Facile Synthesis of COF-Supported Reduced Pd-Based Catalyst for One-Pot Reductive Amination of Aldehydes
Abstract
1. Introduction
2. Results and Discussion
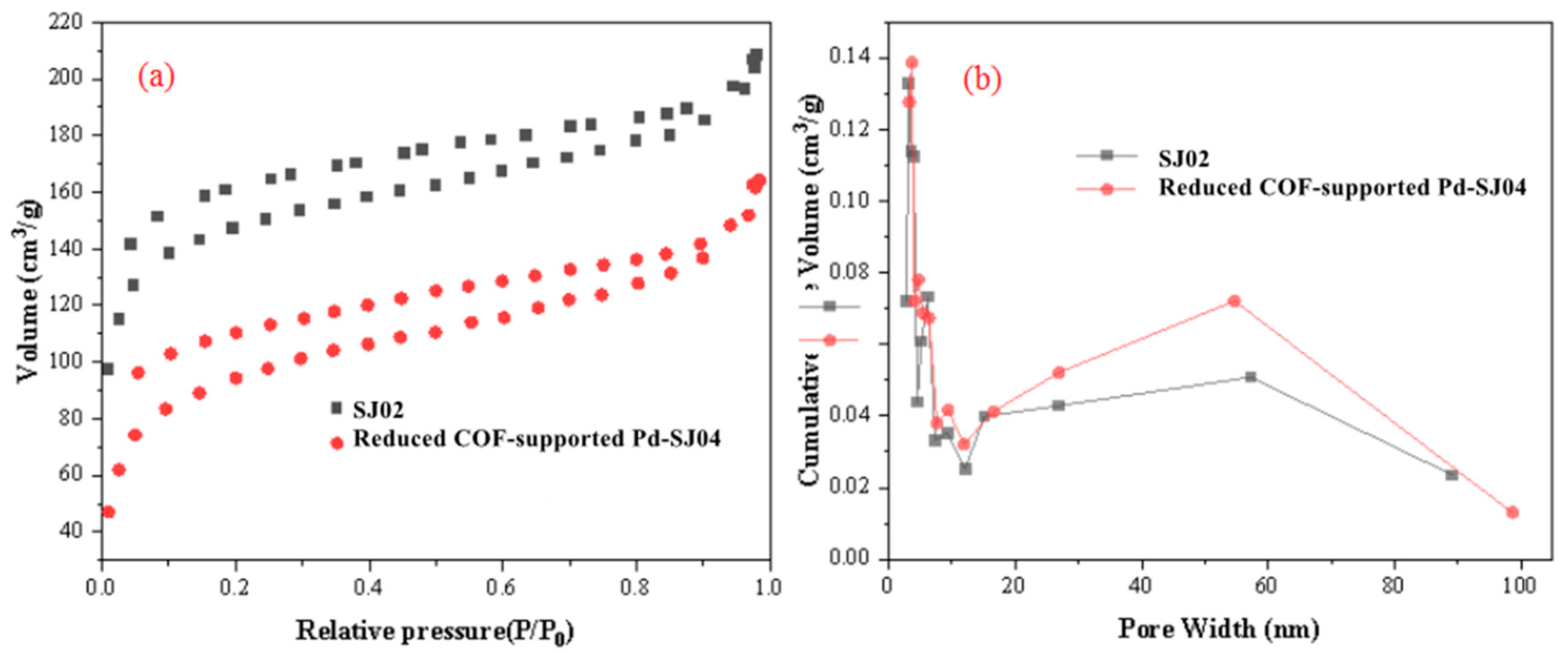
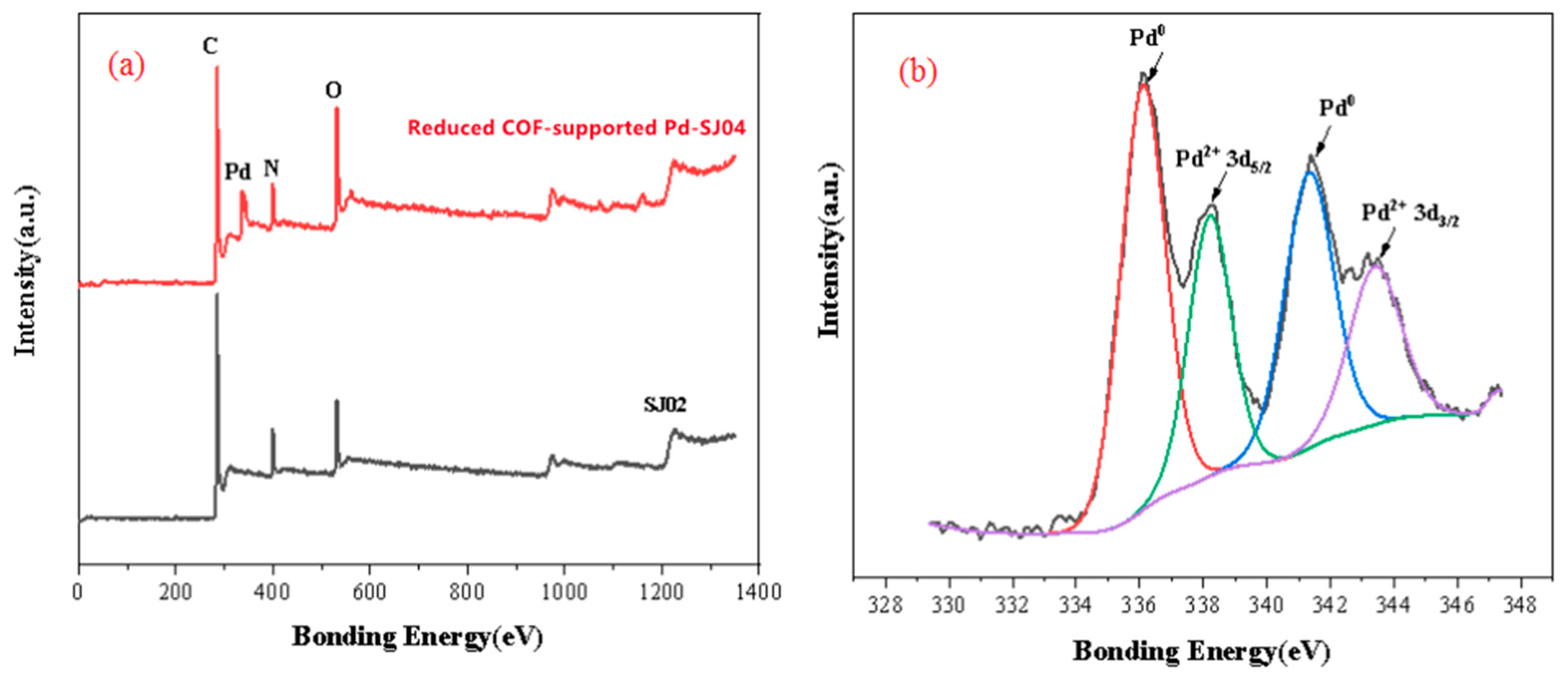
2.1. Characterization of COF Support and Reduced COF-Supported Pd Catalyst
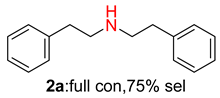
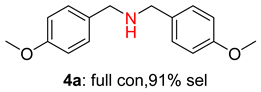
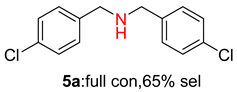
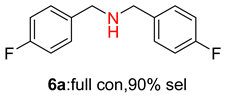
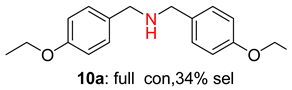
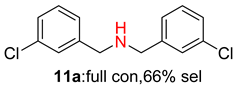
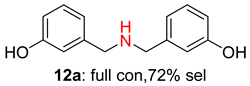
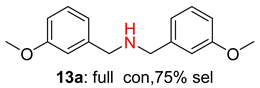
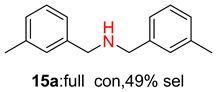
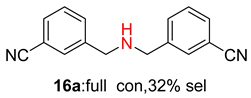
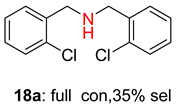
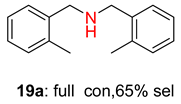
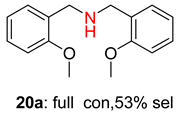
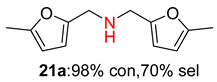
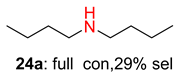
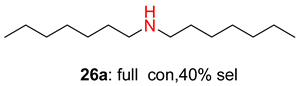
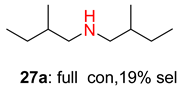
2.2. Reduced COF-Supported Pd Catalyst Reductive Amination
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of COF Material
3.2.2. Synthesis of COF Based Pd Catalyst
3.2.3. Synthesis of the Reduced COF-Supported Pd Catalyst
3.3. Catalyst Characterization
3.4. General Procedure for the Catalytic Reductive Amination Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choucair, B.; Leon, H.; Mire, M.-A.; Lebreton, C.; Mosset, P. Indium- or zinc-mediated one-pot synthesis of homoallylamines, beta-amino esters, and beta-amino nitriles. Org. Lett. 2000, 2, 1851–1853. [Google Scholar] [CrossRef]
- Harrington, P.E.; Jean, D.J.S., Jr.; Clarine, J.; Coulter, T.S.; Croghan, M.; Davenport, A.; Davis, J.; Ghiron, C.; Hutchinson, J.; Kelly, M.G.; et al. The discovery of an orally efficacious positive allosteric modulator of the calcium sensing receptor containing a dibenzylamine core. Bioorg. Med. Chem. Lett. 2010, 20, 5544–5547. [Google Scholar] [CrossRef]
- Qi, F.; Hu, L.; Lu, S.; Cao, X.; Gu, H. Selective synthesis of secondary amines by Pt nanowire catalyzed reductive amination of aldehydes and ketones with ammonia. Chem. Commun. 2012, 48, 9631–9633. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.Q.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of Primary Amines by Reductive Amination of Biomass-Derived Aldehydes/Ketones. Angew. Chem. Int. Ed. 2017, 56, 3050–3054. [Google Scholar] [CrossRef]
- Lu, S.; Xu, P.; Cao, X.; Gu, H.W. A highly active worm-like PtMo nanowire for the selective synthesis of dibenzylamines. RSC Adv. 2018, 8, 8755–8760. [Google Scholar] [CrossRef]
- Li, M.; Pischetola, C.; Cardenas-Lizana, F.; Keane, M.A. Production of benzylamine by tandem dehydrogenation/amination/reduction over Cu and Au catalysts. Appl. Catal. A Gen. 2020, 590, 117368. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Yoon, C.H.; Jung, K.W. Synthesis of secondary amines. Tetrahedron 2001, 57, 7785–7811. [Google Scholar] [CrossRef]
- Kumar, R.; Gleissner, E.H.; Tiu, E.G.V.; Yamakoshi, Y. C-70 as a Photocatalyst for Oxidation of Secondary Benzylamines to Imines. Org. Lett. 2016, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; AI-Dakhil, A.; Lupp, D.; Gholap, S.S.; Lai, Z.P.; Liang, L.C.; Huang, K.W. Cobalt-Catalyzed Selective Hydrogenation of Nitriles to Secondary Imines. Org. Lett. 2018, 20, 6430–6435. [Google Scholar] [CrossRef]
- Pagnoux-Ozherelyeva, A.; Pannetier, N.; Mbaye, M.D.; Gaillard, S.; Renaud, J.L. Knolker’s Iron Complex: An Efficient In Situ Generated Catalyst for Reductive Amination of Alkyl Aldehydes and Amines. Angew. Chem. Int. Ed. 2012, 51, 4976–4980. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chung, Y.K. Hydrogen-Free Cobalt-Rhodium Heterobimetallic Nanoparticle-Catalyzed Reductive Amination of Aldehydes and Ketones with Amines and Nitroarenes in the Presence of Carbon Monoxide and Water. ACS Catal. 2015, 5, 4846–4850. [Google Scholar] [CrossRef]
- Hahn, G.; Kunnas, P.; de Jonge, N.; Kempe, R. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst. Nat. Catal. 2019, 2, 71–77. [Google Scholar] [CrossRef]
- Murugesan, K.; Beller, M.; Jagadeesh, R.V. Reusable Nickel Nanoparticles-Catalyzed Reductive Amination for Selective Synthesis of Primary Amines. Angew. Chem. Int. Ed. 2019, 58, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, T.; Morias, T.; Windels, S.; Marquez, C.; Vankelecom, I.; De Vos, D.E. Ni-Catalyzed reductive amination of phenols with ammonia or amines into cyclohexylamines. Green Chem. 2020, 22, 1884–1893. [Google Scholar] [CrossRef]
- Murugesan, K.; Chandrashehar, V.G.; Senthamarai, T.; Jagadeesh, R.V.; Beller, M. Reductive amination using cobalt-based nanoparticles for synthesis of amines. Nat. Protoc. 2020, 15, 1313–1337. [Google Scholar] [CrossRef]
- Zheng, J.X.; Roisnel, T.; Darcel, C.; Sortais, J.B. Nickel-Catalysed Reductive Amination with Hydrosilanes. ChemSusChem 2013, 5, 2861–2864. [Google Scholar] [CrossRef]
- Tanabe, M.; Yumoto, R.; Osakada, K. Reaction of an alkyne with dinickel-diphenylsilyl complexes. An emissive disilane formed via the consecutive Si-C and Si-Si bond-making processes. Chem. Commun. 2012, 48, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Zhu, S.F.; Zhou, Q.L. Transition Metal-Catalyzed Enantioselective Hydrogenation of Enamines and Imines. Chem. Rev. 2011, 111, 1713–1760. [Google Scholar] [CrossRef]
- Grirrane, A.; Garcia, H.; Corma, A. Supported palladium nanoparticles as heterogeneous ligand-free catalysts for the Hiyama C-C coupling of vinylsilanes and halobenzenes leading to styrenes. J. Catal. 2013, 302, 49–57. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xiao, Z.M.; Wu, L. Metal-nanoparticles Supported on Solid as Heterogeneous Catalysts. Curr. Org. Chem. 2013, 17, 1325–1333. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev. 2016, 312, 99–148. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Jiang, D.L. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.P.; Coupry, D.E.; Addicoat, M.A.; Okushita, K.; Nishimura, K.; Heine, T.; Jiang, D.L. Multiple-component covalent organic frameworks. Nat. Commun. 2016, 7, 12325. [Google Scholar] [CrossRef]
- Yoo, J.; Cho, S.J.; Jung, G.Y.; Kim, S.H.; Choi, K.H.; Kim, J.H.; Lee, C.K.; Kwak, S.K.; Lee, S.Y. COF-Net on CNT-Net as a Molecularly Designed, Hierarchical Porous Chemical Trap for Polysulfides in Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 3292–3300. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.X.; Yan, X.P. Controllable preparation of core-shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem. Commun. 2017, 53, 2511–2514. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, N.; Ma, L.L. Recent Advances in Covalent Organic Frameworks for Catalysis. Chem. Asian J. 2020, 15, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.; Sasmal, H.S.; Basu, A.; Midya, S.P.; Kandambeth, S.; Pachfule, P.; Balaraman, E.; Banerjee, R. Predesigned Metal-Anchored Building Block for in Situ Generation of Pd Nanoparticles in Porous Covalent Organic Framework: Application in Heterogeneous Tandem Catalysis. ACS Appl. Mater. Interfaces 2017, 9, 13785–13792. [Google Scholar] [CrossRef]
- Fiore, A.M.; Romanazzi, G.; Dell’Anna, M.M.; Latronico, M.; Leonelli, C.; Mali, M.; Rizzuti, A.; Mastrorilli, P. Mild and efficient synthesis of secondary aromatic amines by one-pot stepwise reductive amination of arylaldehydes with nitroarenes promoted by reusable nickel nanoparticles. Mol. Catal. 2019, 476, 110507. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, N.; Liu, J.N.; Li, M.; Xu, Y.; Wang, C.G.; Wang, Y.Z.; Zheng, H.Q.; Ma, L.L. The Immobilization of Pd(II) on Porous Organic Polymers for Semihydrogenation of Terminal Alkynes. ACS Appl. Mater. Interfaces 2020, 12, 51428–51436. [Google Scholar] [CrossRef] [PubMed]




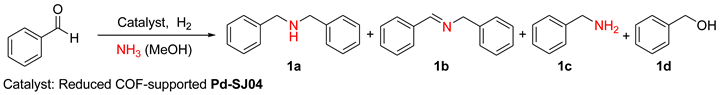 | |||||
|---|---|---|---|---|---|
| Entry | H2 Pressure (bar) | Temperature (°C) | Time (h) | Conversion (%) | Yield of 1a (%) |
| 1 | 10 | 90 | 4 | >99 | 79.1 |
| 2 | 20 | 90 | 4 | >99 | 81.7 |
| 3 | 30 | 90 | 4 | >99 | 78.5 |
| 4 | 20 | 50 | 4 | >99 | 75.6 |
| 5 | 20 | 70 | 4 | >99 | 85.1 |
| 6 | 20 | 90 | 4 | >99 | 81.7 |
| 7 | 20 | 110 | 4 | >99 | 72.0 |
| 8 | 20 | 90 | 2 | >99 | 83.8 |
| 9 | 20 | 90 | 4 | >99 | 81.7 |
| 10 | 20 | 90 | 8 | >99 | 76.9 |
| 11 | 20 | 90 | 12 | >99 | 72.3 |
| 12 | 20 | 70 | 2 | >99 | 87.6 |
 | ||
|---|---|---|
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, M.; Ma, L. Facile Synthesis of COF-Supported Reduced Pd-Based Catalyst for One-Pot Reductive Amination of Aldehydes. Catalysts 2021, 11, 287. https://doi.org/10.3390/catal11020287
Liu J, Zhang M, Ma L. Facile Synthesis of COF-Supported Reduced Pd-Based Catalyst for One-Pot Reductive Amination of Aldehydes. Catalysts. 2021; 11(2):287. https://doi.org/10.3390/catal11020287
Chicago/Turabian StyleLiu, Jianguo, Mingyue Zhang, and Longlong Ma. 2021. "Facile Synthesis of COF-Supported Reduced Pd-Based Catalyst for One-Pot Reductive Amination of Aldehydes" Catalysts 11, no. 2: 287. https://doi.org/10.3390/catal11020287
APA StyleLiu, J., Zhang, M., & Ma, L. (2021). Facile Synthesis of COF-Supported Reduced Pd-Based Catalyst for One-Pot Reductive Amination of Aldehydes. Catalysts, 11(2), 287. https://doi.org/10.3390/catal11020287







