Electrocatalysts for Using Renewably-Sourced, Organic Electrolytes for Redox Flow Batteries
Abstract
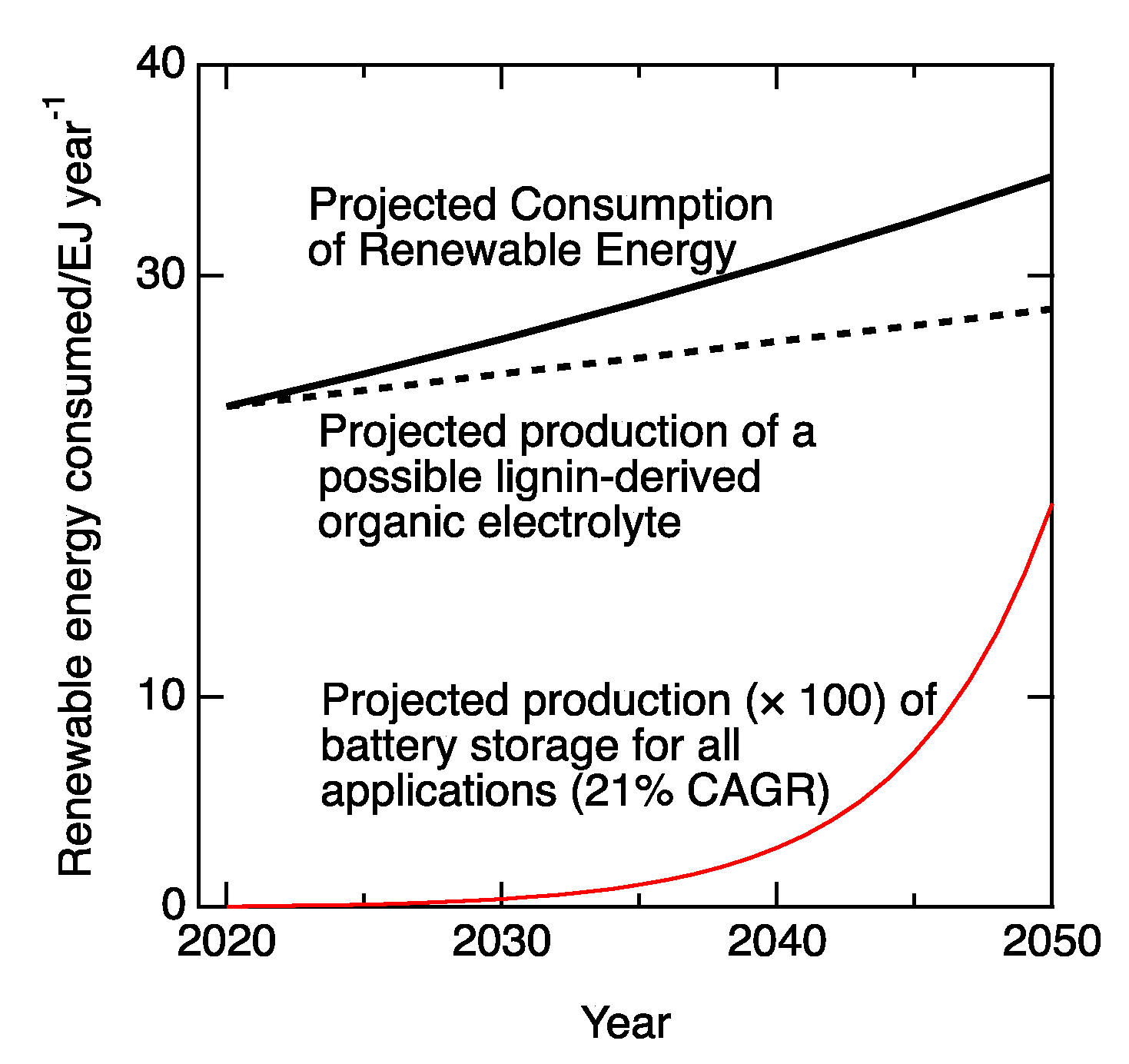
:1. Introduction
2. Flow Batteries
2.1. Renewable Organic Redox Shuttles
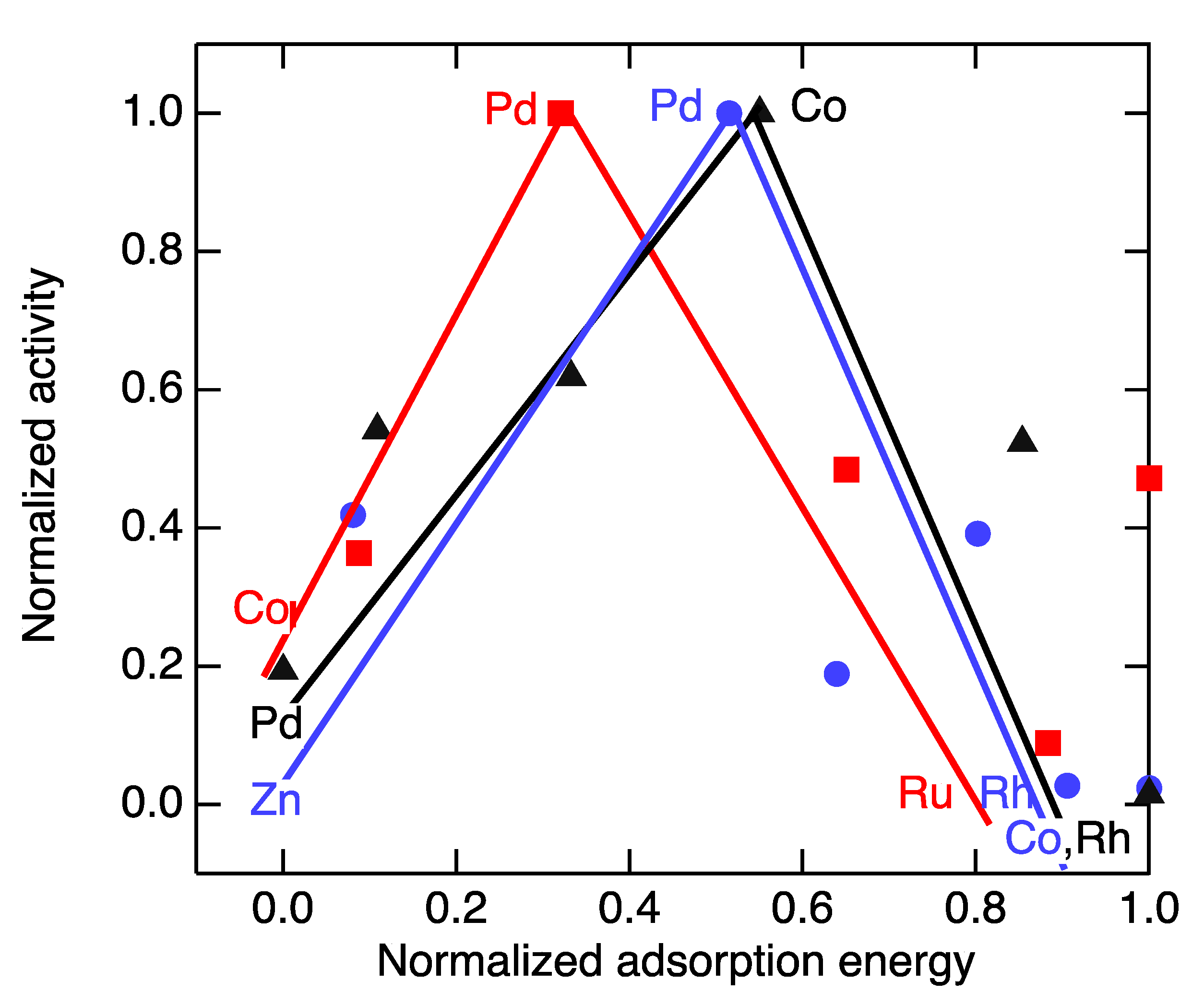
2.2. Choosing/Designing Electrocatalysts for Organic Redox Flow Batteries
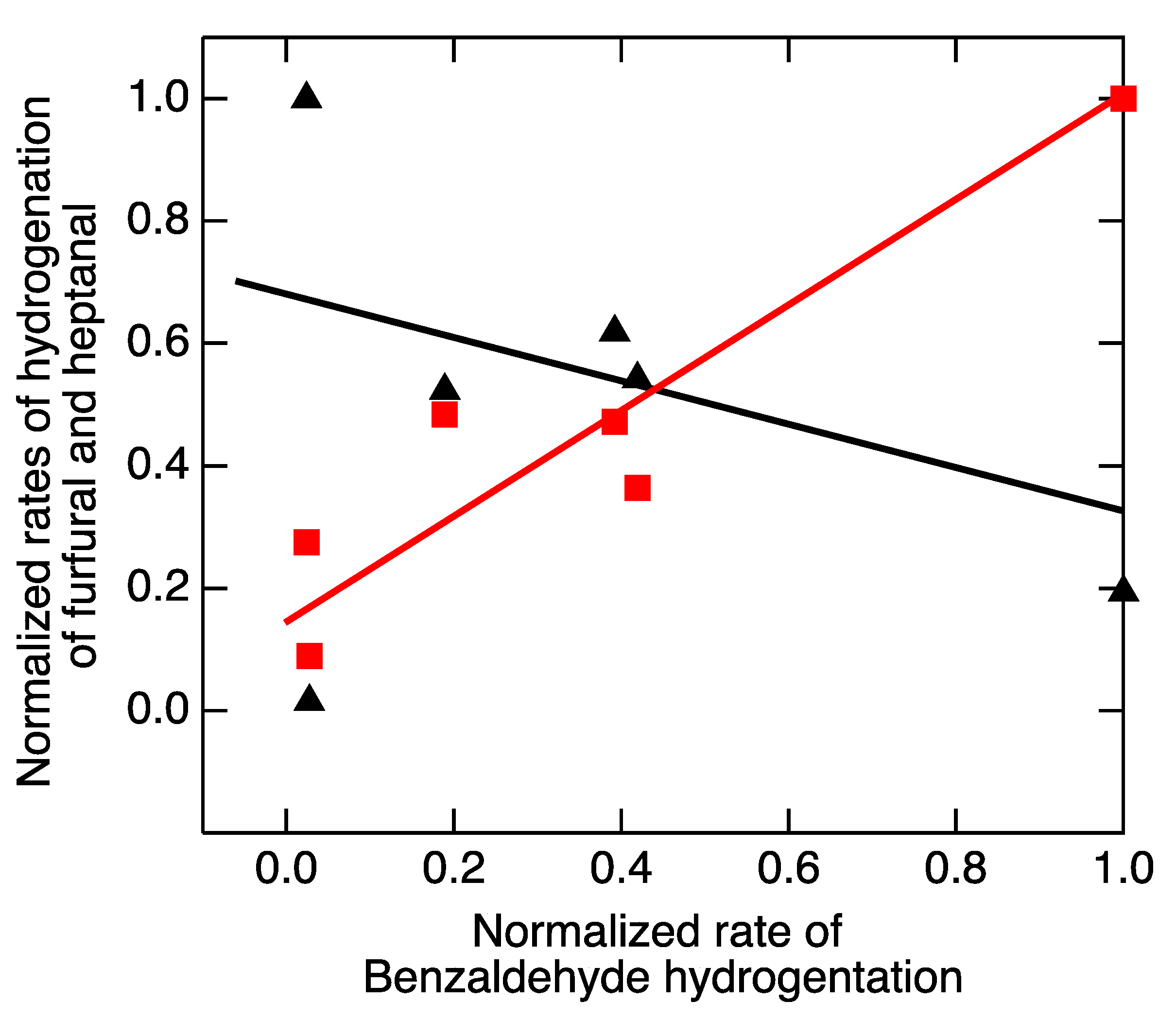
2.3. Electrochemical Hydrogenation of Benzaldehyde as a Fiducial Reaction
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- International Energy Agency. IEA Renewables 2020. 2020. Available online: https://www.iea.org/reports/renewables-2020 (accessed on 10 November 2020).
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Renewable Energy Statistics 2020. Available online: https://irena.org/publications/2020/Mar/Renewable-Capacity-Statistics-2020 (accessed on 28 February 2021).
- US Energy Information Agency. Battery Storage in the United States: An Update on Market Trends. 2020. Available online: https://www.eia.gov/analysis/studies/electricity/batterystorage/pdf/battery_storage.pdf (accessed on 29 November 2020).
- American Wind Energy Association. US Wind Industry Quarterly Market Report, Fourth Quarter 2019. 2019. Available online: https://cleanpower.org/resources/american-clean-power-market-report-q4-2020/ (accessed on 20 April 2020).
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. Engl. 2017, 56, 686–711. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; et al. Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; De León, C.P.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Weber, R.S.; Holladay, J.E.; Jenks, C.; Panisko, E.A.; Snowden-Swan, L.J.; Ramirez-Corredores, M.; Baynes, B.; Angenent, L.T.; Boysen, D. Modularized production of fuels and other value-added products from distributed, wasted, or stranded feedstocks. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, e308. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Pulp and Paper Capacity Survey 2017–2022. 2018. Available online: http://www.fao.org/3/CA1791T/ca1791t.pdf (accessed on 19 May 2020).
- Tabor, D.P.; Gómez-Bombarelli, R.; Tong, L.; Gordon, R.G.; Aziz, M.J.; Aspuru-Guzik, A. Mapping the frontiers of quinone stability in aqueous media: Implications for organic aqueous redox flow batteries. J. Mater. Chem. A 2019, 7, 12833–12841. [Google Scholar] [CrossRef]
- Chen, R. Redox flow batteries for energy storage: Recent advances in using organic active materials. Curr. Opin. Electrochem. 2020, 21, 40–45. [Google Scholar] [CrossRef]
- Xing, X.; Huo, Y.; Wang, X.; Zhao, Y.; Li, Y. A benzophenone-based anolyte for high energy density all-organic redox flow battery. Int. J. Hydrog. Energy 2017, 42, 17488–17494. [Google Scholar] [CrossRef]
- Huo, Y.; Xing, X.; Zhang, C.; Wang, X.; Li, Y. An all organic redox flow battery with high cell voltage. RSC Adv. 2019, 9, 13128–13132. [Google Scholar] [CrossRef] [Green Version]
- Sjöström, E. Wood Chemistry, Fundamentals and Applications, 2nd ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- International Renewable Energy Agency. Renewable Capacity Statisitics 2020; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- US Energy Information Agency. International Energy Outlook 2019. 2019. Available online: https://www.eia.gov/outlooks/aeo/data/browser/#/?id=24-IEO2019&cases=Reference&sourcekey=0, (accessed on 10 April 2020).
- Geigle, P.; Hartwig, J.; Larionov, E.; Baal, E. Redox-Active Compounds and Uses Thereof; CMBlu Projekt AG: Alzenau, Germany, 2006. [Google Scholar]
- Lee, J.; Park, M.J. Tattooing Dye as a Green Electrode Material for Lithium Batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Cantu, D.C.; Padmaperuma, A.B.; Nguyen, M.-T.; Akhade, S.A.; Yoon, Y.; Wang, Y.-G.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R.; Lilga, M.A. A Combined Experimental and Theoretical Study on the Activity and Selectivity of the Electrocatalytic Hydro-genation of Aldehydes. ACS Catal. 2018, 8, 7645–7658. [Google Scholar] [CrossRef]
- Baskin, A.; Prendergast, D. Exploring chemical speciation at electrified interfaces using detailed continuum models. J. Chem. Phys. 2019, 150, 041725. [Google Scholar] [CrossRef]
- Tong, Y.J. In situ electrochemical nuclear magnetic resonance spectroscopy for electrocatalysis: Challenges and prospects. Curr. Opin. Electrochem. 2017, 4, 60–68. [Google Scholar] [CrossRef]
- Singh, N.; Song, Y.; Gutiérrez, O.Y.; Camaioni, D.M.; Campbell, C.T.; Lercher, J.A. Electrocatalytic Hydrogenation of Phenol over Platinum and Rhodium: Unexpected Temperature Effects Resolved. ACS Catal. 2016, 6, 7466–7470. [Google Scholar] [CrossRef]
- Chambrion, P.; Roger, L.; Lessard, J.; Béraud, V.; Mailhot, J.; Thomalla, M. The influence of surfactants on the electrocatalytic hydrogenation of organic compounds in micellar, emulsified, and hydroorganic solutions at Raney nickel electrodes. Can. J. Chem. 1995, 73, 804–815. [Google Scholar] [CrossRef] [Green Version]
- Ilikti, H.; Rekik, N.; Thomalla, M. Electrocatalytic hydrogenation of alkyl-substituted phenols in aqueous solutions at a Raney nickel electrode in the presence of a non-micelle-forming cationic surfactant. J. Appl. Electrochem. 2004, 34, 127–136. [Google Scholar] [CrossRef]
- Jaeger, D.A.; Bolikal, D.; Nath, B. Surfactant Effects on the Electrochemical Reduction of an α,β-Unsaturated Ketone. In Surfactants in Solution; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 149–152. [Google Scholar]
- Moorthy, P.N.; Kishore, K. Electrochemical Studies in Surfactant Solutions. In Surfactants in Solution; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 135–147. [Google Scholar]
- Andrews, E.M.; Egbert, J.D.; Sanyal, U.; Holladay, J.D.; Weber, R.S. Anode-Boosted Electrolysis in Electrochemical Upgrading of Bio-oils and in the Production of H2. Energy Fuels 2020, 34, 1162–1165. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Akhade, S.A.; Cantu, D.C.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R. Electro-reduction of organics on metal cathodes: A multiscale-modeling study of benzaldehyde on Au (111). Catal. Today 2020, 350, 39–46. [Google Scholar] [CrossRef]
- Stonehart, P.; Ross, P.N. The Commonality of Surface Processes in Electrocatalysis and Gas-Phase Heterogeneous Catalysis. Catal. Rev. 1975, 12, 1–35. [Google Scholar] [CrossRef]
- Lund, H. A Century of Organic Electrochemistry. J. Electrochem. Soc. 2002, 149, S21–S33. [Google Scholar] [CrossRef]
- Baizer, M.M. Recent developments in organic synthesis by electrolysis. Tetrahedron 1984, 40, 935–969. [Google Scholar] [CrossRef]
- Weinberg, N.L.; Weinberg, H.R. Electrochemical oxidation of organic compounds. Chem. Rev. 1968, 68, 449–523. [Google Scholar] [CrossRef]
- Lob, W. Electrochemistry of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1906. [Google Scholar]
- Chum, H.L.; Baizer, M.M. The Electrochemistry of Biomass and Derived Materials; American Chemical Society: Washington, DC, USA, 1985. [Google Scholar]
- Fry, A.J. Synthetic Organic Electrochemistry; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Frumkin, A.N.; Érshler, A.B. Progress in Electrochemistry of Organic Compounds; Plenum Press: London, UK, 1971. [Google Scholar]
- Meites, L.; Zuman, P.; Rupp, E.B.; Fenner, T.L.; Spritzer, L. Handbook Series in Organic Electrochemistry; CRC Press: Cleveland, OH, USA, 1976. [Google Scholar]
- Akhade, S.A.; Singh, N.; Gutierrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, U.; Lopez-Ruiz, J.A.; Padmaperuma, A.B.; Holladay, J.; Gutiérrez, O.Y. Electrocatalytic Hydrogenation of Oxygenated Compounds in Aqueous Phase. Org. Process. Res. Dev. 2018, 22, 1590–1598. [Google Scholar] [CrossRef]
- Sanyal, U.; Song, Y.; Singh, N.; Fulton, J.L.; Herranz, J.; Jentys, A.; Gutiérrez, O.Y.; Lercher, J.A. Structure Sensitivity in Hydrogenation Reactions on Pt/C in Aqueous-phase. ChemCatChem 2018, 11, 575–582. [Google Scholar] [CrossRef]
- Song, Y.; Chia, S.H.; Sanyal, U.; Gutiérrez, O.Y.; Lercher, J.A. Integrated catalytic and electrocatalytic conversion of substituted phenols and diaryl ethers. J. Catal. 2016, 344, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Amini, K.; Gostick, J.; Pritzker, M.D. Metal and Metal Oxide Electrocatalysts for Redox Flow Batteries. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Schlemmer, W.; Nothdurft, P.; Petzold, A.; Riess, G.; Fruhwirt, P.; Schmallegger, M.; Gescheidt-Demner, G.; Fischer, R.; Freunberger, S.A.; Kern, W.; et al. 2-Methoxyhydroquinone from Vanillin for Aqueous Redox-Flow Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 22943–22946. [Google Scholar] [CrossRef]
- Wang, H.; Sayed, S.Y.; Luber, E.J.; Olsen, B.C.; Shirurkar, S.M.; Venkatakrishnan, S.; Tefashe, U.M.; Farquhar, A.K.; Smotkin, E.S.; McCreery, R.L.; et al. Redox Flow Batteries: How to Determine Electrochemical Kinetic Parameters. ACS Nano 2020, 14, 2575–2584. [Google Scholar] [CrossRef]
- Lister, T.E.; Diaz, L.A.; Lilga, M.A.; Padmaperuma, P.A.; Lin, Y.J.; Palakkal, V.M.; Arges, C.G. Low-Temperature Electrochemical Upgrading of Bio-oils Using Polymer Electrolyte Membranes. Energy Fuels 2018, 32, 5944–5950. [Google Scholar] [CrossRef]
- Carroll, K.J.; Burger, T.; Langenegger, L.; Chavez, S.; Hunt, S.T.; Román-Leshkov, Y.; Brushett, F.R. Electrocatalytic Hydrogenation of Oxygenates using Earth-Abundant Transition-Metal Nanoparticles under Mild Conditions. ChemSusChem 2016, 9, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-S.; Huang, S.-L.; Chen, M.-L.; Tsai, T.-J.; Lin, Y.-S. Improving Electrochemical Activity in a Semi-V-I Redox Flow Battery by Using a C–TiO2–Pd Composite Electrode. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y. Bismuth Trioxide Modified Carbon Nanotubes as Negative Electrode Catalysts for all Vanadium Redox Flow Batteries. Int. J. Electrochem. Sci. 2020, 7733–7743. [Google Scholar] [CrossRef]
- Cañizares, P.; Saez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical Oxidation of Polyhydroxybenzenes on Boron-Doped Diamond Anodes. Ind. Eng. Chem. Res. 2004, 43, 6629–6637. [Google Scholar] [CrossRef]
- Lips, S.; Waldvogel, S.R. Use of Boron-Doped Diamond Electrodes in Electro-Organic Synthesis. ChemElectroChem 2019, 6, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Peralta-Hernández, J.M.; Méndez-Tovar, M.; Guerra-Sánchez, R.; Martínez-Huitle, C.A.; Nava, J.L. A Brief Review on Envi-ronmental Application of Boron Doped Diamond Electrodes as a New Way for Electrochemical Incineration of Synthetic Dyes. Int. J. Electrochem. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Pleskov, Y.; Evstefeeva, Y.; Krotova, M.; Varnin, V.; Teremetskaya, I. Synthetic semiconductor diamond electrodes: Electrochemical behaviour of homoepitaxial boron-doped films orientated as (111), (110), and (100) faces. J. Electroanal. Chem. 2006, 595, 168–174. [Google Scholar] [CrossRef]
- Salazar-Banda, G.R.; Eguiluz, K.I.; Avaca, L.A. Boron-doped diamond powder as catalyst support for fuel cell applications. Electrochem. Commun. 2007, 9, 59–64. [Google Scholar] [CrossRef]
- Trasatti, S. Work Function, Electronegativity and Electrochemical Behaviour of Metals III. Electrolytic Hydrogen Evolution in Acid Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Lopez-Ruiz, J.A.; Andrews, E.M.; Akhade, S.A.; Lee, M.-S.; Koh, K.; Sanyal, U.; Yuk, S.F.; Karkamkar, A.J.; Derewinski, M.A.; Holladay, J.; et al. Understanding the Role of Metal and Molecular Structure on the Electrocatalytic Hydrogenation of Oxygenated Organic Compounds. ACS Catal. 2019, 9, 9964–9972. [Google Scholar] [CrossRef]
- Barteau, M.A. Linear free energy relationships for C1-oxygenate decomposition on transition metal surfaces. Catal. Lett. 1991, 8, 175–183. [Google Scholar] [CrossRef]
- Quaino, P.; Juarez, F.; Santos, E.; Schmickler, W. Volcano plots in hydrogen electrocatalysis—Uses and abuses. Beilstein J. Nanotechnol. 2014, 5, 846–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvillo, M.; Córdova, I.; Del Valle, M.; Oropeza, M.T. Electrochemical Oxidation of Vanillin and Capsaicin in Hartmann Solution. ECS Trans. 2010, 29, 339–347. [Google Scholar] [CrossRef]
- Parpot, P.; Bettencourt, A.; Carvalho, A.; Belgsir, E. Biomass conversion: Attempted electrooxidation of lignin for vanillin production. J. Appl. Electrochem. 2000, 30, 727–731. [Google Scholar] [CrossRef]
- Schmitt, D.; Regenbrecht, C.; Hartmer, M.; Stecker, F.; Waldvogel, S.R. Highly selective generation of vanillin by anodic degra-dation of lignin: A combined approach of electrochemistry and product isolation by adsorption. Beilstein J. Org. Chem. 2015, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Stecker, F.; Malkowsky, I.M.; Fischer, A.; Waldvogel, S.R.; Regenbrecht, C. Method for Producing Vanillin by Electrochemical Oxidation of Aqueous Lignin Solutions or Suspensions; Rheinisch Friedrich Wilhems Universtät Bonn: Bonn, Germany, 2014. [Google Scholar]
- Moniri, S.; van Cleve, T.; Linic, S. Pitfalls and best practices in measurements of the electrochemical surface area of platinum-based nanostructured electro-catalysts. J. Catal. 2017, 345, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Egbert, J.D.; Lopez-Ruiz, J.A.; Prodinger, S.; Holladay, J.D.; Mans, D.M.; Wade, C.E.; Weber, R.S. Counting surface redox sites in carbon-supported electrocatalysts by cathodic stripping of O deposited from N2O. J. Catal. 2018, 365, 405–410. [Google Scholar] [CrossRef]
- Weber, R.S. Normalizing Hetereogeneous Electrocatalytic and Photocatalytic Rates. ACS Omega 2019, 4, 4109–4112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, R.S. Electrocatalysts for Using Renewably-Sourced, Organic Electrolytes for Redox Flow Batteries. Catalysts 2021, 11, 315. https://doi.org/10.3390/catal11030315
Weber RS. Electrocatalysts for Using Renewably-Sourced, Organic Electrolytes for Redox Flow Batteries. Catalysts. 2021; 11(3):315. https://doi.org/10.3390/catal11030315
Chicago/Turabian StyleWeber, Robert S. 2021. "Electrocatalysts for Using Renewably-Sourced, Organic Electrolytes for Redox Flow Batteries" Catalysts 11, no. 3: 315. https://doi.org/10.3390/catal11030315









