhibiting similar optical properties which portrays the advantage of utilizing ZnIn2S4 in environmental remediation applications. Additionally, ZnIn2S4 can also be beneficial over ZnS with its narrow bandgap value reported between 2.06 eV–2.85 eV [63]. The splendid physical and chemical properties of ZnIn2S4 have attracted immense attention in various applications including H2 generation [37,38,39,40,41,42,43,44,45,46,47,48,49], CO2 reduction [64,65,66], environmental remediation [40] under visible light irradiation. The layer structured semiconductor ZnIn2S4 exhibiting cubic polymorph contains ABC stacking of S atoms with tetrahedral and octahedral coordination of Zn and In atoms respectively, while the hexagonal phase consists of ABABA stacking of S atoms with Zn; half of the In atoms are coordinated tetragonally and the other half In atoms are coordinated octahedrally. The rhombohedral phase exhibits a strong Zn-S and In-S bond in the layer with a weaker S–S bond, with every S atom corresponding to a different layer [66]. The band structure of ZnIn2S4 has also been explored theoretically on the basis of Density Functional Theory (DFT) [55,64,67,68]. Studies suggest that ZnIn2S4 is a direct bandgap semiconductor as both VB and CB of ZnIn2S4 lie on G point of thee Brillouin zone [55]. Valence band consists mainly of S3p and Zn3d orbitals and the conduction band consists of hybridized In5s5p and S3p orbitals. Under photo illumination, electrons would transfer from the valence band to the conduction band leaving behind the photogenerated holes which are beneficial for photo-assisted water splitting [55].
3. Electronic Structure Beneficial for Water Splitting
Photocatalytic water splitting is an energetically uphill reaction (Gibbs energy = 237 kJ/mol) which involves positive energy change and multiple electron transfer similar to naturally occurring photosynthesis. Hence, photocatalytic water splitting is often referred to as “artificial photosynthesis” [69]. Theoretically, a material possessing a minimum bandgap of 1.23 eV is suitable for photochemical water splitting, as explained in Figure 2 [16].
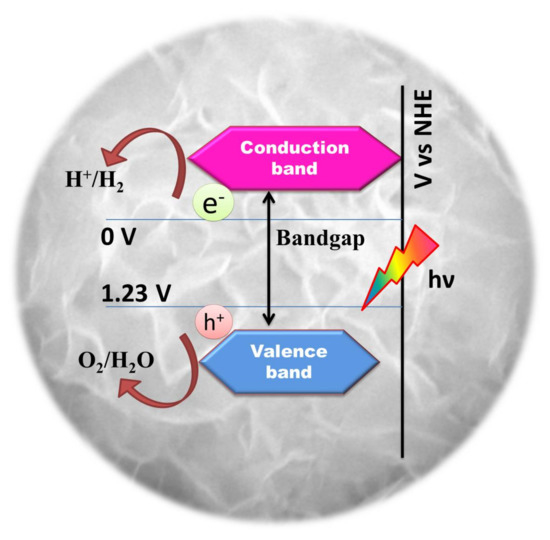
Figure 2.
Schematic illustration of the essential electronic structure of a photocatalyst for water splitting.
Despite the fact that metal oxide photocatalysts are easily available, metal sulfides have attracted a lot of attention for their high absorbance in the ‘hole controlled photocatalysis’. Notably, the photocatalytic activity depends on the quantity of photon absorption. In this regard, high absorbance materials like sulfides show better photocatalytic activity [69,70]. Ionic character in the range of 20–30% is essential for the increased photocatalytic activity of a catalytic material [70]. High ionic character is much needed for water adsorption on the catalyst surface in spite of the fact that the ionic character is responsive to surface corrosion. The majority of the oxide materials provide 50% ionic character attributed to their high electronegativity difference while sulfides also perfectly match this basic requirement [70]. A drawback with the oxide materials is that they face hydrogen embrittlement (metal hydride formation inside the lattice) due to the high affinity of hydrogen with oxygen. This leads to the reduction in catalyst durability in photocatalytic water splitting reactions. Conversely, the rate of hydrogen embrittlement on the sulfide surface is comparatively lesser than the oxide surface [70].
4. Photocatalytic Hydrogen Evolution by ZnIn2S4
ZnIn2S4, a ternary compound of the AB2X4 family has been identified as a visible light photocatalyst with desirable band energy positions to split water photocatalytically. To achieve the same, various experimental synthesis techniques have been explored. Bai et al., [34] synthesized a series of flower-like ZnIn2S4 through surfactant-assisted hydrothermal method where the pH level of reactant played a major role in providing a maximum yield of H2 about 1545 μmol g−1 h−1. Photostable ZnIn2S4 [45] could produce H2 through water reduction for at least up to 150 h. Chaudari et al., [32] have reported excellent photocatalytic activity of ZnIn2S4 for the production of H2 through the splitting of H2S. The quantity of the surfactant (triethylamine), solvent and the synthesis temperature both have a huge impact on the morphology of the product as well as the rate of H2 production [45,50]. ZnIn2S4 synthesized at 160 °C at pH 1 showed 34.3% of apparent quantum yield. Among a series of surfactants used, a sample synthesized with cetyltrimethylammonium bromide (CTAB) is noted to have a larger d(006) space value [47]. It is claimed that larger d(006) space could promote higher separation of photogenerated charge carriers thereby enhancing the photocatalytic performance. Synthesis of ZnIn2S4 through thermal sulfidation at different temperatures provides clear insights into the phase change of the material from cubic to rhombohedral with respect to changes in the synthesis temperature [51]. This study provides a precise understanding of the effect of phase change on optical and photocatalytic properties of ZnIn2S4.
5. Strategies for Enhancing Photocatalytic Performance
In a typical photo-assisted water splitting process, a photocatalyst is excited with energy greater than or equal to the band-gap value to generate electron–hole pairs. These photogenerated charge carriers would migrate to the surface of the photocatalyst and react with the organic pollutant. Photogenerated electrons would reduce H+ to H2 whilst the holes would be consumed by the sacrificial agent added to the system. Though its bandgap lies in the visible region, ZnIn2S4 suffers from recombination of charge carriers as well as poor migration [63]. In order to improve the photocatalytic performance of ZnIn2S4, several approaches have been made including the formation of heterostructure with a suitable photocatalyst, doping with metal ions, surface modification, control of morphology etc. These modifications aid in improving the surface and optical properties of ZnIn2S4 and escalating migration of charge carriers with lower recombination rate resulting in enhanced photocatalytic activity.
5.1. Heterostructure Formation
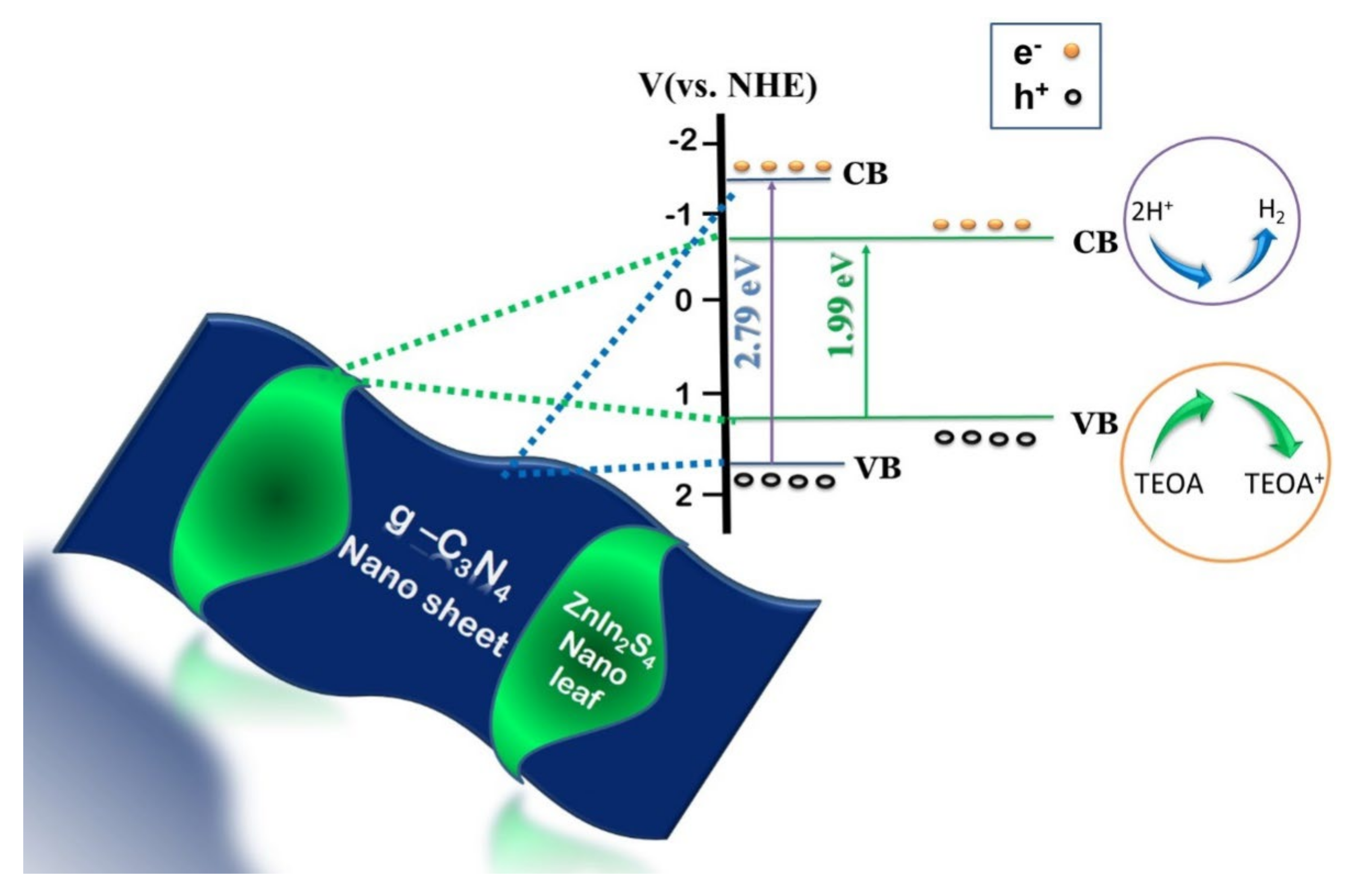
A heterostructured photocatalyst is advantageous in the sense that it assists in extending the light absorption range and accelerates charge transfer. Building a heterojunction between ZnIn2S4 and other suitable semiconductors could facilitate band alignment which could suppress the recombination rate leading to enhanced photocatalytic performance. Lin et al., (2018) designed a heterojunction between g-C3N4/ZnIn2S4 achieving in-situ growth of ZnIn2S4 nano leaves on the surface of g-C3N4 nanosheets (Figure 3) via a one-step surfactant-assisted solvothermal method [37].
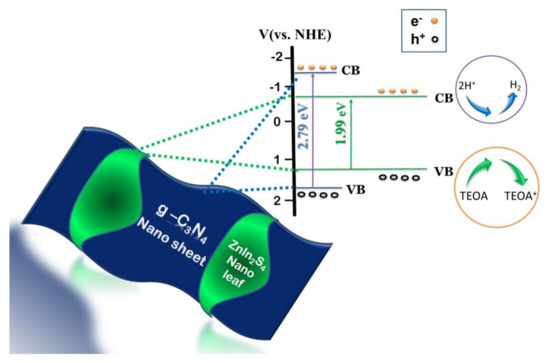
Figure 3.
Schematic representation of in-situ growth of ZnIn2S4 nano leaves over g-C3N4 nanosheet. Figure adapted from [37].
Pudkon et al., (2020) investigated the effect of coupling MoS2 with ZnIn2S4 in H2 generation. An improved H2 generation of 200 μmol g−1 h−1 was observed with 40% loading of MoS2 into ZnIn2S4 lattice due to the enhanced charge separation efficiency and transportation, as well as by charge recombination suppression at the contact interface between ZnIn2S4 and MoS2 [40] Yuan et al., (2016) constructed MoS2-graphene/ZnIn2S4 microarchitectures for the improved H2 generation of 4167 μmol g−1 h−1. The results reveal that the superior activity of the heterostructure is due to the positive synergetic effect between MoS2 and graphene, where MoS2 and graphene act as H2 evolution reaction catalyst and electron transfer bridge respectively [44]. Chai et al., (2012) fabricated a series of composite heterostructures between multiwall carbon nanotubes (MWCNT) and ZnIn2S4 via a facile hydrothermal method. It is envisaged that MWCNT electron-accepting and electron-transporting properties increase the life of electron-hole pairs generated by semiconductor thus helping in enhancing the photocatalytic property [52]. Experimental results suggest that 3% MWCNT embedded in the interior of ZnIn2S4 microspheres shows a maximum H2 production rate of 684 μmol h−1 [52]. Fan et al., (2010) hydrothermally synthesized ZnIn2S4 and deposited it over electrospun poly(HFBA-co-MAA)/PVDF fibers [53]. Here Poly(HFBA-co-MAA) is referred to as Hexafluorobutylacrylate-co-methacrylic acid and PVDF is referred to polyvinylidene fluoride. It is suggested that the use of polymer-carriers has the advantages of being easy recycle, flexibility, excellent weatherability and large surface area. The growth of ZnIn2S4 microspheres on polymer surface (organic carrier) aids in photocorrosion resistance and enhances photocatalytic performance. ZnIn2S4/fluoropolymer fiber composites were able to produce 9.1 mL/h even when recycled up to three runs. On the other hand, Li et al., (2010) synthesized series of ZnS coated ZnIn2S4 via facile solvothermal synthesis using methanol as the solvent [28]. Among the samples synthesized with different mol% of ZnS loading, the sample with 17% ZnS loading showed better photocatalytic activity. In their investigation, glucose is used as a hole scavenger and acts as an electron donor to inhibit photocorrosion on the catalyst surface. Thus H2 production is improved by preventing photocorrosion and recombination of electrons and holes at the semiconductor surface. The superior photocatalytic activity was noted in the presence of glucose and went up to 103 μmol with an irradiation for 10 h whereas the value was just 16 μmol in the absence of glucose.
5.2. Doping as a Strategy to Enhance Photocatalysis
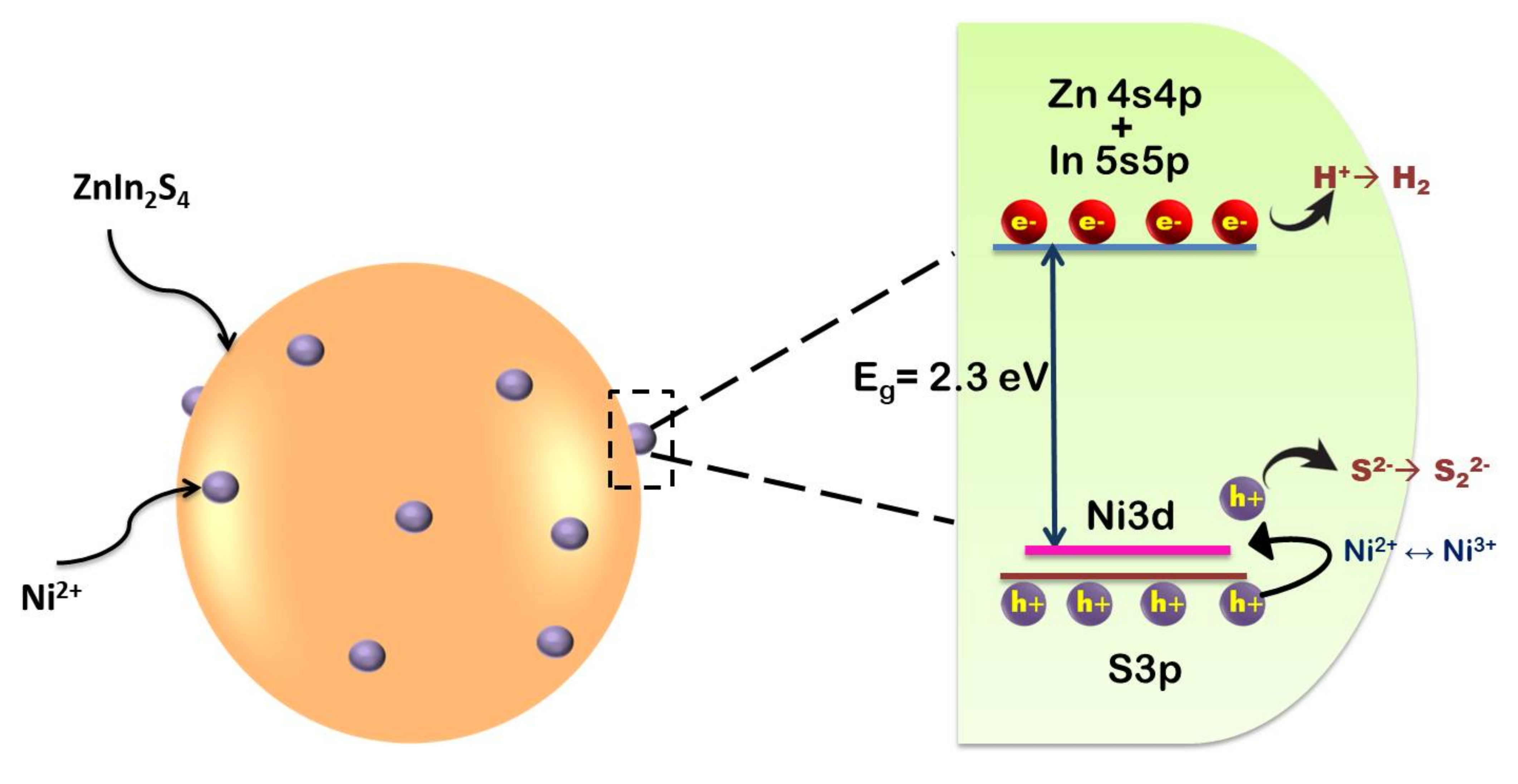
Doping is generally considered a common strategy in boosting up the spectral response of a semiconductor. Commonly, doping an element into a semiconductor could narrow the bandgap and enhance the light-absorption ability [63,68]. Owing to the special electronic configuration of rare earth elements (REEs) with vacant f orbital, doping of rare-earth ions has attracted research interest in the field of photocatalysis. Fien et al., (2014), has doped a series of RE ions (La3+, Ce3+, Er3+, Gd3+, Y3+) into the lattice of ZnIn2S4 [38]. In their study, the photocatalytic H2 production efficiency was observed to increase in the order of La-ZnIn2S4>Ce-ZnIn2S4>Er-ZnIn2S4>Gd-ZnIn2S4>Y-ZnIn2S4 post modification of ZnIn2S4 with RE ions. The decreasing number of electrons in the rare-earth 4f shell was consistent with the increased photocatalytic activity. REEs existed as RE2O3 oxide and modified the lattice of ZnIn2S4, increased its surface area and introduced defects on the catalyst surface, thus inhibiting recombination of photogenerated charge carriers. ZnIn2S4 lattice was also modified with La3+ for H2 evolution activity [39]. Compared to pure ZnIn2S4, the H2 evolution could be increased by 141.6% for 1 wt% La-doped samples. Zhu et al., (2017) on the other hand introduced reduced graphene oxide (RGO) and La into ZnIn2S4 lattice [43] and used Pt as co-catalyst for H2 evolution. Among a series of samples synthesized by them, 1.0 Pt/1.0RGO/1.0 La-ZnIn2S4 gave the highest productivity of 2255 μmol g−1 h−1. It was claimed that RGO could transfer the photoexcited electrons which are easily captured by the surface defects produced by La modification on ZnIn2S4 to generate H2. Shen et al., (2012) modified ZnIn2S4 with series of alkaline earth (AE) metals (Ca, Ba, Sr) [49]. Among the AE-modified samples, only Ca incorporated samples show higher photocatalytic activity than pure ZnIn2S4, while the other two samples perform similar to bare samples. The variation in the photocatalytic performance was analyzed on the basis of UV-Vis spectroscopy and photoluminesce spectra. From UV-Vis absorption spectra it was clear that all samples exhibited the same profile with an intense absorption edge at 500 nm post-incorporation of AE. However, photoluminescence (PL) show decreased emission intensities in the order of ZnIn2S4>Ca– ZnIn2S4>Sr– ZnIn2S4>Ba– ZnIn2S4. It is expected that the introduction of AE ions could produce surface defects acting as trap centers for electrons, improving the charge separation and leading to a higher photocatalytic performance by Ba– ZnIn2S4 sample. However, only Ca- ZnIn2S4 exhibits better performance. This contradiction is explained by the defect emission peaks in the PL spectra at about 400–450 nm. Quenching in the native defect peaks relates to more non-radiative recombination in the system. Hence, both Ba and Sr doped samples possess fewer electrons and holes involved in photocatalysis thereby showing decreased efficiency in H2 evolution [49]. An enhancement in photocatalytic activity post-incorporation of different transition metal (Cr, Mn, Fe, Co) ions into the lattice of ZnIn2S4 [54] is also investigated. The effect of Mn, Cr, Fe and Co doping on photocatalytic activity is analyzed on the basis of band structure and photoluminescence properties. The enhanced performance of the Mn-doped sample is attributed to the increased number of electrons and holes for photocatalysis induced by Mn doping. However, the decreased photocatalytic activity for Fe, Cr, Co-doped samples is attributed to the impurity levels created in the band-gap region which act as non-radiative recombination centers for photogenerated electrons and holes. Shen et al., (2008) analyzed the photocatalytic activity of Cu-doped ZnIn2S4 for H2 evolution application [55]. Incorporating Cu in ZnIn2S4 lattice increased hydrogen evolution up to 151.5 μmol h−1 under visible light irradiation. Similar behavior could be observed upon Ni incorporation (Jing et al., (2010)) Ni2+ existed in NiS state post doping with its energy level lying close to the valence band of S3p orbital (see Figure 4) and acts as trapping sites for photogenerated holes while getting oxidized to Ni3+ state. Due to the instability of Ni3+, it reverts back to Ni2+ state by releasing a hole. Thus, the shallow trapping of holes can extend the lifetime of the charge carrier separation and promote enhancement in the photocatalytic activity [56].
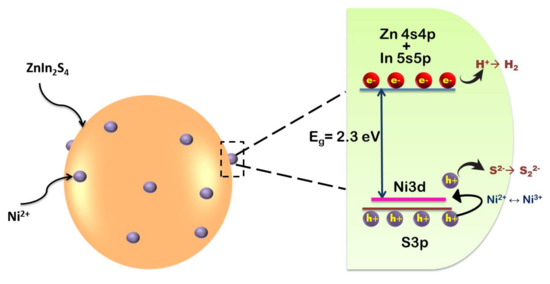
Figure 4.
Schematic for band structure of Ni2+ doped ZnIn2S4. Figure adapted from [56].
5.3. Morphology and Porosity
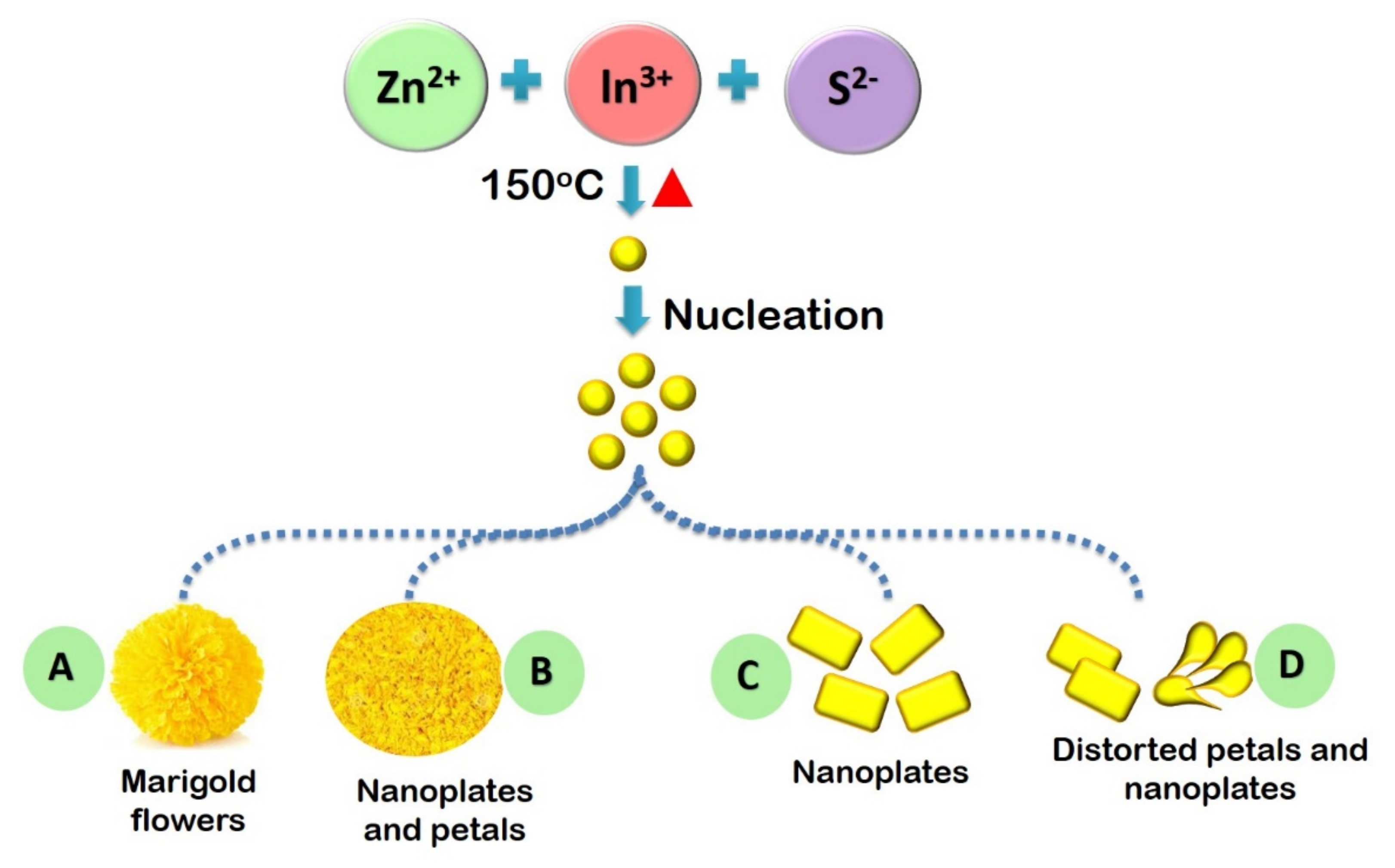
Shape, size and structure of a material have a strong correlation with its physical and chemical properties. Thus tuning the shape and size of semiconductor photocatalyst could be a practical approach in controlling their photocatalytic activity. A simple solution chemistry route has been proposed by Gou et al., (2005) for the shape-controlled synthesis of ZnIn2S4 of various dimensions [31]. With the combination of hydrothermal, solvothermal and surfactant template techniques well-defined morphology of ZnIn2S4 could be achieved. This includes the synthesis of materials with various morphologies such as nanotubes, nanowires, nanoribbons, microspheres etc. For the nanoribbons, the solvothermal route has been used with pyridine as the solvent with a synthesis temperature >180 °C, while for the nanotubes, the synthesis temperature was lowered to <160 °C. Microspheres of ZnIn2S4 composed of irregular sheets were obtained when the solvent is replaced with water. The as-synthesized nano and microstructures exhibit modified optical properties with strong absorption from the UV to the visible range. Chaudhari et al., (2011) has proposed a detailed mechanism to control the microsphere shaped morphology of ZnIn2S4 by varying the solvent concentration (see Figure 5) [32]. In their study, (TEA), the sample exhibited marigold like morphology with curved petals in absence of triethylamine (TEA), while the sample with 0.005 mol of TEA possessed marigold morphology with increased puffiness. On further increasing the concentration of TEA to 0.01 mol, the entire flower-like morphology was drastically suppressed to smaller plates. It is proposed that the excess TEA could increase the bonding strength between TEA and surface atoms of ZnIn2S4 easily breaking down the Van der Waals force exerted between the petals. This accounts for the formation of nanoplates instead of micron-sized flowers. With TEA ~0.015 mol accelerated growth of unidimensional structures, like nanostrips, could also be achieved. Among ZnIn2S4 samples synthesized with various morphologies, H2 evolution was higher (about 5287 μmol h−1) in the case of the sample prepared with 0.01 mol of TEA with flower-like morphology.
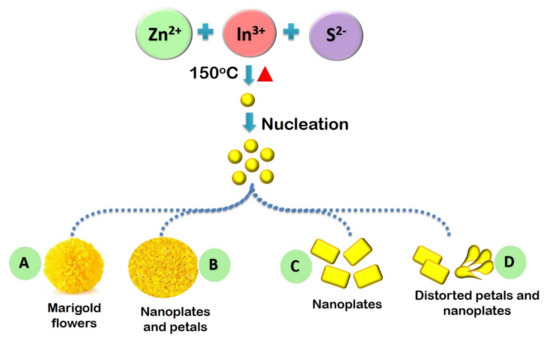
Figure 5.
Schematic illustration of the growth of ZnIn2S4 with different solvent concentrations. Figure adapted from [32].
Lin et al., (2018) designed a heterojunction between 2D g-C3N4 nanosheets and 2D ZnIn2S4 nano leaves via facile surfactant-assisted solvothermal method for enhanced photo-induced H2 generation [37]. From the as-synthesized bulk g-C3N4, thinner g-C3N4 nanosheets are exfoliated through the thermal oxidation process. These exfoliated nanosheets can help in constraining the vertical movement of charge carriers and facilitate recombination resistance [36]. ZnIn2S4 nano leaves were extracted from bulk ZnIn2S4 microspheres with the help of trisodium citrate dihydrate. These structures mimicking leaves from nature are expected to be beneficial for charge separation and promotion of improved photocatalytic H2 generation. A combination of these two 2D structures, could create effective heterojunction with high-speed charge transportation and migration with enhanced photocatalytic activity. The effort of Tian et al., (2014) in modifying ZnIn2S4 with REEs had significant influence on the morphology and porous nature of the material. Unmodified ZnIn2S4 possessed gully-ball like spherical structures with collapsed nanosheets. When Y3+ was added, the surface of ZnIn2S4 was partially open with pores ranging between 0.3 μm to 0.5 μm. The addition of Gd3+ could open up the surface with many porous sheets to a given rose-like structure, while the addition of Er3+ and Ce3+ opened up the entire surface with a reduced gap possessing porous nanosheets ranging from 0.1 μm to 0.2 μm. A regular morphology with a fully opened sphere was observed with the addition of La3+. This shows that the addition of rare earth ions onto the surface of ZnIn2S4 significantly modifies the surface with more regular, stabilized textures arresting agglomeration and maintaining mesopores. Their findings can be related to the fact that the addition of REEs promote-opening of porous structures with a decreased gap between nanosheets, increased surface area and pore volume providing better photocatalytic activity than pure ZnIn2S4 [38]. Solvent mediated synthesis of ZnIn2S4 by Shen et al., (2008) provides new insights on the effect of solvents on the morphology, crystallinity and photocatalytic properties of ZnIn2S4 [50]. Samples synthesized with H2O as solvent presented a flowering-cherry-sphere-like structure with numerous petals. MeOH mediated synthesis resulted in smaller compact spheres with reduced petal length and thickness. Under ethylene glycol mediated condition, the sample did not show flower-like structure but presented clusters of irregular sheets. These results suggested that different organic solvents would hinder the growth of ZnIn2S4 and affect crystallinity. It was observed that the aqueous mediated sample showed regular morphology with good crystallinity and the highest photocatalytic activity among all the samples.
5.4. Role of Sacrificial Agent
Sacrificial agents play a prominent role in photocatalytic H2 production reaction. Many reports suggest that the efficiency of a photocatalyst also relies on the nature of the sacrificial agent. In case of sulfide photocatalyst, amines and sulfide/sulfite-based sacrificial agents can get easily adsorbed on the surface of the catalyst and consume holes when compared to alcohols and sugar. The use of alcohols and sugars produces a neutral pH medium while amines and mixtures of sulfide and sulfite produce a high alkaline medium which is preferential for efficient H2 production [71,72]. Li et al., (2010) in their work have used biomass glucose as an electron donor. From the viewpoint of renewable sources, they claim that glucose from cellulose or starch, when used as an electron donor, is far better when compared to S2− obtained from sulfide/sulfite mixtures. Here, the H2 production experiment with ZnS/ZnIn2S4 has been conducted with and without the usage of glucose as an electron donor. In the absence of glucose, it is noted that the H2 production is saturated after a certain time whereas, a substantial increase in the rate of H2 production is noted in the presence of glucose. In absence of glucose, S2− generated from ZnS photocatalyst acts as a sacrificial agent for H2 generation. In the presence of glucose, a proportional increase of H2 with respect to light irradiation was observed. This is attributed to the fact that glucose acts as a direct hole scavenger inhibiting photo corrosion leading to an enhancement in photoactivity [28]. Similarly, several other hole scavengers such as KOH, triethylamine, lactic acid have also been used for H2 production with sulfide-based photocatalysts [32,35,37,42].
6. Compounds with AB2X4 Structure Other than ZnIn2S4
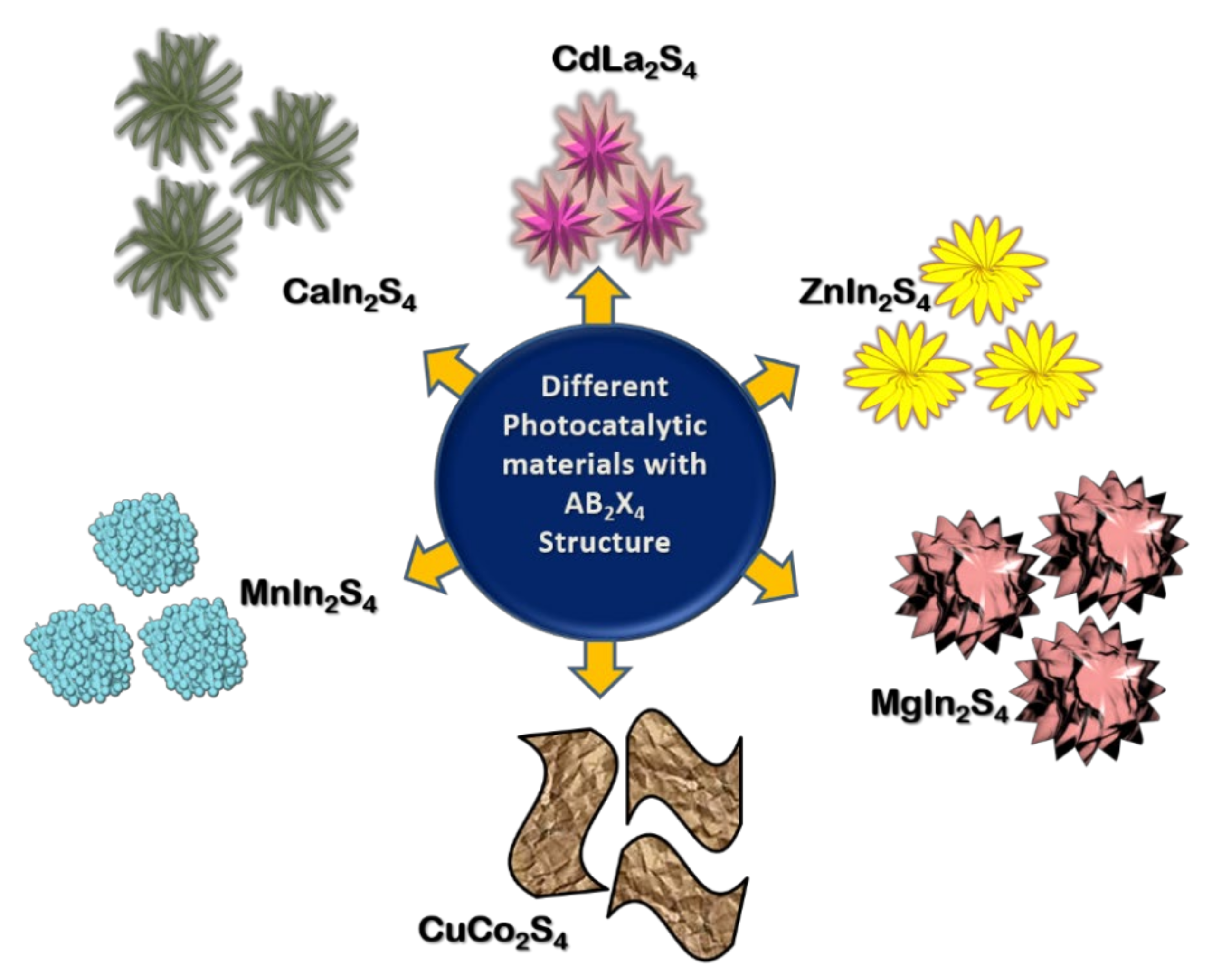
Several multi-metal sulfide photocatalysts have been reported for water splitting applications using various sacrificial agents (see Figure 6). It is mainly attributed to the electronic properties of sulfide materials having conduction band composed of d, s, p orbitals and valence band having S3p orbitals which are negative compared to 2p orbitals of oxide materials. Hence sulfide materials possess band positions negative enough than oxides to reduce water to hydrogen. In addition, their narrow bandgap helps to cover the maximum of the solar spectrum [73]. Table 2 summarizes various such sulfide photocatalysts with AB2X4 structure (besides ZnIn2S4).
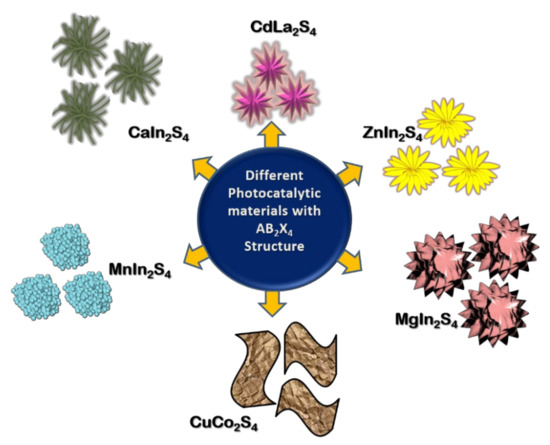
Figure 6.
Representation of the morphologies of photocatalytic materials with AB2X4 structure.
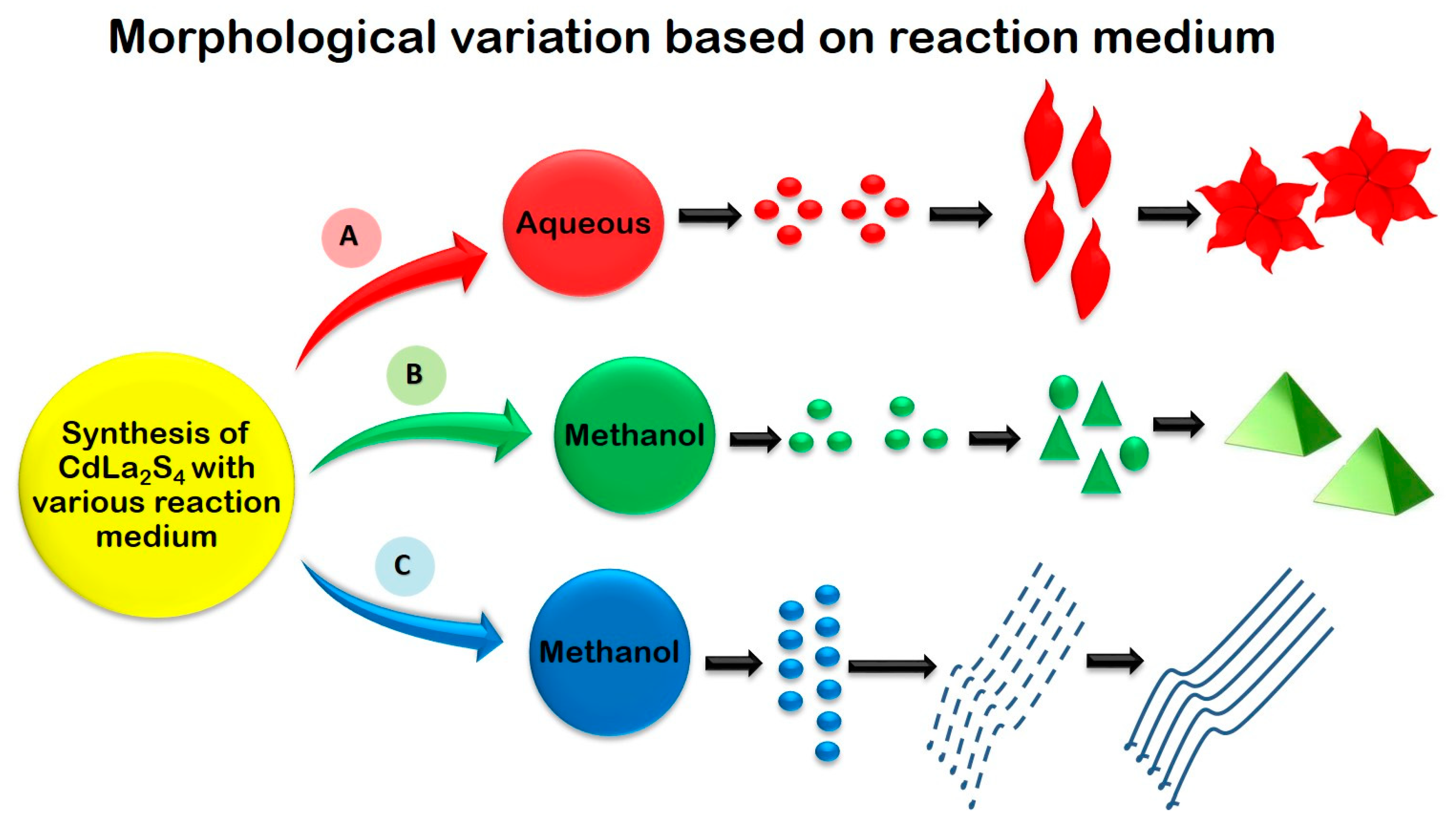
To enhance the performance of sulphide photocataysts, several techniques such as, doping, creating heterojunctions, introducing sacrificial agents and co-catalysts have been followed. CaIn2S4 has been proposed as an efficient photocatalyst for H2 generation by Ding et al., (2013) and a modification of CaIn2S4 with g-C3N4 has been proposed by Jiang et al., (2015) [74,79]. Jiang et al., (2015) elucidates the enhanced photocatalytic performance of CaIn2S4/g-C3N4 heterostructure through H2 production and degradation of textile dye methyl orange. Two-dimensional g-C3N4/cubic CaIn2S4 based heterojunctions provide interfacial contact which promotes charge separation to facilitate enhanced photo-activity. Pt co-catalyst assisted H2 production with 30% CaIn2S4/g-C3N4 nanocomposite resulted in H2 evolution rate of 102 μmol g−1 h−1 (three times higher than that of pristine CaIn2S4) [79]. Kale et al., (2006) synthesized CdIn2S4 with fine marigold-like morphology through aqueous-mediated hydrothermal method and two-dimensional nanotubes morphology with the diameter of 25 nm, through methanol-mediated solvothermal process. In the H2 evolution reaction, a quantum yield of 16.8% was achieved in the case of marigold-like morphology while 17.1% was achieved for CdIn2S4 with nanotube morphology [81]. NiS2 nanoparticles were deposited onto CdLa2S4 nanocrystals as co-catalyst for the enhancement of photocatalytic activity. The NiS2 loaded sample resulted in significant enhancement for H2 production under visible light irradiation. Compared to the pristine CdLa2S4, 2 wt% NiS2 loading sample exhibited three times higher H2 production rate up to 2.5 mmol g−1 h−1 [82]. Interesting morphologies like self-assembled nanohexagon flowers, nanoprisms and nanowires for CdLa2S4 were demonstrated through facile hydrothermal synthesis by varying the reaction medium with water and methanol. A wide variation in morphologies attained from highly crystalline 3D nanoprisms to 1D nanowires showed the influence of the reaction medium during synthesis (Figure 7). The optical band gap of bare nanoprisms, nanowires, nanohexagon flowers and nanoplates of CdLa2S4 range from 2.1 eV to 2.3 eV and are active under the visible region of the solar spectrum. CdLa2S4 with 3D prism morphology generated maximum amount of H2 up to 2552 mmol h−1g−1 [75].
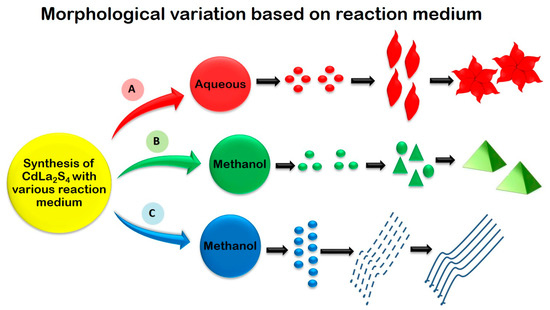
Figure 7.
Schematic representation of various morphologies of CdLn2S4 obtained by controlling the reaction medium and their corresponding concentration. Figure adapted from [74].
CdS nanocrystals incorporated CdLa2S4 microspheres with 0.4 wt% of Pt as co-catalyst showed a high H2-production rate of 2.25 mmol h−1 [80]. Magnesium-based chalcogenide photocatalyst MgIn2S4, on the other hand, when integrated with polyaniline (PANI) proves to be an efficient H2 generating photocatalyst [78]. PANI/MgIn2S4 nanoflower photocatalysts with 1% PANI loading synthesized through facile chemisorption method exhibits a decent H2 evolution of 200.8 μmol g−1 h−1 under visible light irradiation. Chauhan et al., (2019) reported co-catalyst free CuCo2S4 nanosheets as a promising semiconductor photocatalyst for water splitting reactions under visible light irradiation. A simple hydrothermal route was adapted for the synthesis of nanosheets, exhibiting an appropriate band-gap of 2.24 eV. A quantum yield of 2.48% was achieved for the photo-catalytically active CuCo2S4 nanosheets under visible light and it exhibited excellent weight-normalized photoactivity generating H2 at the rate of ~25,900 μmol g−1 h−1. CuCo2S4 nanosheets have been considered important due to the extraordinary long-term operational stability up to 12 h study time without using any co-catalyst [70,71].
7. Conclusions
It is observed from the review that, tremendous efforts have been put up in exploring sulfide photocatalysts and improving their efficiency in the past several decades. It is necessary for an ideal photocatalyst to have higher efficiency with excellent solar spectrum response to be applicable at the industrial level. REEs semiconductors are known for their narrow band gaps and superior photoresponse. Moreover, many promising strategies for enhancing the performance of the catalyst by introducing foreign elements, integrating suitable semiconductors to form heterojunctions, etc are being explored. At this stage, it is necessary to address the key issue of enhancing the H2 production efficiency of sulfur-based photocatalysts. Intensive investigations need to be carried out in improving the behavior of photogenerated electrons and holes which could be affected by crystallinity, surface states, defects and morphology of the semiconductor. The lack of extensive experiments for scaling-up the efficiency of sulfides to promote laboratory research to industrial-scale needs to be overcome through systematic research. Among various sulfide catalysts, ZnIn2S4 is an emerging ternary metal chalcogenide photocatalyst with excellent features such as good crystallinity, porous hierarchical morphology, optical properties, easy fabrication and eco-friendly nature. Despite encouraging properties, ZnIn2S4 based compounds as a photocatalyst face many challenges. However, with its certain superior and moldable properties can be considered as a promising material for photo-assisted applications in the future.
Author Contributions
Conceptualization, R.J.; data collection, R.J. and R.P.V.; Manuscript preparation, R.J., R.P.V., A.R., S.S. and C.-T.C.; Revision, R.J., R.P.V., A.R., S.S. and C.-T.C.; Figure contribution, R.P.V. and A.R.; Schematic design, A.R.; Review, S.S. and C.-T.C.; Funding acquisition, S.S. and C.-T.C. Corresponding author-responsible for ensuring that the descriptions are accurate and agreed by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grant funded from MOST-108-2622-E-197-003-CC3 Taiwan.
Acknowledgments
Shubra Singh would like to acknowledge University Grants Commission (UGC), DST Solar Energy Harnessing Center-DST/TMD/SERI/HUB/1(C) and DST/TMD-EWO/WTI/2K19/EWFH/2019/122. R. Janani would like to acknowledge the SERB project [EMR/2017/000794].
Conflicts of Interest
The author declare no conflict of interest.
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Lui, J.; Chen, W.H.; Tsang, D.C.W.; You, S. A critical review on the principles applications, and challenges of waste to hydrogen technologies. Renew. Sustain. Energy Rev. 2020, 134, 110365. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Nagaraj, P.; Kakarla, S.; Reddy, R. graphene/graphitic carbon notride- based ternary nanohybrids: Synthesis methods, properties, and applications for photocatlytic hydrogen production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- Gupta, N.M. Factors affecting the efficiency of a water splitting photocatalyst: A perspective. Renew. Sustain. Energy Rev. 2017, 71, 585–601. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, R.; Ng, H.Y.; Zhang, P.; Xiang, Q.; Li, X. A review on 2D MoS2 cocatalysts in photocatalytic H2 production. J. Mater. Sci. Technol. 2020, 56, 89–121. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Current trends in strategies to improve photocatalytic performance of perovskites materials for solar to hydrogen production. Renew. Sustain. Energy Rev. 2020, 132, 110073. [Google Scholar] [CrossRef]
- Morillo, E.S.; Toledo, N.M.; Luisa, J.; Fierro, G.; Rufino, M.; Yerga, N. Role of the Sulphur Source in the Solvothermal Synthesis of Ag-CdS Photocatalysts: Effects on the Structure and Photoactivity for Hydrogen Production. Hydrogen 2020, 1, 64–89. [Google Scholar] [CrossRef]
- Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M. Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts 2020, 10, 464. [Google Scholar] [CrossRef]
- Martín, S.S.; Rivero, M.J.; Ortiz, I. Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production. Catalysts 2020, 10, 901. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Z.; Chang, X.; Xu, J.; Wang, D.H. Modified Nano-TiO2 Based Composites for Environmental Photocatalytic Applications. Catalysts 2020, 10, 759. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.J. Recent Progress on Metal Sulfide Composite Nanomaterials for Photo Catalytic Hydrogen Production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef]
- Li, J.; Calvo, P.J.; Paineau, E.; Ghazzal, M.N. Metal Chalcogenides Based Heterojunctions and Novel Nanostructures for Photocatalytic Hydrogen Evolution. Catalysts 2020, 10, 89. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.Z.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Sato, J.; Saito, S.; Nishiyama, H.; Inoue, Y. New Photocatalyst Group for Water Decomposition of RuO2-Loaded p-Block Metal (In, Sn, and Sb) Oxides with d10 Configuration. J. Phys. Chem. B 2001, 105, 6061. [Google Scholar] [CrossRef]
- Ikarashi, K.; Sato, J.; Kobayashi, H.; Saito, S.; Nishiyama, H.; Inoue, Y. Photocatalysis for Water Decomposition by RuO2-Dispersed ZnGa2O4 with d10 Configuration. J. Phys. Chem. B 2002, 106, 9048. [Google Scholar] [CrossRef]
- Sato, J.; Saito, S.; Nishiyama, H.; Inoue, Y. Photocatalytic Activity for Water Decomposition of Indates with Octahedrally Coordinated d10 Configuration. I. Influences of Preparation Conditions on Activity. J. Phys. Chem. B 2003, 107, 7965. [Google Scholar] [CrossRef]
- Sato, J.; Kobayashi, H.; Inoue, Y. Photocatalytic Activity for Water Decomposition of Indates with Octahedrally Coordinated d10 Configuration. II. Roles of Geometric and Electronic Structures. J. Phys. Chem. B 2003, 107, 7970. [Google Scholar] [CrossRef]
- Sato, J.; Kobayashi, H.; Ikarashi, K.; Saito, S.; Nishiyama, H.; Inoue, Y. Photocatalytic Activity for Water Decomposition of RuO2-Dispersed Zn2GeO4 with d10 Configuration. J. Phys. Chem. B 2004, 108, 4369. [Google Scholar] [CrossRef]
- Kadowaki, H.; Saito, N.; Nishiyama, H.; Inoue, Y. RuO2-loaded Sr2+-doped CeO2 with d0 Electronic Configuration as a New Photocatalyst for Overall Water Splitting. Chem. Lett. 2007, 36, 440–441. [Google Scholar] [CrossRef]
- Inoue, Y. Photocatalytic water splitting by RuO2-loaded metal oxides and nitrides with d0- and d10 -related electronic configurations Energy Environ. Science 2009, 2, 364–386. [Google Scholar]
- Chen, Z.; Xing, P.; Chen, P.; Chen, Q.; Wang, Y.; Yu, J.; He, Y. Synthesis of carbon doped KTaO3 and its enhanced performance in photocatalytic H2 generation. Catal. Commun. 2018, 109, 6–9. [Google Scholar] [CrossRef]
- Iwashina, K.; Kudo, A. Rh-Doped SrTiO3 Photocatalyst Electrode Showing Cathodic Photocurrent for Water Splitting under Visible-Light Irradiation. J. Am. Chem. Soc. 2011, 133, 13272–13275. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A Review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Pareek, A.; Gopalakrishnan, A.; Borse, P.H. Efficiency and stability aspects of CdS photoanode for solar hydrogen generation technology. J. Phys. Conf. Ser. 2016, 755, 012006. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Peng, S.; Lu, G.; Li, S. Photocatalytic hydrogen generation in the presence of glucose over ZnS-coated ZnIn2S4 under visible light irradiation. Int. J. Hydrogen Energy 2010, 35, 7116–7126. [Google Scholar] [CrossRef]
- Seo, W.S.; Otsuka, R.; Okuno, H.; Ohta, M.; Koumoto, K. Thermoelectric properties of sintered polycrystalline ZnIn2S4. J. Mater. Res. 1999, 14, 4176–4181. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Zhang, W.; Chen, C.; Li, W.; Sun, M.; He, Y.; Fu, X. Low-Temperature and Template-Free Synthesis of ZnIn2S4Microspheres. Inorg. Chem. 2008, 47, 9766–9772. [Google Scholar] [CrossRef]
- Gou, X.; Cheng, F.; Shi, Y.; Zhang, L.; Peng, S.; Chen, A.J.; Shen, P. Shape-Controlled Synthesis of Ternary Chalcogenide ZnIn2S4 and CuIn(S,Se)2 Nano-/Microstructures via Facile Solution Route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef]
- Chaudhari, N.S.; Bhirud, A.P.; Sonawane, R.S.; Nikam, L.K.; Warule, S.S.; Rane, V.H.; Kale, B.B. Ecofriendly hydrogen production from abundant hydrogen sulfide using solar light-driven hierarchical nanostructured ZnIn2S4 photocatalyst. Green Chem. 2011, 13, 2500–2506. [Google Scholar] [CrossRef]
- Romeo, N.; Dallaturca, A.; Braglia, R.; Sberveglieri, G. Charge storage in ZnIn2S4single crystals. Appl. Phys. Lett. 1973, 22, 21–22. [Google Scholar] [CrossRef]
- Bai, X.; Li, J. Photocatalytic hydrogen generation over porous ZnIn2S4 microspheres synthesized via a CPBr-assisted hy-drothermal method. Mater. Res. Bull. 2011, 46, 1028–1034. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J.; Lu, W.; Zeng, K.; Zhu, H.; Xu, Q.-H.; Ho, G.W. Self-surface charge exfoliation and electrostatically coordinated 2D hetero-layered hybrids. Nat. Commun. 2017, 8, 14224. [Google Scholar] [CrossRef]
- Zhenyi, Z.; Kuichao, L.; Zhiqing, F.; Yanan, B.; Bin, D. Hierarchical Sheet-on-Sheet ZnIn2S4/g-C3N4 Heterostructure with Highly Efficient Photocatalytic H2 production Based on Photoinduced Interfacial Charge Transfer. Sci Rep. 2016, 6, 19221. [Google Scholar]
- Lin, B.; Li, H.; An, H.; Hao, W.; Wei, J.J.; Dai, Y.; Ma, C.; Yang, G. Preparation of 2D/2D g-C3N4 nanosheet@ ZnIn2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B 2018, 220, 542–555. [Google Scholar] [CrossRef]
- Tian, F.; Zhu, R.; He, Y.; Ouyang, F. Improving photocatalytic activity for hydrogen evolution over ZnIn2S4 under visi-ble-light: A case study of rare earth modification. Int. J. Hydrog. Energy 2014, 39, 6335–6344. [Google Scholar] [CrossRef]
- Tian, F.; Zhu, R.; Song, K.; Ouyang, F.; Cao, G. The effects of amount of La on the photocatalytic performance of ZnIn2S4 for hydrogen generation under visible light. Int. J. Hydrog. Energy 2015, 40, 2141–2148. [Google Scholar] [CrossRef]
- Pudkon, W.; Bahruji, H.; Miedziak, P.J.; Davies, T.E.; Morgan, D.J.; Pattisson, S.; Kaowphong, S.; Hutchings, G.J. Enhanced visible-light-driven photocatalytic H2 production and Cr(vi) reduction of a ZnIn2S4/MoS2 heterojunction synthesized by the biomolecule-assisted microwave heating method. Catal. Sci. Technol. 2020, 10, 2838–2854. [Google Scholar] [CrossRef]
- Bai, X.; Danc, W.C.; Peng, W. Photodecomposition of H2S to H2 over CdxZn1−xS composite photocatalysts. Rare Met. 2009, 28, 2. [Google Scholar] [CrossRef]
- Li, W.; Lin, W.; Yang, G. 2D self-assembled MoS2/ ZnIn2S4 heterostructure for efficient photocatalytic hydrogen evolution. Nanoscale 2017, 9, 18290–18298. [Google Scholar] [CrossRef]
- Zhu, R.; Tian, F.; Che, S.; Cao, G.; Ouyang, F. The photocatalytic performance of modified ZnIn2S4 with graphene and La for hydrogen generation under visible light. Renew. Energy 2017, 113, 1503–1514. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Tu, J.R.; Ye, Z.J.; Chen, D.Q.; Hub, B.; Huang, Y.W. MoS2-graphene/ ZnIn2S4 hierarchical microarchitectures with an electron transport bridge between light-harvesting semiconductor and cocatalyst: A highly efficient photocatalyst for solar hydrogen generation. Appl. Catal. B 2016, 188, 13–22. [Google Scholar] [CrossRef]
- Lei, Z.; You, W.; Liu, M.; Zhou, G.; Takata, T.; Hara, M.; Domen, K.; Li, C. Photocatalytic water reduction under visible light on a novel ZnIn2S4 catalyst synthesized by hydrothermal method. Chem. Commun. 2003, 2142–2143. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Zeng, P.; Zhang, X.; Liu, X. Template-Free Hydrothermal Synthesis of ZnIn2S4 Floriated Microsphere as an Efficient Photocatalyst for H2 Production under Visible-Light Irradiation. J. Phys. Chem. C 2011, 115, 6149–6155. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Cetyltrimethylammoniumbromide (CTAB) assisted hydrothermal synthesis of ZnIn2S4 as an efficient visible light drivenphotocatalyst for hydrogen production. Int. J. Hydrog. Energy 2008, 33, 4500–4510. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Crystallite, optical and photocatalytic properties of visible-light-driven ZnIn2S4 photocatalysts synthesized via a surfactant-assisted hydrothermal method. Mater. Res. Bull. 2009, 44, 100–105. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guan, X.; Guo, L. Improving visible-light photocatalytic activity for hydrogen evolution over ZnIn2S4: A case study of alkaline-earth metal doping. J. Phys. Chem. Solids 2012, 73, 79–83. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Morphology, structure and photocatalytic performance of ZnIn2S4 synthesized via a sol-vothermal/hydrothermal route in different solvents. J. Phys. Chem. Solids 2008, 69, 2426–2432. [Google Scholar] [CrossRef]
- Shen, S.; Guo, P.; Zhao, L.; Yuanchang, D.; Liejin, G. Insights into photoluminescence property and photocatalytic ac-tivity of cubic and rhombohedral ZnIn2S4. J. Solid State Chem. 2011, 184, 2250–2256. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Zeng, P.; Zhang, X. Preparation of a MWCNTs/ ZnIn2S4 composite and its enhanced photocatalytic hydrogen production under visible-light irradiation. Dalton Trans. 2012, 41, 1179. [Google Scholar] [CrossRef]
- Fan, W.J.; Zhou, Z.F.; Xu, W.B.; Shi, Z.F.; Ren, F.M.; Ma, H.H.; Huang, S.W. Preparation of ZnIn2S4/fluoropolymer fiber composites and its photocatalytic H2 evolution from splitting of water using Xe lamp irradiation. Int. J. Hydrog. Energy 2010, 35, 6525–6530. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Ren, F.; Kronawitter, C.X.; Mao, S.S.; Guo, L. Solar light-driven photocatalytic hydrogen evolution over ZnIn2S4 loaded with transition-metal sulfides. Nanoscale Res. Lett. 2011, 6, 1–6. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Zhou, Z.; Guo, L. Enhanced Photocatalytic Hydrogen Evolution over Cu-Doped ZnIn2S4 under Visible Light Irradiation. J. Phys. Chem. C 2008, 112, 16148–16155. [Google Scholar] [CrossRef]
- Jing, D.; Liu, M.; Guo, L. Enhanced Hydrogen Production from Water over Ni Doped ZnIn2S4 Microsphere Photocatalysts. Catal. Lett. 2010, 140, 167–171. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C. Recent advances in metal sulfides: From controlled fabrication to elec-trocatalytic photocatalytic and photoelectrochemical water splitting and beyond. Chem.Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Rohrer, G.S. Structure and Bonding in Crystalline Materials; Amsterdam University Press: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Vaughan, D.J.; Corkhill, C.L. Mineralogy of Sulfides. Elements 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Hauck, J.; Mika, K. Ordering of Metal Atoms in Wurtzite and Sphalerite Structures. J. Solid State Chem. 1998, 138, 334–341. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and Strength of M−S Bonds (M = Au, Ag, and Cu) in Binary Alloy Gold Clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef]
- Yina, X.; Sheng, P.; Zhong, F.; Van Nguyen, M.; Cai, Q.; Grimes, C. CdS/ZnIn2S4/TiO2 3D-Heterostructures and Their Photoelectrochemical Properties. New J. Chem. 2016, 40, 6675–6685. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, X.; Jiang, L.; Yu, H.; Zhang, J.; Wang, H.; Guan, R.; Guangming, Z. Recent advances in synthesis, modi-fication and photocatalytic application of micro/nano-structured zinc indium sulfide. Chem. Eng. J. 2018, 354, 407–431. [Google Scholar]
- Chen, J.; Xin, F.; Yin, X.; Xiang, T.; Wang, Y. Synthesis of hexagonal and cubic ZnIn2S4 nanosheets for the photocatalytic reduction of CO2 with methanol. RSC Adv. 2015, 5, 3833. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Shawky, A.; Aljahdali, M.S. Palladium/zinc indium sulfide microspheres: Enhanced photo-catalysts prepare methanol under visible light conditions. J. Taiwan Inst. Chem. Eng. 2016, 65, 498–504. [Google Scholar] [CrossRef]
- Chen, J.; Xin, F.; Niu, H.; Mao, C.J.; Song, J.M. Photocatalytic reduction of CO2 with methanol over Bi2S- ZnIn2S4 nano-composites. Mat. Lett. 2017, 198, 1–3. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Tang, H.M.; Gao, S.-P. Density Functional Theory Study of ZnIn2S4 and CdIn2S4 Polymorphs Using Full-Potential Linearized Augmented Plane Wave Method and Modified Becke—Johnson Potential. Phys. Status Solidi B 2020, 257, 1900485. [Google Scholar] [CrossRef]
- Janani, R.; Priyanga, G.; Behara, S.; Melwin, A.A.; Shaheer, A.; Thomas, T.; Neppolian, B.; Singh, S. Enhanced solar light driven hydrogen generation and environment remediation through Nd incorporated ZnIn2S4. Renew. Energy 2020, 162, 2031–2040. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Chauhan, M.; Soni, K.; Karthik, P.E.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Promising visible-light driven hydrogen production from water on a highly efficient CuCo2S4 nanosheet photocatalyst. J. Mater. Chem. A 2019, 7, 6985–6994. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Ma, J.; Liu, Z.; Li, Y. A bimetallic sulfide CuCo2S4 with good synergistic effect was constructed to drive high performance photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 552, 17–26. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Wahab, A.A. Photocatalytic hydrogen pro-duction: Role of sacrificial agents on the activity of oxide, carbon, and sulfide catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L. Metal sulfide semiconductor for photocatalytic hydrogen production. Catal. Sci. Technol. 2013, 3, 1672–1690. [Google Scholar] [CrossRef]
- Ding, J.; Sun, S.; Yan, W.; Bao, J.; Gao, C. Photocatalytic H2 evolution on a novel CaIn2S4 photocatalyst under visible light irradiation. Int. J. Hydrog. Energy 2013, 30, 13153–13158. [Google Scholar] [CrossRef]
- Kale, B.B.; Baeg, J.-O.; Kong, K.-J.; Moon, S.-J.; Nikam, L.K.; Patil, K.R. Self assembled CdLa2S4 hexagon flowers, nanoprisms and nanowires: Novel photocatalysts for solar hydrogen production. J. Mater. Chem. 2011, 21, 2624–2631. [Google Scholar] [CrossRef]
- Chen, W.; He, Z.-C.; Huang, G.-B.; Wu, C.-L.; Chen, W.-F.; Liu, X.-H. Direct Z-scheme 2D/2D MnIn2S4/g-C3N4 architectures with highly efficient photocatalytic activities towards treatment of pharmaceutical wastewater and hydrogen evolution. Chem. Eng. J. 2019, 359, 244–253. [Google Scholar] [CrossRef]
- Liang, H.; Feng, T.; Tan, S.; Zhao, K.; Wang, W.; Dong, B.; Cao, L. Two-dimensional (2D) MnIn2Se4 nanosheets with porous structure: A novel photocatalyst for water splitting without sacrificial agents. Chem. Commun. 2019, 55, 15061–15064. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Xu, Y.; Xie, M.; Liu, J.; Deng, J.; Huang, L.; Xu, H.; Li, H. Three dimensional polyaniline/MgIn2S4 nanoflower photocatalysts accelerated interfacial charge transfer for the photoreduction of Cr(VI), photodegradation of organic pollution and photocatalytic H2 production. Chem. Eng. J. 2019, 360, 1601–1612. [Google Scholar] [CrossRef]
- Jiang, D.; Li, J.; Xing, C.; Zhang, Z.; Meng, S.; Chen, M. Two-Dimensional CaIn2S4/g-C3N4 Heterojunction Nanocomposite with Enhanced Visible-Light Photocatalytic Activities: Interfacial Engineering and Mechanism Insight. ACS Appl. Mater. Interfaces 2015, 34, 19234–19242. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, C.; Wang, Z.; Jiao, S.; Zhu, H. Hydrothermal synthesis of CdS/CdLa2S4 heterostructures for efficient visi-ble-light driven photocatalytic hydrogen production. RSC Adv. 2012, 2, 10330–10336. [Google Scholar] [CrossRef]
- Kale, B.B.; Baeg, J.O.; Lee, S.M.; Chang, H.; Moon, S.J.; Lee, C.W. CdIn2S4 Nanotubes and “Marigold” Nanostruc-tures: A Visible-Light Photocatalyst. Adv. Funct. Mater. 2006, 16, 1349–1354. [Google Scholar] [CrossRef]
- Yuan, P.Y.; Cao, W.S.; Yin, S.L.; Xu, L.; Xue, C. NiS2 Co-catalyst decoration on CdLa2S4 nanocrystals for efficient photo-catalytic hydrogen generation under visible light irradiation. Int. J. Hydrog. Energ. 2013, 38, 7218–7223. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).